Abstract
Lewy bodies and Lewy neurites are the defining neuropathological characteristics of Parkinson’s disease and dementia with Lewy bodies. They are made of abnormal filamentous assemblies of unknown composition. We show here that Lewy bodies and Lewy neurites from Parkinson’s disease and dementia with Lewy bodies are stained strongly by antibodies directed against amino-terminal and carboxyl-terminal sequences of α-synuclein, showing the presence of full-length or close to full-length α-synuclein. The number of α-synuclein-stained structures exceeded that immunoreactive for ubiquitin, which is currently the most sensitive marker of Lewy bodies and Lewy neurites. Staining for α-synuclein thus will replace staining for ubiquitin as the preferred method for detecting Lewy bodies and Lewy neurites. We have isolated Lewy body filaments by a method used for the extraction of paired helical filaments from Alzheimer’s disease brain. By immunoelectron microscopy, extracted filaments were labeled strongly by anti-α-synuclein antibodies. The morphologies of the 5- to 10-nm filaments and their staining characteristics suggest that extended α-synuclein molecules run parallel to the filament axis and that the filaments are polar structures. These findings indicate that α-synuclein forms the major filamentous component of Lewy bodies and Lewy neurites.
Keywords: ubiquitin, sarkosyl-insoluble filaments, immunoelectron microscopy
Parkinson’s disease (PD) is the second most common neurodegenerative disorder, after Alzheimer’s disease. Clinically, it is a movement disorder that is characterized by tremor, rigidity, and bradykinesia. Neuropathologically, PD is defined by nerve cell loss in the substantia nigra and the presence there of Lewy bodies and Lewy neurites (1, 2). Lewy bodies and Lewy neurites also are found in other brain regions, such as the dorsal motor nucleus of the vagus, the nucleus basalis of Meynert, and the locus coeruleus (reviewed in ref. 3). Brainstem-type Lewy bodies appear as intracytoplasmic inclusions, 5–25 μm in diameter, with a dense eosinophilic core and a clearer surrounding halo. Ultrastructurally, they are composed of a dense core of filamentous and granular material that is surrounded by radially oriented filaments (4, 5).
Abundant Lewy bodies and Lewy neurites in cerebral cortex are the defining neuropathological characteristics of dementia with Lewy bodies (DLB), a common late-life dementia that is clinically similar to Alzheimer’s disease (6–8). Cortical Lewy bodies are spherical intracytoplasmic inclusions that lack a distinctive core and halo, but that are made of filaments with morphologies similar to those of Lewy bodies from brainstem areas (6, 7).
Lewy neurites constitute an important part of the pathology of PD and DLB. They correspond to abnormal neurites that contain filaments similar to those found in Lewy bodies (9). A number of proteins have been identified immunologically by light microscopy in Lewy bodies and Lewy neurites from PD and DLB, which include neurofilaments and ubiquitin (10–15). Although these have been useful as markers for diagnostic purposes, the biochemical composition of Lewy body filaments has remained unknown.
Recently, the discovery of point mutations in the α-synuclein gene as a rare cause of familial PD (16, 17) has led us to the finding that α-synuclein is a component of Lewy bodies and Lewy neurites from idiopathic PD and DLB (18). We show here that antibodies raised against amino-terminal and carboxyl-terminal sequences of the 140-aa α-synuclein protein strongly immunostain Lewy bodies and Lewy neurites and that the number of stained structures exceeds that recognized by anti-ubiquitin antibodies. Antibodies directed against β-synuclein (19) and the BCSG1 protein (γ-synuclein) (20) failed to stain Lewy bodies and Lewy neurites. We also show that 5- to 10-nm filaments extracted from cingulate cortex of patients with DLB are labeled by anti-α-synuclein antibodies, indicating that α-synuclein is the major component of the filaments of Lewy bodies and Lewy neurites.
MATERIALS AND METHODS
Materials.
Substantia nigra, hippocampal formation, and cingulate cortex from eight patients with autopsy-confirmed DLB, some with concomitant Alzheimer’s disease pathology, and substantia nigra from six patients with autopsy-confirmed PD were used. In some experiments the same brain regions from four age-matched controls were also used. The anti-α-synuclein antibody PER2 and the anti-β-synuclein antibody PER3 have been described previously (19). Anti-α-synuclein serum PER1 was raised in a rabbit by using a synthetic peptide corresponding to amino acid residues 11–34 of human α-synuclein as the immunogen and affinity-purified, as described (19). Antiserum PER4 was raised in a rabbit by using full-length recombinant human α-synuclein as the immunogen. Antiserum PER5 was raised in a rabbit by using recombinant human BCSG1 protein (γ-synuclein) (20) as the immunogen. Monoclonal anti-ubiquitin antibody 1510 (21) was purchased from Chemicon. Recombinant human α-synuclein and β-synuclein were expressed and purified as described (19). BCSG1 (γ-synuclein) DNA was amplified from adult human brain cDNA by using PCR and subcloned into the expression plasmid pRK172, followed by expression in Escherichia coli BL21(DE3). The DNA sequence differed in two nucleotides from the published sequence (20), resulting in two amino acid changes (glutamic acid residues at positions 12 and 68 instead of lysines). The same sequence was obtained in three separate PCRs. Recombinant γ-synuclein was purified in the same way as α-synuclein (19).
Immunohistochemistry and Immunoblotting.
Brain tissues from patients with DLB or PD were immersion-fixed in 4% paraformaldehyde. Sections (40 μm) were cut on a freezing microtome and processed free-floating, as described (22). Some tissues were paraffin-embedded and 7-μm sections were cut, followed by immunostaining. In some experiments, sections were double-stained for α-synuclein and ubiquitin by using a published procedure (22). The anti-α-synuclein antibody was used as the first primary antibody and the anti-ubiquitin antibody was used as the second primary antibody. Dilutions were as follows: PER1, PER2, and PER3 were at 1:200; PER4 and PER5 were at 1:500; and the anti-ubiquitin antibody 1510 was at 1:250. Sections were counterstained weakly with hematoxylin. Immunoblot analysis was carried out with affinity-purified antibody PER1 (diluted 1:200), rabbit antisera PER4 (diluted 1:5,000), and PER5 (diluted 1:5,000), and developed by using a Vectastain kit (Vector Laboratories) (19). SDS-polyacrylamide gels (15%) were used to monitor protein purification and for immunoblot analyses.
Extraction of Dispersed α-Synuclein Filaments.
Filaments were extracted from cingulate cortex of patients with DLB by using a procedure developed for the extraction of dispersed paired helical and straight filaments from Alzheimer’s disease brain (23, 24). One-gram tissue was homogenized in 10 mM Tris⋅HCl, pH 7.4, containing 800 mM NaCl, 1 mM EGTA, and 10% sucrose. After centrifugation at 14,000 rpm for 20 min the supernatant was incubated with sarcosyl at a final concentration of 1% for 1 h at room temperature. The solution was spun at 40,000 rpm for 1 h, and the resulting pellet was resuspended in 50 mM Tris⋅HCl, pH 7.4, at 100 μl/g starting material.
Electron Microscopy.
Aliquots of the dispersed filament preparations were placed on carbon-coated 400-mesh grids and stained with 1% lithium phosphotungstate, and micrographs were recorded at a nominal magnification of ×40,000 on a Philips model EM208S microscope as described previously (25). Procedures for immunoelectron microscopy were as described (25). The primary antibodies were used at 1:50 (PER1) and 1:100 (PER4), and after reaction with the appropriate secondary gold-conjugated antibody (Sigma), the grids were stained with 1% lithium phosphotungstate.
RESULTS
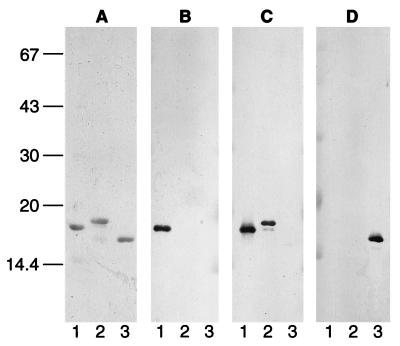
Affinity-purified anti-α-synuclein antibodies PER1 and PER2 were used to stain tissue sections from patients with PD and DLB. PER2 was raised against a synthetic peptide corresponding to amino acids 116–131 of human α-synuclein (19). It recognizes α-synuclein, but not β-synuclein or γ-synuclein. Similarly, affinity-purified antiserum PER1, which was raised against a synthetic peptide corresponding to residues 11–34 of α-synuclein, recognizes α-synuclein, but not β-synuclein or γ-synuclein (Fig. 1). This contrasts with affinity-purified antiserum PER3, which recognizes β-synuclein specifically (19) and antiserum PER5, which recognizes only γ-synuclein. Antiserum PER4 recognizes both α-synuclein and β-synuclein, but not γ-synuclein. It recognizes the carboxyl-terminal region of α-synuclein (unpublished data) and reacts more strongly with α-synuclein than with β-synuclein (Fig. 1). Identical results were obtained with the synuclein antibodies on an extract of cerebral cortex from control brain (not shown).
Figure 1.
Specificity of anti-synuclein sera PER1, PER4, and PER5 for recombinant synucleins. Gels were stained with Coomassie brilliant blue (A) or immunoblotted with PER1 (B), PER4 (C), and PER5 (D). Lanes: 1, recombinant α-synuclein; 2, recombinant β-synuclein; 3, recombinant γ-synuclein. Molecular mass is shown to the left in Mr × 10−3.
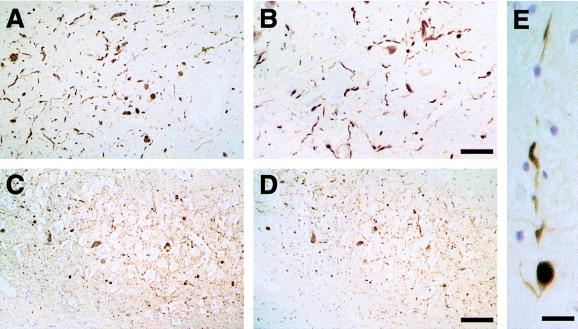
Staining of substantia nigra sections from patients with DLB revealed staining for PER1 and PER2 (Fig. 2 A and B). Large numbers of Lewy bodies and Lewy neurites were strongly immunoreactive, and similar numbers of structures were stained by each antibody. Most immunopositive Lewy bodies were located in neuronal perikarya, but the rarer intraneuritic Lewy bodies also were strongly stained (Fig. 2E), as were extracellular Lewy bodies.
Figure 2.
Tissue from patients with dementia with Lewy bodies immunostained for α-synuclein (antibodies PER1 and PER2). (A and B) α-Synuclein-positive Lewy bodies and Lewy neurites in substantia nigra stained with PER1 (A) or PER2 (B). [Bar = 100 μm (in B for A and B).] (C and D) α-Synuclein-positive Lewy neurites in hippocampus stained with antibodies PER1 (C) or PER2 (D). [Bar = 80 μm (in D for C and D).] (E) α-Synuclein-positive intraneuritic Lewy body in a Lewy neurite in substantia nigra stained with PER2. (Bar = 40 μm.)
In hippocampus from patients with DLB, strong staining of Lewy neurites was observed. Staining of adjacent tissue sections with PER1 and PER2 showed that the same structures were stained by each antibody (Fig. 2 C and D). No specific staining of Lewy bodies or Lewy neurites was observed with antibodies PER3 and PER5, showing that β-synuclein and γ-synuclein are not present. Lewy bodies and Lewy neurites in substantia nigra from PD patients also were strongly immunoreactive for PER1 and PER2 (Fig. 3E and F). Antibody PER4 gave a staining pattern identical to that obtained with PER1 and PER2 (data not shown).
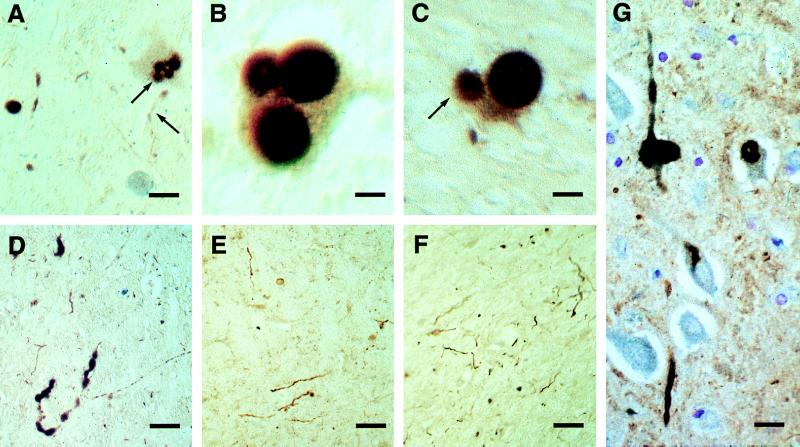
Figure 3.
Substantia nigra from patients with Parkinson’s disease (A–F) and cingulate cortex from a patient with dementia with Lewy bodies (G) immunostained for α-synuclein (antibodies PER1 and PER2, in brown) and ubiquitin (in blue). Where only α-synuclein is stained, the color appears as pale reddish-brown, but where ubiquitin also is stained, the superposition of color gives a dark black and brown appearance. The blue staining is not seen on its own, because all the ubiquitin-immunoreactive structures are also α-synuclein-positive. (A) Nerve cell with four Lewy bodies, three of which are double-stained for α-synuclein (antibody PER2) and ubiquitin, whereas one is immunoreactive only for α-synuclein (arrow). A Lewy neurite is stained only for α-synuclein (arrow). (Bar = 30 μm.) (B) Nerve cell with three Lewy bodies that are double-stained for α-synuclein (antibody PER2) and ubiquitin. The halo of each Lewy body is strongly immunoreactive for ubiquitin, whereas both the core and the halo of each Lewy body are immunoreactive for α-synuclein. (Bar = 10 μm.) (C) Nerve cell with two Lewy bodies, one of which is double-stained for α-synuclein (antibody PER1) and ubiquitin, whereas one is immunoreactive only for α-synuclein (arrow). (Bar = 13 μm.) (D) Lewy neurite double-stained for α-synuclein and ubiquitin. (Bar = 90 μm.) (E and F) Lewy neurites single-stained for α-synuclein by using antibodies PER1 (E) and PER2 (F). (Bar = 100 μm.) (G) Intraneuronal and intraneuritic Lewy bodies and Lewy neurites double-stained for α-synuclein (antibody PER1) and ubiquitin. (Bar = 18 μm.)
In some experiments double-labeling immunohistochemistry was used to study colocalization of α-synuclein and ubiquitin (Fig. 3). Most Lewy bodies and Lewy neurites in substantia nigra (Fig. 3 A–C) and cingulate cortex (Fig. 3 D and G) from DLB patients were immunoreactive for both α-synuclein and ubiquitin. However, the α-synuclein staining was always more extensive. Thus, some Lewy bodies and Lewy neurites were stained for α-synuclein, but not for ubiquitin (Fig. 3 A and C). In brainstem-type Lewy bodies immunoreactive for both α-synuclein and ubiquitin, the ubiquitin antibody stained mostly the halo of the Lewy body, whereas α-synuclein antibodies stained both the halo and the core (Fig. 3 A–C). No structures were seen to be ubiquitin-positive and α-synuclein-negative.
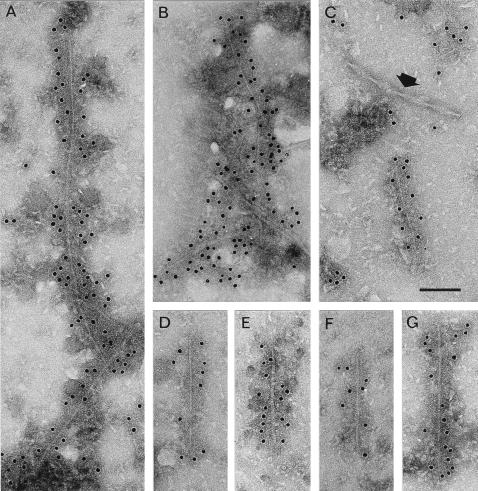
To identify assembled structures containing α-synuclein, cingulate cortex from patients with DLB was extracted by a method widely used for the isolation of dispersed paired helical and straight filaments from Alzheimer’s disease brain (23, 24). It enriches for filaments that are insoluble in sarcosyl, whereas the bulk of SDS-resistant filaments is removed in the first centrifugation step (23). The resuspended pellets from sarcosyl treatment were immunolabeled with anti-α-synuclein antibodies. Slender filamentous structures were clearly labeled by the PER4 antiserum (Fig. 4). Small clumps of filaments associated with amorphous material were sometimes visualized (Fig. 4 A and B), but mostly the labeled structures corresponded to single, isolated fragments (Fig. 4 C–G) that were 50–700 nm long. Paired helical filaments present in some preparations clearly were not labeled by the PER4 antiserum (Fig. 4C). The labeled structures showed various morphologies, including a uniform, approximately 5-nm-wide filament (Fig. 4D), a somewhat less regular, 10-nm-wide filament with a dark stain-penetrated center line (Fig. 4E), a filament of twisted appearance with a width varying between 5 and 10 nm with a crossover spacing of about 90 nm (Fig. 4F), and a 10-nm filament with slender 5-nm extensions at one or both ends (Fig. 4 C and G). The majority of filaments had a 10-nm width. Similar results were obtained when PER2 was used instead of PER4.
Figure 4.
Filaments from cingulate cortex of patients with dementia with Lewy bodies labeled with anti-α-synuclein antibody PER4. The gold particles conjugated to the second antibody appear as black dots. (A and B) Small clumps of labeled α-synuclein filaments. (C) A labeled α-synuclein filament and an unlabeled paired helical filament (arrow). The labeled filaments have various morphologies, including 5-nm filament (D); 10-nm filament with dark stain-penetrated center line (E); twisted filament showing alternating width (F); 10-nm filament with slender 5-nm extensions at ends (G, also C). [Bar = 100 nm (C).]
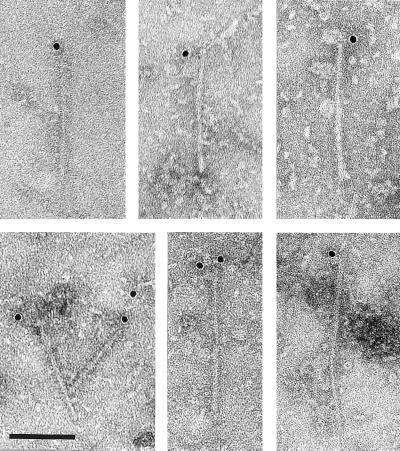
A quite different pattern of labeling was shown by the PER1 antibody (Fig. 5). In this case one or occasionally two gold particles were observed at one but never at both ends of filaments. α-Synuclein filaments were found in all cases of DLB, but not in cingulate cortex from age-matched control cases extracted in the same manner. No filaments were labeled by β-synuclein or γ-synuclein antibodies.
Figure 5.
Filaments from cingulate cortex of patients with dementia with Lewy bodies labeled with anti-α-synuclein antibody PER1. The gold particles conjugated to the second antibody appear as black dots. In this case one or sometimes two gold particles are associated with one but never two ends of the filament. (Bar = 100 nm.)
DISCUSSION
The present findings show that Lewy bodies and Lewy neurites from PD and DLB are strongly immunoreactive for α-synuclein, in agreement with our previous report and two subsequent studies (18, 26, 27). We show here that antibodies recognizing amino-terminal and carboxyl-terminal α-synuclein regions give identical staining patterns, indicating that full-length or close to full-length α-synuclein is present in Lewy bodies and Lewy neurites. α-Synuclein antibodies stain Lewy bodies in their entirety, with strong staining of core and corona of brainstem-type Lewy bodies.
Currently, staining for ubiquitin is the most sensitive means of detecting Lewy bodies and Lewy neurites. Double-staining immunohistochemistry for α-synuclein and ubiquitin showed that staining for α-synuclein is more extensive than staining for ubiquitin, indicating that accumulation of α-synuclein precedes ubiquitination. In particular, the number of Lewy neurites stained for α-synuclein was much larger than that immunoreactive for ubiquitin. It follows that staining for α-synuclein will probably replace staining for ubiquitin as the preferred means of identifying Lewy bodies and Lewy neurites. Antibodies directed against β-synuclein and γ-synuclein did not stain Lewy bodies or Lewy neurites. Thus, of the known brain synucleins, only α-synuclein is found in the Lewy body pathology. These findings suggest but do not prove that α-synuclein is a major component of the abnormal filaments that make up Lewy bodies and Lewy neurites.
This was investigated directly by immunoelectron microscopy of sarcosyl-insoluble filaments extracted from cingulate cortex of patients with DLB. Filaments had a diameter of 5–10 nm, in keeping with previously reported dimensions of filaments in Lewy bodies and Lewy neurites, based on heavy-metal-stained tissue sections and isolated Lewy bodies (3–5, 7, 9, 11, 12, 28–32). Antiserum PER4, which recognizes the carboxyl-terminal region of α-synuclein, labeled filaments along their entire lengths, indicating that they contain α-synuclein as a major component. The labeled structures had various morphologies, including a 5-nm straight filament and 10-nm filaments both straight and twisted, with the 10-nm filaments being more numerous. These appearances would be consistent with a model in which the α-synuclein molecules assembled to form a 5-nm protofilament, two of which could associate to produce a variably twisted filament. Some 10-nm filaments were unraveled, revealing slender extensions at one or both ends. These various morphologies suggest that α-synuclein molecules, which are believed to be extended and relatively unstructured (33), may run parallel to the filament axis. This differs from the packing of tau protein in filaments from Alzheimer’s disease and other tauopathies, where individual tau molecules are believed to run mainly perpendicular to the filament axis (25, 34). Antibody PER1, which recognizes residues 11–34 of α-synuclein, only ever labeled one filament end. This suggests first that the PER1 epitope is buried in the body of the filament and exposed only at the end, and second, that the filaments are polar structures. The situation is akin to that in microtubules, where an anti-α-tubulin antibody labels only the minus end of the tubule (35). The polar nature of the α-synuclein filaments would be consistent with the characteristic spherically radiating focal architecture of Lewy bodies, particularly those in the brainstem. Although PER1 labeled only one end of each α-synuclein filament, it stained the Lewy body pathology very strongly. This suggests the presence of a pool of unassembled α-synuclein in Lewy bodies and Lewy neurites. Antibodies directed against the microtubule-binding region of tau protein show a similar behavior, in that they strongly stain the neurofibrillary lesions of Alzheimer’s disease, but fail to decorate isolated tau filaments (24, 36).
Future studies with additional α-synuclein antibodies of known epitope will test the validity of the proposed arrangement of α-synuclein in Lewy body filaments. Meanwhile, in conjunction with the discovery of mutations in α-synuclein in some familial cases of PD (16, 17), our findings suggest that the presence of α-synuclein filaments may be the cause of nerve cell death and that idiopathic PD and DLB are α-synucleinopathies.
Acknowledgments
We thank Dr. N. J. Cairns and Professor P. L. Lantos (Medical Research Council Brain Bank, Department of Neuropathology, Institute of Psychiatry, London) and Dr. J. Xuereb (Medical Research Council Brain Bank, Department of Psychiatry, University of Cambridge, Cambridge, U.K.) for kindly providing the tissues from patients with Parkinson’s disease and dementia with Lewy bodies. We thank C. Villa for help with photography. M.H. is supported by a postdoctoral fellowship from Innogenetics Inc.
ABBREVIATIONS
- PD
Parkinson’s disease
- DLB
dementia with Lewy bodies
References
- 1.Lewy F H. Handbuch der Neurologie. Vol. 3. 1912. pp. 920–933. [Google Scholar]
- 2.Trétiakoff M C. Ph.D. thesis. University of Paris; 1919. [Google Scholar]
- 3.Forno L S. J Neuropathol Exp Neurol. 1996;55:259–272. doi: 10.1097/00005072-199603000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Duffy P E, Tennyson V M. J Neuropathol Exp Neurol. 1965;24:398–414. [PubMed] [Google Scholar]
- 5.Roy S, Wolman L. J Pathol. 1969;99:39–44. doi: 10.1002/path.1710990106. [DOI] [PubMed] [Google Scholar]
- 6.Okazaki H, Lipkin L E, Aronson S M. J Neuropathol Exp Neurol. 1961;20:237–244. doi: 10.1097/00005072-196104000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Kosaka K, Oyanagi S, Matsushita M, Hori A, Iwase S. Acta Neuropathol. 1976;36:221–233. doi: 10.1007/BF00685366. [DOI] [PubMed] [Google Scholar]
- 8.Ince P G, Perry E K, Morris C M. Brain Pathol. 1998;8:299–324. doi: 10.1111/j.1750-3639.1998.tb00156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dickson D W, Ruan D, Crystal H, Mark M H, Davies P, Kress Y, Yen S-H. Neurology. 1991;41:1402–1409. doi: 10.1212/wnl.41.9.1402. [DOI] [PubMed] [Google Scholar]
- 10.Goldman J E, Yen S-H, Chiu F C, Peress N S. Science. 1983;221:1082–1084. doi: 10.1126/science.6308771. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt M L, Murray J, Lee V M-Y, Hill W D, Wertkin A, Trojanowski J Q. Am J Pathol. 1991;139:53–65. [PMC free article] [PubMed] [Google Scholar]
- 12.Pollanen M S, Dickson D W, Bergeron C. J Neuropathol Exp Neurol. 1993;52:183–191. doi: 10.1097/00005072-199305000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Kuzuhara S, Mori H, Izumiyama N, Yoshimura M, Ihara Y. Acta Neuropathol. 1988;75:345–353. doi: 10.1007/BF00687787. [DOI] [PubMed] [Google Scholar]
- 14.Lowe J, Blanchard A, Morrell K, Lennox G, Reynolds L, Billett M, Landon M, Mayer R J. J Pathol. 1988;155:9–15. doi: 10.1002/path.1711550105. [DOI] [PubMed] [Google Scholar]
- 15.Manetto V, Perry G, Tabaton M, Mulvihill P, Fried V A, Smith H T, Gambetti P, Autilio-Gambetti L. Proc Natl Acad Sci USA. 1988;85:4501–4505. doi: 10.1073/pnas.85.12.4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polymeropoulos M H, Lavedan C, Leroy E, Ide S E, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, et al. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 17.Krüger R, Kuhn W, Müller T, Woitalla D, Graeber M, Kösel S, Przuntek H, Epplen J T, Schöls L, Riess O. Nat Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 18.Spillantini M G, Schmidt M L, Lee V M-Y, Trojanowski J Q, Jakes R, Goedert M. Nature (London) 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 19.Jakes R, Spillantini M G, Goedert M. FEBS Lett. 1994;345:27–32. doi: 10.1016/0014-5793(94)00395-5. [DOI] [PubMed] [Google Scholar]
- 20.Ji H, Liu J E, Jia T, Wang M, Liu J, Xiao G, Joseph B K, Rosen C, Shi Y E. Cancer Res. 1997;57:759–764. [PubMed] [Google Scholar]
- 21.Shaw G, Chau V. Proc Natl Acad Sci USA. 1988;85:2854–2858. doi: 10.1073/pnas.85.8.2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spillantini M G, Goedert M, Jakes R, Klug A. Proc Natl Acad Sci USA. 1990;87:3947–3951. doi: 10.1073/pnas.87.10.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greenberg S G, Davies P. Proc Natl Acad Sci USA. 1990;87:5827–5831. doi: 10.1073/pnas.87.15.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goedert M, Spillantini M G, Cairns N J, Crowther R A. Neuron. 1992;8:159–168. doi: 10.1016/0896-6273(92)90117-v. [DOI] [PubMed] [Google Scholar]
- 25.Crowther R A. Proc Natl Acad Sci USA. 1991;88:2288–2292. doi: 10.1073/pnas.88.6.2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wakabayashi K, Matsumoto K, Takayami K, Yoshimoto M, Takahashi H. Neurosci Lett. 1997;239:45–48. doi: 10.1016/s0304-3940(97)00891-4. [DOI] [PubMed] [Google Scholar]
- 27.Takeda A, Mallory M, Sundsmo M, Honer W, Hansen L, Masliah E. Am J Pathol. 1998;152:367–372. [PMC free article] [PubMed] [Google Scholar]
- 28.Forno L S, Norville R L. Acta Neuropathol. 1976;34:183–197. doi: 10.1007/BF00688674. [DOI] [PubMed] [Google Scholar]
- 29.Gibb W R G, Esiri M M, Lees A J. Brain. 1985;110:1131–1153. doi: 10.1093/brain/110.5.1131. [DOI] [PubMed] [Google Scholar]
- 30.Wakabayashi K, Takahashi H, Takeda S, Ohama E, Ikuta F. Acta Neuropathol. 1988;76:217–221. doi: 10.1007/BF00687767. [DOI] [PubMed] [Google Scholar]
- 31.Galloway P G, Mulvihill P, Perry G. Am J Pathol. 1992;140:809–822. [PMC free article] [PubMed] [Google Scholar]
- 32.Iwatsubo T, Yamaguchi H, Fujimoro M, Yokosawa H, Ihara Y, Trojanowski J Q, Lee V M-Y. Am J Pathol. 1996;148:1517–1529. [PMC free article] [PubMed] [Google Scholar]
- 33.Weinreb P H, Zhen W, Poon A W, Conway K A, Lansbury P T. Biochemistry. 1996;35:13709–13715. doi: 10.1021/bi961799n. [DOI] [PubMed] [Google Scholar]
- 34.Crowther R A, Wischik C M. EMBO J. 1985;4:3661–3665. doi: 10.1002/j.1460-2075.1985.tb04132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fan J, Griffiths A D, Lockhart A, Cross R A, Amos L A. J Mol Biol. 1996;259:325–330. doi: 10.1006/jmbi.1996.0322. [DOI] [PubMed] [Google Scholar]
- 36.Goedert M, Spillantini M G, Jakes R, Rutherford D, Crowther R A. Neuron. 1989;3:519–526. doi: 10.1016/0896-6273(89)90210-9. [DOI] [PubMed] [Google Scholar]