Abstract
Objective:
Serum uric acid (SUA) is associated with cardiovascular disease (CVD). However it is still disputed whether the relationship is mediated by other risk factors such as obesity, dyslipidaemia, hypertension and insulin resistance. We explored the association of the uric acid level with carotid intima-media thickness (IMT), a well known marker of CVD, in postmenopausal healthy women.
Methods:
We consecutively enrolled postmenopausal women undergoing a screening for health evaluation. After an accurate clinical examination, and a biochemical evaluation, the enrolled subjects underwent B mode ultrasonography to assess common carotid intima media thickness.
Results:
Among 234 women aged 45–70 years, the uric acid level is associated with carotid IMT independently of other prognostic factors (p=0.03). In particular, women in the highest tertiles of uric acid level have a greater IMT than women in the lowest tertile (p=0.007).
Conclusions:
Independently of other cardiovascular risk factors, SUA levels are associated with carotid IMT even in subjects without the metabolic syndrome. This confirms and expands the role of uric acid in the determinism of CVD. Prospective trials would be useful to evaluate interventions aimed at lowering the uric acid level.
Key words: Carotid atherosclerosis, Serum uric acid, Postmenopausal women, Cardiovascular disease, Cerebrovascular disease
Introduction
The positive association between serum uric acid (SUA) and cardiovascular diseases (CVD) has been recognised by several epidemiological studies [1–3]. However, whether uric acid is an independent risk factor for cardiovascular (CV) mortality is still disputed, as many studies suggest that hyperuricaemia is associated with CVD because of confounding factors such as obesity, dyslipidaemia, hypertension, use of diuretics and insulin resistance [4, 5]. Elevated SUA levels are commonly seen in association with glucose intolerance, hypertension and dyslipidaemia, a cluster of disorders that characterise the metabolic syndrome [6–8]. Studies performed in healthy volunteers suggest that the link between the metabolic syndrome and a high SUA is related to insulin action in stimulating urate reabsorption in the renal proximal tubule [9]. High-resolution ultrasound is a reliable, non-invasive method to detect early structural and functional atherosclerotic changes in the arterial wall. Increased carotid intima-media thickness (IMT) is a structural marker of early atherosclerosis related to vascular risk factors, and predicting CV events in different population groups [10, 11]. Finally, the relation between SUA level and CVD has been investigated mainly in men. Therefore we evaluated the relationship of IMT and SUA in postmenopausal women.
Methods
We consecutively enrolled postmenopausal women undergoing menopause health-screening tests in our University hospital. They were all Caucasian, aged 45–75 years, in postmenopausal status, defined as no natural menses for at least one year, and with a serum FSH level more than 40 IU/l. Women with a history of CVD were excluded from the study. The study was approved by the local ethics committee, and all subjects provided informed consent. An evaluation including collection of demographic information, risk factors for CVD, medical history, medication use, alcohol use, tobacco use and a physical examination to assess blood pressure and anthropometric measurements were performed. Venous blood was collected after overnight fasting, into vacutainer tubes (Becton & Dickinson), and centrifuged within 4 h. Serum glucose, uric acid, creatinine, total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C) and triglycerides were measured by standard laboratory techniques. Quality controls were assessed daily for all determinations. Low-density lipoprotein cholesterol (LDL-C) was calculated by the Friedewald formula.
The ATP III criteria [12] were used to classify study participants as affected or not affected by the metabolic syndrome. In brief, we based metabolic syndrome diagnosis on the presence of ≥3 of the following factors: (1) waist circumference >88 cm, (2) fasting triglycerides >150 mg/dl, (3) HDL-C <50 mg/dl, (4) hypertension (systolic blood pressure >130 mmHg, diastolic blood pressure ≥85 mmHg), and (5) fasting glucose ≥110 mg/dl. The following criteria were used to define the CV risk factors: diabetes: fasting blood glucose ≥126 mg/dl or antidiabetic treatment; hyperlipidaemia: TC >200 mg/dl or triglycerides >200 mg/dl or lipid-lowering drugs use; hypertension: systolic blood pressure >140 mmHg or diastolic blood pressure >90 mmHg or antihypertensive treatment; tobacco use: present smokers.
Vascular ultrasound
The subjects underwent B-mode ultrasonography of the extracranial carotid arteries by use of a duplex system (a high-resolution ultrasound instrument ATL, HDI 5000 with a 5–12-MHz linear array multifrequency transducer). All the examinations were performed by the same ultrasonographer blinded to clinical information. The right and left common carotid arteries (CCA) were evaluated with the subjects in supine position, with the head turned away from the sonographer, and the neck extended with mild rotation. The IMT, defined as the distance between the intima-luminal interface and the media-adventitial interface, was measured as previously described [13]. Briefly, in the posterior approach and with the sound beam set perpendicular to the arterial surface, 1 cm from the bifurcation, three longitudinal measurements of IMT were completed on the right and left CCAs’ far wall, at sites free of any discrete plaques. The mean of the 3 right and left longitudinal CCA-IMT measurements was then calculated. The coefficient variation of the methods was 3.3%. We evaluated only CCA because it is easier to image, with no missing images; moreover the examination reproducibility is the best among the different segments [14].
Statistical analysis
Differences in baseline demographic and clinical characteristics among the groups were assessed by χ2-tests for categorical variables and unpaired Student’s t-test or ANOVA for continuous variables. Pearson correlation coefficient was used to evaluate variables associated to IMT. Multiple regression analysis was used to obtain the relationship between IMT and SUA. Covariates in adjusted models include age, and those significantly correlated to carotid IMT. All reported p values are two-sided. Significant differences were assumed to be present at p<0.05. All comparisons were performed using the statistical package SPSS 11.0 for Windows.
Results
We enrolled 234 postmenopausal women; the mean age was 56.4 years. All subjects completed the protocol study. Demographic, clinical and biochemical characteristics and prevalence of evaluated risk factors of the study population are shown in Table 1. Only 3% of our population had carotid IMT greater than 1 mm, a value known to predict high CV risk in the elderly. No subjects had a history of gout or a creatinine level >1.2 mg/dl. On the univariate analysis age, TC, glucose, systolic blood pressure, smoking and uric acid were positively correlated to IMT (Table 2), therefore we performed a stepwise multiple regression analysis. After this analysis the variables significantly correlated to carotid IMT were age (beta=0.330, p(0.001), systolic blood pressure (beta=0.143, p=0.025) and SUA (beta=0.133, p=0.030).
Table 1.
Clinical and biochemical characteristics
| Mean±SD | |
|---|---|
| Age (years) | 56.41±7.08 |
| Body mass index (kg/m2) | 28.45±5.27 |
| Waist circumference (cm) | 90.3±12.2 |
| Systolic blood pressure (mmHg) | 127.9±16.5 |
| Diastolic blood pressure (mmHg) | 77.9±8.7 |
| Glucose (mg/dl) | 98.5±21.8 |
| Creatinine (mg/dl) | 0.78±0.1 |
| TC (mg/dl) | 231.8±42.1 |
| LDL-C (mg/dl) | 145.96±35.8 |
| HDL-C (mg/dl) | 60.7±14.8 |
| Triglycerides (mg/dl) | 138.7±126.8 |
| SUA (mg/dl) | 4.2±1.1 |
| IMT (mm) | 0.69±0.15 |
| Smokers (%) | 20 |
| Hypertension (%) | 35 |
| Hyperlipidaemia (%) | 48 |
| Diabetes (%) | 5 |
| Metabolic syndrome (%) | 20 |
Table 2.
Pearson correlation coefficient and probability levels (ρ) between risk factors or HRT and diuretic use and carotid IMT
| Correlation coefficient | p | |
|---|---|---|
| Age | 0.411 | 0.000 |
| Body mass index | 0.107 | 0.102 |
| SBP | 0.288 | 0.000 |
| DBP | 0.098 | 0.137 |
| Glucose | 0.131 | 0.045 |
| Total cholesterol | 0.156 | 0.017 |
| LDL cholesterol | 0.114 | 0.101 |
| HDL cholesterol | −0.097 | 0.155 |
| Triglycerides | 0.049 | 0.464 |
| Serum uric acid | 0.242 | 0.000 |
| Creatinine | 0.060 | 0.321 |
| Smoking | 0.168 | 0.01 |
| HRT use | 0.070 | 0.222 |
| Diuretic use | 0.069 | 0.296 |
After the population was divided into tertiles according to SUA level, the IMT mean not adjusted for age (p=0.000) and age adjusted (p=0.007) was significantly greater in the second and third (highest) tertiles vs. the first (lowest) tertile (Fig. 1).
Fig. 1.
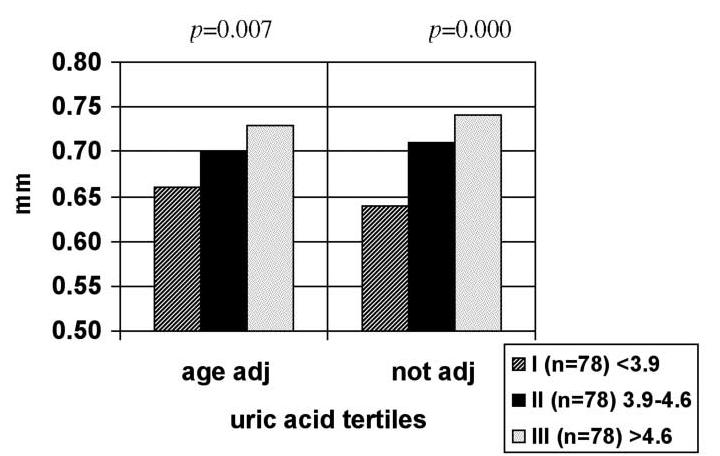
Not adjusted and age-adjusted carotid intima-media thickness mean according to serum uric acid tertiles (I, lowest tertile; III, highest tertile)
We performed a multiple stepwise regression analysis excluding subjects affected by metabolic syndrome (n=47, 20%), a syndrome linked to insulin resistance, as it was suggested that the association between uric acid and CVD is mediated by insulin resistance [15], and we found that only age (beta=0.390, p<0.001) and SUA (beta=0.182, p=0.006) are correlated to IMT. We also performed this analysis in non-drinking women (n=124), as there is a known relationship with alcohol intake [16], and the results remain unchanged (data not shown). Finally we performed a stepwise regression analysis excluding diuretics users (n=26, 11%), as diuretics increase uric acid concentrations, and the correlations do not change.
Discussion
The main result of our study was to establish an association between subclinical carotid atherosclerosis and uric acid in postmenopausal women. Increased uric acid could play a causative role in carotid and coronary atherosclerosis. It is well recognised that because of the loss of protective effect of oestrogens, postmenopausal women have an increased prevalence of CVD. Wingrove et al. [17] demonstrated that the uric acid concentration is significantly higher in postmenopausal vs. premenopausal women, moreover Nicholls et al. [18] found that oestrogen therapy is able to reduce SUA levels. Therefore the increase of uric acid as a consequence of oestrogen falls in menopause could be one of the possible explanations of an increased incidence of CVD in these women. In fact much, but not all, epidemiological research identifies hyperuricaemia as an independent risk factor for the development of CV and renal disease, particularly in patients with hypertension or congestive heart failure, and in women [1–5, 19]. Specifically in postmenopausal women it is found that the mean value of uric acid increases linearly with the numerical increase in coronary arteries with stenosis [20].
Recent findings from the LIFE study are consistent with a role of uric acid in the development of CV disease. In the cited study, up to one third of the CV benefit of losartan vs. atenolol could be ascribed to the differences in effect of these agents on SUA levels [21].
Our results confirm that the relationship between uric acid and carotid atherosclerosis should be considered as independent as the exclusion of subjects affected by the metabolic syndrome should be able to avoid interference with the known confounding variables (obesity, dyslipidaemia, hypertension, insulin resistance).
Several possible pathologic mechanisms linking SUA to CV disease have been proposed, including deleterious effects on endothelial function, oxidative metabolism, platelet adhesiveness, haemorheology and aggregation [22].
Moreover it is suggested that generation of uric acid during ischaemia reperfusion contributes to atherogenesis and intima proliferation following arterial injury [23].
Finally, increased amounts of uric acid are found in carotid plaques, especially in those obtained from women [24]. This result supports a role in the development of cerebral atherosclerosis, also confirmed by the findings that, not only in diabetic patients, but also in the general population [25], uric acid is an independent predictor of stroke. Our results seem to confirm this latter hypothesis, with uric acid directly promoting atherosclerosis by wall injury and consequent wall thickening. However, because these explanations are beyond the aim of the present clinical study, more elaborate studies are required to assess the potential negative role of uric acid on atherosclerosis.
Study limitations
There are some study limitations. Firstly, the data analysis was restricted to an observational study. Only a prospective study can confirm the interdependencies of changes in SUA levels and CVD incidence. Secondly, no serum insulin levels were measured to obtain an index of insulin resistance. As insulin resistance is believed to play a major role in the metabolic syndrome, the inclusion of this variable in our statistical analysis would have been useful. On the other hand, the analysis performed after exclusion of subjects with the metabolic syndrome should ensure that the association is present even in subjects without insulin resistance.
Conclusions
Our study, together with others’ studies, suggests that SUA may be a tool to help to identify patients at high risk of CVD, especially in postmenopausal women in apparent good health, and in the absence of the metabolic syndrome. SUA should therefore be considered, along with other risk factors, such as obesity, hyperlipidaemia, hyperglycaemia, hypertension and smoking in the assessment of overall CV risk. The remaining key questions are whether uric acid has a causal relation to CV disease, whether a reduction can prevent carotid artery disease, and whether uric acid can be reduced to an optimal level whereby it no longer imposes an increased risk for CVD. These questions would be best approached through randomised clinical trials.
References
- 1.Fang J, Alderman MH (2000) Serum uric acid and cardiovascular mortality. The NHANES I epidemiologic follow-up study, 1971–1992. JAMA 283:2404–2410 [DOI] [PubMed]
- 2.Liese AD, Hense HW, Löwel H et al (1999) Association of serum uric acid with all-cause and cardiovascular disease mortality and incident myocardial infarction in the MONICA Augsburg cohort. Epidemiology 10:391–397 [DOI] [PubMed]
- 3.Brand FN, McGee DL, Kannel WB, Stokes III J, Castelli WP (1985) Hyperuricemia as a risk factor of coronary heart disease: the Framingham study. Am J Epidemiol 121:11–18 [DOI] [PubMed]
- 4.Culleton BF, Larson MG, Kannel WB, Levy D (1999) Serum uric acid and risk for cardiovascular disease and death: The Framingham Heart Study. Ann Intern Med 131:7–13 [DOI] [PubMed]
- 5.Burnier M, Brunner HR (1999) Is hyperuricemia a predictor of cardiovascular risk? Curr Opin Nephrol Hypertens 8:167–172 [DOI]
- 6.Bonora E, Targher G, Zenere MB et al (1996) Relationship of uric acid concentration to cardiovascular risk factors in young men. The role of obesity and central fat distribution. The Verona Young Men Atherosclerosis Risk Factors Study. Int J Obes Relat Metab Disord 20:975–980 [PubMed]
- 7.Vuorinen-Markkola H, Yki-Järvinen H (1994) Hyperuricemia and insulin resistance. J Clin Endocrinol Metab 78:25–29 [DOI] [PubMed]
- 8.Facchini F, Chen YD, Hollenbeck CB, Reaven GM (1991) Relationship between resistance to insulin-mediated glucose uptake, urinary uric acid clearance and plasma uric acid concentration. JAMA 266:3008–3011 [DOI] [PubMed]
- 9.Galvan AQ, Natali A, Baldi S et al (1995) Effect of insulin on uric acid excretion in humans. Am J Physiol Endocrinol Metab 268:E1–E5 [DOI] [PubMed]
- 10.O’Leary DH, Polak JF, Kronmal RA et al (1999) Carotidartery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. N Engl J Med 340:14–22 [DOI] [PubMed]
- 11.Bots ML, Hoes AW, Koudstaal PJ, Hofman A, Grobbee DE (1997) Common carotid intima-media thickness and risk of stroke and myocardial infarction: the Rotterdam Study. Circulation 96:1432–1437 [DOI] [PubMed]
- 12.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (2001) Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 285:2486–2497 [DOI] [PubMed]
- 13.Pujia A, Gnasso A, Irace C, Colonna A, Mattioli PL (1994) Common carotid arterial wall thickness in NIDDM subjects. Diabetes Care 17:1330–1336 [DOI] [PubMed]
- 14.van Swijndregt AD, De Lange EE, De Groot E, Ackerstsff RG (1999) An in vivo evaluation of reproducibility of intima-media thickness measurements of the carotid artery segments using B-mode ultrasound. Ultrasound Med Biol 25:323–330 [DOI] [PubMed]
- 15.Rathmann W, Funkhouser E, Dyer AR, Roseman JM (1998) Relations of hyperuricemia with the various components of the insulin resistance syndrome in young black and white adults: the CARDIA study. Coronary Artery Risk Development in Young Adults. Ann Epidemiol 8:250–261 [DOI] [PubMed]
- 16.Faller J, Fox IH (1982) Ethanol-induced hyperuricemia, evidence for increased urate production by activation of adenine nucleotide turnover. N Engl J Med 307:1598–1602 [DOI] [PubMed]
- 17.Wingrove CS, Walton C, Stevenson JC (1998) The effect of menopause on serum uric acid levels in non-obese healthy women. Metabolism 47:435–438 [DOI] [PubMed]
- 18.Nicholls A, Snaith ML, Scott JT (1973) Effect of estrogen therapy on plasma and urinary levels of uric acid. Br Med J 1:449–451 [DOI] [PMC free article] [PubMed]
- 19.Alderman MH, Cohen H, Madhavan S, Kivlighn S (1999) Serum uric acid and cardiovascular events in successfully treated hypertensive patients. Hypertension 34:144–150 [DOI] [PubMed]
- 20.Kotake H, Sawada Y, Hoshio A et al (1992) Relation between serum uric acid and angiographically defined coronary artery disease in postmenopausal women. J Med 23:409–415 [PubMed]
- 21.Hoieggen A, Alderman MH, Kjeldsen SE, Julius S, Devereux RB, De Faire U, Fyhrquist F, Ibsen H, Kristianson K, Lederballe-Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Wedel H, Chen C, Dahlof B, LIFE Study Group (2004) The impact of serum uric acid on cardiovascular outcomes in the LIFE study. Kidney Int 65:1041–1049 [DOI] [PubMed]
- 22.Leyva F, Anker S, Swan JW, Godsland IF, Wingrove CS, Chua TP, Stevenson JC, Coats AJ (1997) Serum uric acid as an index of impaired oxidative metabolism in chronic heart failure. Eur Heart J 18:858–865 [DOI] [PubMed]
- 23.Rao GN, Corson MA, Berk BC (1991) Uric acid stimulates vascular smooth muscle cell proliferation by increasing platelet-derived growth factor A-chain expression. J Biol Chem 266:8604–8608 [PubMed]
- 24.Patetsios P, Song M, Shutze WP, Pappas C, Rodino W, Ramirez JA, Panetta TF (2001) Identification of uric acid and xanthine oxidase in atherosclerotic plaque. Am J Cardiol 88:188–191 [DOI] [PubMed]
- 25.Mazza A, Pessina AC, Pavei A, Scarpa R, Tikhonoff V, Casiglia E (2001) Predictors of stroke mortality in elderly people from the general population. Eur J Epidemiol 17:1097–1104 [DOI] [PubMed]


