Abstract
Rheumatoid arthritis (RA) is a common autoimmune disease characterized by persistent inflammation of joints resulting in progressive destruction of cartilage and bone. Recently, biological agents that suppress the activities of proinflammatory cytokines have shown efficacy as antirheumatic drugs, but require frequent administration, and often result in systemic immune suppression. Thus, gene transfer approaches are being developed as an alternative approach for targeted, more efficient, and sustained delivery of inhibitors of inflammatory cytokines as well as other therapeutic agents. Several gene therapy approaches have been established in preclinical animal models. In these models, autoantigen-specific T cells have been demonstrated to be ideal gene delivery vehicles for the local delivery of “immunoregulatory molecules” because these cells have tissue-specific homing and retention properties. Indeed, bioluminescence studies in an animal model of inflammatory arthritis revealed that these cells accumulated in and remained in inflamed joints. Transfer of genetically modified dendritic cells (DCs) may also have interesting effects. We conclude that modifying antigen-specific T cells or autologous DCs by retroviral transduction for local expression of regulatory proteins is a promising therapeutic strategy for the treatment of RA.
Key words: Antigen-specific T cell, Cell trafficking, Dendritic cell (DC), Local delivery, Regulatory T cell
Introduction
Rheumatoid arthritis (RA) is an autoimmune disease characterized by the infiltration of various leukocyte subpopulations into both the developing pannus and synovial space. The chronic nature of this disease results in multiple joint inflammation with subsequent destruction of joint and cartilage and erosion of bone. The exact pathogenesis of RA remains largely elusive; however, cumulative evidence has suggested that T-cell-mediated autoimmune responses play a crucial role in the pathogenesis of RA.1 Autoantigens that lead to autoimmune arthritis have not been identified, clonotypic analysis of T-cell receptors (TCR) from infiltrating T cells in synovial tissue suggests the existence of an autoantigen recognized by autoreactive T cells.2 Although there are many drugs on the market that alleviate some of the abnormal immune responses, including T-cell functions associated with autoimmune arthritis, current treatments for RA often result in nonspecific toxicity and systemic immune suppression. The local delivery of “regulatory proteins” that can modulate an autoimmune response would be a desirable new approach for the treatment of RA. Autoantigen-specific T cells have tissue-specific homing properties, suggesting that these cells may be ideal vehicles for the local delivery of “immunoregulatory molecules”. This review focuses on the potential therapeutic utility of immune cells, mainly autoantigen-specific T cells, as gene delivery vehicles that locally manipulate the abnormal immune response in RA.
Current therapeutic strategies for the treatment of rheumatoid arthritis
Because the etiology of RA is unknown, the treatment options remain limited. The goal of current therapies for RA is elimination of joint pain by the inhibition of chronic inflammation, and improvement of the activity of daily living by the suppression of joint destruction.3 Although nonsteroidal antiinflammatory drugs (NSAIDs) are frequently prescribed as an antiinflammatory, the joint destruction is not inhibited by the use of NSAIDs. Treatment of RA has been improved with the advent of disease-modifying antirheumatic drugs (DMARDs) such as methotrexate (MTX), sulfasalazine, and others. However, it is still difficult to suppress the progression of joint destruction even by the use of DMARDs. Recent advances in the field of immunology and rheumatology have revealed that inflammatory cytokines such as tumor necrosis factor (TNF), interleukin (IL)-1β, and IL-6 play crucial roles in the synovial inflammation in RA. Therefore, to increase the specificity of therapies for RA, emphasis has shifted to targeting cytokines and their receptors. Neutralization of pro-inflammatory cytokines by monoclonal antibodies (mAbs) or soluble receptors can efficiently control RA.4–7 Importantly, unlike usual DMARDs, joint destruction has been shown to be significantly suppressed by TNF blockade. Thus, modulation of cytokine balance is a promising strategy for controlling RA. However, arthritis therapies that employ biological agents are currently limited by possible systemic side effects such as the occurrence and re-emergence of viral and bacterial infections, as well as their exorbitant expense. Most importantly, the best of current regimens still fail to help a significant percentage of patients with severe, destructive disease. As a result of the clear need for alternative, novel therapies for treating arthritis, gene transfer has been evaluated as an approach to circumvent the inherent impediments associated with delivery of therapeutic proteins.
Role of T cells in rheumatoid arthritis
It is well accepted that CD4+ T cells play a critical role in the pathogenesis of several autoimmune diseases including RA.1 Therefore, therapeutic strategies have focused on the modulation of CD4+ T cells. Depletion of CD4+ T cells was effective in treating a mouse model of RA, type II collagen (CII)-induced arthritis.8 Unfortunately, the same treatment seemed less effective in human RA.9 Furthermore, a recent experiment has raised the question of whether T cells contribute to the pathogenesis of collagen-induced arthritis.10 There is a continuing argument on the involvement of T cells in the pathogenesis of RA. Several reports have indicated that the major pathogenic factors in RA involve the macrophage and fibroblast-like synovial cells.11 There is significant supporting evidence for such a hypothesis, and most inflammatory events, which include joint destruction, are clearly mediated by these cells. Nevertheless, the production of significant amounts of T-cell-derived cytokines, the major histocompatibility complex (MHC) association with RA, and the response to therapies directed or partly directed at T cell, such as cyclosporine A, tacrolimus and leflunomide, suggest that the involvement of T cells in the pathogenic process can not be easily abandoned. Further support for such involvement stems from data showing the involvement of cell contact between T cells and macrophages as one mechanism for the secretion of proinflammatory cytokines.12 Recent observations in the course of clinical trials seem to support the notion of a potential role of T cells in the initiation and perpetuation of RA. A fusion protein comprised of CTLA4 fused to an immunoglobulin Fc portion (CTLA-4Ig), which binds to the costimulatory molecules B7-1 (CD80) and B7-2 (CD86) and thus prevents CD28/B7 interaction and subsequent activation of the CD28 receptor on T cells, appears to be highly effective in treating RA.13 Although these data will have to be confirmed in further trials, not only do these results suggest that therapies directed at interfering with T-cell activities may be as effective as other DMARDs, but also that T cells are involved in the pathogenesis of RA.
Autoantigen-specific CD4+ T cells can transfer organspecific autoimmune disease in mice, and CD4+ T cells can be found in target organs in both human and mouse models of autoimmunity; thus, autoantigen-specific CD4+ T cells have tissue-specific homing properties. In fact, it has been reported that T cells that express specific chemokine receptor were found in the synovial tissue of RA patients.14,15 These results also raise the possibility that arthritogenic CD4+ T cells, retrovirally transduced to express immunoregulatory proteins, may be ideal candidates for the local “gene therapy”.
Autoantigen-specific T-cell-mediated gene therapy in rheumatoid arthritis
Local gene transfer approaches are being developed as an alternative approach for targeted, more efficient and sustained delivery of inhibitors of abnormal immune responses. There are several different approaches that can be utilized for the treatment of arthritis.16,17 The first approach involves the direct injection of the vector at the site where expression is needed, allowing genetic modification of cells following injection (Fig. 1c). The second approach involves the genetic manipulation of cells such as fibroblast or synovial cells in culture followed by injection of the modified cells locally such as intra-articular injection (Fig. 1b). However, the possibility of treating each affected joint may prove difficult in a clinical setting because of the number of joints involved. Hence, alternative approaches have been developed to deliver therapeutic agents. Localized gene delivery of a preselected amount of a regulatory protein to a specific site should ensure maximum therapeutic effect in the area of inflammation while minimizing the exposure of nontargeted organs to the gene products, and markedly reducing the risk of undesirable systemic side effects (Fig. 1a). Antigen-specific CD4+ T cells and T-cell hybridomas have been used to deliver immunoregulatory cytokines and cytokine inhibitors to autoimmune lesions, showing that T cells are suitable vehicles for targeted immunotherapy.18,10 We and others have demonstrated effective prevention of collagen-induced arthritis (CIA), an animal models of rheumatoid arthritis, by adoptive cellular gene therapy using autoantigen-specific T cells and T-cell hybridomas retrovirally transduced to express IL-12/23 receptor blocker IL-12p40,20 the regulatory cytokine IL-4,21 and TNF-antagonizing anti-TNF scFv.22 Amelioration of autoimmune arthritis by antigen-specific immunoregulatory proteins such as IL-12p40 and/or IL-4-transduced CD4+ T cells was due to local delivery of immunoregulatory protein to the inflamed joint. This was demonstrated by three points as follows. First, analysis of the cytokine profile, isotype profiles of antitype II collagen antibodies, and the proliferative responses to antigen of draining lymph node cells and spleen cells was unaffected in the treatment groups compared with untreated and vector-only treated animals. This finding is also consistent with a good safety profile of the adoptive cellular gene therapy approach in terms of low systemic side effects. Second, antigen recognition was required by the transduced T cells to ameliorate CIA. To this effect, when retrovirally transduced CII-reactive and MBP-reactive Tcell hybridomas that express equivalent amounts of immune regulatory protein were injected into both CIA and experimental autoimmune encephalomyelitis (EAE) mice, only CII-reactive T-cell hybridomas were therapeutic in CIA and only MBP-reactive T cells were therapeutic in EAE.20,23 Interestingly, both cell types were found to migrate to inflamed paws as confirmed by in vivo real-time bioluminescence imaging and polymerase chain reaction (PCR) detection of YFP marker mRNA in joint tissue homogenates. However, bioluminescence imaging showed that only CII-specific cells persisted in the inflammatory lesions, while the homing of MBP-specific cells was transient. Of note, the CII-specific cells persisted in the joints long-term because YFP mRNA could be detected by PCR up to 55 days after adoptive cell transfer. Based on the chemokine receptor expression profile of our antigenspecific T-cell hybridomas and their ability to migrate in vitro in response to chemokines that were found to be expressed in inflamed joints in CIA,21 we hypothesize that these vehicle cells home to sites of inflammation by means of chemotaxis independent of their antigen specificity. Retention in inflammatory lesions, however, occurs only upon recognition of antigen by the specific T-cell receptor (TCR). Third, PCR analysis of joint tissue homogenates showed that in animals that received anti-TNF scFv expressing T-cell hybridomas, IL-6 transcription was suppressed.22 Taken together, these results strongly suggest that T cell-mediated adoptive cellular gene therapy works based on site-specific homing and retention of the vehicle cells and local effects of the delivered immune-modulating molecules.
Fig. 1.
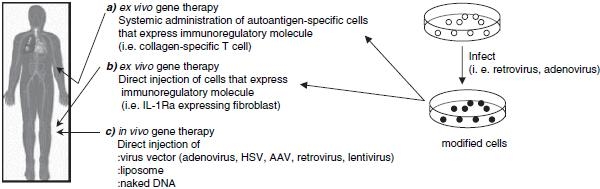
Approaches and vectors used for local gene transfer to joints. IL, interleukin; HSV, herpes simplex virus; AAV, adeno-associated virus
Previous barriers of retroviral transduction for the application of gene therapy have included low proviral integration frequency in immune cells, proviral promoter shutdown, and inadequate isolation and expansion of transduced cells. In fact, only 3%–5% transduction efficiency was obtained in previous studies using retrovirus-mediated TGF-β or IL-10 gene transduction into T cells.24,25 However, Costa et al. have developed a novel retroviral construct that contains an internal ribosomal entry site (IRES).26 This bicistronic expression vector allows coexpression of two genes, the gene of interest and a fluorescent-markerencoding gene on one mRNA transcript. Using this novel construct we and others have been able to stably and durably transduce primary T cells and T-cell hybridomas with up to 80% transduction efficiency that has made retroviral-mediated adoptive T-cell-mediated gene therapy easier in murine models of autoimmune disease. Adoptive T cell-mediated gene therapy may have great advantages over other gene delivery tools for treating autoimmunity because autoantigen reactive T cells can target the inflamed autoantigen-expressing tissues, while other methods of gene transfer such as adenovirus-mediated gene therapy may be limited by strong immunogenicity, making repeated injections difficult.27
However, potential problems of autoantigen-specific T-cell-mediated retrovirus gene therapy remain to be solved. For instance, in the clinical setting, autoantigen-specific T cells are not easy to obtain because the autoantigen may differ in different patients and may change with time in the same patient due to epitope spreading. To circumvent these potential problems, T cells could be engineered to recognize a joint antigen with an appropriate MHC restriction via TCR gene transfer to obtain artificial autoreactive T cells.28,29 A recent clinical trial has raised the serious question of retrovirus-mediated gene therapy. Hacein-Bey-Abina et al. reported that uncontrolled exponential clonal proliferation of mature T cells has occurred in patients who had retrovirus-mediated gene therapy.30 Therefore, new challenges are needed to develop even more specific gene therapy interventions.
Candidate molecules to generate regulatory T cells by gene transduction
Numerous studies have demonstrated successful prevention or amelioration of autoimmune diseases by blocking T-cell-derived cytokines with specific antagonists or by counteracting the inflammatory response with immune regulatory cytokines. Systemic administrations of TGF-β, interleukin-1 receptor antagonist (IL-1β), IL-4, IL-10, IL-12p40, anti-IL-15, anti-IL-17, or anti-TNF serve as effective therapies in models of autoimmune disease such as CIA.31,32 Some of these protocols appear to work by shifting the cytokine balance away from Th1 dominance. Indeed, cytokine-induced immune deviation has been investigated as potential therapy for autoimmune diseases because cytokines present at the time of activation may alter the pathogenicity of effector T cells.33,34 These results suggest that antiinflammatory cytokines or specific antagonists of T-cell-derived cytokines would be good candidates for antigen-specific T-cell-mediated gene therapy.
A still growing body of evidence has demonstrated that specific T-cell populations that have suppressive/regulatory properties (Tregs) tightly control autoaggressive immune responses. Therefore, generation of Treg by gene introduction would be a novel cell-mediated therapy for autoimmune arthritis. Among the CD4+ Treg cells basically two different subsets of Treg cells can be differentiated by their distinct suppressive mechanisms. The suppressive capacity of the first subset, Th3 and type 1T regulatory (Tr1) cells, is contact independent and is based mainly on cytokines such as IL-10 and TGF-β.35,36 The possibility of using one or the other of these two cytokines as candidates was described above. The second T-cell subset with suppressive functions that limits the outcome of autoimmune responses, was described as a subset of peripheral CD4+ T cells, which coexpress CD25, that is critical for the control of autoreactive T cells in vivo.37,38 Naturally occurring CD4+CD25+ Treg cells exert their suppressive effects obviously via cell contact by membrane-bound molecules although the nature of these molecules is still elusive. CD4+CD25+ Treg cells are characterized by the constitutive expression of several activation markers including CTLA-4 (CD152),39 glucocorticoid-induced TNFR (GITR) family-related protein,40 membrane-bound TGF-β, OX40 (CD134),41 and L-selectin (CD62L).42
Regarding the development and function of CD4+CD25+ Treg cells, the transcription factor FoxP3 was found to play a key role.43 Originally, it was demonstrated that mice expressing a naturally occurring loss-of-function mutation of FoxP3 (scurfy mice) rapidly develop a fatal lymphoproliferative disease similar to that seen in mice lacking CTLA-4 or TGF-β.44 FoxP3 is exclusively expressed in murine CD4+CD25+ Treg cells and ectopic expression of FoxP3 in conventional CD4+CD25− cells conferred suppressor function to this T-cell subset. Recently, Anandasabapathy et al. have identified a new gene named GRAIL (gene related to anergy in lymphocytes).45 They have demonstrated that retrovirally transduced T-cell hybridomas that expressed GRAIL showed a blockade in Il-2 transcription, and CD4+CD25+ Treg cells also express GRAIL after activation (Ref. 46, and unpublished data). The significance of these molecules with respect to the suppressive properties of CD4+CD25+ Treg cells is currently controversial; however, these molecules would be candidates to generate antigen-specific regulatory T cells. Taken together, these observations will provide several candidate molecules for T-cell-mediated gene therapy of autoimmune arthritis.
Using dendritic cells as gene delivery vehicles
A simple method for targeted gene delivery is the local injection of either naked DNA or viral vectors into, for example, inflamed joints. Interestingly, however, studies by Lechman et al. in various models of arthritis show a remarkable “contralateral effect” after intra-articular injection of adenoviral vectors encoding antiinflammatory cytokines and cytokine antagonists; that is, disease amelioration was observed not only in the injected but also in the non-injected contralateral joints.47 These authors suggested that modified activity of DCs may be a possible mechanism underlying this phenomenon. Therefore, DCs would also be a good candidate to deliver immunoregulatory molecules.
Dendritic cells (DCs) are the most effective antigenpresenting cells (APCs) in the induction of primary immune responses.48 A growing understanding of heterogeneous immunoregulatory functions of DCs prompted several investigators to consider DC-based immunotherapies for autoimmune diseases. Genetic modification of DCs with genes encoding immunoregulatory molecules is an attractive strategy for generation of immunoregulatory DCs. This challenging approach has been tried for the control of allograft rejection in transplantation where Fas ligand-transduced DCs prolonged cardiac allograft survival in mice.49
In arthritis animal models, Kim et al. investigated the use of genetically modified DCs in the therapy of autoimmune disease.50 They demonstrated that intravenous injection into mice with established CIA of immature DCs infected with adenovirus encoding IL-4 resulted in almost complete suppression of disease, with no recurrence of disease for up to 4 weeks after treatment. These studies suggested that DCs could prove to be powerful vehicles for effective, long-term gene therapy of autoimmune disease. We have found that DCs transduced to express either IL-12p40 or IL-10 are also effective in suppressing CIA (Nakajima et al., unpublished data). These data are in excellent agreement with Morita et al., who demonstrated reduced CIA disease incidence and severity by injecting bone marrow-derived DCs retrovirally transduced to express IL-4 before disease onset.51 These experiments raise the exciting possibility of using DCs for adoptive cellular gene therapy of autoimmune disease. Regarding the mechanism of DC action, adoptive transfer of IL-4-expressing DCs lead to suppression of Th1-type immune responses in the lymph nodes and spleen and diminished the associated humoral immune responses. The authors concluded that the therapeutic DCs migrated to the lymphoid tissues and modulated T-cell immune responses by expression of the regulatory cytokine IL-4 through specific DC-T-cell interactions. A recent study demonstrated interesting results targeting the specific DC-T-cell interactions. Transfer of DCs genetically engineered to express TNF related apoptosis inducing ligand (TRAIL) could inhibit CIA.52 These modified DCs induced antigen-specific T-cell apoptosis by the interaction between TRAIL on DC and TRAIL receptor expressed on T cells. Our own studies of bone marrow-derived DC migration in CIA using bioluminescence imaging suggested that DCs injected intravenously not only homed to lymphoid organs, but also accumulated in inflamed joints and therefore could be used to deliver antiinflammatory molecules directly to the site of inflammation (Nakajima, unpublished data). Taken together, these results indicate that the use of genetically engineered DCs is a very promising approach for adoptive cellular gene therapy of autoimmune disease.
Bioluminescence imaging of immune cell trafficking in vivo
Lymphocytes are highly mobile cells that travel throughout the body in response to a tremendous variety of stimuli. Understanding lymphocyte trafficking patterns in vivo is a necessary prelude to adoptive cellular gene therapy. To directly examine whether CII-specific T cells home to the site of inflammation, we transduced a GFP-luciferase fusion protein gene into CII-specific T-cell hybridomas and tested the patterns of cell trafficking using whole body bioluminescence imaging of the labeled cells in living animals.20,21 This novel technique has been used to monitor bacterial colonization and tumor cell growth in vivo and has demonstrated excellent sensitivity,53,54 and should allow visualization of the trafficking of antigen-specific CD4+ T cells in vivo.55 Before imaging, mice were anesthetized and received a solution of the substrate luciferin into the peritoneal cavity 5 min before imaging. The enzymatic reaction between luciferase and luciferin causes emissions of photons from within the animal. Photons emitted from luciferase within the animal, transmitted through the tissue were detected by a cooled charge-coupled device camera. A pseudocolor image representing light intensity of the emission is superimposed on a gray-scale body-surface reference image collected under real illumination, and the data are acquired and analyzed using appropriate software. This bioluminescence study revealed that antigen-specific “immunoregulatory protein”-producing cells inhibited CIA development by homing to and remaining in the inflamed joints and suppressing inflammation locally (Fig. 2).
Fig. 2.
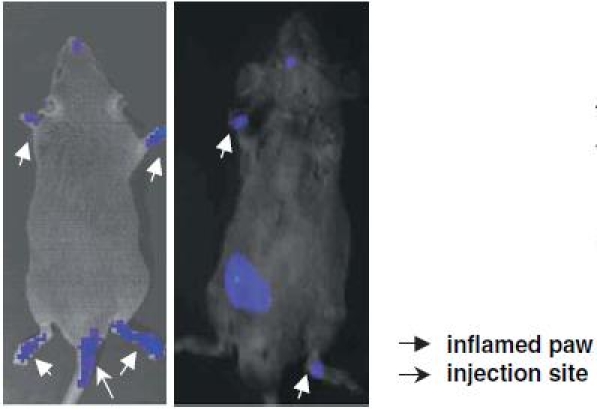
Visualization of lymphocyte trafficking in vivo. CII-immunized DBA/1 LacJ mice received CII-specific CD4+ T-cell hybridomas expressing a GFP-luciferase reporter gene (1 × 106/mouse) intravenously. The images were obtained on day 14 after the cell transfer
Concluding remarks
The main challenge for gene therapy in arthritis is to be able to provide a cost-effective long-term safe treatment that will dramatically improve the current therapeutic outcomes provided by protein therapy and provide for repair of joint tissue and function. Retroviral-mediated adoptive cellular gene therapy has been developed as an efficient novel strategy for the therapy of RA. The clinical trials pioneered by the group in Pittsburgh using autologous synoviocytes constitutively expressing IL-1Ra from a retroviral vector demonstrated that such approach is safe and feasible.56 Whether such another ex vivo approach using these immobile cells will ultimately lead to a long-term benefit needs further investigation. Thus, antigen-specific T cells and DCs hold promise as effective gene delivery vehicles for adoptive cellular gene therapy as shown by our studies described above. As a further step to enhance homing capacity, novel engineered chemokine receptor-bearing cells that can more specifically follow chemokine gradients to inflammatory lesions may be required in the future. Finally, some very encouraging results have been obtained in studies combining various gene products and/or gene delivery strategies; thus, future research should look further into additional potential targets.
Stem cells have been proposed as potent therapeutic means for tissue repair in arthritic disease.57 These cells provide a new cell-mediated therapy for advanced RA. While mesenchymal stem cells capable of differentiating into cartilage and bone can be isolated from bone marrow and be genetically manipulated in vitro, it will be necessary to develop means to regulate their differentiation pathway in vivo as well as assuring their homing to damaged areas for appropriate repair. These cells may also need to be engineered to stop the inflammatory reactions occurring in the diseased joint and or directed at the transferred cells themselves.
In conclusion, improved adoptive cellular gene therapy delivering optimized combinations of immune-regulatory molecules will undoubtedly be informative in characterizing the underlying immune mechanisms in organ-specific autoimmune diseases, and should lead to new therapeutic options for treating human autoimmune diseases.
References
- 1.Panayi GS. T cell-dependent pathways in rheumatoid arthritis. Curr Opin Rheumatol. 1997;9:236–40. doi: 10.1097/00002281-199705000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Hakoda M, Ishimoto T, Yamamoto K, Inoue K, Kamatani N, Miyasaka N, et al. Clonal analysis of T cell infiltrates in synovial tissue of patients with rheumatoid arthritis. Clin Immunol Immunopathol. 1990;57:387–98. doi: 10.1016/0090-1229(90)90113-5. [DOI] [PubMed] [Google Scholar]
- 3.Guidelines for the management of rheumatoid arthritis. Arthritis Rheum 2002;46:328-6 [DOI] [PubMed]
- 4.Moreland LW, Baumgartner SW, Schiff MH, Tindall EA, Fleisdhmann RM, Weaver AL, et al. Treatment of rheumatoid arthritis with a recombinant human tumor necrosis factor receptor (p75)-Fc fusion protein. N Engl J Med. 1997;337:141–7. doi: 10.1056/NEJM199707173370301. [DOI] [PubMed] [Google Scholar]
- 5.Lipsky PE, van der Heijde DMFM, St. Clair EW, Furst DE, Breedveld FC, Kalden JR, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. N Engl J Med. 2000;343:1594–602. doi: 10.1056/NEJM200011303432202. [DOI] [PubMed] [Google Scholar]
- 6.Cohen S, Hurd E, Cush J, Schiff M, Weinblatt ME, Moreland LW, et al. Treatment of rheumatoid arthritis with anakinra, a recombinant human interleukin-1 receptor antagonist, in combination with methotrexate. Arthritis Rheum. 2002;46:614–24. doi: 10.1002/art.10141. [DOI] [PubMed] [Google Scholar]
- 7.Breedveld FC, Weisman MC, Kavanaugh AF, Cohen SB, Pavelka K, Van Vollenhoven R, et al. The PREMIER study. A multicenter, ramdomized, double-blind clinical trial of combination therapy with adalinumab plus methotrexate alone or adalinumab alone in patients with early, appressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006;54:26–37. doi: 10.1002/art.21519. [DOI] [PubMed] [Google Scholar]
- 8.Liblau RS, Singer SM, McDevitt HO. Th1 and Th2 CD4+ T cells in the pathogenesis of organ-specific autoimmune diseases. Immunol Today. 1995;16:34–8. doi: 10.1016/0167-5699(95)80068-9. [DOI] [PubMed] [Google Scholar]
- 9.Epstein WV. Expectation bias in rheumatoid arthritis clinical trials. The anti-CD4 monoclonal antibody experience. Arthritis Rheum. 1996;39:1773–80. doi: 10.1002/art.1780391102. [DOI] [PubMed] [Google Scholar]
- 10.Plows D, Kontogeorgos G, Kollias G. Mice lacking mature T and B lymphocytes develop arthritic lesions after immunization with type II collagen. J Immunol. 1999;162:1018–23. [PubMed] [Google Scholar]
- 11.Firestein GS, Zvaifler NJ. How important are T cells in chronic rheumatoid synovitis? II. T cell-independent machanisms from beginning to end. Arthritis Rheum. 2002;46:298–308. doi: 10.1002/art.502. [DOI] [PubMed] [Google Scholar]
- 12.Burger D, Dayer JM. Cytokines, acute-phase proteins, and hormones: IL-1 and TNF-alpha production in contact-mediated activation of monocytes by T lymphocytes. Ann NY Acad Sci. 2002;966:464–73. doi: 10.1111/j.1749-6632.2002.tb04248.x. [DOI] [PubMed] [Google Scholar]
- 13.Genovese MC, Becker JC, Schiff M, Luggen M, Sherrer Y, Kremer J, et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. N Engl J Med. 2005;353:1114–23. doi: 10.1056/NEJMoa050524. [DOI] [PubMed] [Google Scholar]
- 14.Qin S, Rottman JB, Myers P, Kassam N, Weinblatt M, Loetscher M, et al. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest. 1998;101:746–54. doi: 10.1172/JCI1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suzuki N, Nakajima A, Yoshino S, Matsushima K, Yagita H, Okumura K. Selective accumulation of CCR5+ T lymphocytes into inflamed joint of rheumatoid arthritis. Int Immunol. 1999;11:553–9. doi: 10.1093/intimm/11.4.553. [DOI] [PubMed] [Google Scholar]
- 16.Robbins PD, Evans CH, Chernajovsky Y. Gene therapy for arthritis. Gene Ther. 2003;10:902–11. doi: 10.1038/sj.gt.3302040. [DOI] [PubMed] [Google Scholar]
- 17.Chernajovsky Y, Gould DJ, Podhajcer OL. Gene therapy for autoimmune diseases: quo vadis? Nat Rev Immunol. 2004;4:800–11. doi: 10.1038/nri1459. [DOI] [PubMed] [Google Scholar]
- 18.Shaw MK, Lorens JB, Dhawan A, DalCanto R, Tse HY, Tran AB, et al. Local delivery od interleukin 4 by retrovirus-transduced T lymphocytes ameliorates experimental autoimmune encephalomyelitis. J Exp Med. 1997;185:1711–4. doi: 10.1084/jem.185.9.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mathisen PM, Yu M, Johnson JM, Drazba JA, Touhy VK. Treatment of experimental autoimmune encephalomyelitis with genetically modified memory T cells. J Exp Med. 1997;186:159–64. doi: 10.1084/jem.186.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakajima A, Seroogy CM, Sandora MR, Tarner IH, Costa GL, Taylor-Edwards C, et al. Antigen-specific T cell-mediated gene therapy in collagen-induced arthritis. J Clin Invest. 2001;107:1293–301. doi: 10.1172/JCI12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tarner IH, Nakajima A, Seroogy CM, Erman J, Levicnik A, Contag CH, et al. Retroviral gene therapy of collagen-induced arthritis by local delivery of IL-4. Clin Immunol. 2002;105:304–14. doi: 10.1006/clim.2002.5299. [DOI] [PubMed] [Google Scholar]
- 22.Smith R, Tarner IH, Hollenhorst M, Lin C, Levicnik AU, Fathman CG, et al. Localized expression of an anti-TNF single-chain antibody prevents development of collagen-induced arthritis. Gene Ther. 2003;10:1248–57. doi: 10.1038/sj.gt.3301980. [DOI] [PubMed] [Google Scholar]
- 23.Costa GL, Sandora M, Nakajima A, Nguyen E, Taylor-Edwards C, Slavin AJ, et al. Adoptive immunotherapy of experimental autoimmune encephalomyelitis via T cell delivery of the IL-12 p40 subunit. J Immunol. 2001;167:2379–87. doi: 10.4049/jimmunol.167.4.2379. [DOI] [PubMed] [Google Scholar]
- 24.Chen LZ, Hochwald GM, Dakin G, Cheng C, Simmons WJ, Dranoff G, et al. Gene therapy in allergic encephalomyelitis using myelin basic protein-specific T cells engineered to express latent transforming growth factor-b1. Proc Natl Acad Sci USA. 1998;95:12516–21. doi: 10.1073/pnas.95.21.12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Setoguchi K, Misaki Y, Araki Y, Fujio K, Kawahata K, Kitamura T, et al. Antigen-specific T cells transduced with IL-10 ameliorate experimentally induced arthritis without impairing the systemic immune response to the antigen. J Immunol. 2000;165:5980–6. doi: 10.4049/jimmunol.165.10.5980. [DOI] [PubMed] [Google Scholar]
- 26.Costa GL, Benson JM, Seroogy CM, Achacoso P, Fathman CG, Nolan GP. Targeting rare populations of murine antigen-specific T lymphocytes by retroviral transduction for potential application in gene therapy for autoimmune disease. J Immunol. 2000;164:3581–90. doi: 10.4049/jimmunol.164.7.3581. [DOI] [PubMed] [Google Scholar]
- 27.Evans CH, Ghivizzani SC, Oligino TA, Robbins PD. Future of adenoviruses in the gene therapy of arthritis. Arthritis Res. 2001;3:142–6. doi: 10.1186/ar291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kessels HW, Wolkers MC, van den Boom MD, Van der Valk MA, Schumacher TN. Immunotherapy through TCR gene transfer. Nat Immunol. 2001;2:957–61. doi: 10.1038/ni1001-957. [DOI] [PubMed] [Google Scholar]
- 29.Stanislawski T, Voss RH, Lotz C, Sadovnikova E, Willemsen RA, Kuball J, et al. Circumventing tolerance to a human MDM2-derived tumor antigen by TCR gene transfer. Nat Immunol. 2001;2:962–70. doi: 10.1038/ni1001-962. [DOI] [PubMed] [Google Scholar]
- 30.Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–9. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- 31.Slavin AJ, Tarner IH, Nakajima A, Urbanek-Ruiz I, McBride J, Contag CH, et al. Adoptive cellular gene therapy of autoimmune disease. Autoimmun Rev. 2002;1:213–9. doi: 10.1016/S1568-9972(02)00051-4. [DOI] [PubMed] [Google Scholar]
- 32.Tarner IH, Slavin AJ, McBride J, Levicnik A, Smith R, Nolan GP, et al. Treatment of autoimmune disease by adoptive cellular gene therapy. Ann NY Acad Sci. 2003;998:512–9. doi: 10.1196/annals.1254.067. [DOI] [PubMed] [Google Scholar]
- 33.Racke MK, Bonomo A, Scott DE, Cannella B, Levine A, Raine CS, et al. Cytokine-induced immune deviation as a therapy for inflammatory autoimmune disease. J Exp Med. 1994;180:1961–6. doi: 10.1084/jem.180.5.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakajima A, Hirose S, Yagita H, Okumura K. Roles of IL-4 and IL-12 in the development of lupus in NZB/W F1 mice. J Immunol. 1997;158:1466–72. [PubMed] [Google Scholar]
- 35.Chen Y, Kuchroo VK, Inobe J, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994;265:1237–40. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- 36.Groux H, O’Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MGA. CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–42. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 37.Bluestone JA, Tang Q. How do CD4+CD25+ regulatory T cells control autoimmunity? Curr Opin Immunol. 2005;17:638–42. doi: 10.1016/j.coi.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 38.Randolph DA, Fathman CG. Cd4(+)Cd25(+) regulatory T cells and their therapeutic potential. Annu Rev Med. 2006;57:381–402. doi: 10.1146/annurev.med.57.121304.131337. [DOI] [PubMed] [Google Scholar]
- 39.Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, et al. Immunologic self-tolerance maintained by CD25+CD4+ regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–10. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25+CD4+ regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol. 2002;3:135–42. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- 41.McHugh RS, Whitters MJ, Piccirillo CA, Young DA, Shevach EM, Collins M, et al. CD4+CD25+ immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity. 2002;16:311–23. doi: 10.1016/S1074-7613(02)00280-7. [DOI] [PubMed] [Google Scholar]
- 42.Lepault F, Gagnerault MC. Characterization of peripheral regulatory CD4+ T cells that prevent diabetes onset in nonobese diabetic mice. J Immunol. 2000;164:240–7. doi: 10.4049/jimmunol.164.1.240. [DOI] [PubMed] [Google Scholar]
- 43.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 44.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 45.Anandasabapathy N, Ford GS, Bloom D, Holness C, Paragas V, Seroogy C, et al. GRAIL: an E3 ubiquitin ligase that inhibits cytokine gene transcription is expressed in anergic CD4+ T cells. Immunity. 2003;18:535–47. doi: 10.1016/S1074-7613(03)00084-0. [DOI] [PubMed] [Google Scholar]
- 46.Ermann J, Szanya V, Ford GS, Paragas V, Fathman CG, Lejon K. CD4(+)CD25(+) T cells facilitate the induction of T cell anergy. J Immunol. 2001;167:4271–5. doi: 10.4049/jimmunol.167.8.4271. [DOI] [PubMed] [Google Scholar]
- 47.Lechman ER, Keravala A, Nash J, Kim SH, Mi Z, Robbins PD. The contralateral effect conferred by intra-articular adenovirus-mediated gene transfer of viral IL-10 is specific to the immunizing antigen. Gene Ther. 2003;10:2029–35. doi: 10.1038/sj.gt.3302109. [DOI] [PubMed] [Google Scholar]
- 48.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 49.Min W-P, Gorczynski R, Huang X-Y, Kushida M, Kim P, Obataki M, et al. Dendritic cells genetically engineered to express Fas ligand induce donor-specific hyporesponsiveness and prolong allograft survival. J Immunol. 2000;164:161–7. doi: 10.4049/jimmunol.164.1.161. [DOI] [PubMed] [Google Scholar]
- 50.Kim HS, Kim S, Evans CH, Ghivizzani SC, Oligino T, Robbins PD. Effective treatment of established murine collagen-induced arthritis by systemic administration of dendritic cells genetically modified to express IL-4. J Immunol. 2001;166:3499–505. doi: 10.4049/jimmunol.166.5.3499. [DOI] [PubMed] [Google Scholar]
- 51.Morita Y, Yang J, Gupta R, Shimizu K, Shelden EA, Endres J, et al. Dendritic cells genetically engineered to express IL-4 inhibit murine collagen-induced arthritis. J Clin Invest. 2001;107:1275–84. doi: 10.1172/JCI11490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu Z, Xu X, Hsu H-C, Tousson A, Yang P-A, Wu Q, et al. CII-DC-AdTRAIL cell gene therapy inhibits infiltration of CII-reactive T cells and CII-induced arthritis. J Clin Invest. 2003;112:1332–41. doi: 10.1172/JCI19209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Contag PR, Olomu IN, Stevenson DK, Contag CH. Bioluminescent indicator in living mammals. Nat Med. 1998;4:245–7. doi: 10.1038/nm0298-245. [DOI] [PubMed] [Google Scholar]
- 54.Edinger M, Hoffmann P, Ermann J, Drago K, Fathman CG, Strober S, et al. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med. 2003;9:1144–50. doi: 10.1038/nm915. [DOI] [PubMed] [Google Scholar]
- 55.Hardy J, Edinger M, Bachmann MH, Negrin RS, Fathman CG, Contag CH. Bioluminescence imaging of lymphocyte trafficking in vivo. Exp Hematol. 2001;29:1353–60. doi: 10.1016/S0301-472X(01)00756-1. [DOI] [PubMed] [Google Scholar]
- 56.Evans CH, Robbins PD, Ghivizzani SC, Herndon JH, Kang R, Bahnson AB, et al. Clinical trial to assess the safety, feasibility, and efficacy of transferring a potentially anti-arthritic cytokine gene to human joints with rheumatoid arthritis. Hum Gene Ther. 1996;20:1261–80. doi: 10.1089/hum.1996.7.10-1261. [DOI] [PubMed] [Google Scholar]
- 57.Jorgensen C, Noel D, Apparailly F, Sany J. Stem cells for repair of cartilage and bone: the next challenge in osteoarthritis and bone: the next challenge in osteoarthritis and rheumatoid arthritis. Ann Rheum Dis. 2001;60:305–9. doi: 10.1136/ard.60.4.305. [DOI] [PMC free article] [PubMed] [Google Scholar]


