Abstract
Acute ischemic stroke may trigger an inflammatory response that leads to increased levels of C-reactive protein (CRP). High levels of CRP may be associated with poor outcome because they reflect either an inflammatory reaction or tissue damage. We evaluated the prognostic value of CRP within 12 h of onset of ischemic stroke. Levels of CRP were routinely obtained within 12 h of symptom onset in 561 patients with ischemic stroke. CRP values were dichotomized as <7 or ≥7 mg/L. The full range of CRP values was used to detect a possible level-risk relationship. We studied the relation between CRP values and poor outcome (modified Rankin Scale score >2) or death at 3 months. A multiple logistic regression model was applied to adjust for age, sex, NIHSS score, current cigarette smoking, diabetes mellitus, hypertension, statin use, and stroke subtype. After adjustment for potential confounders, patients with CRP levels ≥7 mg/L had a significantly increased risk of poor outcome (adjusted OR 1.6, 95% CI 1.1–2.4) or death (adjusted OR 1.7, 95% CI 1.0–2.9) at 3 months. In addition, the risk of poor outcome or death at 3 months increased with higher levels of CRP. CRP within 12 h of ischemic stroke is an independent prognostic factor of poor outcome at 3 months.
Keywords: C-reactive protein, Ischemic stroke, Clinical outcome, Prognostic factor, Inflammation
Background
Elevated serum levels of C-reactive protein (CRP) are found in up to three quarters of patients with ischemic stroke [8, 26]. Increases in CRP may reflect a systemic inflammatory response following ischemic stroke, the extent of tissue injury, or concurrent infections. Moreover, in animal models of focal cerebral ischemia, CRP increased secondary brain damage through activation of the complement system [13, 22].
Several studies have assessed the value of CRP in the very early phase of stroke as a prognostic factor of functional outcome. These studies were either small, included a selected group of patients, or tested only the relation between CRP and mortality instead of functional outcome. The findings were inconclusive as some found a positive association [6, 18, 20], but others not [27, 30].
Verification of the role of CRP as an early prognostic factor of functional outcome after ischemic stroke may be of clinical importance, because it is an easily-measured and readily available inflammatory marker. The aim of our study was therefore to determine the prognostic value of CRP measured in the very early phase of ischemic stroke for poor functional outcome and death in a large sample of patients with acute ischemic stroke.
Methods
Study design
All patients included in the present study participated in the Paracetamol (Acetaminophen) In Stroke (PAIS) trial, a multicenter, randomized, placebo-controlled clinical trial of high-dose paracetamol in patients with acute stroke. The study protocol has been published earlier [7, 28]. In short, patients with ischemic stroke or intracerebral hemorrhage within 12 h of symptom onset with no history of liver disease or pre-stroke impairment (modified Rankin Scale (mRS) score <2) [29] were included in this study. Patients with ischemic stroke included in the PAIS trial between March 2003 and March 2007 in centers where CRP was measured as a part of routine laboratory assessment on admission were included in the present study if venous blood sampling for CRP was accomplished within 12 h of symptom onset.
Baseline variables
Baseline clinical information was extracted from the trial records. This included body temperature on admission and 24 h later, stroke subtype according to the TOAST classification [1], stroke severity as assessed with the NIH Stroke Scale (NIHSS) [5], and cardiovascular risk factors. In addition, the occurrence of infections during hospitalization was assessed. Body temperature was measured with either tympanic or rectal thermometers.
Blood samples and CRP assay
Blood samples for assessment of CRP and white blood cell count were taken on admission. The time of CRP measurement relative to stroke onset was recorded in all patients.
Levels of CRP were determined with a clinically validated assay. Participating centers used different analyzers (Dade Behring, Beckman LX-20, Beckman Synchron, Beckman Coulter DXC, Olympus 640, Ortho Vitros, Roche Cobas 6000, Roche Cobas Integra, Roche Hitachi 917, Roche Modular) to establish CRP levels. Intra- and inter-assay variation of all participating centers was evaluated by means of the external quality control scheme of the Dutch Foundation for Quality Assessment in Clinical Laboratories (SKML). If the intra-assay and/or inter-assay coefficient of variation was higher than 7.5%, patients from these centers were excluded from further analysis. All centers were subjected to quality review by the Dutch Foundation for Quality Assessment in Clinical Laboratories (SKML (http://www.skml.nl).
Some centers did not report exact values of CRP below a certain cut-off point, as values below these cut-off points were considered “normal” values. These cut-off points varied between 1 and 7 mg/L.
As extremely high levels of CRP likely reflect an infection at the time of blood sampling, patients in whom the level of CRP was higher than 2 SD above the mean were excluded from further analysis.
Outcome measures
Poor outcome was defined as a score of more than 2 on the mRS, including death, at 3 months from stroke onset. A secondary outcome was death at 3 months. Outcome was assessed without knowledge of baseline CRP levels.
Statistical analysis
Statistical analyses were performed with Stata/SE 8.2 for Windows (Statacorp, College Station, Texas, USA). The relation between outcome and dichotomized CRP levels was expressed as an odds ratio (OR), with a corresponding 95% confidence interval (CI), through logistic regression.
CRP values were dichotomized at 7 mg/L. This cut-off point was selected, as this was the highest cut-off point below which values were considered normal and not reported as exact values by some centers. In order to study a possible level-risk relationship, all CRP values were recoded with 7 mg/L as the lower bound. Odds ratios were expressed per unit increase in logarithmically transformed CRP levels. Adjustments for the impacts of age, sex, baseline NIHSS score, cigarette smoking status, diabetes mellitus, hypertension, statin use, and stroke subtype (cardioembolic stroke versus non-cardioembolic stroke) was made with multiple logistic regression. We performed additional analyses to account for the potential confounding effect of antipyretic treatment by stratifying for treatment with acetaminophen.
Results
Between 1 March 2003 and 1 March 2007, 1187 patients were included in the PAIS trial. Sixteen of the 29 participating centers routinely performed CRP measurements on admission for acute stroke. In these 16 centers, 897 patients where included in the PAIS trial in the period under study. Of these patients, 336 were excluded from the present study because of hemorrhagic stroke (n = 117), CRP measurement not accomplished within 12 h of stroke onset or time of assessment unknown (n = 54), CRP measurements with analyzers with a variation coefficient >7.5% (n = 56), or CRP levels over 110 mg/L (2 SD; n = 9).
The median CRP level was 5 mg/L (IQR 2–8) and 33% of patients had CRP levels of 7 mg/L or above.
Table 1 shows the baseline characteristics of the patients with CRP levels <7 or ≥7 mg/L. Median time from onset of symptoms to CRP measurement was 137 min (range 0–696). Patients with CRP ≥7 mg/L were older, had higher scores on the NIHSS, had a slightly higher body temperature on admission, were more often smokers, more frequently had hypertension, diabetes mellitus, or atrial fibrillation, and less often used statins than patients with low CRP levels. Cardioembolic strokes were observed more often in patients with CRP ≥7 mg/L.
Table 1.
Baseline characteristics of the study population
| CRP < 7 mg/L | CRP ≥ 7 mg/L | p | |
|---|---|---|---|
| Demographics | |||
| N | 377 | 184 | 0.07 |
| Age (years), mean (SD) | 69.1 (13.4) | 70.9 (13.8) | 0.98 |
| Male | 226 (60%) | 110 (60%) | |
| Stroke severitya | |||
| NIHSS, mean (SD) | 7.5 (6.0) | 8.9 (6.6) | 0.02 |
| Risk factors | |||
| Arterial hypertension | 183 (49%) | 107 (58%) | 0.03 |
| Atrial fibrillation | 47 (12%) | 47 (26%) | 0.0005 |
| Diabetes mellitus | 50 (13%) | 37 (20%) | 0.03 |
| Current cigarette smoking | 108 (29%) | 70 (38%) | 0.03 |
| Hypercholesterolemia | 104 (28%) | 42 (23%) | 0.30 |
| History | |||
| Stroke | 84 (22%) | 40 (22%) | 0.68 |
| Myocardial infarction | 47 (12%) | 25 (14%) | 0.92 |
| Peripheral vascular disease | 25 (7%) | 21 (11%) | 0.09 |
| Physical examination | |||
| Systolic blood pressure, mean (SD) | 169 (31) | 171 (34) | 0.63 |
| Diastolic blood pressure, mean (SD) | 89 (18) | 89 (21) | 0.81 |
| Body temperature, mean (SD) | 36.9 (0.6) | 37.0 (0.6) | 0.08 |
| Laboratory assessments | |||
| Time until CRP measurement (min), median (range) | 140 (2–696) | 129 (0–681) | 0.64 |
| Leukocytes 109/L, mean (SD) | 8.2 (2.6) | 9.4 (3) | 0.0001 |
| Stroke type (TOAST)b | |||
| Undetermined | 189 (50%) | 81 (44%) | 0.09 |
| Large vessel disease (≥50% stenosis) | 38 (10%) | 28 (15%) | 0.06 |
| Cardiac source of embolism | 60 (16%) | 44 (24%) | 0.02 |
| Small vessel occlusion | 49 (13%) | 24 (13%) | 0.88 |
| Other determined etiology | 41 (11%) | 7 (4%) | 0.003 |
| Treatment | |||
| RtPA | 95 (25%) | 50 (27%) | 0.42 |
| Ace inhibitor | 65 (17%) | 38 (21%) | 0.46 |
| Statin | 101 (27%) | 36 (20%) | 0.001 |
Sixteen patients with CRP ≥7 mg/L (9%) and 15 patients (4%) with CRP <7 mg/L developed an infection during hospitalization. Two patients with CRP ≥7 mg/L had an infection (both pneumonia) within 24 h of stroke onset. No infections within 24 h were reported in patients with CRP <7 mg/L.
No association between CRP levels and the body temperature at 24 h after study enrollment was found.
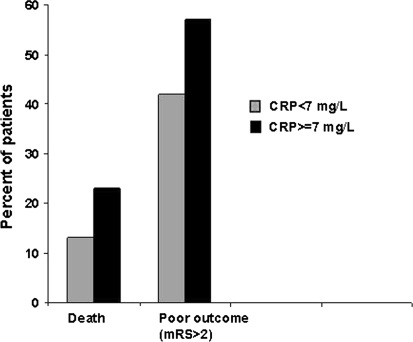
Patients with CRP levels ≥7 mg/L more often had a poor outcome (57 versus 42%; p = 0.006) or died (23 versus 13%; p = 0.0007) than patients with lower CRP levels (Fig. 1). Adjustment for potential confounders did not change these results (Table 2).
Fig. 1.
Association of CRP levels on admission with poor outcome or death at 3 months
Table 2.
Association between increased CRP (≥7 mg/L) at baseline and outcome after ischemic stroke
| OR (95% CI) | Adjusted OR (95% CI)a | |
|---|---|---|
| Poor outcome (mRS > 2) | 1.9 (1.3–2.7) | 1.6 (1.1–2.4) |
| Death | 2.0 (1.3–3.2) | 1.7 (1.0–2.9) |
aAdjusted for age, sex, NIHSS score, cigarette smoking, diabetes mellitus, hypertension, statin use, and stroke subtype
An additional stratified analysis to account for the potential confounding effect of treatment with acetaminophen did not affect the association.
A level-risk relationship was observed between CRP and poor outcome or death at 3 months. The relation between CRP and poor outcome was attenuated after adjustment for potential confounders (Table 3).
Table 3.
Level-risk relationship CRP and outcome
| ORa (95% CI) | Adjusted ORa, b (95% CI) | |
|---|---|---|
| Poor outcome (mRS > 2) | 1.6 (1.2–2.2) | 1.3 (0.9–1.9) |
| Death | 2.1 (1.5–3.0) | 1.9 (1.2–2.8) |
aORs were expressed per 1 unit increase in logarithmically transformed CRP levels
bAdjusted for age, sex, NIHSS score, cigarette smoking, diabetes mellitus, hypertension, statin use, and stroke subtype
After exclusion of patients who developed an infection during the first 2 weeks after stroke onset, the adjusted odds ratio for poor outcome was 1.5 (95% CI 1.0–2.3; p = 0.07), and for death 1.9 (95% CI 1.1–3.4).
Discussion
In this study, patients with CRP levels ≥7 mg/L within 12 h of ischemic stroke onset had a significantly increased risk of poor functional outcome or death at 3 months, even after adjustment for potential confounders. In addition, a level-risk relationship was found between CRP and poor outcome and death.
Several studies have found an association between increased CRP levels and clinical outcome in the time window between 12 and 72 h after ischemic stroke [6, 8, 9, 15, 18, 20, 21, 25–27, 30]. The results of previous studies that have aimed to assess the prognostic value of CRP in the very early phase of stroke are ambiguous. Two prospective studies did not observe a relation between CRP levels obtained within 6 or 12 h after symptom onset and death or dependency at follow-up [27, 30]. Both studies were rather small (127 and 111 patients) and may therefore have lacked the power to detect an association. In addition, one of these studies included only patients treated with rt-PA [27].
In line with our results, three other studies found an association between CRP and outcome. In one of these studies, CRP was measured within 24 h of stroke onset, and 25% of the patients had a transient ischemic attack (TIA) instead of a stroke, which may have influenced the observed association [6]. In addition, only the relation between CRP and mortality, and not disability, was studied. Other studies included only patients aged 75 years or older [18], or patients who had a middle cerebral artery occlusion and received rt-PA [20]. In the latter, an association with mortality was found, but functional outcome was not assessed [20].
The strengths of the present study are its large sample size, robust outcome measures and early CRP measurement, which increase the relevance of our findings. Furthermore, detailed information on confounders was available.
Some methodological limitations should be discussed. First, this study was part of a larger clinical trial, and not designed to evaluate the prognostic value of CRP with regard to clinical outcome in acute ischemic stroke. As a consequence, a detailed history of inflammatory conditions was not recorded.
Levels were determined with different analyzers, which may have resulted in a systematic error. For this reason, measurements performed with analyzers with an inter-assay coefficient of variation ≥7.5% or that demonstrated bias relative to the other participating centers were excluded from further analysis. Secondly, the lowest detection limit for CRP varied from 1 to 7 mg/L among the centers. Therefore, CRP levels had to be dichotomized. This makes it more difficult to compare our results with previous studies. However, in three of the five studies that assessed the relation between CRP levels in the very early phase of acute stroke and clinical outcome, a similar cut-off point level was selected [20, 27, 30]. Furthermore, all CRP values were recoded with 7 as the lower bound, in order to study a possible level-risk relationship.
A fourth issue might be that rather few patients with very severe stroke were included, which may affect the generalisability of the results.
In patients with ischemic stroke, increased levels of CRP may reflect a pre-existing degree of atherosclerosis or the presence of vascular risk factors. We found that patients with higher levels of CRP were more often smokers and more frequently had hypertension, diabetes mellitus, or atrial fibrillation. Furthermore, cardioembolic strokes were observed more often in patients with higher levels of CRP. Moreover, patients with CRP ≥7 mg/L used statins less often than patients with low CRP levels, suggesting that drugs in this class reduce levels of CRP. These findings are supported by previous studies [2, 10, 17, 18, 23, 24].
Why could CRP concentration be a prognostic factor for poor outcome and death? Early after onset of ischemic stroke, increased CRP levels may reflect an accompanying inflammatory reaction. Inflammatory processes play an important role in the pathophysiology of ischemic stroke [19]. Cerebral ischemia triggers an inflammatory response characterized by activation and release of acute phase proteins such as C-reactive protein (CRP) and cytokines [4, 16, 19]. The inflammatory processes may start within 2 h after stroke onset and sustain for days [25], and may contribute to ischemic brain damage even in that early stage [11].
Elevated CRP levels may be a reflection of the extent of brain injury. In our study, patients with CRP levels ≥7 mg/L had higher NIHSS scores on admission. Previous studies have shown that patients with increased CRP levels have larger infarctions [25]. Although patients with CRP levels ≥7 mg/L taken as a group had worse outcomes at 3 months as assessed with the mRS, multiple logistic regression modeling indicated that the adverse outcome in this group remained even after adjustment for initial stroke severity by NIHSS score and multiple other risk factors for poor outcome. This suggests that CRP not only reflects the amount of tissue damage, but may also indicate a state of enhanced risk due to increased inflammation or cytokine excess. Interestingly, recent experimental studies have shown that CRP itself may contribute to secondary brain damage after focal cerebral ischemia, possibly via a complement-mediated exacerbation of tissue injury [13, 22]. In rats, treatment with human CRP after middle cerebral artery occlusion resulted in larger infarcts [13]. Similar results have been observed in experimental models of myocardial infarction [14].
It is therefore conceivable that increased levels of CRP following stroke are not only a consequence of brain infarction, but contribute to ischemic damage as well.
Increased CRP levels following ischemic stroke may also reflect concurrent infections. Secondary infections are common in the first week of stroke and are associated with poor outcome [3, 11, 12], but they usually occur more than 12 h after stroke onset. In our study, only two clinically overt infections were reported within 24 h after stroke onset. In addition, we excluded patients in whom the CRP level exceeded 2 SD above the mean, who may have had an infection before stroke onset. Furthermore, exclusion of patients developing an early infection during hospitalization did not substantially affect the relation between CRP levels and poor functional outcome or death.
The use of biomarkers as predictors of stroke lesion evolution and prognosis is becoming increasingly important, as they may be valuable tools in the search for an optimal management of stroke patients. The present study confirms results from previous studies that have advocated CRP as a powerful prognostic marker in patients with ischemic stroke [6, 8, 9, 15, 18, 20, 21, 25, 30]. It may provide important prognostic information beyond conventional clinical parameters. Further studies are needed to assess whether CRP is also a predictor of outcome after stroke.
Moreover, the fact that animal studies have found that CRP might exacerbate brain tissue damage following ischemic stroke, might stimulate research into the underlying pathogenetic mechanisms and development of new, more targeted, medical treatments for acute ischemic stroke.
Conclusion
Elevated CRP levels in the very early phase of acute ischemic stroke are independent prognostic factors for poor outcome at 3 months.
Acknowledgments
The PAIS trial was sponsored by the Netherlands Heart Foundation, grant number 2002B148. We are grateful to all patients, secretaries, and neurologists who contributed to this study. All patients participated in the PAIS trial; written informed consent was obtained from all patients or from their legal representatives for inclusion in the trial and for follow-up. The study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
Conflict of interest statement
The authors declare no conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Appendix: Participating centres with names of investigators
Meander MC Amersfoort (HMA van Gemert), Erasmus MC Rotterdam (DWJ Dippel), UMC Utrecht (HB van der Worp), Sint Franciscus Gasthuis Rotterdam (FH Vermeij), Slotervaart Ziekenhuis Amsterdam (VIH Kwa), Gelre ziekenhuizen Apeldoorn (HP Bienfait), TweeSteden ziekenhuis Tilburg (BPW Jansen), Sint Elisabeth Ziekenhuis Tilburg (PLM de Kort), Westfriesgasthuis Hoorn (TC van der Ree), Maasstad ziekenhuis Rotterdam (R Saxena), Ziekenhuis Bethesda Hoogeveen (PG Oomes), Dirksland Ziekenhuis Dirksland (UW Huisman), Sint Lucas Andreas Ziekenhuis Amsterdam (EJ Wouda), Martini Ziekenhuis Groningen (C Bouwsma), Catharina Ziekenhuis Eindhoven (K Keizer) Albert Schweitzer ziekenhuis Dordrecht (RP Kleyweg/H Kerkhoff), Amphia Ziekenhuis Breda (HBC Verbiest/SLM Bakker), Rijnland Ziekenhuis Leiderdorp (ELLM De Schryver), Diakonessenhuis Utrecht (RCJM Donders), Slingeland Ziekenhuis Doetinchem (RA van der Kruijk), IJsselland Ziekenhuis Capelle aan den Ijssel (J Heerema), Jeroen Bosch Ziekenhuis Den Bosch (RAJAM Bernsen), Ziekenhuis Hilversum Hilversum (D Herderschêe), Diaconessenhuis Meppel (EJW Keuter), Vlietlandziekenhuis Schiedam (WC Baart), Spaarne Ziekenhuis Hoofddorp (RJ Meijer), Beatrixziekenhuis Gorinchem (RB Alting van Geusau), Streekziekenhuis Koningin Beatrix Winterswijk (JP de Ruiter), Wilhelmina Ziekenhuis Assen (JN Wessel/AE Bollen).
References
- 1.Adams HP, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 2.Albert MA, Danielson E, Rifai N, et al. Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. JAMA. 2001;286:64–70. doi: 10.1001/jama.286.1.64. [DOI] [PubMed] [Google Scholar]
- 3.Aslanyan S, Weir CJ, Diener HC, et al. Pneumonia and urinary tract infection after acute ischaemic stroke: a tertiary analysis of the GAIN International trial. Eur J Neurol. 2004;11:49–53. doi: 10.1046/j.1468-1331.2003.00749.x. [DOI] [PubMed] [Google Scholar]
- 4.Barone FC, Feuerstein GZ. Inflammatory mediators and stroke: new opportunities for novel therapeutics. J Cereb Blood Flow Metab. 1999;19:819–834. doi: 10.1097/00004647-199908000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Brott T, Adams HP, Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20:864–870. doi: 10.1161/01.str.20.7.864. [DOI] [PubMed] [Google Scholar]
- 6.Christensen H, Boysen G. C-reactive protein and white blood cell count increases in the first 24 h after acute stroke. Cerebrovasc Dis. 2004;18:214–219. doi: 10.1159/000079944. [DOI] [PubMed] [Google Scholar]
- 7.den Hertog HM, van der Worp HB, van Gemert HM, et al. Correction: PAIS: paracetamol (acetaminophen) in stroke; protocol for a randomized, double blind clinical trial. [ISCRTN74418480] BMC Cardiovasc Disord. 2008;8:29. doi: 10.1186/1471-2261-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Napoli M, Papa F, Bocola V. C-reactive protein in ischemic stroke: an independent prognostic factor. Stroke. 2001;32:917–924. doi: 10.1161/01.str.32.4.917. [DOI] [PubMed] [Google Scholar]
- 9.Di Napoli M, Papa F, Bocola V. Prognostic influence of increased C-reactive protein and fibrinogen levels in ischemic stroke. Stroke. 2001;32:133–138. doi: 10.1161/01.str.32.1.133. [DOI] [PubMed] [Google Scholar]
- 10.Di Napoli M, Schwaninger M, Cappelli R, et al. Evaluation of C-reactive protein measurement for assessing the risk and prognosis in ischemic stroke: a statement for health care professionals from the CRP Pooling Project members. Stroke. 2005;36:1316–1329. doi: 10.1161/01.STR.0000165929.78756.ed. [DOI] [PubMed] [Google Scholar]
- 11.Emsley HC, Hopkins SJ. Acute ischemic stroke and infection: recent and emerging concepts. Lancet Neurol. 2008;7:341–353. doi: 10.1016/S1474-4422(08)70061-9. [DOI] [PubMed] [Google Scholar]
- 12.Emsley HC, Smith CJ, Gavin CM, et al. An early and sustained peripheral inflammatory response in acute ischemic stroke: relationships with infection and atherosclerosis. J Neuroimmunol. 2003;139:93–101. doi: 10.1016/S0165-5728(03)00134-6. [DOI] [PubMed] [Google Scholar]
- 13.Gill R, Kemp JA, Sabin C, Pepys MB. Human C-reactive protein increases cerebral infarct size after middle cerebral artery occlusion in adult rats. J Cereb Blood Flow Metab. 2004;24:1214–1218. doi: 10.1097/01.WCB.0000136517.61642.99. [DOI] [PubMed] [Google Scholar]
- 14.Griselli M, Herbert J, Hutchinson WL, et al. C-reactive protein and complement are important mediators of tissue damage in acute myocardial infarction. J Exp Med. 1999;190:1733–1740. doi: 10.1084/jem.190.12.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kocer A, Canbulat C, Gozke E, et al. C-reactive protein is an indicator for fatal outcomes in first-time stroke patients. Med Sci Monit. 2005;11:CR540–CR544. [PubMed] [Google Scholar]
- 16.Kushner I, Agrawal A. CRP can play both pro-inflammatory and anti-inflammatory roles. Mol Immunol. 2007;44:670–671. doi: 10.1016/j.molimm.2006.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ladenvall C, Jood K, Blomstrand C, et al. Serum C-reactive protein concentration and genotype in relation to ischemic stroke subtype. Stroke. 2006;37:2018–2023. doi: 10.1161/01.STR.0000231872.86071.68. [DOI] [PubMed] [Google Scholar]
- 18.Masotti L, Ceccarelli E, Forconi S, et al. Prognostic role of C-reactive protein in very old patients with acute ischaemic stroke. J Intern Med. 2005;258:145–152. doi: 10.1111/j.1365-2796.2005.01514.x. [DOI] [PubMed] [Google Scholar]
- 19.McColl BW, Allan SM, Rothwell NJ. Systemic inflammation and stroke: aetiology, pathology and targets for therapy. Biochem Soc Trans. 2007;35:1163–1165. doi: 10.1042/BST0351163. [DOI] [PubMed] [Google Scholar]
- 20.Montaner J, Fernandez-Cadenas I, Molina CA, et al. Poststroke C-reactive protein is a powerful prognostic tool among candidates for thrombolysis. Stroke. 2006;37:1205–1210. doi: 10.1161/01.STR.0000217744.89208.4e. [DOI] [PubMed] [Google Scholar]
- 21.Muir KW, Weir CJ, Alwan W, et al. C-reactive protein and outcome after ischemic stroke. Stroke. 1999;30:981–985. doi: 10.1161/01.str.30.5.981. [DOI] [PubMed] [Google Scholar]
- 22.Pepys MB, Hirschfield GM, Tennent GA, et al. Targeting C-reactive protein for the treatment of cardiovascular disease. Nature. 2006;440:1217–1221. doi: 10.1038/nature04672. [DOI] [PubMed] [Google Scholar]
- 23.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 24.Ridker PM, Rifai N, Pfeffer MA, et al. Long-term effects of pravastatin on plasma concentration of C-reactive protein. The Cholesterol and Recurrent Events (CARE) Investigators. Circulation. 1999;100:230–235. doi: 10.1161/01.cir.100.3.230. [DOI] [PubMed] [Google Scholar]
- 25.Smith CJ, Emsley HC, Gavin CM, et al. Peak plasma interleukin-6 and other peripheral markers of inflammation in the first week of ischaemic stroke correlate with brain infarct volume, stroke severity and long-term outcome. BMC Neurol. 2004;4:2. doi: 10.1186/1471-2377-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith CJ, Emsley HC, Vail A, et al. Variability of the systemic acute phase response after ischemic stroke. J Neurol Sci. 2006;251:77–81. doi: 10.1016/j.jns.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 27.Topakian R, Strasak AM, Nussbaumer K, et al. Prognostic value of admission C-reactive protein in stroke patients undergoing iv thrombolysis. J Neurol. 2008;255:1190–1196. doi: 10.1007/s00415-008-0866-y. [DOI] [PubMed] [Google Scholar]
- 28.van Breda EJ, van der Worp HB, van Gemert HM, et al. PAIS: paracetamol (acetaminophen) in stroke; protocol for a randomized, double blind clinical trial [ISRCTN 74418480] BMC Cardiovasc Disord. 2005;5:24. doi: 10.1186/1471-2261-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Swieten JC, Koudstaal PJ, Visser MC, et al. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–607. doi: 10.1161/01.str.19.5.604. [DOI] [PubMed] [Google Scholar]
- 30.Winbeck K, Poppert H, Etgen T, et al. Prognostic relevance of early serial C-reactive protein measurements after first ischemic stroke. Stroke. 2002;33:2459–2464. doi: 10.1161/01.STR.0000029828.51413.82. [DOI] [PubMed] [Google Scholar]