Abstract
Background
The Disability of the Arm, Shoulder and Hand (DASH) questionnaire is a region-specific self-administered questionnaire that consists of a disability/symptom (DASH-DS) scale, and two optional modules, the work (DASH-W) and the sport/music (DASH-SM) modules. The DASH was cross-culturally adapted and developed by the Impairment Evaluation Committee, Japanese Society for Surgery of the Hand. The purpose of this study was to test the reliability, validity, and responsiveness of the Japanese version of DASH (DASH-JSSH).
Methods
A series of 72 patients with upper extremity disorders completed the DASH-JSSH, the medical outcomes study 36-item short-form health survey (SF-36), and the Visual Analog Scale (VAS) for pain. Thirty-eight of the patients were reassessed for test-retest reliability 1 or 2 weeks later. Reliability was investigated by reproducibility and internal consistency. To analyze the validity, a principal component analysis and correlation coefficients between the DASH-JSSH and the SF-36 were obtained. Responsiveness was examined by calculating the standardized response mean (mean change/SD) and effect size (mean change/SD of baseline value) after carpal tunnel release of the 17 patients with carpal tunnel syndrome.
Results
Cronbach’s alpha coefficients in the DASH-DS and DASH-W were 0.962 and 0.967, respectively. The intraclass correlation coefficients for the same were 0.82 and 0.85, respectively. The unidimensionality of the DASH-DS and DASH-W were confirmed. The correlations between the DASH-DS score and the subscale of the SF-36 scale ranged from −0.29 to −0.73. The correlation coefficient between the DASH-DS and the DASH-W was 0.79. The standardized response mean/effect size of DASH-DS, DASH-W, and VAS for pain were −0.48/−0.26, −0.68/−0.41, and −0.40/−0.40, respectively. DASH-DS and DASH-W were as moderately sensitive as VAS for pain.
Conclusion
The DASH-DS and DASH-W Japanese version have evaluation capacities equivalent to those of the original and other language versions of the DASH.
Introduction
Upper extremity disorder can limit the activities of sufferers and have negative effects on their quality of life (QOL) and work activity. It is a common disorder and a costly health problem in the general population and in the workplace.1,2 Numerous epidemiological studies as well as those on psychosocial factors of upper extremity disorders in the workplace have been systematically reviewed and summarized.1,2
Health status is an important patient outcome used to evaluate medical (especially surgical3) intervention and in ergonomic studies.4 Several measures for the evaluation of upper extremity function have been developed.5–10 Most of them are joint-specific5,10 or disease-specific.7,8 Others are intended to evaluate the function of the entire upper extremity using a regionspecific measure.6,9 The Disability of the Arm, Shoulder, and Hand (DASH) questionnaire was devised as a region-specific measure by the American Academy of Orthopaedic Surgeons (AAOS) in collaboration with a number of other organizations.6 The rationale for use of this measure is that the upper extremity is a functional unit or kinetic chain.11 Therefore, the DASH is suitable for measuring health status outcome because it is mainly a measure of disability.
The DASH is now available in several languages,12–18 and studies of its reliability and validity have been published for not only the original version3 but also for the Swedish,12 German,14 Spanish,15 Dutch,16 Italian,17 and Chinese18 versions. However, one of the optional DASH modules, the work module, has not been studied except in the Italian and Chinese versions.17,18 We, the Impairment Evaluation Committee (Japanese Society for Surgery of the Hand), have completed cross-cultural adaptation and developed the DASH Japanese version (DASH-JSSH). The purpose of this study was to test the reliability, validity, and responsiveness of the DASH-JSSH (including the work module) and to make the DASH-JSSH available for use in Japan.
Material and methods
In accordance with published guidelines,19,20 we organized the DASH-JSSH committee consisting of translators, researchers, a methodologist, and a Japanese-language expert.
Adaptation process
The English version of the DASH (version 2.0) was translated into Japanese by two translators whose first language was Japanese. One of them had no medical background, and the other did. Their two “forward” translations were synthesized into one after being reviewed and discussed by the committee. This Japanese version (prefinal version) was translated back into English by two other translators whose first language was English. One of them was blinded to the concepts being investigated and had no medical background. The other had a medical background. We collected pilot test data and submitted it with the DASH-JSSH (prefinal version) to the AAOS in 2002. They suggested that the DASH-JSSH (prefinal version) needed to be modified in terms of cultural adaptation. Following their suggestions, our committee modified the DASH-JSSH prefinal version into a new, final version. The final DASH-JSSH version was then evaluated with regard to reliability, validity, and responsiveness.
DASH questionnaire
The main part of the DASH is a 30-item disability/symptom (DASH-DS) scale concerning the patient’s upper extremity.3,6 Each item has five response choices, ranging from “no difficulty or no symptom” to “unable to perform activity or very severe symptom.” It is scored on a scale of 1–5. The items ask about the severity of each of the symptoms of pain, activity-related pain, tingling, weakness, and stiffness (five items: numbers 24–28); the degree of difficulty when performing various physical activities because of an arm, shoulder, or hand problem (21 items: numbers 1–21); the effect of the upper extremity problem on social activities, work, and sleep (three items: numbers 22, 23, 29); and the psychological effect on self-image (one item: number 30). These provide the DASH disability/symptom (DASH-DS) score ranging from 0 (no disability) to 100 (the severest disability), after summation of the scores from all items and transformation.
The DASH also contains two optional modules concerning the ability to work and the ability to perform sports or play musical instruments. These two optional modules each consists of four items, each of which is rated on a scale of 1–5. These provide the DASH work (DASH-W) score and the DASH sport/music (DASH-SM) score ranging from 0 (no disability) to 100 (the severest disability) after summation of the scores from all items and transformation.
Patients and setting
A series of 73 patients with upper extremity disorders were seen on an outpatient basis in five orthopedic surgery departments in Japan. Exclusion criteria were age below 18 years and relevant co-morbidity (e.g., connective tissue disease). One patient with a co-morbidity of systemic lupus erythematosus (SLE) was excluded. The study was thus conducted on 72 patients (17 men, 55 women) who were suffering from carpal tunnel syndrome (38 patients), rotator cuff disease (10 patients), cubital tunnel syndrome (7 patients), thoracic outlet syndrome (4 patients), or other problems (13 patients). The mean age was 54.1 years (SD 14.9 years, range 20–81 years). After informed consent was obtained from the patients to participate in this study, they answered the questionnaire. The DASH-JSSH questionnaire, the official Japanese version of the 36-item short-form health survey (SF-36, version 1.2)21,22 and the Visual Analog Scale (VAS) (0–10 scale) for pain. The data collected from the 72 patients were used as a baseline value. Among the 72 patients, the 38 who had no therapy during the consecutive outpatient visits were readministered the DASH-JSSH questionnaire and VAS for pain 1 or 2 weeks later. The 17 patients with carpal tunnel syndrome who underwent carpal tunnel release by three hand surgeons answered the DASH-JSSH questionnaire and the VAS for pain twice preoperatively and postoperatively (3 months after surgery). The protocol of this study was reviewed and approved by the institutional review board prior to this implementation.
Assessment of reliability, validity, and responsiveness
Reliability was investigated by looking at the reproducibility and internal consistency based on the test-retest method. The following analysis was conducted to examine the validity. A principal component analysis was conducted to examine the construct validity and the unidimensionality of the DASH-JSSH disability/symptom (DASH-JSSH-DS) and DASH-JSSH work (DASH-JSSH-W) scales. Completeness of item responses of the DASH-JSSH and VAS for pain was examined. Correlation coefficients between the DASH-JSSH and the SF-36 were obtained, and the following hypotheses were examined to investigate concurrent validity: (1) “Physical functioning” (SF-36-PF) or “role-physical” (SF-36-RP) would exhibit the strongest association. (2) “Bodily pain” (SF-36-BP) would exhibit the next strongest association. (3) “Mental health” (SF-36-MH) and “vitality” (SF-36-VT) would exhibit the weakest association.
Correlation coefficients between the DASH-JSSH-DS and the DASH-JSSH-W or the DASH-JSSH sport/music (DASH-JSSH-SM) scales were also obtained. Correlation coefficients between the DASH-JSSH-DS and the SF-36 were obtained. Correlation coefficients between the DASH-JSSH and VAS for pain were obtained, and the criterion-based validity investigation looked at the following hypothesis: The correlation between the DASH-JSSH and VAS for pain would be high. The responsiveness was examined by calculating the standardized response mean (SRM) (mean change/SD)23 and effect size (mean change/SD of baseline value)24 after carpal tunnel release of the patients with carpal tunnel syndrome.
Statistical analysis
Kolmogorov-Smirnov and Liliefors probability tests were used to assess distribution of the DASH-JSSH, SF-36, VAS for pain, ages of the subjects, time required to fill out the questionnaires of the DASH-JSSH and SF-36. The interval measurements [DASH-JSSH-DS, DASH-JSSH-SM, SF-36-BP, general health subscale of SF-36 (SF-36-GH), SF-36-VT, SF-36-MH, VAS, age, time required], were normally distributed, and therefore correlation was assessed using a parametric test (Pearson’s correlation). The other interval measurements [DASH-JSSH-W, SF-36-PF, SF-36-RP, social functioning subscale of SF-36 (SF-36-SF) and roleemotional subscale of SF-36 (SF-36-RE)] were not normally distributed, and therefore correlation was assessed using a nonparametric test (Spearman’s correlation). Crohnbach’s alpha was used to assess internal consistency. Instrument test-retest reliability was assessed with the intraclass correlation coefficient (ICC).
All statistical analyses were conducted using the Statistical Package for Social Science (SPSS) version 12.0J software. The critical values for significance were set at P < 0.05.
Results
Completeness of item responses
No patients had difficulty completing the DASH-JSSH questionnaire. It took them 8 min 1 s, on average, to finish the questionnaire: 95% confidence interval (95% CI): 7 min 2 s to 9 min 1 s. Most of the patients considered all the items of the DASH-JSSH-DS section to be clear. Of the 72 patients, 9 (nonrespondent group) did not answer one or more items of DASH-JSSH-DS. None of them failed to answer more than three items. Seven patients did not answer item 21 regarding sexual activity. Three patients did not respond to item 19. Five patients did not respond to items 2, 7, 8, 18, and 25, with each item unanswered by one of them. The mean age (64 ± 12 years) of the nonrespondent group (n = 9) was significantly higher than the mean age (53 ± 15 years) of the respondent group (n = 63) who completed all the items (P = 0.038). Of the 72 patients, 55 (76%) answered the DASH-JSSH-W. However, only 15 of the 72 (21%) patients responded to the DASH-JSSH-SM.
The mean DASH-JSSH scores, mean SF-36 subscale scores, mean VAS, and their ranges are shown in Table 1. The numbers of ceiling and floor scores of the DASH-JSSH questionnaire, SF-36 subscales and VAS are shown in Table 2. No patients recorded the minimum disability score of 0 on the DASH-JSSH-DS (ceiling) or the maximum disability score of 100 on the DASH-JSSH-DS (floor). Twelve and four patients had the maximum scores, and three and four patients had the minimum scores, for the DASH-JSSH-W and the DASH-JSSH-SM, respectively.
Table 1.
Scores for DASH-JSSH, SF-36, and VAS
| Score | ||||
|---|---|---|---|---|
| DDASH-JSSH-DS | 72 | 28 (21) | 25 | 3–93 |
| DASH-JSSH-W | 55 | 33 (32) | 25 | 0–100 |
| DASH-JSSH-SM | 15 | 47 (43) | 44 | 0–100 |
| SF-36-PF | 72 | 72 (24) | 80 | 0–100 |
| SF-36-RP | 72 | 48 (41) | 50 | 0–100 |
| SF-36-BP | 72 | 47 (23) | 42 | 0–100 |
| SF-36-GH | 72 | 50 (21) | 49 | 5–97 |
| SF-36-VT | 72 | 56 (25) | 57 | 0–100 |
| SF-36-SF | 72 | 76 (26) | 88 | 13–100 |
| SF-36-RE | 72 | 55 (43) | 67 | 0–100 |
| SF-36-MH | 72 | 61 (25) | 60 | 8–100 |
| VAS [0–10] | 72 | 4.7 (2.8) | 5.0 | 0–10 |
DASH-JSSH-DS, disability/symptom scale of DASH-JSSH; DASH-JSSH-W, work module of DASH-JSSH; DASH-JSSH-SM, sport/music module of DASH-JSSH; SF-36-PF, physical functioning subscale of the 36-item Short-Form Health Survey (SF-36); SF-36-RP, rolephysical subscale of SF-36; SF-36-BP, bodily pain subscale of SF-36; SF-36-GH, general health subscale of SF-36; SF-36-VT, vitality subscale of SF-36; SF-36-SF, social functioning subscale of SF-36; SF-36-RE, role-emotional subscale of SF-36; SF-36-MH, mental health subscale of SF-36; VAS [0–10], Visual Analog Scale for pain [0–10 scale]
Table 2.
Ceiling and floor scores for DASH-JSSH, SF-36, and VAS
| Instrument scale | No. | No. ceiling scoresa | No. floor scoresb |
|---|---|---|---|
| DASH-JSSH-DS | 72 | 0 | 0 |
| DASH-JSSH-W | 55 | 12 (22%) | 3 (6%) |
| DASH-JSSH-SM | 15 | 4 (27%) | 4 (27%) |
| SF-36-PF | 72 | 4 (6%) | 1 (1%) |
| SF-36-RP | 72 | 18 (25%) | 24 (34%) |
| SF-36-BP | 72 | 3 (4%) | 2 (3%) |
| SF-36-GH | 72 | 0 | 0 |
| SF-36-VT | 72 | 1 (1%) | 2 (3%) |
| SF-36-SF | 72 | 31 (43%) | 0 |
| SF-36-RE | 72 | 29 (41%) | 23 (32%) |
| SF-36-MH | 72 | 2 (3%) | 0 |
| VAS [0–10] | 72 | 4 (6%) | 2 (3%) |
a Maximum health status scores
b Minimum health status scores
Reliability
Internal consistency was assessed by use of Cronbach’s alpha coefficient. The alpha coefficient for the 30 items in the DASH-JSSH-DS was high (0.962). When the alpha coefficient was calculated for each of the 30 items by eliminating each item, one by one, the range was 0.959–0.963; and no items were found to change the internal consistency substantially. The alpha coefficients for the four items in the DASH-JSSH-W and DASH-MS were also high (0.967 and 0.985, respectively). When the alpha coefficients were calculated for each of the four items by eliminating each item, one by one, the ranges were 0.952–0.963 and 0.971–0.989, respectively. No items were found to change the internal consistency substantially.
Instrument test-retest reliability was assessed with the intraclass correlation coefficient (ICC). There were 38 patients for the test-retest reliability, and the period between the first and second tests was a mean of 9.2 days (range 6-17 days). The ICC for the DASH-JSSH-DS was 0.82 (95% CI 0.69–0.90). The ICCs for the DASH-JSSH-W and DSSH-JSSH-SM were 0.85 (95% CI 0.70–0.93) and 0.91 (95% CI 0.64–0.98), respectively. All ICCs for the DASH-JSSH indicate sufficient reproducibility.
Validity
A principal component analysis was conducted to confirm the unidimensionality of the DASH-JSSH-DS. The first factor had an eigenvalue (amount of variation in the total sample accounted for by that factor)16 of 14.65, which explained the 49% total variance of the DASH-JSSH-DS scores of the patients. The unidimensionality was found to be strong as a result of a substantial difference between the first and the second factors (eigenvalue 2.80) (Fig. 1). When looking at the first factor loading for each item, all items except item 24 exhibited loading (the correlation with the total score) of 0.4 or higher (Table 3) and communalities of more than 0.6, except question 30 (communality 0.47). The unidimensionality of the DASH-JSSH-W was also confirmed by a principal component analysis. The eigenvalue for the first factor was 3.652, which explained the 91% total variance of the DASH-JSSH-W scores of the patients. Furthermore, the unidimensionality of the combined items for the DASH-JSSH-DS and the DASH-JSSH-W was also confirmed by a principal component analysis (Fig. 2). The eigenvalue for the first factor was 17.94, which explained the 53% total variance of the combined scores of the patients. The unidimensionality was found to be strong as a result of a substantial difference between the first and second factors (eigenvalue 2.68). All combined items exhibited first loading (the correlation with the total score) of more than 0.4 and communalities of more than 0.7.
Fig. 1.
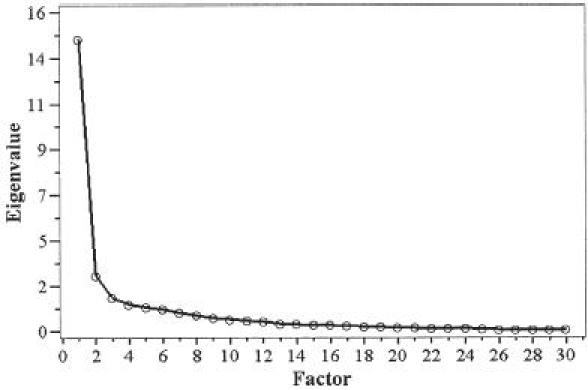
Scree plot of the principal components in the disability/symptom scale of Disability of the Arm, Shoulder, and Hand — Japanese Society for Surgery of the Hand (DASH-JSSH-DS)
Table 3.
Factor loading (unrotated) of principal components of DASH-JSSH-DS
| Item no. | Loading |
|---|---|
| 1 | 0.631 |
| 1 | 0.631 |
| 2 | 0.707 |
| 3 | 0.752 |
| 4 | 0.770 |
| 5 | 0.817 |
| 6 | 0.763 |
| 7 | 0.814 |
| 8 | 0.843 |
| 9 | 0.820 |
| 10 | 0.683 |
| 11 | 0.797 |
| 12 | 0.831 |
| 13 | 0.767 |
| 14 | 0.731 |
| 15 | 0.632 |
| 16 | 0.702 |
| 17 | 0.745 |
| 18 | 0.722 |
| 19 | 0.759 |
| 20 | 0.585 |
| 21 | 0.708 |
| 22 | 0.621 |
| 23 | 0.787 |
| 24 | 0.323 |
| 25 | 0.404 |
| 26 | 0.612 |
| 27 | 0.667 |
| 28 | 0.497 |
| 29 | 0.545 |
| 30 | 0.606 |
Fig. 2.
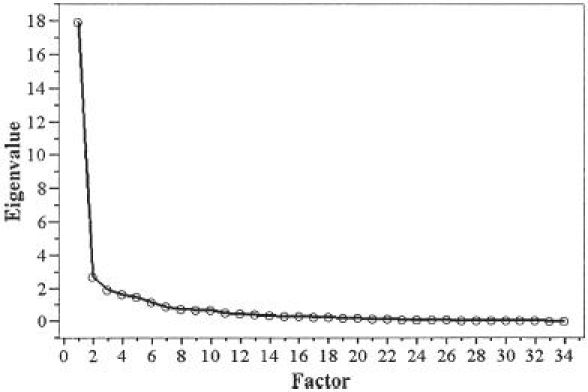
Scree plot of the principal components in combined items of DASH-JSSH-DS and the work module of DASH-JSSH (DASH-JSSH-W)
The correlations between the DASH-JSSH-DS score and the subscales of the SF-36 scale ranged from −0.29 to −0.73 (Table 4). The strongest correlation was observed in “role-physical” followed by “physical functioning” and “bodily pain.” The correlation between DASH-JSSH-DS and “mental health” or “vitality” was somewhat weak. These results support the hypothesis set down in advance except the strongest correlation (Table 4). Correlation coefficients between the DASH-JSSH-DS and the DASH-JSSH-W or the DASH-JSSH-SM were 0.79 and 0.82, respectively (Table 4) (P < 0.01). These results indicate strong correlations between the DASH-JSSH-DS and the DASH-JSSH-W and between the DASH-JSSH-DS and the DASH-JSSH-SM.
Table 4.
DASH-JSSH, SF-36, VAS, and their correlations
| Correlation coefficients with | ||||
|---|---|---|---|---|
| Instrument scale | No. | DASH-JSSH-DS‡ | DASH-JSSH-W# | DASH-JSSH-SM‡ |
| DASH-JSSH-DS‡ | 72 | — | — | — |
| DASH-JSSH-W# | 55 | 0.79** | — | — |
| DASH-JSSH-SM‡ | 15 | 0.82** | 0.58* | — |
| SF-36-PF# | 72 | −0.62** | −0.45** | −0.65** |
| SF-36-RP# | 72 | −0.73** | −0.77** | −0.72** |
| SF-36-BP‡ | 72 | −0.61** | −0.47** | −0.24 |
| SF-36-GH‡ | 72 | −0.44** | −0.52** | 0.19 |
| SF-36-VT# | 72 | −0.36** | −0.42** | −0.15 |
| SF-36-SF# | 72 | −0.29** | −0.38* | 0.12 |
| SF-36-RE# | 72 | −0.53** | −0.55** | −0.04 |
| SF-36-MH‡ | 72 | −0.38** | −0.42** | −0.17 |
| VAS [0–0]‡ | 72 | 0.47** | 0.64** | 0.45 |
* P < 0.05; **P < 0.01; ‡ Pearson’s correlation; # Spearman’s correlation
The correlations between the DASH-JSSH-W score and the subscales of the SF-36 scale ranged from −0.38 to −0.77 (Table 4). These correlations were similar to the correlations between the DASH-JSSH-DS score and the subscales of the SF-36 scale. The correlations between the DASH-JSSH-SM score and the subscales of the SF-36 scale were weak and ranged from 0.12 to −0.65 (Table 4).
No statistical difference in age was found between men and women (P = 0.161). The DASH-JSSH-DS mean score was 49.9 (SD 4.7) for men and 55.4 (SD 1.8) for women. There was no statistical difference between them (P = 0.194). This result supports our hypothesis. The correlation between the DASH-JSSH-DS score and age was weak (r = 0.296, P < 0.05).
The correlation between the DASH-JSSH-DS score and the degree of pain was examined and observed as moderate for the SF-36 bodily pain score and the VAS for pain (Table 4). The correlation between the DASH-JSSH-W score and the degree of pain was also examined and observed as moderate for the SF-36 bodily pain score and the VAS for pain (Table 4).
Responsiveness
Of 38 patients with carpal tunnel syndrome, 17 underwent carpal tunnel release. They completed the DASH-JSSH and VAS for pain 3 months after the surgery. The mean subject age was 57 years (SD 10 years; range 48–78 years). There were 16 females and one male. Of the 17 patients, 13 completed the DASH-JSSH-W, and those scores were included for this study. On the other hand, only 3 of the 17 patients completed the DASH-JSSH-SM, so this score was excluded for this study. All scores for DASH-JSSH-DS, DASH-JSSH-W, and VAS for pain were normally distributed. The calculated SRM and effect size of DASH-JSSH-DS, DASH-JSSH-W, and VAS for pain were −0.48/−0.26, −0.68/−0.41, and −0.40/−0.40, respectively. There was no statistical difference between the mean values of preoperative and postoperative DASH-JSSH-DS scores (P = 0.068). There was a statistical difference between the mean values of preoperative and postoperative DASH-JSSH-W scores (P = 0.030). There was no statistical difference between the mean value of preoperative and postoperative VAS scores for pain (P = 0.119). DASH-JSSH-DS and DASH-JSSH-W were as moderately sensitive as VAS for pain.
Discussion
Japanese adaptation of the DASH questionnaire was performed following a systematic standardized approach. 19,20 The purpose of this study was to examine the psychometric qualities of the DASH-JSSH by assessing its psychometric standards in the area of the reliability, validity, and responsiveness.
The DASH-JSSH consists of a 30-item scale and two optional 4-item scale modules. It took patients a similar amount of time to complete the DASH-JSSH compared with the time to complete the other language versions.13 This indicated that the questionnaire was easy to understand. Elderly patients left no more than three items unanswered, and those were thought to be pertaining to specific activities, such as sexual and recreational activities, not performed by those individuals.
The lack of floor and ceiling effects assure the authors of the validity of this version of the DASH-JSSH-DS.
The validation process of the DASH-JSSH scale has shown that it has reliability similar to that of the other language versions12–18 including the original one.25 This scale, as was the original DASH scale, was developed for clinical assessment of a patient group, not of individual patients.22 Internal consistency needs to be higher than 0.95 if a scale is to be used for tracking individual patients.26 Most translated versions of the DASH12,14–17 and the original one6 had internal consistency higher than 0.95. Thus, most language versions of the DASH, including the Japanese version, could be used with caution for daily assessment of individual patient status over time.
The validation process of the DASH-JSSH questionnaire has shown that it has validity similar to that of the other language versions,12,14–18 including the original DASH.25 The strong correlations between the DASHJSSH-DS and SF-36 subscales (role of physical health, physical functioning, and bodily pain) support this validity and demonstrate results similar to those of the validation papers for the other language versions including the original DASH.
The strong correlations between the DASH-JSSH-W and SF-36 subscales (role of physical health, physical functioning, and bodily pain) support this validity and demonstrate results similar to those of the validation paper for the Italian version.17 The correlations between the DASH-JSSH-SM and SF-36 subscales were weak and demonstrated results similar to those of the validation paper for the Italian version17 because of the small sample size. This area needs further investigation. These results demonstrated that the DASH-JSSH (especially DASH-JSSH-DS and DASH-JSSH-W) measures the important elements that make up healthrelated QOL.
The DASH-JSSH-DS scale exhibited high unidimensionality, and there was no low item-scale correlation. These results were similar to those of the Dutch version.16 The DASH-JSSH-W scale also exhibited high unidimensionality, and there was no low itemscale correlation. The communalities of these two scales (DASH-JSSH-DS and DASH-JSSH-W) were high. These results show that the DASH-JSSH-W has a high quality of validation.
Cohen’s rule-of-thumb for interpreting the “effect size index” — a value of 0.2 is small, 0.5 is moderate, and 0.8 or greater is large — can be applied to the SRM.23 The responsiveness of the DASH-JSSH-DS and DASH-JSSH-W for the patients with carpal tunnel syndrome was moderate 3 months after carpal tunnel release operation, although other studies showed a higher responsiveness of the DASH-DS than our results.27 There was no statistical difference between the mean value of preoperative and postoperative DASH-JSSHDS scores. This is thought to be due to the small sample size and requires further investigation.
We believe the strengths of this study are that the DASH-JSSH-W and DASH-JSSH-DS scales demonstrated good reproducibility, consistency, and validity. Moreover, both of them had moderate responsiveness.
A limitation of this present study is that we cannot successfully validate the DASH-JSSH-SM because the sample size was relatively small and the patient’s response rate was low. The samples of this study are not representative of the general population.
Conclusions
We have concluded that the DASH-DS and DASH-W Japanese version have evaluation capacities equivalent to those of the other language versions of the DASH. We expect that use of these scales in Japan to assess treatment by the patients themselves will contribute to meaningful improvement of outcome for patients with upper extremity disorders.
Footnotes
An erratum to this article is available at http://dx.doi.org/10.1007/s00776-006-1094-x.
References
- 1.Musculoskeletal disorders and the workplace: low back and upper extremities. Washington, DC: National Academy Press; 2001. pp. 38–64. [PubMed] [Google Scholar]
- 2.Bernard BP, editor. Musculoskeletal disorders and workplace factors: a critical review of epidemiologic evidence for work-related musculoskeletal disorders of the neck, upper extremity, and low back. Bethesda, MD: US Department of Health and Human Services; 1997. [Google Scholar]
- 3.Dawson J, Carr A. Outcomes evaluation in orthopaedics. J Bone Joint Surg Br. 2001;83:313–5. doi: 10.1302/0301-620X.83B3.12148. [DOI] [PubMed] [Google Scholar]
- 4.Brouwer B, Mazzoni C, Pearce GW. Tracking ability in subjects symptomatic of cumulative trauma disorder: does it relate to disability? Ergonomics. 2001;44:443–56. doi: 10.1080/00140130010017868. [DOI] [PubMed] [Google Scholar]
- 5.Constant CR, Murley AHG. A clinical method of functional assessment of the shoulder. Clin Orthop. 1987;214:161–4. [PubMed] [Google Scholar]
- 6.Hudak PL, Amadio PC, Bombardier C, the Upper Extremity Collaborative Group (UECG) Development of an upper extremity outcome measure: the DASH (disabilities of the arm, shoulder and hand) [corrected] Am J Ind Med. 1996;29:602–8. doi: 10.1002/(SICI)1097-0274(199606)29:6<602::AID-AJIM4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 7.Kirkley A, Griffin S, McLintock H, Ng L. The development and evaluation of a disease-specific quality of life measurement tool for shoulder instability: the Western Ontario Shoulder Instability Index (WOSI) Am J Sports Med. 1998;26:764–72. doi: 10.1177/03635465980260060501. [DOI] [PubMed] [Google Scholar]
- 8.Levine DW, Simmons BP, Koris MJ, Daltroy LH, Hohl GG, Fossel AH, et al. A self-administered questionnaire for the assessment of severity of symptoms and functional status in carpal tunnel syndrome. J Bone Joint Surg Am. 1993;75:1585–92. doi: 10.2106/00004623-199311000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Martin DP, Engelberg R, Agel J, Swiontrowski MF. Comparison of the Musculoskeletal Function Assessment Questionnaire with the Short Form 36, the Western Ontario and McMaster Universities Osteoarthritis Index, and the Sickness Impact Profile Health Status Measures. J Bone Joint Surg Am. 1997;79:1323–35. doi: 10.2106/00004623-199709000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Research Committee, American Shoulder and Elbow Surgeons. Richards R, An KN, Bigliani LU, Friedman RJ, Gartsman GM, Gristina AG, et al. A standardized method for the assessment of shoulder function. J Shoulder Elbow Surg. 1994;3:347–52. doi: 10.1016/S1058-2746(09)80019-0. [DOI] [PubMed] [Google Scholar]
- 11.Davis AM, Beaton DE, Hudak P, Amadio P, Bombardier C, Cole D, et al. Measuring disability of the upper extremity: a rationale supporting the use of a regional outcome measure. J Hand Ther. 1999;12:269–74. doi: 10.1016/s0894-1130(99)80063-5. [DOI] [PubMed] [Google Scholar]
- 12.Atroshi I, Gummesson C, Andersson B, Dahlgren E, Johansson A. The disabilities of the arm, shoulder and hand (DASH) outcome questionnaire: reliability and validity of the Swedish version evaluated in 176 patients. Acta Orthop Scand. 2000;71:613–8. doi: 10.1080/000164700317362262. [DOI] [PubMed] [Google Scholar]
- 13.Dubert T, Voche P, Dumontier C, Dinh A. Le questionnaire DASH: adaptation française d’un outil d’évaluation international. Chir Main. 2001;20:294–302. doi: 10.1016/S1297-3203(01)00049-X. [DOI] [PubMed] [Google Scholar]
- 14.Offenbaecher M, Ewert T, Sangha O, Stucki G. Validation of a German version of the Disabilities of Arm, Shoulder, and Hand questionnaire (DASH-G) J Rheumatol. 2002;29:401–2. [PubMed] [Google Scholar]
- 15.Rosales RS, Delgado EB, De La Lastra-Bosch ID. Evaluation of the Spanish version of the DASH and carpal tunnel syndrome health-related quality-of-life instruments: cross-cultural adaptation process and reliability. J Hand Surg [Am] 2002;27:334–43. doi: 10.1053/jhsu.2002.30059. [DOI] [PubMed] [Google Scholar]
- 16.Veehof MM, Sleegers EJA, van Veldhoven NHMJ, Schuurman AH, van Meeteren NLU. Psychometric qualities of the Dutch language version of the Disabilities of the Arm, Shoulder, and Hand questionnaire (DASH-DLV) J Hand Ther. 2002;15:347–354. doi: 10.1016/s0894-1130(02)80006-0. [DOI] [PubMed] [Google Scholar]
- 17.Padua R, Padua L, Ceccarelli E, Romanini E, Zanoli G, Amadio PC, et al. Italian version of the Disability of the Arm, Shoulder and Hand (DASH) questionnaire: cross-cultural adaptation and validation. J Hand Surg [Br] 2003;28:179–86. doi: 10.1016/s0266-7681(02)00303-0. [DOI] [PubMed] [Google Scholar]
- 18.Lee EWC, Lau JSY, Chung MMH, Li APS, Lo SK. Evaluation of the Chinese version of the Disability of the Arm, Shoulder and Hand (DASH-HKPWH): cross-cultural adaptation process, internal consistency and reliability study. J Hand Ther. 2004;17:417–23. [PubMed] [Google Scholar]
- 19.Beaton DE, Bombardier C, Guillemin F, Ferraz MB. Guidelines for the process of cross-cultural adaptation of self-report measures. Spine. 2000;25:3186–91. doi: 10.1097/00007632-200012150-00014. [DOI] [PubMed] [Google Scholar]
- 20.Guillemin F, Bombardier C, Beaton D. Cross-cultural adaptation of health-related quality of life measures: literature review and proposed guidelines. J Clin Epidemiol. 1993;46:1417–32. doi: 10.1016/0895-4356(93)90142-N. [DOI] [PubMed] [Google Scholar]
- 21.Fukuhara S, Bito S, Green J, Hsiao A, Kurokawa K. Translation, adaptation, and validation of the SF-36 health survey for use in Japan. J Clin Epidemiol. 1998;51:1037–44. doi: 10.1016/S0895-4356(98)00095-X. [DOI] [PubMed] [Google Scholar]
- 22.Fukuhara S, Ware JE, Kosinski M, Wada S, Gandek B. Psychometric and clinical tests of validity of the Japanese SF-36 health survey. J Clin Epidemiol. 1998;51:1045–53. doi: 10.1016/S0895-4356(98)00096-1. [DOI] [PubMed] [Google Scholar]
- 23.Liang MH, Fossel AH, Larson MG. Comparisons of five health status instruments for orthopedic evaluation. Med Care. 1990;28:632–42. doi: 10.1097/00005650-199007000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Kazis LE, Anderson JJ, Meenan RF. Effect sizes for interpreting changes in health status. Med Care. 1989;27(Suppl):S178–89. doi: 10.1097/00005650-198903001-00015. [DOI] [PubMed] [Google Scholar]
- 25.Beaton DE, Katz JN, Fossel AH, Wright JG, Tarasuk V. Measuring the whole or the parts? Validity, reliability, and responsiveness of the Disabilities of the Arm, Shoulder and Hand outcome measure in different regions of the upper extremity. J Hand Ther. 2001;14:128–46. [PubMed] [Google Scholar]
- 26.McHorney CA, Tarlov AR. Individual-patient monitoring in clinical practice: are available health status surveys adequate? Qual Life Res. 1995;4:293–307. doi: 10.1007/BF01593882. [DOI] [PubMed] [Google Scholar]
- 27.Gay RE, Amadio PC, Johnson JC. Comparative responsiveness of the Disabilities of the Arm, Shoulder, and Hand, the Carpal Tunnel Questionnaire, and the SF-36 to clinical change after carpal tunnel release. J Hand Surg [Am] 2003;28:250–4. doi: 10.1053/jhsu.2003.50043. [DOI] [PubMed] [Google Scholar]


