Abstract
The production of autoantibodies to citrullinated type II collagen and the citrullination of type II collagen were analyzed in rheumatoid arthritis. Autoantibodies to citrullinated type II collagen were detected in 78.5% of serum samples from 130 rheumatoid arthritis patients. Autoantibodies to native noncitrullinated type II collagen were detected in 14.6% of serum samples, all of which were positive for anti-citrullinated type II collagen antibodies. Serum samples were also positive for anti-citrullinated type II collagen antibodies in 1 of 31 systemic lupus erythematosus patients and 2 of 55 patients with osteoarthritis of the knee. In contrast, sera samples from 24 systemic sclerosis patients, 21 dermatomyositis/polymyositis patients, 21 ankylosing spondylitis patients, and 18 psoriatic arthritis patients were all negative for anti-citrullinated type II collagen antibodies. Anti-citrullinated type II collagen antibodies and fragments of citrullinated type II collagen were found in the synovial fluid obtained from affected knee joints of 15 rheumatoid arthritis patients. Moreover, anti-citrullinated type II collagen antibodies were isolated from the synovium of affected knee joints in 8 rheumatoid arthritis patients using antigen/antibody immunocomplex dissociation buffer but not by using standard buffers. These findings indicate that autoantibodies that react with citrullinated type II collagen are specifically produced and that immunocomplexes composed of fragments of citrullinated type II collagen and autoantibodies are deposited in the inflamed articular synovium in rheumatoid arthritis patients. Assaying for the presence of anti-citrullinated type II collagen antibodies may therefore be useful for diagnosing rheumatoid arthritis, and the deposition of these immunocomplexes in the articular synovium may be involved in pathogenesis.
Key words: Autoantibody, Citrullination, Collagen type II, Rheumatoid arthritis (RA)
Introduction
Rheumatoid arthritis (RA) is associated with citrullination, which is the conversion of arginine residues into citrulline residues. This process is catalyzed by the enzyme peptidylarginine deiminase (PADI). Citrullination plays an important role in protein degradation, as molecular interactions that are mediated by positively charged arginine residues are lost upon citrullination.1,2 Citrullinated proteins have been found in the synovium of RA patients.3 Moreover, four single nucleotide polymorphisms (SNPs) have been identified in the exons of the PADI type 4 (PADI-4) gene in RA patients. Susceptible PADI-4 messenger RNA (mRNA) including four SNPs is significantly more stable than nonsusceptible mRNA.4,5 PADI-4 is primarily expressed in granulocytes, where expression is generally induced by nonspecific inflammation and lasts for a few days.2 PADI-4 protein presumably leaks out of granulocytes following cell death and then goes on to citrullinate proteins or degraded peptides in affected RA joints.
Individuals with RA frequently have autoantibodies to citrullinated peptides derived from filaggrin or fibrin.6–9 However, the involvement of these autoantibodies in RA pathogenesis remains unclear, since filaggrin is not present in articular joints and fibrin is not a joint-specific molecule. Because type II collagen is a major and specific molecule in articular joints, we investigated whether this protein is citrullinated and whether autoantibodies react with citrullinated type II collagen in sera and joint fluids of RA patients.
Patients and methods
Subjects
Serum samples were obtained from patients with RA and were stored at −80°C until use. Diagnosis and anatomical staging of the disease were established according to the American Rheumatism Association (ARA) criteria. Of 130 patients (24 men and 106 women; mean age, 56.2 ± 12.4 years), 24 were classified as stage I, 46 as stage II, 41 as stage III, and 19 as stage IV according to the Steinbrocker classification. Serum samples were also obtained from 14 patients with polyarticular-type juvenile rheumatoid arthritis (JRA), 31 systemic lupus erythematosus (SLE) patients, 24 systemic sclerosis patients, 21 dermatomyositis/polymyositis patients, 21 ankylosing spondylitis patients, 18 psoriatic arthritis patients, and 55 patients with osteoarthritis (OA) of the knee. Diagnoses of these patients were established according to their respective clinical criteria. Forty healthy donors (11 men and 29 women; mean age, 53.8 ± 16.4 years) provided serum samples as controls.
Synovial fluid samples were obtained from 15 patients with RA (3 men and 12 women; mean age, 61.2 ± 8.7 years; 11 stage II, 4 stage III [Steinbrocker classification]), and 41 patients with OA (9 men and 32 women; mean age, 68.3 ± 5.1 years) by aspiration from knee joints at the outpatient clinic of the Jikei University hospital, and were stored at −80°C until use. Synovial samples were obtained from 8 patients with RA (2 men and 6 women; mean age, 52.7 ± 5.5 years; all stage II [Steinbrocker classification]) and 11 patients with OA (3 men and 8 women; mean age, 63.6 ± 5.4 years) by resection from supra-patella pouches of the knee joints during synovectomy performed in the Jikei University hospital, and were stored at −80°C until use. Informed consent was obtained from all donors and patients, and approval by the ethical committee of the university and the institutional review board were also obtained.
Preparation of citrullinated type II collagen
Human type II collagen was isolated from articular cartilage as described by Miller.10 Purity of isolated type II collagen was confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), by Coomassie blue (Bio-Rad) staining, and by Western blotting with antihuman type II collagen antibody (Chemicon International, Temecula, CA, USA) (Fig. 1). Isolated human type II collagen was dissolved with 0.5 M AcOH by vortexing at a concentration of 1.0 mg/ml, and the solution was then dissolved in reaction buffer (0.1 M Tris-HCl [pH 7.6], 10 mM CaCl2, and 5 mM DTT) at a concentration of 30μg/ml. Type II collagen was then citrullinated by incubation with peptidylarginine deiminase (PADI) (Sigma, St. Louis, MO, USA) at a concentration of 1 unit/ml for 1h at 50°C. After citrullination, the PADI was excluded by spin-column. Citrullination of type II collagen was confirmed by Western blotting with anti-citrulline antibody (Biogenesis, Poole, UK) (Fig. 1).
Fig. 1A–C.
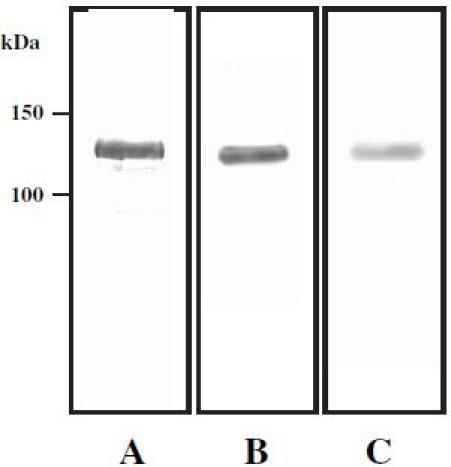
Human type II collagen was isolated from articular cartilage as described in Patients and methods. Purity of the isolated type II collagen was confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis using Coomassie blue staining (A) and Western blotting with anti-human type II collagen antibody (B). Isolated human type II collagen was citrullinated as described in Patients and methods. Citrullination of human type II collagen was confirmed by Western blotting using anti-citrulline antibody (C)
Assay for anti-citrullinated type II collagen antibodies
Detection of anti-citrullinated type II collagen antibodies was performed by enzyme-linked immunosorbent assay (ELISA) using buffered normal rabbit serum (NRS) as a blocking agent to diminish nonspecific binding of human IgG to the plastic plates, as previously described.11 Each well of the ELISA plate (Serocluster U plates; Costar, Cambridge, MA, USA) was coated with citrullinated type II collagen by incubation with 100μl solution dissolved in phosphate buffer (pH 7.6) at a concentration of 5μg/ml overnight at 4°C. Serum, fluid samples, or synovial extracts were diluted 1 : 50 in buffered NRS and added to the plates. The secondary antibody, peroxidase-conjugated rabbit antihuman IgG (Fc specific; Cooper Biochemical, Cochranville, PA, USA) was diluted 1:2000 with 25% NRS. The assay was performed with the standard technique and the plates were read at a wavelength of 490 nm using 630 nm as a reference. All samples were tested in duplicate, and the results were averaged. For the sera assay, the median and standard deviation (SD) of the OD490 values of sera from healthy donors were determined after the distribution of OD490 values was standardized using BoxCox conversion. Sera with an OD490 that was greater than the median + 2 × SD of controls were considered positive. The cutoff value was 0.16. For the joint fluid and synovial extract assays, OD490 values in OA samples were used as controls and the cutoff values were 0.2 and 0.10, respectively. Control serum was included on all plates to monitor plate-to-plate variation. Variation never exceeded 5%, and values were therefore not corrected.
Inhibition studies were performed by preincubating sera with buffered NRS containing citrullinated type II collagen at a concentration of 20 μg/ml overnight at 4°C. The OD490 signal of the blank was defined as 100% inhibition, and the original OD490 signal in the ELISA was defined as 0% inhibition.
Isolation of anti-citrullinated type II collagen antibodies from the synovium
Synovial samples were cut into small pieces and washed with Tris buffer containing 0.1% Tween-20. Synovial pieces were then homogenized with ImmunoPure Gentle Ag/Ab Elution Buffer (Pierce, Rockford, IL, USA) at neutral pH to dissociate the antigen/antibody immunocomplexes deposited in the synovium or were homogenized with Tris buffer or phosphate buffer as a negative control. After centrifugation and collection of supernatants, ImmunoPure Gentle Ag/Ab Elution Buffer was substituted with Tris buffer using a Centricon Centrifugal Filter Device (Millipore, Bedford, MA, USA). Liquid samples were then concentrated at 10μl/1.0g of wet weight volume of synovium using a Microcon Centrifugal Filter Device (Millipore). The procedure described above was performed at 4°C. The anti-citrullinated type II collagen antibodies in the synovial extracts were detected by ELISA as described above.
Isolation and detection of citrullinated type II collagen fragments in synovial fluids
Synovial fluid samples were applied to an affinity column conjugated with rabbit polyclonal antibodies directed against human type II collagen (Chemicon International.) after hyaluronidase treatment; this was followed by centrifugation to remove debris. ImmunoPure Gentle Ag/Ab Elution Buffer was applied onto the column after washing with Tris buffer, and the elution buffer was then collected and desalted using a Centricon Centrifugal Filter Device. The elution buffer was then freeze-dried and completely solubilized in the sample buffer (50 mM Tris-HCl, pH 6.8, 2% (w/v) SDS, 10% (w/v) glycerol, with 6% β-mercaptoethanol). Samples were subjected to SDS-PAGE (10% (w/v) gel) and were transferred to nitrocellulose membranes. Staining was performed in the manner of standard technique. Briefly, the blotted membrane was reacted with rabbit anti-citrulline antibodies (Biogenesis) or with rabbit anti-type I collagen antibodies as control for 1h at room temperature after washing with Tris buffer. Then, the membrane was reacted with the secondary anti-rabbit IgG antibodies conjugated with alkaline phosphatase (Chemicon International) for 30 min at room temperature after washing with Tris buffer three times. The immune complexes were visualized with the NBT/BCIP staining solution (Roche; Mannheim, Germany) according to the manufacturer’s instructions after washing with Tris buffer three times. Ten synovial fluid samples obtained from 10 of 15 RA patients (9 stage II and 1 stage III [Steinbrocker classification]) were analyzed. Fluid samples obtained from OA patients were not analyzed in the present study.
Results
Anti-citrullinated type II collagen antibodies in RA sera
Of the 130 RA serum samples assayed, 102 (78.5%) were positive for autoantibodies that reacted with citrullinated human type II collagen (Fig. 2). The reactivity of these sera with citrullinated type II collagen was more than 80% inhibited by the antigen, with the exception of one sample, of which reactivity was the strongest compared to the other samples (Fig. 2). No significant differences were observed in the incidence of anti-citrullinated type II collagen antibodies between sera from patients with different stages of RA. Antibodies to native noncitrullinated type II collagen were detected in 19 (14.6%) serum samples from 130 RA patients. All these positive samples were positive for anti-citrullinated type II collagen antibodies. Anti-citrullinated type II collagen antibodies were also positive in 11 (78.6%) of 14 sera from patients with polyarticular type JRA. Of the sera from 31 patients with SLE, one sample was positive for autoantibodies, while 2 of the 55 sera samples from patients with OA of the knee were positive for autoantibodies. All sera samples from the 24 systemic sclerosis patients, 21 dermatomyositis/polymyositis patients, 21 ankylosing spondylitis patients, and 18 psoriatic arthritis patients were negative for anti-citrullinated type II collagen autoantibodies (Fig. 3).
Fig. 2.
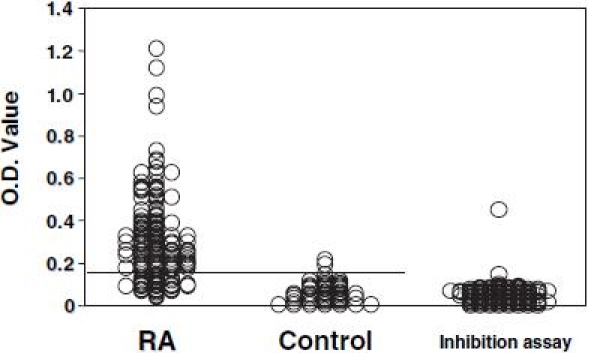
The assay for detecting anti-citrullinated type II collagen antibodies was performed in 130 serum samples from patients with rheumatoid arthritis (RA) and 40 serum samples from healthy donors. The transverse line shows the cutoff value. For the positive sera from RA patients, an inhibition assay was performed using citrullinated type II collagen as antigen. O.D., OD490 value
Fig. 3.
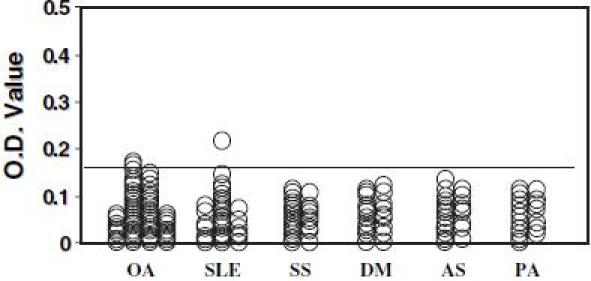
The assay for detecting anti-citrullinated type II collagen antibodies was performed in 55 serum samples from patients with osteoarthritis (OA) of the knee, 31 systemic lupus erythematosus (SLE) patients, 24 systemic sclerosis (SS) patients, 21 dermatomyositis/polymyositis (DM) patients, 21 ankylosing spondylitis (AS) patients, and 18 psoriatic arthritis (PA) patients. The transverse line shows the cutoff value
Anti-citrullinated type II collagen antibodies in synovial fluids
Of the 15 RA synovial fluid samples assayed, 13 (86.7%) were positive for anti-citrullinated type II collagen antibodies, and the reactivity of these samples with citrullinated type II collagen was more than 80% inhibited by the antigen (Fig. 4). Anti-citrullinated type II collagen antibodies were positive in all sera obtained from the 15 RA patients who donated synovial fluids.
Fig. 4.
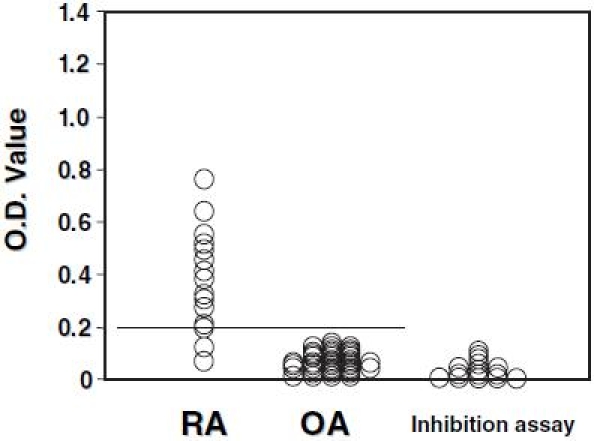
The assay for detecting anti-citrullinated type II collagen antibodies was performed in 15 synovial fluid samples obtained from affected knee joints of rheumatoid arthritis (RA) patients and 41 synovial fluid samples obtained from knees of osteoarthritis (OA) patients. The transverse line shows the cutoff value. For all samples from RA knees, an inhibition assay was performed using the citrullinated type II collagen as antigen
Anti-citrullinated type II collagen antibodies in synovial extracts
All eight synovial extracts from RA patients that were homogenized with the antigen/antibody immunocomplex dissociation buffer were positive for anti-citrullinated type II collagen autoantibodies, and the reactivity of these synovial extracts with citrullinated type II collagen was more than 80% inhibited by the antigen (Fig. 5). Synovial extracts from RA synovium homogenized with Tris or phosphate buffer were all negative. Anti-citrullinated type II collagen antibodies were all positive in sera obtained from the eight RA patients from whom synovium was extracted.
Fig. 5.
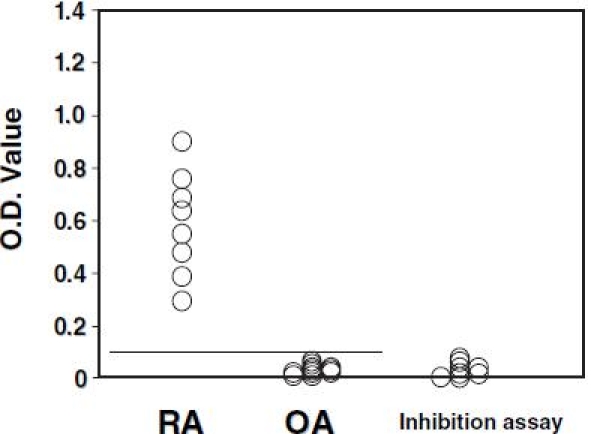
Extracts were obtained from the synovium of affected knees of 8 rheumatoid arthritis (RA) patients and 11 osteoarthritis (OA) patients using the buffer for the dissociation of antigen/antibody immunocomplex, and the assay for anti-citrullinated type II collagen antibodies was performed. The transverse line shows the cutoff value. For all samples from RA patients, an inhibition assay was performed using citrullinated type II collagen as antigen
Fragments of citrullinated type II collagen in synovial fluids
Affinity chromatography using polyclonal anti-human type II collagen antibodies revealed protein bands corresponding to a molecular weight of approximately 55 kDa and less than 20 kDa that reacted with anti-citrulline antibodies in all 10 synovial fluid samples isolated from the knee joints of RA patients (Fig. 6).
Fig. 6A,B.
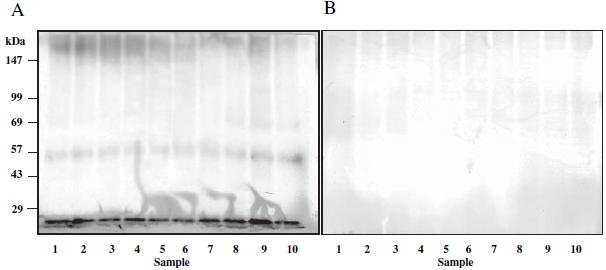
Fragments of type II collagen were isolated from the synovial fluid of knees of 10 RA patients by affinity chromatography using polyclonal antibodies against human type II collagen. These isolated fragments were then separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. A Western blotting was performed using anti-citrulline antibodies. Fragments of citrullinated type II collagen were found in all 10 samples at a molecular weight of approximately 55 kDa and less than 20 kDa. B Western blotting was performed with anti-type I collagen antibodies as control
Discussion
Type II collagen is a major and specific molecule in articular cartilage. In the present study, we found that type II collagen was citrullinated in the affected joints of patients with RA and that autoantibodies to citrullinated type II collagen were specifically produced in these patients. Moreover, autoantibodies to citrullinated type II collagen were isolated from the inflamed articular synovium of RA patients using antigen/antibody immunocomplex dissociation buffer but not by standard Tris or phosphate buffer. These findings indicate that immunocomplexes composed of fragments of citrullinated type II collagen and autoantibodies were formed and deposited in the inflamed articular synovium of RA patients. Although it is unclear whether these immunocomplexes are involved in the induction of arthritis, it is known that systemic administration of a mixture of monoclonal anti-type II collagen antibodies induces arthritis in mice,12 that major cytokines expressed in the articular synovium of RA patients, interleukin-1 and tumor necrosis factor-α, are predominantly expressed in the arthritic joints of these mice,13 and that the immunocomplexes deposited in these tissues activate the complement cascade, which is one of the first steps in the induction of inflammation. Therefore, these findings support the idea that the formation and subsequent deposition of citrullinated type II collagenautoantibody immunocomplexes in the synovium are involved in disease pathogenesis in RA patients, although there is a possibility that the immunocomplexes including the other citrullinated peptides except type II collagen also correlate the disease pathogenesis of RA.
Recently, it has been reported that the serological detection of antibodies to the cyclic citrullinated peptide (CCP), which is a modified peptide variant of a citrullinated filaggrin epitope, is quite significant in RA diagnosis.8,14,15 An assay of CCP antibody of second generation was later established and the sensitivity and specificity of the assay were 66%–88% and 89%–98%, respectively.16–20 The sensitivity and specificity of the anti-citrullinated type II collagen antibody assay were approximately 79% and 95%, respectively. Thus, the anti-citrullinated type II collagen antibody assay may become one of the significant tools for diagnosis of RA, although the sensitivity of the assay is less than that of the CCP antibody assay of second generation.
Acknowledgment
We are grateful to all the members of the Department of Orthopaedic Surgery, The Jikei University School of Medicine, for the collection of samples.
References
- 1.Venrooij WJ, Pruijn GJM. Citrullination: a small change for a protein with great consequences for rheumatoid arthritis (commentary) Arthritis Res. 2000;2:249–51. doi: 10.1186/ar95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou Z, Ménard HA. Autoantigenic posttranslational modifications of proteins: does it apply to rheumatoid arthritis? Curr Opin Rheumatol. 2002;14:250–3. doi: 10.1097/00002281-200205000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Baeten D, Peene I, Union A, Meheus L, Sebbag M, Serre G, et al. Specific presence of intracellular citrullinated proteins in rheumatoid arthritis synovium. Arthritis Rheum. 2001;44:2255–62. doi: 10.1002/1529-0131(200110)44:10<2255::AID-ART388>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki A, Yamada R, Chang X, Tokuhiro S, Sawada T, Suzuki M, et al. Functional haplotypes of PADI4, encoding citrullinating enzyme peptidylarginine deiminase 4, are associated with rheumatoid arthritis. Nat Genet. 2003;34:395–402. doi: 10.1038/ng1206. [DOI] [PubMed] [Google Scholar]
- 5.Yamada R, Suzuki A, Chang X, Yamamoto K. Peptidylarginine deiminase type 4: identification of a rheumatoid arthritis-susceptible gene. Trends Mol Med. 2003;9:503–8. doi: 10.1016/j.molmed.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 6.Schellekens GA, de Jong BA, van den Hoogen FH, Van De Putte LB, Van Venrooij WJ. Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies. J Clin Invest. 1998;101:273–81. doi: 10.1172/JCI1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Girbal-Neuhauser E, Durieux J-J, Arnaud M, Dalbon P, Sebbag M, Vincent C, et al. The epitopes targeted by the rheumatoid arthritis-associated antifilaggrin autoantibodies are posttranslationally generated on various sites of (pro)filaggrin by deimination of arginine residues. J Immunol. 1999;162:585–94. [PubMed] [Google Scholar]
- 8.Schellekens GA, Visser H, de Jong BA, van den Hoogen FH, Hazes JM, Breedveld FD, et al. The diagnostic properties of rheumatoid arthritis antibodies recognizing a cyclic citrullinated peptide. Arthritis Rheum. 2000;43:155–63. doi: 10.1002/1529-0131(200001)43:1<155::AID-ANR20>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 9.Masson-Bessière C, Sebbag M, Girbal-Neuhauser E, Nogueira L, Vincent C, Senshu T, et al. The major synovial targets of the rheumatoid arthritis-specific antifilaggrin autoantibodies are deiminated forms of the α- and β-chains of fibrin. J Immunol. 2001;166:4177–84. doi: 10.4049/jimmunol.166.6.4177. [DOI] [PubMed] [Google Scholar]
- 10.Miller EJ. Structural studies on cartilage collagen employing limited cleavage and solubilization with pepsin. Biochemistry. 1973;11:4903–9. doi: 10.1021/bi00776a005. [DOI] [PubMed] [Google Scholar]
- 11.Fujii K, Tsuji M, Murota K, Terato K, Shimozuru Y, Nagai Y. An improved enzyme-linked immunosorbent assay of anti-collagen antibodies in human serum. J Immunol Methods. 1989;124:63–70. doi: 10.1016/0022-1759(89)90186-5. [DOI] [PubMed] [Google Scholar]
- 12.Terato K, Hasty KA, Reife RA, Cremer MA, Kang AH, Stuart JM. Induction of arthritis with monoclonal antibodies to collagen. J Immunol. 1992;148:2103–8. [PubMed] [Google Scholar]
- 13.Kagari T, Doi H, Shimozato T. The importance of IL-1β and TNF-α, and the noninvolvement of IL-6, in the development of monoclonal antibody-induced arthritis. J Immunol. 2002;169:1459–66. doi: 10.4049/jimmunol.169.3.1459. [DOI] [PubMed] [Google Scholar]
- 14.Zeng X, Ai M, Tian X, Gan X, Shi Y, Song Q, et al. Diagnostic value of anti-cyclic citrullinated peptide antibody in patients with rheumatoid arthritis. J Rheumatol. 2003;30:1451–5. [PubMed] [Google Scholar]
- 15.Bas S, Genevay S, Meyer O, Gabay C. Anti-cyclic citrullinated peptide antibodies, IgM and IgA rheumatoid factors in the diagnosis and prognosis of rheumatoid arthritis. Rheumatology. 2003;42:677–80. doi: 10.1093/rheumatology/keg184. [DOI] [PubMed] [Google Scholar]
- 16.van Venrooij WJ, Hazes JM, Visser H. Anticitrullinated protein/peptide antibody and its role in the diagnosis and prognosis of early rheumatoid arthritis. Neth J Med. 2002;60:383–8. [PubMed] [Google Scholar]
- 17.Vasishta A. Diagnosing early-onset rheumatoid arthritis: the role of anti-CCP antibodies. Am Clin Lab. 2002;21:34–6. [PubMed] [Google Scholar]
- 18.Lee DM, Schur PH. Clinical utility of the anti-CCP assay in patients with rheumatic diseases. Ann Rheum Dis. 2003;62:870–4. doi: 10.1136/ard.62.9.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suzuki K, Sawada T, Murakami A, Matsui T, Tohma S, Nakazono K, et al. High diagnostic performance of ELISA detection of antibodies to citrullinated antigens in rheumatoid arthritis. Scand J Rheumatol. 2003;32:197–204. doi: 10.1080/03009740310003677. [DOI] [PubMed] [Google Scholar]
- 20.Pinheiro GC, Scheinberg MA, Aparecida-da Silva M, Maciel S. Anti-cyclic citrullinated peptide antibodies in advanced rheumatoid arthritis. Ann Intern Med. 2003;139:234–5. doi: 10.7326/0003-4819-139-3-200308050-00021. [DOI] [PubMed] [Google Scholar]


