Abstract
Glucocorticoids (GCs, cortisol in human) are associated with impairments in declarative memory retrieval. Brain regions hypothesized to mediate these effects are the hippocampus and prefrontal cortex (PFC). Our aim was to use fMRI in localizing the effects of GCs during declarative memory retrieval. Therefore, we tested memory retrieval in 21 young healthy males in a randomized placebo-controlled crossover design. Participants encoded word lists containing neutral and emotional words 1 h prior to ingestion of 20 mg hydrocortisone. Memory retrieval was tested using an old/new recognition paradigm in a rapid event-related design. It was found that hydrocortisone decreased brain activity in both the hippocampus and PFC during successful retrieval of neutral words. These observations are consistent with previous animal and human studies suggesting that glucocorticoids modulate both hippocampal and prefrontal brain regions that are crucially involved in memory processing.
Electronic Supplementary Material
The online version of this article (doi:10.1007/s11682-007-9003-2) contains supplementary material, which is available to authorized users.
Keywords: fMRI, Glucocorticoids, Hippocampus, Memory retrieval, PFC
Introduction
It is well established that one of the mechanisms through which stress influences memory and cognition are elevations in cortisol level (Wolf 2003). In healthy populations, investigations into chronic stress have shown that long term administration of GCs impairs declarative memory (Young et al. 1999; Newcomer et al. 1999). The effects of acute stress on declarative memory have been investigated either by elevating cortisol levels with psychosocial stress (Elzinga et al. 2005; Abercrombie et al. 2006; Kuhlmann et al. 2005b), or with one-off GC administration (Abercrombie et al. 2003; Buchanan and Lovallo 2001; Kirschbaum et al. 1996; de Quervain et al. 2000). In contrast to chronic studies, acute stress studies into effects of cortisol made it possible to disentangle the effects of GCs on encoding, consolidation and retrieval (see for a review on acute effects Lupien and McEwen 1997). Quervain et al. (2000) were the first to target specific memory phases by pre-learning, post-learning and pre-retrieval administration of 25 mg of cortisone to healthy participants in a randomized crossover design. In their study, GCs administered 1 h before retrieval significantly impaired free recall of words learned 24 h earlier. The same dose given before or after learning had no effect on encoding or consolidation of words. In several psychosocial stress studies it was found that participants who showed a pronounced cortisol increase in response to the stressor performed poorer in memory retrieval compared to participants who only had a mild or no cortisol response, which suggests that the results obtained with GC administration are comparable to those with psychosocial stressors (Wolf et al. 2001b; Buchanan et al. 2006; Kirschbaum et al. 1996).
Since the study of de Quervain et al. (2000), several modulators of the effects of GCs on memory performance have been uncovered, such as dose (e.g., Abercrombie et al. 2003), gender (Wolf et al. 2001b), age (Wolf et al. 2001a; Lupien et al. 2002), and time of day (Het et al. 2005; Maheu et al. 2005). Also, arousal experienced during encoding (Abercrombie et al. 2006) and arousing properties of the declarative material itself (Buchanan and Lovallo 2001; Wolf et al. 2004) appeared to influence GC effects on memory performance. For instance, GC administration (Kuhlmann and Wolf 2006a) or stress (Cahill et al. 2003) is associated with enhanced memory consolidation of emotionally arousing material, but not of neutral material. In contrast, acute psychosocial stress is associated with greater impairments in memory retrieval of emotionally arousing than more neutral information (Kuhlmann et al. 2005b). Moreover, GC administration before retrieval lowered the tendency to recall emotional words better than neutral words (Kuhlmann et al. 2005a) (for a review on the opposing effects of GCs on consolidation and retrieval processes, see Roozendaal 2002).
As of yet, it remains unclear how GCs exert their influence on memory in the human. Glucocorticoid receptors (GRs) have been found in several brain areas of relevance for memory, specifically the hippocampus and the PFC. On the basis of animal and human studies it has been proposed that the effects of GCs on cognitive functioning depend (at least in part) on activation of glucocorticoid receptors in the hippocampus (Roozendaal 2002; Roozendaal et al. 2003) or the differential activation of mineralocorticoid and glucocorticoid receptors in the hippocampus and prefrontal cortex (Oitzl et al. 1995; Lupien and Lepage 2001; Lupien et al. 2002). Cortisolrelated retrieval impairments usually appear during ‘hippocampus-dependent’ tasks that require conscious recollection of the study episode, such as free recall (Buchanan et al. 2006; de Quervain et al. 2000; Kuhlmann et al. 2005a) and cued recall tasks (de Quervain et al. 2003). Occasionally, cortisol-related impairment in recognition memory has been reported (Domes et al. 2004), which is considered to be partly recollection- and partly familiarity-based.
Apart from the hippocampus, the prefrontal cortex (PFC) plays a general role in retrieval of declarative memories. Recognition, cued recall and free recall of previously studied words have all shown to activate the PFC (Buckner and Wheeler 2001). In addition, functional magnetic resonance imaging (fMRI) studies have consistently reported activation in both the medial temporal lobe and PFC during memory retrieval, and there is evidence of interactions between these brain regions during memory retrieval (for reviews see Buckner and Wheeler 2001; Simons and Spiers 2003). The PFC as well as the hippocampus are known to be significant targets of circulating glucocorticoids (Lupien and Lepage 2001). Moreover, cortisol-related impairment in prefrontal-dependent working memory tasks has been reported (Lupien et al. 1999; Elzinga and Roelofs 2005; Young et al. 1999; Oei et al. 2006; Wolf et al. 2001a). Therefore, apart from the hippocampus, GC-effects on the PFC could be expected during memory retrieval.
So far, one H215O-positron emission tomography (PET) imaging study investigated the effects of GC administration on memory retrieval in young healthy men (de Quervain et al. 2003). In that study, 25 mg cortisone impaired cued recall of neutral word pairs learned 24 h earlier and this was associated with reduced blood flow in the right parahippocampal gyrus, left visual cortex and cerebellum. Surprisingly, no cortisol-related changes were found in prefrontal brain regions. It is possible that the low temporal resolution of PET has masked the cortisol effects in the PFC: PET typically localizes sustained averaged activation during a 60–90 min window, and cannot compare brain activation patterns associated with specific events, such as correct and incorrect retrieval and correct rejections. These activations can be determined with event-related (ER)-fMRI.
To our knowledge, GC-effects on memory retrieval have never been localized using fMRI. The aim of the present study was to localize the differential effects of GC administration on retrieval of neutral and emotional words in young healthy men. Here we used ER-fMRI in a double blind placebo-controlled randomized crossover design during retrieval of words in a recognition paradigm. We expected to find a GC-induced decrease in the blood oxygen level dependent (BOLD) response in the hippocampus and PFC during correct retrieval of previously learned words compared to placebo conditions. In addition, we investigated whether treatment interacts with the arousing properties of the words by using negative emotional and neutral items.
Materials and methods
Participants
Twenty-eight male university students volunteered to participate in this study. Screening led to the exclusion of six volunteers: four for medical or psychiatric reasons and two for left-handedness. One participant did not show up for the second scan session, leaving a total of 21 young (mean age: 22.8±2.9 years, mean BMI: 22.3±1.8), healthy, right-handed participants. Due to technical problems fMRI data of one participant were lost so that imaging data of 20 participants could be analysed, whereas data of 21 participants were available for behavioral analysis.
Participants gave written informed consents. All received 75 Euros for their participation. Criteria for inclusion were: a body mass index (BMI=kg/m2) between 18 and 25, no medical and psychiatric history, determined by a brief version of the Amsterdam Biographical Interview (ABV; Wilde 1963) and the Dutch version of the Symptom Checklist-90 (Arrindell and Ettema 1986), using norm scores for a healthy population. Exclusion criteria included use of medication or psychotropic drugs within three months prior to the test sessions, blood pressure over 140/90 mmHg, diabetes, current and past psychiatric problems, the use of remedies containing corticosteroids, and lefthandedness. Females were not included in this study to avoid interactions of hormones due to menstrual cycle or birth control pill and cortisol, or other specific sex differences in stress effects on memory (Wolf et al. 2001b). The study was approved by the medical ethical committee of the Free University Medical Center (VUMC), and carried out according to the standards of the Declaration of Helsinki (Declaration of Helsinki 2000).
Design
In a double blind, randomised placebo-controlled crossover design, participants aged 19–28 years (mean age, 22.8±2.9) received 20 mg hydrocortisone (Hoechst) or placebo. With regard to the dose and timings, the design was very similar to the behavioural study of Wolf et al. (2001a), who found GC-induced retrieval impairments in delayed free recall. Tablets were ingested 1 h prior to scanning in order to achieve high cortisol levels. Cortisol level was monitored throughout the study using Salivettes (Kirschbaum and Hellhammer 1994).
Recognition task
The recognition task was adapted from a paradigm by de Ruiter (2005). During each scan session, the task consisted of an encoding-phase (outside the scanner) of 80 target words (40 emotional negative, e.g., terrorist, and 40 neutral, e.g., architect) and a recognition-phase (inside the scanner). Words were selected from a pool of words validated in a perceptual clarification task (Ter Laak, unpublished Master's thesis), in which these words were recognized most consistently and rapidly as neutral and negative words under minimal presentation conditions. This task was considered to be an intentional encoding task, because participants were casually informed that ‘later on’ they would be tested for word recall. Participants were instructed to view randomly presented words, and to indicate the emotional value of the word by pressing either ‘0’ (neutral) or ‘1’ (emotional) on a laptop keyboard. In the recognition-phase of the task, the 80 encoded targets were shown together with 80 foils (40 new emotional negative and 40 new neutral words), and 40 fillers. All stimuli were projected on a back-projection screen located at the head end of the scanner table via an LCD projector located outside the scanner room. Subjects viewed all stimuli on a screen through a mirror placed on the head coil.
Participants had to push a button as fast as possible to indicate whether they consciously recollected a given word from the learning phase (‘remember’), whether they were less certain of having learned the word (‘know’), or whether they had no recollection of the word (‘not seen before’). Therefore, on each trial, one of the target words or foils was shown against a grey background, while response options were indicated at the bottom of the screen by black arrows pointing to the left and to the right (“⋘not seen < know > remember⋙”). Fillers consisted of the instruction ‘⋘press left button’ or ‘press right button⋙’, and were used as baseline. After the response was made a 1–2 second inter-stimulus-interval (grey screen) started, from stimulus offset until stimulus onset. All stimuli were presented in a self-paced manner, in order to prevent distress or boredom of the participants, and to automatically induce jitter. A time limit of 3 s was maintained in case of non-responses. Four word lists were used, two for each session that contained emotional and neutral words matched for valence, frequency, and length. List order was balanced across participants, so that half of the participants had list 1 or 2 as targets and list 2 or 1 as foils during the first session, and list 3 and 4 as targets or foils at the second session. List 3 and 4 were administered to the other participants during their first session. Stimulus order within the lists was pseudo randomized. There was no significant effect of List Order (p=0.35).
MRI scanning
Imaging was carried out on a 1.5 T Sonata MR scanner (Siemens, Erlangen, Germany), using a standard circularly polarized head coil with foam padding to restrict head motion. In both scan sessions, a T1-weighted structural MRI-scan was obtained (repetition time 2700 ms, inversion time 950 ms, echo time 3.97 ms, flip angle 8°, 160 coronal slices, voxel size 1 × 1.5 × 1 mm3). For fMRI, gradient echo and echo planar images (EPI) sensitive to BOLD contrast were obtained in the axial direction (echo time 60 ms, flip angle 90°, isotropic voxels of 3.3 mm, 36 slices, repetition time 2.85 s). The scan procedure on both scan sessions consisted of a structural scan (10 min), echo planar imaging (EPI) during the recognition task (9–14 min), EPI to investigate resting states (10 min), and EPI during a picture encoding task (±12 min), (both EPI resting states and EPI picture encoding task data will be published elsewhere), and a high resolution gradient echo EPI scan (echo time 45 ms, flip angle 90°, 1.64×1.64×2.20 mm voxels, 64 slices) (0.5 min).
Procedure
Participants were invited to the VUMC on three occasions; two scan sessions, 2 weeks apart from each other, and a third time, 8 days after the last scan session. Participants had been asked to refrain from any caffeine or sugar containing drinks, and to have exactly the same light breakfast on both scan days. Furthermore, they were asked to refrain from food intake 2 h before each fMRI procedure. Participants were scanned at exactly the same time during the two appointments, somewhere between 1100 and 1400 h, to keep baseline cortisol levels for each participant as equal as possible. On both scan sessions, exactly the same procedure was followed. First, participants were seated in front of a laptop in a separate room for the encoding of wordlists. Next, they filled out the state-version of the State-Trait Anxiety Inventory (STAI) and were seated for an hour in a waiting room. Seventy-five minutes after arrival, 1 h after the word encoding (to prevent direct effects of pill-ingestion on consolidation), participants were given a tablet containing hydrocortisone 20 mg or a similar looking placebo. One hour after pill ingestion, recognition was tested in the scanner. Saliva was sampled four times on both scan sessions, immediately after arrival (‘pre-baseline’), before pill ingestion (‘baseline’), immediately before entering the scanner (‘prescan’), and immediately after the scanning session (‘postscan’). Participants filled out an exit-interview, and were paid and thanked for their participation.
Data analyses
Word task Proportions of correct hits (CH), false rejections (FR) of old words, and False hits (FH) and correct rejections (CR) of new words (‘foils’) were calculated (total raw scores per category/(40)). Because the number of ‘know’ responses was small, these were categorized as CHs, since modelling these as events of no interest would have resulted in too few CHs in about half of our subjects. Old/new discrimination accuracy (D') was calculated as P (CH)-P(FH) (Snodgrass & Corwin 1988). To check for order and learning effects, RTs of correct hits and rejections and proportion of correct and false hits were analysed separately with repeated measures ANOVAs with Treatment Order as between-subjects factor, and Session, Response Type (new vs. old words), Correctness of Response (correct vs. false hits) and Valence (emotional vs. neutral) as within-subjects.
Effects of Treatment on retrieval and discrimination accuracy were analyzed separately in 2 (treatment) × 2 (Valence [neutral/emotional]) repeated measures ANOVAs, all two-tailed, with proportions of hits (correct and false hits) and D’ as dependent variables. Finally, RTs were analysed using a repeated measures ANOVA with Treatment, Hit Type (CH vs FH) and Valence as within-subjects factors.
Cortisol
Free cortisol levels in saliva were measured using a commercially available chemiluminescence assay kit (IBL, Hamburg, Germany). Both inter- and intra-assay variance was below 10%.
Results
Salivary cortisol levels
As expected, pre-baseline (mean±SD, hydrocortisone 16.1±9.5 nmol/l; placebo 15.1±8.8 nmol/l) and baseline values (mean±SD, hydrocortisone 9.23±7.1 nmol/l; placebo 7.7±4.2 nmol/l) did not differ (pre-baseline, t=0.32, df=40, p=0.75, two-tailed; baseline, t=0.87, df=40, p=0.39, two-tailed). Pre-scan (mean±SD, hydrocortisone 107.5±76.8 nmol/l; placebo 6.4±3.0 nmol/l) and post-scan values (mean±SD, hydrocortisone 72.6±46.4 nmol/l; placebo 9.1±6.2 nmol/l) were significantly higher in the hydrocortisone- condition than in the placebo-condition (prescan: t=5.88, df=19.06, p<0.0005; postscan: t=6.22, df=20.74, p<0.0005, equal variances not assumed for both t-tests). The McNemar test using binomial distribution showed that participants were not able to tell whether they had received placebo or hydrocortisone: four participants (out of 21) correctly indicated noticing an effect of hydrocortisone, whereas four others (out of 21) incorrectly noticed effects of placebo (n=21, exact p=1.00).
Behavioral results
Proportion of hits
Mean proportions of hits (CHs and FHs) are shown in Table 1. For correct hits, there was a significant effect of Session (F[1, 19]=4.59, p=0.045), with better performance during the second session, irrespective of treatment or group (CHs, session 1: M±SE=0.68±0.04; session 2: M±SE=0.76±0.03). There was a trend towards a significant between-subjects effect of Treatment Order (F[1, 19]=3.41, p=0.08, with the group that had placebo first performing less well than the group that received hydrocortisone first (CHs: placebo first, M±SE=0.67±0.04, vs. hydrocortisone first, M±SE=0.78±0.04), on recall of emotional words on session 1 only (see supplementary analyses). For false hits there was no significant between-subjects effect of Treatment Order, and no Session by Treatment Order interaction, both ps>0.22.
Table 1.
Recognition performance
| Probability | Reaction times | ||||
|---|---|---|---|---|---|
| Treatment | CH | FH | CH | FH | CR |
| M | M | M | M | M | |
| (SEM) | (SEM) | (SEM) | (SEM) | {SEM) | |
| Cortisol | |||||
| Emo | 0.82 | 0.50 | 1,374.85 | 1,643.52 | 1,464.78 |
| (0.03) | (0.05) | (81.64) | (101.41) | (78.79) | |
| Neu | 0.66 | 0.26 | 1,534.34 | 1,839.43 | 1,426.43 |
| (0.04) | (0.04) | (112.39) | (125.77) | (74.87) | |
| Placebo | |||||
| Emo | 0.77 | 0.45 | 1,478.94 | 1,884.14 | 1,625.45 |
| (0.05) | (0.05) | (76.39) | (112.18) | (77.21) | |
| Neu | 0.65 | 0.26 | 1,593.06 | 1,875.52 | 1,520.64 |
| (0.05) | (0.05) | (99.16) | (107.11) | (79.19) | |
Mean probability of hits (and standard error), expressed in proportions, and mean reaction times (and standard error) in both cortisol and placebo condition. CH correct hits, FH false hits, CR correct rejections, Emo emotional words, Neu neutral words.
When analyzing treatment effects, no within-subjects effect of Treatment on correct and false hits was found (ps >0.24). A within-subjects effect of Valence showed that emotional words were more often categorized as previously presented compared to neutral words, regardless of whether the word was old (correct hits, F [1, 20]=22.57, p<0.0005) or new (false hits, F [1, 20]=152.74, p<0.0005). There was no main effect of treatment on discrimination accuracy (p=0.74). Discrimination accuracy of emotional words tended to be lower than of neutral words (F [1, 20]=3.60, p=0.07). No Treatment by Valence interaction emerged on correct and false hits or discrimination accuracy (ps>0.3).
Reaction times
Mean reaction times (RTs) are shown in Table 1. No between-subjects effect of Treatment Order was found, F (1, 14)=0.01, p=0.92. However, there was a significant main effect of Time (F [1, 14]=15.37, p=0.002): Both groups responded significantly faster during the second session, possibly due to practice. The group that first had placebo was slower on its first session and faster on the second session compared to the group that had hydrocortisone first, an effect that was also reflected by the analyses of the proportion of hits.
The RT data (CHs and CRs) were further analyzed for treatment effects on separate sessions. Although mean scores in the Hydrocortisone condition show faster responses than the Placebo condition, separate ANOVAs of the RTs on separate sessions showed that this was not a significant effect of Treatment (Session 1: F (1, 18)=0.65, p=0.43; Session 2: F (1, 19)=0.55, p=0.47).
fMRI results
Placebo
Left prefrontal and left parietal regions were significantly activated during correct retrieval (CH-CR) (see Table 2). These regions are typically activated during successful episodic memory retrieval (Konishi et al. 2000; Buckner and Wheeler 2001). Activation was also found in the right hippocampus. Separate analyses for neutral and emotional words demonstrated that mainly neutral words accounted for these effects (see Fig. 1a for activations with z>3.1, uncorrected p). Since there were no activations associated with presentation of the emotional words, we left this contrast out of further analyses.
Table 2.
Maxima of regions showing significant (p<0.05, clustercorrected) during successful retrieval
| Region of activation | Left/Right | BA | x, y, z (mm) | Z value | ||
|---|---|---|---|---|---|---|
| Placebo | ||||||
| CH-CR | ||||||
| Superior frontal gyrus | L | 10 | −26 | 68 | −2 | 4.66 |
| Inferior parietal lobe | L | 40 | −48 | −50 | 56 | 4.64 |
| Medial frontal gyrus | L | 9 | −4 | 36 | 28 | 4.53 |
| Precuneus | L | 7 | −2 | −74 | 44 | 4.50 |
| Caudate nucleus | L | −10 | 10 | 2 | 4.24 | |
| Cingulate gyrus | L | 23 | −2 | −16 | 32 | 3.85 |
| Hippocampus | R | 16 | −4 | −12 | 2.381 | |
| Neu CH- Neu CR | ||||||
| Midfrontal gyrus | L | 9 | −46 | 22 | 30 | 4.41 |
| Superior frontal gyrus | L | 10 | −22 | 66 | 0 | 4.61 |
| Inferior parietal lobe | L | 40 | −48 | −52 | 58 | 4.70 |
| Medial frontal gyrus | L | 9 | −4 | 36 | 28 | 4.26 |
| Precuneus | L | 7 | −4 | −72 | 42 | 4.14 |
| Caudate nucleus | L | −12 | 10 | 2 | 4.54 | |
| Hippocampus | R | 34 | −24 | −10 | 2.491 | |
| Cortisol | ||||||
| CH-CR | ||||||
| Superior parietal lobe | L | 7 | −44 | −62 | 54 | 4.89 |
| Midfrontal gyrus | L | 9/46 | −48 | 24 | 28 | 3.97 |
| Midfrontal gyrus | L | 10/47 | −42 | 56 | −4 | 3.97 |
| Neu CH-NeuCR | ||||||
| Superior parietal lobe | L | 7 | −46 | −62 | 54 | 4.73 |
| Midfrontal gyrus | L | 9/46 | −50 | 26 | 26 | 3.82 |
| Midfrontal gyrus | L | 10/47 | −44 | 56 | −4 | 4.09 |
| Cortisol < Placebo | ||||||
| CH-CR | ||||||
| Hippocampus | R | 16 | −4 | −12 | 2.711 | |
| Neu CH-Neu CR | ||||||
| Superior frontal gyrus | R | 10 | 24 | 66 | −04 | 3.64 |
| Putamen | L | −16 | 10 | −06 | 3.37 | |
| Precuneus | R | 7 | 12 | −76 | 46 | 3.50 |
| Hippocampus | L | −26 | −20 | −14 | 2.731 | |
| Hippocampus | R | 34 | −24 | −10 | 2.671 | |
CH-CR correct hits (“old”) contrasted with correct rejections (“new”) of the pooled words, NeuCH-NeuCR correct hits contrasted with correct rejections of neutral words, BA Brodmann area, x, y, z coordinates of local maxima are listed according to the Talairach coordinate system (Talairach and Tournoux 1988).
1 Uncorrected p<0.01
Fig. 1.
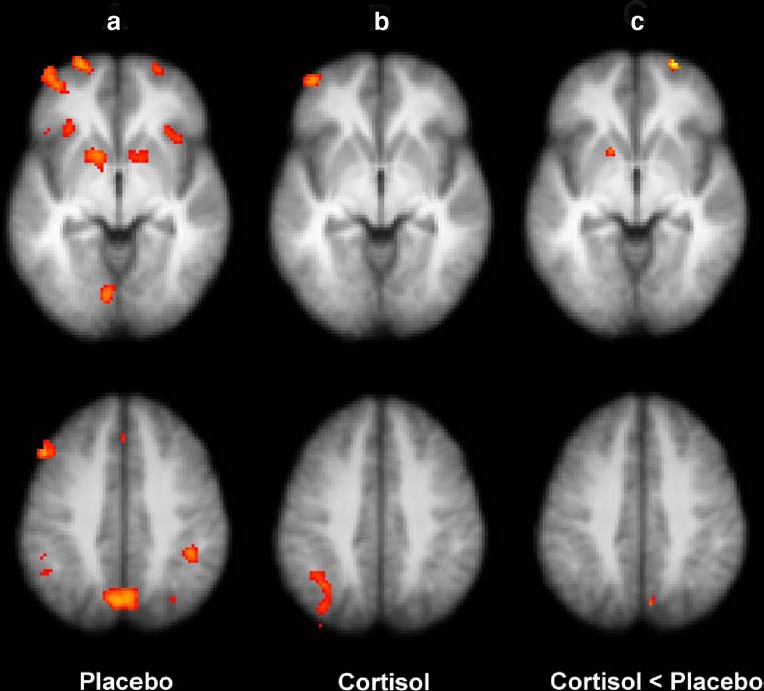
The contrast neutral correct hits vs. neutral correct rejections a showing areas active during placebo treatment, thresholded z value of 3.1 for display purposes; b showing activity during cortisol treatment (z>3.1), see Table 2 for those maxima of clusters that survived cluster-correction; and c showing areas in the superior frontal gyrus, putamen and precuneus in which activity was significantly decreased during hydrocortisone as compared with placebo treatment (cluster-corrected z>3.1, p<0.05). The up-per and lower horizontal slices correspond to resp. z=34 and z=57 in the Talairach coordinate system (Talairach and Tournoux 1988). Left in the image is left in the brain
Hydrocortisone
Significant brain activity during successful retrieval of all old words (CHs vs. CRs) was found in the left superior parietal lobe and left midfrontal gyri during hydrocortisone treatment (see Table 2). This was entirely attributable to the activation associated with retrieval of neutral words (NeuCH vs. NeuCR) (see Fig. 1b).
Treatment effects
Relative to placebo condition, in the contrast CH-CR (pooled words) hydrocortisone treatment led to significantly less activation in the right hippocampus (Table 2). When neutral words were analyzed separately (neuCH-neuCR), significantly less activation was found during hydrocortisone treatment in the right superior frontal gyrus, left putamen and right precuneus compared to the placebo condition (see Fig. 1c). In addition, significantly less activation was found bilaterally in the hippocampus during hydrocortisone treatment compared to placebo condition (see Fig. 2).
Fig. 2.
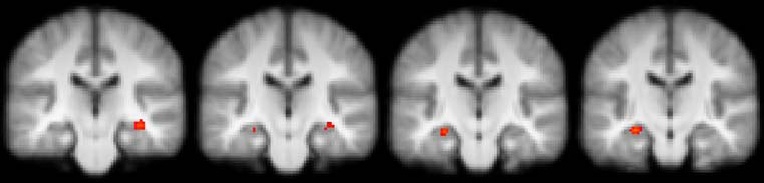
Coronal slices showing significant hydrocortisone-induced decreases in activation as compared with placebo in left and right hippocampus (z>2.3, uncorrected p). Left in the image is left in the brain
Discussion
In the present study for the first time GC-induced decreases in brain activity during successful declarative memory retrieval were localized using ER-fMRI. Under placebo conditions, robust activations showed up during successful retrieval in areas consistently found as part of an episodic retrieval network, including hippocampus, left parietal and predominantly left prefrontal areas (Konishi et al. 2000; Wagner et al. 2005; Buckner and Wheeler 2001; Shannon and Buckner 2004). Consistent with our hypothesis, GC administration reduced brain activation in both the PFC and hippocampus. Although a lenient criterion for finding decreases in hippocampal activation was used, our data converge with Quervain et al. (2003), who found support for right MTL involvement in mediating cortisol effects on declarative memory retrieval which increases the validity of our data. These findings are consistent with the view that GCs may affect declarative memory retrieval through mediation by both hippocampus and PFC (Lupien and Lepage 2001). The present data extend the results of de Quervain et al. (2003).
Declarative memory is known to rely on the integrity of the hippocampus. However, the PFC is also involved with declarative memory retrieval (Lepage et al. 2000) irrespective of whether task type is free recall, cued recall or recognition (Buckner and Wheeler 2001). Furthermore, MTL and PFC are known to interact during retrieval (Simons and Spiers 2003). The PFC as well as the hippocampus are known to be significant targets of circulating glucocorticoids (Lupien and Lepage 2001). In addition, GC-associated memory impairments on a behavioural level are well established. For instance, Wolf et al. (2001a) found GC-induced impairments of free recall of neutral words in a design similar to the present study. Clearly, complex effortful retrieval, such as free recall, makes greater demands on processes of organization, strategic search, monitoring and verification, relative to more automatic remembering like recognition. The interactions between frontal and temporal regions may therefore be more important in effortful tasks, such as free recall (Simons and Spiers 2003), making it more sensitive to the effects of GCs on a behavioral level. Nonetheless, because the participants in our study hardly ever gave ‘know’ answers, our task mainly tapped from the recollective aspects (‘remember’) of recognition, that appear to depend more on (the integrity of) the hippocampus, than the familiarity components (‘know’) of recognition (see for a review (Brown and Aggleton 2001). Unfortunately, the number of ‘know’ answers was too small for separate analyses, so we could not differentiate between ‘remember’ and ‘know’ components. Nevertheless, since both the hippocampus and PFC were affected by GCs in this study, it is likely that in more demanding tasks reduced activation in the PFC will be associated with GC-induced retrieval impairments. Free recall protocols fit for fMRI or the use of more difficult (associative) recognition tasks would be a promising direction for further replication of behavioural studies.
Apart from decreases in activation in MTL and PFC, reduced activation during successful retrieval was also found in the precuneus and putamen. The parietal lobe has also found to contribute to declarative memory retrieval (Shannon and Buckner 2004; Lundstrom et al. 2005). It has been suggested that frontal and parietal regions provide a general signal of retrieval success, probably by indicating that information is ‘old’ (Buckner and Wheeler 2001), or perceived as ‘old’ (Wheeler and Buckner 2003). The posterior parietal cortex and precuneus might contribute to retrieval through its connections to the MTL (Wagner et al. 2005). Perhaps, the reduction in activation of the precuneus that we have found is a consequence of decreased activity in the hippocampus, or it could be a direct effect of GCs on the precuneus. Since working memory is dependent on prefrontal and parietal regions our findings may also be relevant in understanding the acute impairing effects of high cortisol levels on working memory (Lupien et al. 1999).
Several limitations of the present study should be noted as well when interpreting the present findings. First, no treatment effects on task performance were found. Although the study had sufficient power to detect differences in brain activation between hydrocortisone and placebo treatment, no differences were found in recognition memory performance. This could be explained by the number of participants in our study. The only (behavioral) study that has reported cortisol-induced retrieval impairments in recognition memory included 60 male participants (Domes et al. 2004), which is far more than the number of participants in our study. Also, as stated before, the effects of cortisol on a behavioural level may be stronger in more demanding memory tasks, such as free recall. Furthermore, we used intentional encoding (the participants consciously knew that they had to recall the words later on and possibly elaborated words deeper), instead of incidental encoding (so that participants are unaware at the time of encoding that they have to recall words later). With our design we had to keep sessions similar, and therefore we were not able to do the incidental encoding, which is often used in retrieval studies. Moreover, the present study was limited by an effect of order. This order effect appeared to be caused by deviating emotional word retrieval data on the first session of the group that had placebo on the first session. Only on the second session numerically less emotional words were recognized in the hydrocortisoneas compared with the placebo condition, which was in the direction of our expectations even though this was not a significant difference. Future memory retrieval studies should be aware of order effects in crossover designs and should consider including a third (control) group with two placebo conditions.
This study could also not provide information about the differential effects of GCs on neutral and emotional declarative memory, since the results with regard to the emotional words were difficult to interpret. What we can conclude is that we did find indications for reduced neural activation in the neutral category, which is in line with other studies that found an association between cortisol and impaired retrieval of neutral material (de Quervain et al. 2003; de Quervain et al. 2000; Wolf et al. 2001a). With regard to the emotional category, De Ruiter (2005), who used a similar word recognition task and the same contrasts, also found that it looked like emotional stimuli recruited less cortical networks than emotional stimuli. However, by using the subtraction method in fMRI analyses the effects of emotional stimuli may have been cancelled out. Affective valence does have an effect on the tendency to respond ‘old’ to new emotional items, so that even when participants correctly identify new emotional items, brain activation resembling activity to old items may have been elicited. Also in an event-related potential (ERP) study differences in magnitude of the old/new effect for negative and neutral words were found, that were attributable to the increased positivity of the ERPs elicited by new negative items relative to the new neutral items (Maratos et al. 2000). Taken together, with the old/new effect it could not be clearly determined to what extent emotional stimuli had activated the retrieval network, and consequently the effect of hydrocortisone on emotional retrieval remains to be resolved. Clearly, more fMRI studies that use other paradigms are necessary to investigate the effects of GCs on emotional memory retrieval (see for instance Strange and Dolan 2004).
So far, only a small number of studies have been published on the interaction of cortisol and stimuli with different valence or arousal properties. In one study GCs were administered before memory retrieval testing in female participants only (Kuhlmann et al. 2005a), and it was found that cortisol diminished the advantage of better remembrance that emotional words generally have. Our behavioural data cannot be directly compared to that study, however, since cortisol administration may have a very different effect in males than in females, as sex-steroids greatly influence the response to cortisol administration (Kuhlmann and Wolf 2005; Wolf et al. 2001b), and also because men and women respond differently to emotional stimuli (Yang et al. 2007). Therefore, studies that include both men and women should be conducted. Other studies examined the effects of cortisol elevations during memory retrieval induced by psychosocial stress (Kuhlmann et al. 2005b; Domes et al. 2004; Oei et al. 2006). Domes et al. (2004) found that cortisol impaired recognition of emotionally arousing positive words, whereas the other two studies found cortisol-induced impaired memory for emotionally negative stimuli. Direct comparisons between stress-induced cortisol and cortisol administration are problematic for several reasons, however. For instance, results in stress-studies could be related to adrenergic mechanisms that may act in concert with GCs (Roozendaal 2003; Roozendaal et al. 2004; Murchison et al. 2004). Human studies need to be performed to further clarify these putative interacting mechanisms.
This line of research may provide important data regarding the processing of traumatic memories or memory dysfunction in stress-related psychiatric disorders. There are indications that GCs could lessen traumatic memory retrieval, and as such, be useful to PTSD treatment (Schelling et al. 2004). In patients who have experienced traumatic stress, alterations in the hypothalamic-pituitary-adrenal axis, changes in hippocampal function and structure and associated declarative (hippocampus-dependent) memory deficits, have been found repeatedly (Bremner 2006). There are also indications that patients with posttraumatic stress disorder (PTSD) have altered central brain sensitivity to glucocorticoids (GCs, cortisol in humans) with healthy individuals (Grossman et al. 2006; Vythilingam et al. 2006 but see Bremner et al. 2004b; Bremner et al. 2004a). Moreover, cortisol administration has found to impair phobic fear memories (Soravia et al. 2006). Because noradrenergic mechanisms are also implicated in — for instance — PTSD and depression, future studies should incorporate noradrenergic measures to elucidate the interactions between noradrenergic and GC effects, and be aware of possible habituation within crossover designs (Okuda et al. 2004; Kuhlmann and Wolf 2006b).
To summarize, using fMRI we show for the first time in the human that acute cortisol elevation is associated with decreased brain activity in the PFC and hippocampus during declarative memory retrieval. This observation is in line with previous animal studies as well as behavioral human studies. The finding of differential effects on emotional material awaits further research. The in vivo localization of the effects of this key stress hormone in the human brain opens up an important new avenue for research in cognitive neuroscience, which ultimately should lead to a better understanding of stress associated psychiatric disorders.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Analyses of Order and Learning Effects (DOC 38.0 kb)
Acknowledgements
We thank Hanane El Hachioui for helping with the data collection. The study was supported by a grant from Hersenstichting Nederland (project nr. 11F03(2).55).
Footnotes
Electronic Supplementary Material
The online version of this article (doi:10.1007/s11682-007-9003-2) contains supplementary material, which is available to authorized users.
References
- Abercrombie, H. C., Kalin, N. H., Thurow, M. E., Rosenkranz, M. A., & Davidson, R. J. (2003). Cortisol variation in humans affects memory for emotionally laden and neutral information. Behavioral Neuroscience, 117, 505–516. [DOI] [PubMed]
- Abercrombie, H. C., Speck, N. S., & Monticelli, R. M. (2006). Endogenous cortisol elevations are related to memory facilitation only in individuals who are emotionally aroused. Psychoneuroendocrinology, 31, 187–196. [DOI] [PubMed]
- Arrindell, W. A., & Ettema, J. H. M. (1986). SCL-90. Handleiding bij een multidimensionele psychopathologie-indicator. Lisse: Swets & Zeitlinger B.V.
- Bremner, J. D. (2006). Stress and brain atrophy. CNS & Neorological Disorders Drug Targets, 5, 503–512. [DOI] [PMC free article] [PubMed]
- Bremner, J. D., Vythilingam, M., Vermetten, E., Afzal, N., Nazeer, A., Newcomer, J. W., et al. (2004a). Effects of dexamethasone on declarative memory function in posttraumatic stress disorder. Psychiatry Research, 129, 1–10. [DOI] [PubMed]
- Bremner, J. D., Vythilingam, M., Vermetten, E., Anderson, G., Newcomer, J. W., & Charney, D. S. (2004b). Effects of glucocorticoids on declarative memory function in major depression. Biological Psychiatry, 55, 811–815. [DOI] [PubMed]
- Brown, M. W., & Aggleton, J. P. (2001). Recognition memory: What are the roles of the perirhinal cortex and hippocampus? Nature Reviews Neuroscience, 2, 51–61. [DOI] [PubMed]
- Buchanan, T. W., & Lovallo, W. R. (2001). Enhanced memory for emotional material following stress-level cortisol treatment in humans. Psychoneuroendocrinology, 26, 307–317. [DOI] [PubMed]
- Buchanan, T. W., Tranel, D., & Adolphs, R. (2006). Impaired memory retrieval correlates with individual differences in cortisol response but not autonomic response. Learning and Memory, 13, 382–387. [DOI] [PMC free article] [PubMed]
- Buckner, R. L., & Wheeler, M. E. (2001). The cognitive neuroscience of remembering. Nature Reviews Neuroscience, 2, 624–634. [DOI] [PubMed]
- Cahill, L., Gorski, L., & Le, K. (2003). Enhanced human memory consolidation with post-learning stress: Interaction with the degree of arousal at encoding. Learning and Memory, 10, 270–274. [DOI] [PMC free article] [PubMed]
- Declaration of Helsinki. 52nd WMA General Assembly, Edinburgh, Scotland, Oct. 2000.
- de Quervain, D. J., Henke, K., Aerni, A., Treyer, V., McGaugh, J. L., Berthold, T., et al. (2003). Glucocorticoid-induced impairment of declarative memory retrieval is associated with reduced blood flow in the medial temporal lobe. European Journal of Neuroscience, 17, 1296–1302. [DOI] [PubMed]
- de Quervain, D. J., Roozendaal, B., Nitsch, R. M., McGaugh, J. L., & Hock, C. (2000). Acute cortisone administration impairs retrieval of long-term declarative memory in humans. Nature Neuroscience, 3, 313–314. [DOI] [PubMed]
- de Ruiter, M. B. (2005). Neural correlates of nonclinical dissociation. Doctoral dissertation. University of Amsterdam.
- Domes, G., Heinrichs, M., Rimmele, U., Reichwald, U., & Hautzinger, M. (2004). Acute stress impairs recognition for positive words-association with stress-induced cortisol secretion. Stress, 7, 173–181. [DOI] [PubMed]
- Elzinga, B. M., Bakker, A., & Bremner, J. D. (2005). Stress-induced cortisol elevations are associated with impaired delayed, but not immediate recall. Psychiatry Research, 134, 211–223. [DOI] [PubMed]
- Elzinga, B. M., & Roelofs, K. (2005). Cortisol-induced impairments of working memory require acute sympathetic activation. Behavioral Neuroscience, 119, 98–103. [DOI] [PubMed]
- Forman, S. D., Cohen, J. D., Fitzgerald, M., Eddy, W. F., Mintun, M. A., & Noll, D. C. (1995). Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster-size threshold. Magnetic Resonance in Medicine, 33, 636–647. [DOI] [PubMed]
- Friston, K. J., Worsley, K. J., Frackowiak, R. J. S., Mazziotta, J. C., & Evans, A. C. (1994). Assessing the significance of focal activations using their spatial extent. Human Brain Mapping, 1, 210–220. [DOI] [PubMed]
- Grossman, R., Yehuda, R., Golier, J., McEwen, B., Harvey, P., & Maria, N. S. (2006). Cognitive effects of intravenous hydrocortisone in subjects with PTSD and healthy control subjects. Annals of the New York Academy of Sciences, 1071, 410–421. [DOI] [PubMed]
- Het, S., Ramlow, G., & Wolf, O. T. (2005). A meta-analytic review of the effects of acute cortisol administration on human memory. Psychoneuroendocrinology, 30, 771–784. [DOI] [PubMed]
- Jenkinson, M., Bannister, P., Brady, M., & Smith, S. (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage, 17, 825–841. [DOI] [PubMed]
- Jenkinson, M., & Smith, S. (2001). A global optimisation method for robust affine registration of brain images. Medical Image Analysis, 5, 143–156. [DOI] [PubMed]
- Kirschbaum, C., & Hellhammer, D. H. (1994). Salivary cortisol in psychoneuroendocrine research: Recent developments and applications. Psychoneuroendocrinology, 19, 313–333. [DOI] [PubMed]
- Kirschbaum, C., Wolf, O. T., May, M., Wippich, W., & Hellhammer, D. H. (1996). Stress- and treatment-induced elevations of cortisol levels associated with impaired declarative memory in healthy adults. Life Sciences, 58, 1475–1483. [DOI] [PubMed]
- Konishi, S., Wheeler, M. E., Donaldson, D. I., & Buckner, R. L. (2000). Neural correlates of episodic retrieval success. NeuroImage, 12, 276–286. [DOI] [PubMed]
- Kuhlmann, S., Kirschbaum, C., & Wolf, O. T. (2005a). Effects of oral cortisol treatment in healthy young women on memory retrieval of negative and neutral words. Neurobiology of Learning and Memory, 83, 158–162. [DOI] [PubMed]
- Kuhlmann, S., Piel, M., & Wolf, O. T. (2005b). Impaired memory retrieval after psychosocial stress in healthy young men. Journal of Neuroscience, 25, 2977–2982. [DOI] [PMC free article] [PubMed]
- Kuhlmann, S., & Wolf, O. T. (2005). Cortisol and memory retrieval in women: Influence of menstrual cycle and oral contraceptives. Psychopharmacology (Berl), 183, 65–71. [DOI] [PubMed]
- Kuhlmann, S., & Wolf, O. T. (2006a). Arousal and cortisol interact in modulating memory consolidation in healthy young men. Behavioral Neuroscience, 120, 217–223. [DOI] [PubMed]
- Kuhlmann, S., & Wolf, O. T. (2006b). A non-arousing test situation abolishes the impairing effects of cortisol on delayed memory retrieval in healthy women. Neuroscience Letters, 399(3), 268–272. [DOI] [PubMed]
- Lepage, M., Ghaffar, O., Nyberg, L., & Tulving, E. (2000). Prefrontal cortex and episodic memory retrieval mode. Proceedings of the National Academy of Sciences United States of America, 97, 506–511. [DOI] [PMC free article] [PubMed]
- Lundstrom, B. N., Ingvar, M., & Petersson, K. M. (2005). The role of precuneus and left inferior frontal cortex during source memory episodic retrieval. NeuroImage, 27, 824–834. [DOI] [PubMed]
- Lupien, S. J., Gillin, C. J., & Hauger, R. L. (1999). Working memory is more sensitive than declarative memory to the acute effects of corticosteroids: A dose-response study in humans. Behavioral Neuroscience, 113, 420–430. [DOI] [PubMed]
- Lupien, S. J., & Lepage, M. (2001). Stress, memory, and the hippocampus: can’t live with it, can’t live without it. Behavioural Brain Research, 127, 137–158. [DOI] [PubMed]
- Lupien, S. J., & McEwen, B. S. (1997). The acute effects of corticosteroids on cognition: integration of animal and human model studies. Brain Research: Brain Research Reviews, 24, 1–27. [DOI] [PubMed]
- Lupien, S. J., Wilkinson, C. W., Briere, S., Menard, C., Ng Ying Kin, N. M., & Nair, N. P. (2002). The modulatory effects of corticosteroids on cognition: Studies in young human populations. Psychoneuroendocrinology, 27, 401–416. [DOI] [PubMed]
- Maheu, F. S., Collicutt, P., Kornik, R., Moszkowski, R., & Lupien, S. J. (2005). The perfect time to be stressed: A differential modulation of human memory by stress applied in the morning or in the afternoon. Progress in Neuropsychopharmacology & Biological Psychiatry, 29, 1281–1288. [DOI] [PubMed]
- Maratos, E. J., Allan, K., & Rugg, M. D. (2000). Recognition memory for emotionally negative and neutral words: An ERP study. Neuropsychologia, 38, 1452–1465. [DOI] [PubMed]
- Murchison, C. F., Zhang, X. Y., Zhang, W. P., Ouyang, M., Lee, A., & Thomas, S. A. (2004). A distinct role for norepinephrine in memory retrieval. Cell, 117, 131–143. [DOI] [PubMed]
- Newcomer, J. W., Selke, G., Melson, A. K., Hershey, T., Craft, S., Richards, K., et al. (1999). Decreased memory performance in healthy humans induced by stress-level cortisol treatment. Archives of General Psychiatry, 56, 527–533. [DOI] [PubMed]
- Oei, N. Y., Everaerd, W. T., Elzinga, B. M., van Well, S., & Bermond, B. (2006). Psychosocial stress impairs working memory at high loads: An association with cortisol levels and memory retrieval. Stress, 9, 133–141. [DOI] [PubMed]
- Oitzl, M. S., van Haarst, A. D., Sutanto, W., & De Kloet, E. R. (1995). Corticosterone, brain mineralocorticoid receptors (MRs) and the activity of the hypothalamic-pituitary-adrenal (HPA) axis: The Lewis rat as an example of increased central MR capacity and a hyporesponsive HPA axis. Psychoneuroendocrinology, 20, 655–675. [DOI] [PubMed]
- Okuda, S., Roozendaal, B., & McGaugh, J. L. (2004). Glucocorticoid effects on object recognition memory require training-associated emotional arousal. Proceedings of the National Academy of Sciences United States of America, 101, 853–858. [DOI] [PMC free article] [PubMed]
- Roozendaal, B. (2002). Stress and memory: opposing effects of glucocorticoids on memory consolidation and memory retrieval. Neurobiology of Learning and Memory, 78, 578–595. [DOI] [PubMed]
- Roozendaal, B. (2003). Systems mediating acute glucocorticoid effects on memory consolidation and retrieval. Progress in Neuropsychopharmacology & Biological Psychiatry, 27, 1213–1223. [DOI] [PubMed]
- Roozendaal, B., de Quervain, D. J., Schelling, G., & McGaugh, J. L. (2004). A systemically administered beta-adrenoceptor antagonist blocks corticosterone-induced impairment of contextual memory retrieval in rats. Neurobiology of Learning and Memory, 81, 150–154. [DOI] [PubMed]
- Roozendaal, B., Griffith, Q. K., Buranday, J., de Quervain, D. J., & McGaugh, J. L. (2003). The hippocampus mediates glucocorticoid-induced impairment of spatial memory retrieval: Dependence on the basolateral amygdala. Proceedings of the National Academy of Sciences United States America, 100, 1328–1333. [DOI] [PMC free article] [PubMed]
- Schelling, G., Roozendaal, B., & de Quervain, D. J. (2004). Can posttraumatic stress disorder be prevented with glucocorticoids? Annals of the New York Academy of Sciences, 1032, 158–166. [DOI] [PubMed]
- Shannon, B. J., & Buckner, R. L. (2004). Functional-anatomic correlates of memory retrieval that suggest nontraditional processing roles for multiple distinct regions within posterior parietal cortex. Journal of Neuroscience, 24, 10084–10092. [DOI] [PMC free article] [PubMed]
- Simons, J. S., & Spiers, H. J. (2003). Prefrontal and medial temporal lobe interactions in long-term memory. Nature Reviews Neuroscience, 4, 637–648. [DOI] [PubMed]
- Smith, S. M. (2002). Fast robust automated brain extraction. Human Brain Mapping, 17, 143–155. [DOI] [PMC free article] [PubMed]
- Snodgrass, J.G. & Corwin, J. (1988). Pragmatics of measuring recognition memory: applications to dementia and amnesia. Journal of Experimental psychology: General, 177(1), 34–50. [DOI] [PubMed]
- Soravia, L. M., Heinrichs, M., Aerni, A., Maroni, C., Schelling, G., Ehlert, U., et al. (2006). Glucocorticoids reduce phobic fear in humans. Proceedings of the National Academy of Sciences United States America, 103(14), 5585–5590. [DOI] [PMC free article] [PubMed]
- Strange, B. A. & Dolan, R. J. (2004). Beta-adrenergic modulation of emotional memory-evoked human amygdala and hippocampal responses. Proceedings of the National Academy of Sciences United States America, 101, 11454–11458. [DOI] [PMC free article] [PubMed]
- Talairach, J., & Tournoux, P. (1988). Co-planar stereotaxic atlas of the human brain. 3-dimensional proportional system: An approach to cerebral imaging. Stuttgart, Germany: Thieme.
- Vythilingam, M., Lawley, M., Collin, C., Bonne, O., Agarwal, R., Hadd, K., et al. (2006). Hydrocortisone impairs hippocampal-dependent trace eyeblink conditioning in post-traumatic stress disorder. Neuropsychopharmacology, 31, 182–188. [DOI] [PubMed]
- Wagner, A. D., Shannon, B. J., Kahn, I., & Buckner, R. L. (2005). Parietal lobe contributions to episodic memory retrieval. Trends in Cognitive Sciences, 9, 445–453. [DOI] [PubMed]
- Wheeler, M. E., & Buckner, R. L. (2003). Functional dissociation among components of remembering: Control, perceived oldness, and content. Journal of Neuroscience, 23, 3869–3880. [DOI] [PMC free article] [PubMed]
- Wilde, G.J.S. (1963). Neurotische labiliteit gemeten volgens de vragenlijstmethode. Amsterdam: Van Rossen. [PubMed]
- Wolf, O. T. (2003). HPA axis and memory. Best Practice & Research Clinical Endocrinology & Metabolism, 17, 287–299. [DOI] [PubMed]
- Wolf, O. T., Convit, A., McHugh, P. F., Kandil, E., Thorn, E. L., de Santi, S. et al. (2001a). Cortisol differentially affects memory in young and elderly men. Behavioral Neuroscience, 115, 1002–1011. [DOI] [PubMed]
- Wolf, O. T., Kuhlmann, S., Buss, C., Hellhammer, D. H., & Kirschbaum, C. (2004). Cortisol and memory retrieval in humans: Influence of emotional valence. Annals of the New York Academy of Sciences, 1032, 195–197. [DOI] [PubMed]
- Wolf, O. T., Schommer, N. C., Hellhammer, D. H., McEwen, B. S., & Kirschbaum, C. (2001b). The relationship between stress induced cortisol levels and memory differs between men and women. Psychoneuroendocrinology, 26, 711–720. [DOI] [PubMed]
- Woolrich, M. W., Behrens, T. E., Beckmann, C. F., Jenkinson, M., & Smith, S. M. (2004). Multilevel linear modelling for FMRI group analysis using Bayesian inference. NeuroImage, 21, 1732–1747. [DOI] [PubMed]
- Woolrich, M. W., Ripley, B. D., Brady, M., & Smith, S. M. (2001). Temporal autocorrelation in univariate linear modeling of FMRI data. NeuroImage, 14, 1370–1386. [DOI] [PubMed]
- Worsley, K. J., Evans, A. C., Marrett, S., & Neelin, P. (1992). A three-dimensional statistical analysis for CBF activation studies in human brain. Journal of Cerebral Blood Flow and Metabolism, 12, 900–918. [DOI] [PubMed]
- Yang, H., Zhou, Z., Liu, Y., Ruan, Z., Gong, H., Luo, Q., et al. (2007). Gender difference in hemodynamic responses of prefrontal area to emotional stress by near-infrared spectroscopy. Behavioural Brain Research, 178, 172-176. [DOI] [PubMed]
- Young, A. H., Sahakian, B. J., Robbins, T. W., & Cowen, P. J. (1999). The effects of chronic administration of hydrocortisone on cognitive function in normal male volunteers. Psychopharmacology (Berl), 145, 260-266. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Below is the link to the electronic supplementary material.
Supplementary Analyses of Order and Learning Effects (DOC 38.0 kb)


