Abstract
Background
Radicals have important physiological functions, for example, in immune defense and vasoprotection. However, they are also potentially dangerous waste products of cellular metabolism and they can contribute to the development of many different diseases.
Method
Selective literature review.
Results
The scientific understanding of radicals has not yet led to any therapeutic application. For many years, scavenging already formed radicals with antioxidants was considered to be the most promising therapeutic approach, but clinical trials based on this principle have yielded mostly negative results. Thus, entirely new approaches are needed. The goal should be to prevent the formation of harmful radicals, or to treat radical-related damage if it has already occurred. New diagnostic tools have the potential to identify those patients that are most likely to benefit from this form of treatment, as well as to document its success.
Conclusions
A new generation of cardiovascular drugs is being developed for the prevention or the mechanism-based treatment of vascular damage caused by oxidative stress. This new therapy should go hand in hand with new diagnostics, in accordance with the principle of individualized medicine.
Keywords: oxidative stress, antioxidants, nitric oxide, vascular diagnostics, radicals
Free radicals, oxidative stress, and antioxidants are the basis for many hypotheses about the development of diseases and their prevention. The key assumption is that radicals are harmful and therefore—in reverse—that eliminating them prevents or cures disease. However, radicals also have essential functions. Current, quite plausible, attempts to remove radicals have been mostly clinically ineffective. In this article, the authors will discuss 5 key questions:
What are radicals?
Which effects do they have?
Why do antioxidants not work?
What alternative approaches exist?
What is feasible today?
Recent advances in the area of free radicals reinforce the potential of discovering new diagnostic and therapeutic options for many diseases by means of this pathomechanism. Individualized medicine is likely to enable treating cardiovascular disease not on the basis of symptoms or in a population based manner, but to provide individually based treatment for each patient and the disease mechanism that applies to that patient. Newly developed medications do not treat laboratory or standard parameters but the pathomechanisms that are relevant for each individual patient. In oncology, this is in part already clinical practice. For this reason, this review article also touches on the topic of individualized medicine.
The authors conducted a selective literature search in Medline, using the search terms “vascular oxidative stress”, “vascular NADPH oxidases”, “soluble guanylate cyclase activator/stimulator”, “antioxidants”, “diet and nitric oxide”, and “personalized medicine”. The results were supplemented with literature retrieved by reviewing the reference lists of the identified studies. Selection was based on relevance as well as the authors’ own experiences.
What are radicals?
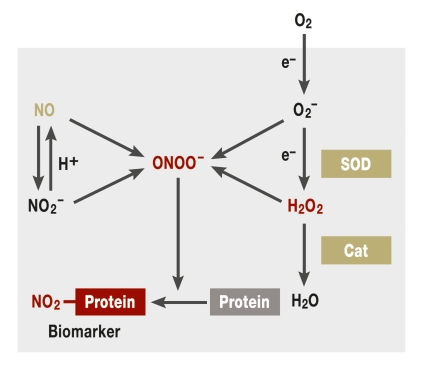
Radicals are compounds with an unpaired—and therefore highly reactive—electron. They can occur, for example, in lipids, amino acids, nucleotides, and oxygen compounds,. Oxygen radicals are of particular importance as they can trigger the formation of all other radicals. Other oxygen containing species that are chemically not radicals also have high reactivity with biological substances. Jointly, these substances are known as reactive oxygen species (ROS) (figure 1).
Figure 1.
Reactive oxygen species (ROS). Oxygen can be activated in several reductive steps into the superoxide radical O2– or hydrogen peroxide (H2O2). The second step can occur spontaneously or be catalyzed by superoxide dismutase (SOD). Hydrogen peroxide is detoxified by catalase (Cat) into oxygen and water. If superoxide and hydrogen peroxide interact with nitric oxide (NO) or nitrite (NO2–), peroxynitrite (ONOO–) is generated. Peroxynitrite can oxidize different cellular components and nitrate proteins. Nitrated proteins are biomarkers for oxidative stress. Red indicates disease promoting proteins or compounds; green, protective factors; arrows, reactions or transformations; a box indicates a protein—for example, an enzyme or receptor. The processes shown within the grey area occur naturally in the body. Oxygen needs to be obtained exogenously by respiration.
In addition to ROS, there are radicals that contain an additional nitrogen atom—for example, nitric oxide (NO). NO can be generated enzymatically by the so-called NO synthases (NOS) or non-enzymatically by nitrite (NO2–). NO has important signaling and protective functions; in 1998, a Nobel Prize was awarded for their discovery. NO/nitrite and ROS, in turn, can react with each other. This generates peroxynitrite (ONOO–), the most reactive compound of all ROS, which can oxidize and nitrite proteins, lipids, and nucleic acids (1).
How do radicals work?
Radicals are a two-edged sword. On the one hand, they have important physiological functions. In addition to NO, which is an important protective factor in the vasculature and a neurotransmitter in the nervous system (2), oxygen radicals are, for example, essential in the immune defense, as well as in the regulation of cellular growth and gene expression (3). But too much of a good thing can literally be harmful, because radicals are also highly dangerous by-products of the cellular metabolism.
Undesirable effects include inactivation of NO as a result of a direct chemical reaction with ROS and oxidative damage of cell components such as DNA and proteins (3). These effects are potentially involved in the development of cardiovascular diseases, neurodegeneration, and cancer (figure 2).
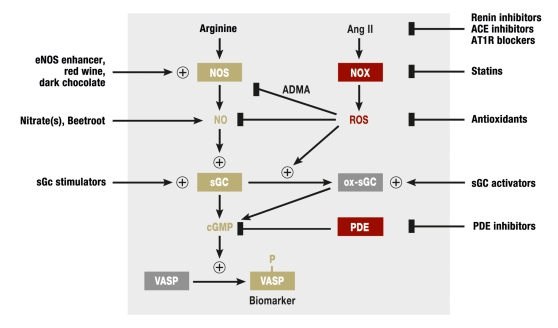
Figure 2.
Enzymatic sources of ROS, their effects and pharmacological modulation. NO synthases (NOS) generate nitric oxide (NO) from the amino acid arginine. NO activates soluble guanylate cyclase (sGC), which mediates its protective effects. sGC generates the second messenger cyclic GMP (cGMP). The phosphorylation of the cGMP dependent protein kinase substrate vasodilator stimulated phosphoprotein (VASP) is a biomarker for this signaling pathway. Angiotensin II (Ang II) induces oxidative stress by activating NADPH oxidases (NOX). ROS generated by NOX result, among others, in accumulation of the arginine derivate asymmetric dimethyl arginine (ADMA), an endogenous inhibitor of NOS. Furthermore, NOS is oxidized and “uncoupled” by ROS. Uncoupled NOS generates ROS instead of NO. Additionally, ROS oxidize the NO receptor sGC (ox sGC). In this process, the heme group of the sGC is oxidized first, and in a second step this group is then released from sGC resulting in heme free (apo-)sGC, which cannot be activated by NO. Apo-sGC can be reactivated by sGC activators. sGC stimulators affect non-oxidized sGC. They allow maximum sGC activity even in the presence of reduced NO concentrations. Red indicates disease promoting proteins or compounds; green, protective factors; arrows, reactions or transformations; arrows with +, stimulation or activation; a line with a rectangle, inhibition; a box indicates a protein—for example, and enzyme or a receptor. The processes within the grey area occur in the body endogenously; the ones outside thee grey area indicate exogenous factors or drugs.
The development and prognosis of cardiovascular diseases are often associated with endothelial dysfunction, meaning a functional impairment of the vascular endothelium that is caused by a disruption of the protective NO signaling pathway (4). Different factors can cause endothelial dysfunction and increased production of ROS is thought to contribute. Three mechanisms of interaction between ROS and the NO signaling pathway have been proven (figure 2):
ROS inactivate NO in a direct reaction
ROS damage the NO-forming enzyme NOS in endothelial cells
ROS damage the NO receptor.
To produce NO, NO synthases oxidize a nitrogen atom of the amino acid L-arginine; to do so they require the sensitive cofactor tetrahydrobiopterin (BH4). Three NOS isoforms exist:
neuronal NOS (nNOS),
immunologically inducible NOS (iNOS), and
endothelial NOS (eNOS).
Because of their high expression in inflammatory tissue, iNOS produces NO in amounts that have toxic effects and are transformed into nitrite peroxides in an acidic milieu. Inhibition of iNOS possibly makes sense in such a scenario, although respective clinical studies have shown negative results (5, e1, e2).
NO released by eNOS mediates vasoprotective effects. It relaxes blood vessels, for example, thus lowering blood pressure. It inhibits thrombocyte aggregation and the growth of smooth muscle cells (2). ROS can oxidize the cofactor BH4 and thus “uncouple” NOS (6), which then forms oxygen radicals themselves (4). Furthermore, in conditions of oxidative stress, increased amounts of an arginine metabolite (asymmetric dimethyl-L-arginine; ADMA) occur in plasma and cells. ADMA inhibits eNOS by binding to NOS instead of L-arginine (7). Since ADMA is increased in patients with endothelial dysfunction, it has been suggested as a novel biomarker for cardiovascular diseases (7).
Why do antioxidants not work?
Specialized antioxidant enzymes (superoxide dismutase, catalase, and peroxidases) physiologically regulate the optimal balance between ROS formation and breakdown (figure 1). An imbalance can lead to oxidative stress.
Although the hypothesis that oxidative stress is involved in the pathogenesis and development of many diseases is plausible, the majority of clinical studies using antioxidant therapies have yielded negative results (8) (table). Administration of vitamin E or beta-carotene may even be harmful and increase overall mortality (8, 9). The health promoting effects of exercise can be partially negated by vitamin C (10). In the long term, radicals formed during exercise are likely to have the same effects against oxidative stress as a vaccine would. Antioxidants can suppress this vaccination effect (10). A high dose of prophylactic vitamin E for healthy persons or a therapeutic dose for patients with cardiovascular disease can therefore no longer be justified (8). It is not known whether selective supplementation is effective in individuals with confirmed vitamin E deficiency or oxidative stress. However, no valid marker exists for local or systemic stress (8). This underlines the importance of developing new diagnostic tests along the avenue to individualized medicine.
Table. Selected review articles and meta-analyses on the effects of antioxidant supplements.
| Publication | Study type | Antioxidants studied | Objectives and end points | Conclusion |
| Vivekananthan, et al. 2003 (e15) | Meta-analysis | Beta-carotene and vitamin E | Effects of vitamins E and beta-carotene on CVD associated and overall mortality and morbidity | No evidence for efficacy of vitamin E on mortality or morbidity, beta-carotene probably harmful |
| Bjelakovic, et al. 2004 (e16) | Meta-analysis | Beta-carotene, vitamins A, C, E, selenium | Effects of AO supplementation on incidence of colon cancer and mortality | Selenium may be beneficial in preventing cancer; high doses of vitamin E are likely to increase mortality; other AO neither harmful nor beneficial |
| Kris-Etherton, et al. 2004 (e17) | Review article | Vitamin E, beta-carotene and AO cocktail | Summarizing the effects of AO on primary and secondary prevention of CVD | Data do not justify supplementation of AO at higher than recommended dosages for dietary intake |
| Miller, et al. 2005 (e18) | Meta-analysis | Vitamin E alone and in combination | Evaluation of dose-response effects of vitamin E supplementation on mortality | Supplementation of high dose vitamin E probably increases mortality and should be avoided |
| Shekelle, et al. 2004 (e19) | Meta-analysis | Vitamin E | Evaluation of CVD associated end points and lipid concentrations | Supplementation with vitamin E has no influence on CVD associated end points |
| Eidelman, et al. 2004 (e20) | Meta-analysis | Vitamin E alone and in combination | Evaluation of odds ratio CVD associated end points | Vitamin E supplementation neither beneficial nor harmful |
| Bjelakovic, et al. 2007 (e21) | Meta-analysis | Beta-carotene, vitamins A, C, E, and selenium | Evaluation of overall mortality and quality of life | Supplementation of beta-carotene, vitamin A, and vitamin E is likely to increase mortality |
| Dotan, et al. 2007 (8) | Markov model | Vitamin alone and in combination | Evaluation of effects of vitamin E supplementation on morbidity and quality of life | Non-selective administration of high dosage vitamin E is not beneficial |
| Gallicchio, et al. 2008 (9) | Review article | Carotinoids | Evaluation of the association between carotinoids and lung cancer | Supplementation of beta-carotene does not result in reduced risk of lung cancer and increases the risk for smokers |
AO, antioxidants; CVD, cardiovascular diseases; CHD, coronary heart disease
One reason for the lack of effect of antioxidants might be that their bioavailability is too low precisely in those locations where ROS concentrations are elevated. Oxidative stress is for the most part not a systemic phenomenon but is limited to individual organs, tissues, and cells, or even subcellular compartments. Antioxidant supplementation, however, is more likely to work systemically. Is it actually possible that after oral administration, every cell in the body receives the optimal concentration of the antioxidant at the right time, so as to scavenge every pathological radical, but to leave those that are physiologically necessary? It is also questionable whether ROS, once formed, can be removed by means of chemical reactions before they trigger harmful effects. And what would happen during such a reaction? Antioxidants themselves can turn into radicals that initiate new radical chain reactions (11).
These considerations, coupled with the negative clinical data, give rise to the suspicion that the “oxidative stress hypothesis” does not apply. Nevertheless, entirely different approaches may be required to treat oxidative stress.
Alternative approaches
Inhibiting the sources of radicals
Oxidative stress is caused in most cases by overproduction of ROS, less so by their reduced breakdown (3). For this reason, it is thought that inhibition of ROS production, which aims to prevent oxidative stress or to reverse it, has great potential for future therapies of cardiovascular diseases.
Thus far, only one enzyme family is known whose sole function it is to generate ROS: NADPH oxidases (figure 2) (3). Other enzymes that generate ROS (xanthine oxidases, cyclo-oxygenases, lipoxygenases, uncoupled NO synthase, cytochrome P450 enzymes, and enzymes of the mitochondrial respiratory chain) primarily have different biochemical functions and generate ROS only as a byproduct or when in a dysfunctional state. Interestingly, NADPH oxidases produce “kindling” radicals that uncouple eNOS and upregulate xanthine oxidases (e3). The cytochrome isoform CYP 2C9 generates eicosanoids, which have a vasodilatory effect on healthy vessels, and is transformed into a ROS source in the vasculature of patients with coronary heart disease (12).
NADPH oxidases were discovered in phagocyte cells, where they cause the so-called respiratory burst—the release of large quantities of ROS by immune cells during the immune response (3, 13). Non-phagocytic NADPH oxidases have been identified in virtually every organ, including blood vessels. They are involved in a multitude of physiological processes—for example, signal transduction, regulation of gene expression, and cell differentiation (3). NADPH oxidases consist of several subunits. The catalytic subunits (NOX) are membrane proteins that transfer electrons from NADPH to oxygen and thus release ROS. Five NOX isoforms exist (NOX1–5) (13). Of particular interest is the isoform NOX5, the only isoform whose activity is directly regulated by calcium (3). Through this isoform, calcium overload of blood vessels may be directly coupled with oxidative stress. The amount of NOX5 protein is raised in the coronary arteries of patients with coronary heart disease, for example (14).
One strategy that may be more successful than administering antioxidants consists of inhibiting defined ROS generators, such as NADPH oxidases. Specific pharmacological inhibitors for NADPH oxidases are currently in their very early stages of development; any associated hopes are limited to the future. Currently, the challenge lies in treating the sequelae of years of oxidative stress in patients with cardiovascular diseases. Such treatments should be individually tailored and differ by stage of disease.
Increasing protective NO
One strategy to correct reduced NO synthesis is to supplement with the NOS substrate L-arginine. Short term supplementation with L-arginine (at least 3 g/day) improves endothelial function (measured as flow-mediated vasodilatation of the brachial artery; FMD) in patients with endothelial dysfunction before the start of L-arginine treatment (15). This resulted, for example, in improved exercise capacity in patients with peripheral occlusive disease (e4). This finding fits in with the concept of individualized medicine, whereby in each patient, the presence of a relevant pathomechanism (here: endothelial dysfunction) should be measured before targeted treatment is initiated. Currently, however, accurate diagnostic methods for endothelial function that quantify relevant biochemical markers in the blood are lacking, however. The only study of the long term effects of L-arginine administration yielded negative results (16), which hints at the development of “arginine resistance.” NO synthesis can possibly be improved further by administering the cofactor BH4 (17).
An alternative approach to increasing NO synthesis lies in using NOS enhancers, which enhances the expression of eNOS. Such a molecule, AVE 9488, has conferred protection against ischemia-reperfusion damage in the mouse model (18), but it has not yet been clinically investigated.
Little NO also works
Many of the physiological functions of NO are mediated by the NO receptor soluble guanylate cyclase (sGC).
sGC is a heme containing enzyme that generates the intracellular messenger substance cyclic guanosine monophosphate (cGMP) from GTP when NO binds to sGC (19).
The pharmacological activation of sGC by organic nitrates that release NO has been a therapeutic approach for 100 years and is used in the acute treatment of angina pectoris and heart failure. The chronic use of nitrates is, however, subject to limitations as nitrate tolerance develops. Nitrates induce ROS production, possibly via NADPH oxidases, which results in uncoupling of eNOS (20).
A new strategy to increase cGMP is the use of sGC stimulators, which are currently in clinical development.
This novel class of substances binds to sGC and potentiates the activation of heme containing sGC by NO (19). In this way, sGC is maximally stimulated even at reduced NO concentrations. The sGC stimulator riociguat (BAY 63–2521) is currently in phase III clinical trials for the oral therapy of pulmonary hypertension. In a preceding phase II study, riociguat improved the exercise capacity, the stroke volume of the heart, and the resistance of pulmonary vessels (21).
ROS can also damage sGC by oxidizing the sGC heme group (ox-sGC). As a result the heme is released. Both ox-sGC and heme-free sGC (apo-sGC) are elevated in cardiovascular diseases that are accompanied by oxidative stress (19). Apo-sGC cannot be activated by NO and is thus lost for physiological NO signal transmission.
This discovery has resulted in the development of the so called sGC activators. In contrast to sGC stimulators, which synergize with NO, sGC activators activate sGC independently of NO, and they act only on the NO insensitive apo-sGC form. Given in combination with NO donors they have an additive effect (19). sGC activators are effective only when sGC is subject to oxidative damage. The development of a diagnostic method for apo-sGC should thus make it possible to target those patients in whom treatment with sGC activators will be effective.
An already established biomarker (BNP; B-type natriuretic peptide) is functionally linked to another, membrane bound guanylate cyclase. BNP measures the severity of heart failure. However, it probably cannot be used for monitoring of drug therapies (e5, e6).
Indeed, the relaxation of isolated vessels in diabetes patients by means of an sGC activator is more pronounced than that of the vessels of healthy subjects (22). Apo-sGC is therefore raised in the pathologically altered vasculature, whereas sGC activators probably have a selective effect. The potential clinical efficacy of the sGC activator Cinaciguat for the treatment of heart failure was supported by the findings of a non-placebo controlled phase IIb study. The stroke volume, for example, improved (23). The sGC activator Ataciguat (HMR1766) is currently in clinical development for the treatment of neuropathic pain.
What is feasible today?
Statins and RAS inhibitors
Interestingly, many pathological stimuli—including angiotensin II, glucose, and oxidized LDL (3)—activate NADPH oxidases in vascular cells (figure 2). Part of the clinical effectiveness of angiotensin converting enzyme (ACE) inhibitors and angiotensin II receptor antagonists is probably due to inhibition of NADPH oxidases (3). Experiments have shown that deletion of the NOX1 gene reduces angiotensin II induced hypertension in mice (3). Furthermore, the pleiotropic effects of statins that cannot be explained by cholesterol lowering may be mediated in part by NADPH oxidase inhibition, since statins reduce the isoprenylation of an NADPH oxidase protein subunit (24).
PDE inhibitors
PDE5 inhibitors constitute an additional therapeutic option. These enhance the effects of NO by inhibiting the breakdown of cGMP. Therapy with the PDE5 inhibitor sildenafil has been clinically tested in pulmonary hypertension, for example. However, sildenafil is not effective in all patients. One possible reason may be a scenario of such severely lowered NO concentrations that cGMP cannot be sufficiently raised by sildenafil (21).
Beetroot, red wine, and dark chocolate
Some foods may confer additional protection, which is presumed to be based on interactions with free radicals. These foods include nitrate rich vegetables. For example, beetroot juice acutely lowers blood pressure in healthy subjects, prevents endothelial dysfunction induced by acute ischemia of the forearm, and reduces platelet aggregation (e7). These effects of nitrates are assumed to be due to their transformation into plasma nitrite and finally NO (e7, e8). Perhaps this is even a mechanism that confers cardioprotective effects of vegetables (e7, e9). However, this is hypothetical, and long term results are lacking. Whether a chronic high intake of nitrites may be toxic is the subject of controversial discussion. Epidemiological studies have, however, not shown a correlation between dietary nitrate/nitrite and stomach cancer (e9). Dark, flavonoid rich chocolate also has blood pressure lowering effects, which is (at least in part) mediated by NO (e10, e11). An intake of only 30 g/day of dark chocolate is sufficient to achieve this effect (e10). The extent of the blood pressure lowering effect of cocoa containing foods is clinically relevant; it is comparable with that of monotherapy with a beta blocker or ACE inhibitor (e12).
Polyphenols in red grapes also stimulate the production of NO and inhibit NADPH oxidases, at least in animal and cell models. These effects may explain the cardioprotective effect of moderate consumption of red wine (e11). It is unlikely that all these effects are conferred by the antioxidant effects of the food ingredients, because administration of isolated antioxidants does not acutely lower blood pressure. Furthermore, tea apparently does not have a blood pressure lowering effect, although it is equally rich in antioxidants (e12). Rather, some—but not all—of the ingredients that are classed as antioxidants influence the expression of protective or harmful genes (e11, e13). Last, but not least, the effects and complex interactions of different food ingredients cannot simply be pressed into a tablet.
Lifestyle
A committed physician is required to ensure prophylaxis; someone who continuously persuades and reminds the patient to eat a balanced diet, engage in physical exercise, keep a reasonable weight, and not to smoke (e14). The authors’ hypothesis is that no medical drug will ever be able to confer better protection and more benefit than these measures.
Conclusion
sGC stimulators and activators are future approaches in the treatment of cardiovascular diseases. The main focus is not on the symptom but on the disease triggering mechanism. In contrast to organic nitrates, continuous long term therapy with these new substances may be possible. Further, inhibition of NADPH oxidases is potentially a more effective strategy for the prevention and therapy of oxidative stress and the resultant cardiovascular diseases than antioxidants. Clinical proof, however, is still awaited. To identify pathomechanisms (here: oxidative stress) that are relevant for the individual patient and to treat these in a targeted manner is an example of individualized medicine applied in the future: new drugs, combined with novel diagnostic tests, will treat the pathomechanisms that are relevant for the individual patient and thus increase the chance of therapeutic success (box). This therapeutic concept is in contrast to the current “one drug fits all” therapies with “blockbuster” drugs and their limitations and financial risks (25). Independently of these future drug developments, the best rule is still: prevention is better than cure.
Box. Individualized medicine.
An individualized approach in medicine allows physicians to treat the individually relevant mechanism of disease in each patient. For example, administering L-arginine may not be beneficial in every patient with coronary heart disease or peripheral arterial occlusive disease, but only for those with raised ADMA concentrations; administration of sGC activators is indicated only if sGC is oxidatively damaged, and NADPH inhibitors only if oxidative stress is actually present in the vascular wall and NADPH oxidases are the relevant source. Plasma nitrotyrosine and other biomarkers may gain in importance for the diagnosis.
What distinguishes the pharmacological activation of sGC from, for example, pharmacological blockade of the beta-adrenoreceptor? A defined pharmacological mechanism is known for both classes of drugs, but only sGC activators treat a clearly defined disease mechanism. Activation of sGC works only if sGC is oxidatively damaged. This occurs physiologically to a certain extent, but under disease conditions it is more prevalent. A disease specificity thus arises, and the pathomechanism (oxidation, loss of heme, loss of NO sensitivity, lowered cGMP concentrations) is disrupted: sGC activators raise cGMP concentrations in conditions where NO remains ineffective. By contrast, it is known that beta-adrenoreceptor blockers block the respective receptors in a specific manner, but it is unclear by means of which mechanism they are effective. It is not known either whether beta-adrenoreceptors are even overstimulated under disease conditions. Only then, however, would beta-adrenoreceptor blockers interfere directly with a pathomechanism.
Only such medications that are effective via a clearly defined pathomechanism make it possible to determine—in combination with suitable diagnostics—whether this pathomechanism is relevant in the individual case and whether the drug is therefore indicated for this particular patient. If the pathomechanism under examination is not relevant—if, for example, sGC is not oxidized to an increasing extent—it is clear from the outset that an sGC activator is probably not the optimal therapeutic option for this particular patient. For beta-adrenoreceptor antagonists, no individual inclusion or exclusion criteria exist so far. But this will probably change in the future. Recently, activating antibodies of the beta-adrenoreceptor have been discovered in the blood of cardiovascular patients (e22). Measuring these antibodies may therefore in future help to predict the effect of beta-adrenoreceptor blockers on an individual basis. The discovery of these autoimmune antibodies could also lead to the development of new, mechanism based therapeutic strategies—namely, the neutralization of these antibodies.
Key Messages.
Radicals and reactive oxygen species are physiologically and pathophysiologically relevant. This might explain why antioxidant supplements that unspecifically scavenge radicals confer no clinical benefit.
New concepts are required to treat oxidative stress. This includes novel drugs that target the patho-mechanism, rather than merely normalize pathological symptoms or laboratory parameters.
Examples of a future generation of mechanism based medications are novel vasodilators that stimulate or activate cGMP synthesis that is impaired as a result of oxidative stress.
Further examples of such drugs are inhibitors of radical generating enzymes, which thus prevent the formation of harmful radicals; however, these have not been tested in clinical trials.
A healthy lifestyle may be more beneficial for preventing cardiovascular and other chronic diseases than medical drugs. This includes a healthy diet, sufficient physical exercise, and avoidance of excess weight and smoking.
Acknowledgments
Translated from the original German by Dr Birte Twisselmann.
Footnotes
Conflict of interest statement
Dr Wingler was in the past employed by Vasopharm GmbH (Ltd). Professor Schmidt has worked as an adviser for Vasopharm GmbH (Ltd) and BayerHealthcare and has received research support from BayerHealthcare and Servier.
References
- 1.Beckman JS. Understanding peroxynitrite biochemistry and its potential for treating human diseases. Archives of Biochemistry and Biophysics. 2009;484:114–116. doi: 10.1016/j.abb.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vanhoutte PM. How we learned to say NO. Arterioscler Thromb Vasc Biol. 2009;29:1156–1160. doi: 10.1161/ATVBAHA.109.190215. [DOI] [PubMed] [Google Scholar]
- 3.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 4.Schulz E, Jansen T, Wenzel P, Daiber A, Munzel T. Nitric oxide, tetrahydrobiopterin, oxidative stress, and endothelial dysfunction in hypertension. Antioxid Redox Signal. 2008;10:1115–1126. doi: 10.1089/ars.2007.1989. [DOI] [PubMed] [Google Scholar]
- 5.Cobb JP. Nitric oxide synthase inhibition as therapy for sepsis: a decade of promise. Surg Infect. 2001;2:93–100. doi: 10.1089/109629601750469410. discussion -1. [DOI] [PubMed] [Google Scholar]
- 6.Pou S, Pou WS, Bredt DS, Snyder SH, Rosen GM. Generation of superoxide by purified brain nitric oxide synthase. J Biol Chem. 1992;267:24173–24176. [PubMed] [Google Scholar]
- 7.Böger GI, Rudolph TK, Maas R, Schwedhelm E, Dumbadze E, Bierend A, et al. Asymmetric dimethylarginine determines the improvement of endothelium-dependent vasodilation by simvastatin effect of combination with oral L-arginine. J Am Coll Cardiol. 2007;49:2274–2282. doi: 10.1016/j.jacc.2007.02.051. [DOI] [PubMed] [Google Scholar]
- 8.Dotan Y, Pinchuk I, Lichtenberg D, Leshno M. Decision analysis supports the paradigm that indiscriminate supplementation of vitamin E does more harm than good. Arterioscler Thromb Vasc Biol. 2009;29:1304–1309. doi: 10.1161/ATVBAHA.108.178699. [DOI] [PubMed] [Google Scholar]
- 9.Gallicchio L, Boyd K, Matanoski G, Tao XG, Chen L, Lam TK, et al. Carotenoids and the risk of developing lung cancer: a systematic review. Am J Clin Nutr. 2008;88:372–383. doi: 10.1093/ajcn/88.2.372. [DOI] [PubMed] [Google Scholar]
- 10.Ristow M, Zarse K, Oberbach A, Kloting N, Birringer M, Kiehntopf M, et al. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci USA. 2009;106:8665–8670. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schafer ZT, Grassian AR, Song L, Jiang Z, Gerhart-Hines Z, Irie HY, et al. Antioxidant and oncogene rescue of metabolic defects caused by loss of matrix attachment. Nature. 2009;461:109–113. doi: 10.1038/nature08268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fichtlscherer S, Dimmeler S, Breuer S, Busse R, Zeiher AM, Fleming I. Inhibition of cytochrome P450 2C9 improves endothelium-dependent, nitric oxide-mediated vasodilatation in patients with coronary artery disease. Circulation. 2004;109:178–183. doi: 10.1161/01.CIR.0000105763.51286.7F. [DOI] [PubMed] [Google Scholar]
- 13.Lambeth JD. NOX enzymes, ROS, and chronic disease: an example of antagonistic pleiotropy. Free Radic Biol Med. 2007;43:332–347. doi: 10.1016/j.freeradbiomed.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guzik TJ, Chen W, Gongora MC, Guzik B, Lob HE, Mangalat D, et al. Calcium-dependent NOX5 Nicotinamide Adenine Dinucleotide Phosphate Oxidase contributes to vascular oxidative stress in human coronary artery disease. Journal of the American College of Cardiology. 2008;52:1803–1809. doi: 10.1016/j.jacc.2008.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bai Y, Sun L, Yang T, Sun K, Chen J, Hui R. Increase in fasting vascular endothelial function after short-term oral L-arginine is effective when baseline flow-mediated dilation is low: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2009;89:77–84. doi: 10.3945/ajcn.2008.26544. [DOI] [PubMed] [Google Scholar]
- 16.Schulman SP, Becker LC, Kass DA, Champion HC, Terrin ML, Forman S, et al. L-arginine therapy in acute myocardial infarction: the Vascular Interaction With Age in Myocardial Infarction (VINTAGE MI) randomized clinical trial. JAMA. 2006;295:58–64. doi: 10.1001/jama.295.1.58. [DOI] [PubMed] [Google Scholar]
- 17.Maier W, Cosentino F, Lutolf RB, Fleisch M, Seiler C, Hess OM, et al. Tetrahydrobiopterin improves endothelial function in patients with coronary artery disease. J Cardiovasc Pharmacol. 2000;35:173–178. doi: 10.1097/00005344-200002000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Frantz S, Adamek A, Fraccarollo D, Tillmanns J, Widder JD, Dienesch C, et al. The eNOS enhancer AVE 9488: a novel cardioprotectant against ischemia reperfusion injury. Basic Res Cardiol. 2009 Jun 23; doi: 10.1007/s00395-009-0041-3. (Epub ahead of print). DOI: 10.1007/s00395-009-0041-3. [DOI] [PubMed] [Google Scholar]
- 19.Evgenov OV, Pacher P, Schmidt PM, Hasko G, Schmidt HH, Stasch JP. NO-independent stimulators and activators of soluble guanylate cyclase: discovery and therapeutic potential. Nat Rev Drug Discov. 2006;5:755–768. doi: 10.1038/nrd2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Munzel T, Wenzel P, Daiber A. Do we still need organic nitrates? J Am Coll Cardiol. 2007;49:1296–1298. doi: 10.1016/j.jacc.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 21.Mittendorf J, Weigand S, Alonso-Alija C, Bischoff E, Feurer A, Gerisch M, et al. Discovery of riociguat (BAY 63-2521): a potent, oral stimulator of soluble guanylate cyclase for the treatment of pulmonary hypertension. ChemMedChem. 2009;4:853–865. doi: 10.1002/cmdc.200900014. [DOI] [PubMed] [Google Scholar]
- 22.Stasch JP, Schmidt PM, Nedvetsky PI, Nedvetskaya TY, H SA, Meurer S, et al. Targeting the heme-oxidized nitric oxide receptor for selective vasodilatation of diseased blood vessels. J Clin Invest. 2006;116:2552–2561. doi: 10.1172/JCI28371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lapp H, Mitrovic V, Franz N, Heuer H, Buerke M, Wolfertz J, et al. Cinaciguat (BAY 58-2667) improves cardiopulmonary hemodynamics in patients with acute decompensated heart failure. Circulation. 2009;119:2781–2788. doi: 10.1161/CIRCULATIONAHA.108.800292. [DOI] [PubMed] [Google Scholar]
- 24.Vecchione C, Brandes RP. Withdrawal of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors elicits oxidative stress and induces endothelial dysfunction in mice. Circ Res. 2002;91:173–179. doi: 10.1161/01.res.0000028004.76218.b8. [DOI] [PubMed] [Google Scholar]
- 25.Jackson G. Torcetrapib: when global risk reduction goes ’off target’. Int J Clin Pract. 2008;62:173–174. doi: 10.1111/j.1742-1241.2007.01687.x. [DOI] [PubMed] [Google Scholar]
- e1.Brouckaert P, Cauwels A, Thoonen R, Buys E, Bloch KD, Sips P, et al. Phenotypes of sGC mutant mice in basic conditions, disease and shock. BMC Pharmacology. 2009;9 [Google Scholar]
- e2.Singh D, Richards D, Knowles RG, Schwartz S, Woodcock A, Langley S, O’Connor B. Selective inducible nitric oxide synthase inhibition has no effect on allergen challenge in asthma. Am J Respir Crit Care Med. 2007;176:988–993. doi: 10.1164/rccm.200704-588OC. [DOI] [PubMed] [Google Scholar]
- e3.McNally JS, Davis ME, Giddens DP, Saha A, Hwang J, Dikalov S, et al. Role of xanthine oxidoreductase and NAD(P)H oxidase in endothelial superoxide production in response to oscillatory shear stress. Am J Physiol Heart Circ Physiol. 2003;285:H2290–H2297. doi: 10.1152/ajpheart.00515.2003. [DOI] [PubMed] [Google Scholar]
- e4.Maxwell AJ, Anderson BE, Cooke JP. Nutritional therapy for peripheral arterial disease: a double-blind, placebo-controlled, randomized trial of HeartBar. Vasc Med. 2000;5:11–19. doi: 10.1177/1358836X0000500103. [DOI] [PubMed] [Google Scholar]
- e5.Pfisterer M, Buser P, Rickli H, Gutmann M, Erne P, Rickenbacher P, et al. BNP-guided vs symptom-guided heart failure therapy: the trial of intensified vs standard medical therapy in elderly patients with congestive heart failure (TIME-CHF) randomized trial. JAMA. 2009;301:383–392. doi: 10.1001/jama.2009.2. [DOI] [PubMed] [Google Scholar]
- e6.Pina IL, O’Connor C. BNP-guided therapy for heart failure. JAMA. 2009;301:432–434. doi: 10.1001/jama.2009.3. [DOI] [PubMed] [Google Scholar]
- e7.Webb AJ, Patel N, Loukogeorgakis S, Okorie M, Aboud Z, Misra S, et al. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension. 2008;51:784–790. doi: 10.1161/HYPERTENSIONAHA.107.103523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e8.Gladwin MT, Schechter AN, Kim-Shapiro DB, Patel RP, Hogg N, Shiva S, et al. The emerging biology of the nitrite anion. Nat Chem Biol. 2005;1:308–314. doi: 10.1038/nchembio1105-308. [DOI] [PubMed] [Google Scholar]
- e9.Wink DA, Paolocci N. Mother was right: eat your vegetables and do not spit! When oral nitrate helps with high blood pressure. Hypertension. 2008;51:617–619. doi: 10.1161/HYPERTENSIONAHA.107.106617. [DOI] [PubMed] [Google Scholar]
- e10.Taubert D, Roesen R, Lehmann C, Jung N, Schomig E. Effects of low habitual cocoa intake on blood pressure and bioactive nitric oxide: a randomized controlled trial. JAMA. 2007;298:49–60. doi: 10.1001/jama.298.1.49. [DOI] [PubMed] [Google Scholar]
- e11.Schmitt CA, Dirsch VM. Modulation of endothelial nitric oxide by plant-derived products. Nitric Oxide. 2009;21:77–91. doi: 10.1016/j.niox.2009.05.006. [DOI] [PubMed] [Google Scholar]
- e12.Taubert D, Roesen R, Schomig E. Effect of cocoa and tea intake on blood pressure: a meta-analysis. Arch Intern Med. 2007;167:626–634. doi: 10.1001/archinte.167.7.626. [DOI] [PubMed] [Google Scholar]
- e13.Park DW, Baek K, Kim JR, Lee JJ, Ryu SH, Chin BR, et al. Resveratrol inhibits foam cell formation via NADPH oxidase 1- mediated reactive oxygen species and monocyte chemotactic protein-1. Exp Mol Med. 2009;41:171–179. doi: 10.3858/emm.2009.41.3.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e14.Ford ES, Bergmann MM, Kroger J, Schienkiewitz A, Weikert C, Boeing H. Healthy living is the best revenge: findings from the European Prospective Investigation Into Cancer and Nutrition-Potsdam study. Arch Intern Med. 2009;169:1355–1362. doi: 10.1001/archinternmed.2009.237. [DOI] [PubMed] [Google Scholar]
- e15.Vivekananthan DP, Penn MS, Sapp SK, Hsu A, Topol EJ. Use of antioxidant vitamins for the prevention of cardiovascular disease: meta-analysis of randomized trials. Lancet. 2003;361:2017–2023. doi: 10.1016/S0140-6736(03)13637-9. [DOI] [PubMed] [Google Scholar]
- e16.Bjelakovic G, Nikolova D, Simonetti RG, Gluud C. Antioxidant supplements for prevention of gastrointestinal cancers: a systematic review and meta-analysis. Lancet. 2004;364:1219–1228. doi: 10.1016/S0140-6736(04)17138-9. [DOI] [PubMed] [Google Scholar]
- e17.Kris-Etherton PM, Lichtenstein AH, Howard BV, Steinberg D, Witztum JL. Antioxidant vitamin supplements and cardiovascular disease. Circulation. 2004;110:637–641. doi: 10.1161/01.CIR.0000137822.39831.F1. [DOI] [PubMed] [Google Scholar]
- e18.Miller ER, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- e19.Shekelle PG, Morton SC, Jungvig LK, Udani J, Spar M, Tu W, et al. Effect of supplemental vitamin E for the prevention and treatment of cardiovascular disease. J Gen Intern Med. 2004;19:380–389. doi: 10.1111/j.1525-1497.2004.30090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e20.Eidelman RS, Hollar D, Hebert PR, Lamas GA, Hennekens CH. Randomized trials of vitamin E in the treatment and prevention of cardiovascular disease. Arch Intern Med. 2004;164:1552–1556. doi: 10.1001/archinte.164.14.1552. [DOI] [PubMed] [Google Scholar]
- e21.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;297:842–857. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- e22.Jahns R, Boivin V, Hein L, Triebel S, Angermann CE, Ertl G, Lohse MJ. Direct evidence for a beta 1-adrenergic receptor-directed autoimmune attack as a cause of idiopathic dilated cardiomyopathy. J Clin Invest. 2004;113:1419–1429. doi: 10.1172/JCI20149. [DOI] [PMC free article] [PubMed] [Google Scholar]