Abstract
The majority of human cervical cancers are associated with the high-risk human papillomavirus (HPV) types. In mouse models for HPV-associated cancers, estrogen is required for the development of cervical and vaginal cancers. The estrogen receptor α (ERα) also is required in mice for these cancers to arise. These data are consistent with the observation in women that long-term use of oral contraceptives or multiple pregnancies significantly increases the risk for cervical cancer in HPV-positive women. In the present study, we examined whether drugs that interfere with the function of ERα are effective in treating and/or preventing cervical cancer in mice. We provide evidence that a complete ER antagonist, ICI 182,780 (ICI), as well as a selective ER modulator, raloxifene, efficiently clear cancer and its precursor lesions in both the cervix and the vagina. Furthermore, ICI was capable of preventing the onset of cancers in mice bearing precursor lesions. These findings point to the potential value of ER antagonists in controlling gynecological disease in the lower reproductive tracts in women.
Keywords: cervix, papillomavirus, SERM
High-risk human papillomavirus (HPV) types, particularly HPV16, are causally associated with human malignancies, including cervical and vaginal cancers in women (1). New prophylactic HPV vaccines can prevent infections by a subset of these HPVs; however, they will not eliminate preexisting HPV infections, new infections by high-risk HPVs not targeted by the vaccines, or cervical cancers and precancerous lesions that arise from those HPV infections (2). Traditional therapeutic approaches (i.e., surgery, radiotherapy, and chemotherapy) are of limited value to patients with advanced or recurrent cervical cancer. Consequently, cervical cancer remains the second leading cause of death by cancer among women worldwide (1, 2). New, more effective therapeutic strategies are clearly needed. This preclinical study identifies a potent new therapeutic approach that not only effectively treats preexisting cervical and vaginal cancers but also can prevent their onset in a mouse model for HPV-associated cervical carcinogenesis.
The uterine cervix is highly responsive to steroidal hormones, such as estrogen. Correspondingly, cervical cancers most commonly arise in the third to fifth decade (i.e., premenopausal period) of life in women (3). Furthermore, use of oral contraceptives or high parity has been shown to significantly increase the risk for cervical cancer in HPV-infected women (4, 5). These observations raise the possibility that steroidal hormones, such as estrogen, might affect cancers of the cervix, much like that of other hormonally responsive female organs (3, 6). Estrogen replacement therapy alone, however, does not increase the risk for cervical cancer, and tamoxifen, a well-known estrogen receptor (ER) antagonist in breast, has no beneficial effect on cervical cancer (7, 8). Unfortunately, neither of these studies controlled for infections with high-risk HPVs that are prerequisite for cervical cancer (1, 2). Furthermore, tamoxifen has an ER agonistic rather than antagonistic effect in human cervix (9). Studies have shown a protective effect on cervical cancer by indole-3-carbinol, a compound found in cruciferous vegetables that favorably alters the metabolism of estrogen; however, this drug has also been shown to inhibit the genesis of other tumors that are not believed to depend on estrogen (10–12). Thus, the evidence for or against a role of estrogen in cervical carcinogenesis in humans remains limited in nature and inconclusive.
To elucidate mechanisms by which HPV oncogenes promote cervical cancer and by which cofactors contribute to cervical carcinogenesis, we have generated transgenic mouse models that express HPV16 E6 and/or E7 under the control of human keratin 14 promoter that drives gene expression in stratified squamous epithelia, natural targets for HPV infection. The progressive disease that arises in these mouse models recapitulates various aspects of human cervical disease, including the multiple stages of cervical carcinogenesis, the anatomical location and histopathological nature of the cancers, and the expression patterns of various biomarkers (13, 14), validating the relevance of these preclinical models to cervical disease in women. By using these mouse models, we have demonstrated that estrogen is required for development of cervical cancer and that ERα is crucial for the development of atypical squamous metaplasia (ASM), which has been proposed to be the very first step of cervical carcinogenesis preceding the development of cervical intraepithelial neoplasia (CIN), a precancerous cervical lesion that can progress to cervical cancer (14–19). It is still unclear, however, whether ER-dependent as opposed to ER-independent pathway is crucial for the progression of CIN lesions to invasive cancer and maintenance of cervical cancer. In the current study, we demonstrate that inhibition of ER is effective in both treating and preventing cervical cancer in mice.
Results and Discussion
Selection of ER Antagonists and Treatment Regimen.
Our goal was to use ER antagonists to evaluate the role of ER in cervical carcinogenesis. Selective ER modulators (SERMs) are compounds that modulate activities of ER by competitively binding to ER and altering recruitment of coactivators and/or corepressors. Most SERMs are known to have ER agonistic, antagonistic, or neutral effects, depending on tissue context, and thus a SERM can have opposite effects in different tissue contexts (20). As far as we could learn from the literature, none of the Food and Drug Administration-approved SERMs had been tested for their effect on the murine cervix. The selective ER down-regulator, ICI 182,780 (ICI; also known as fulvestrant), inhibits nuclear transport of ER and induces proteosomal degradation of ER. Thus, in contrast to SERMs, ICI functions as an ER antagonist in all tissues and is commonly referred to as a “complete” ER antagonist (20). To determine whether ER is crucial for continued growth of cervical cancer, we decided first to investigate the activities of the complete ER antagonist, ICI, in a preclinical mouse model for HPV-associated cervical cancer. Specifically, we used K14E6/K14E7 double-transgenic mice expressing the HPV16 E6 and E7 oncogenes, which, when treated for 6 months with exogenous estrogen (E2) at a physiological level sufficient to induce continuous estrus, develop cervical cancer at high penetrance (15, 19). The progressive neoplastic disease arising in these mice that culminates in cervical cancer is histopathologically indistinguishable from that observed in HPV16-infected women or from that observed in K14HPV16 transgenic mice that harbor the whole HPV16 early region, consistent with the fact that E6 and E7 are the major oncogenes responsible for cervical carcinogenesis (1, 19).
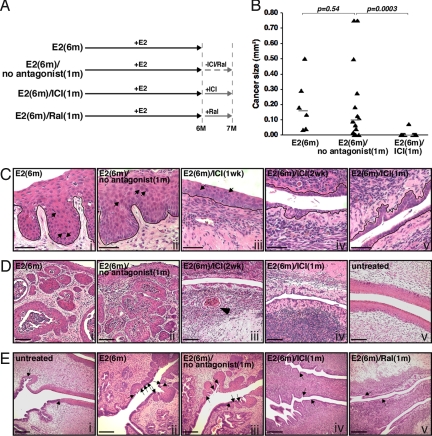
We have shown that K14E6/K14E7 double-transgenic mice that are treated with exogenous estrogen for 6 months and maintained for an additional 3 months without exogenous estrogen have reduced tumor multiplicity and tumor size compared with those treated with exogenous estrogen for 9 months (15). The majority of mice (82%), however, still bear cancers. It is unclear whether tumors in these mice are still dependent on endogenous estrogen or are estrogen-independent. Therefore, we decided to treat the double-transgenic mice with exogenous estrogen for 6 months (the vast majority of these mice are expected to develop cervical cancer) and then treat them with an ER antagonist for 1 month without exogenous estrogen (Fig. 1A). This treatment regimen, if translatable to women, would be relevant to cervical cancer patients who are not under continuous hormonal stimulation, such as oral contraceptives, hormone replacement therapy, or pregnancy.
Fig. 1.
Therapeutic treatment with ICI or raloxifene for 1 month results in regression of cervical disease. (A) Treatment regimens for the therapeutic approach are depicted. See Materials and Methods for details. ICI and Ral stand for ICI 182,780 (fulvestrant) and raloxifene, respectively. (B) Comparison of cervical cancer size in indicated treatment groups. Cancer size (largest cross-sectional area of each cancer) was determined as previously described (19). Mice that did not have cancers were assigned a value of 0. Gray bar represents median value for each group. P values from two-sided Wilcoxon rank sum test are shown. (C) Shown are high-magnification images of representative H&E-stained endocervical sections from indicated groups of mice. Arrows point to some dysplastic cells with dark, enlarged nuclei. Note that cervical dysplasias (CIN) are evident in mice not treated with ICI (i and ii) or treated with ICI for 1 week (iii) but are absent in mice treated with ICI for a longer period (iv and v). The black line delineates the basement membrane that separates epithelium from underlying stroma. (Scale bar, 50 μm.) (D) Shown are low-magnification images of representative H&E-stained endocervical sections from indicated groups of mice. Note that entire fields show cancer in mice not treated with ICI (i and ii). Also note that epithelium of ICI-treated mice (iii and iv) is much thinner than that of untreated mice (v). The arrowhead points to the small cancer remaining in mice treated with ICI for 2 weeks (iii). (Scale bar, 100 μm.) (E) Transition from columnar epithelium to squamous epithelium is present only once in normal cervix (i; normal squamous metaplasia). In ASM, however, it occurs multiple times, and thus patches of squamous epithelium are embedded within columnar epithelium (ii and iii). Note that ASM is absent in mice treated with ICI (iv) or raloxifene (v). Arrows point to transition from columnar epithelium to squamous epithelium. (Scale bar, 200 μm.)
Efficacy of ICI in Treating Cervical Cancer.
K14E6/K14E7 double-transgenic mice were initially divided into three groups: E2(6m) group was treated with exogenous estrogen for 6 months and immediately killed; E2(6m)/no antagonist(1m) group was treated with estrogen for 6 months, as described for the E2(6m) group, but then maintained for one additional month without further treatment; E2(6m)/ICI(1m) group was treated for 6 months with estrogen, then treated for 1 month with ICI (Fig. 1A).
Consistent with previous results (15, 19), all of the control K14E6/K14E7 female mice [i.e., those treated for 6 months with E2; the E2(6m) group] developed cervical cancer (Table 1). Thus, at the time point that ICI was delivered to the E2(6m)/ICI(1m) group (see Fig. 1A), all mice had cancer. In the secondary control group [E2(6m)/no antagonist(1m)], which was maintained for an additional month without further treatment with either E2 or any antagonists, the majority of mice (79%) retained cervical cancer, and their median cancer size (0.10 mm2) was slightly reduced compared with that (0.16 mm2) of the E2 group (Fig. 1B and Table 1). These small reductions in the incidence and size of these cancers were not significantly different (P > 0.5). Cervical cancer multiplicity (2.3 ± 1.4) in the E2(6m)/no antagonist(1m) group was also reduced compared with that (3.3 ± 1.1) of the E2(6m) group, but this was not statistically significant (P = 0.09; Table 1). These modest reductions in tumor burden after removal of exogenous E2 for 1 month are consistent with prior observations that the withdrawal of E2 for a longer period (3 months) leads to a significant reduction in tumor burden (15).
Table 1.
Comparison of cervical/vaginal disease in E2-treated K14E6/K14E7 mice with or without therapeutic ICI/raloxifene treatment
| Treatment group | Group size, n | No disease |
Dysplasia only |
Cancer and dysplasia |
Cancer incidence, % |
Tumor multiplicity, mean ± SD |
||
|---|---|---|---|---|---|---|---|---|
| Cervix (vagina) | CIN1 (VIN1) | CIN2 (VIN2) | CIN3 (VIN3) | Cervix (vagina) | Cervix (vagina) | Cervix (vagina) | ||
| E2(6m) | 6* | 0 (0) | 0 (0) | 0 (1) | 0 (2) | 6 (3) | 100 (50) | 3.3 ± 1.1 (0.5 ± 0.5) |
| E2(6m)/no antagonist(1m) | 14 | 0 (0) | 0 (0) | 0 (3) | 3 (6) | 11 (5) | 79 (36) | 2.3 ± 1.4 (0.4 ± 0.6) |
| E2(6m)/ICI(1m) | 13 | 12 (12) | 0 (0) | 0 (0) | 0 (0) | 1 (1) | 8 (8) | 0.2 ± 0.5 (0.1 ± 0.3) |
| E2(6m)/Ral(1m) | 7 | 7 (6) | 0 (0) | 0 (0) | 0 (1) | 0 (0) | 0 (0) | 0.0 ± 0.0 (0.0 ± 0.0) |
CIN1 (VIN1) indicates low-grade dysplasia; CIN2(VIN2), midgrade dysplasia; CIN3(VIN3), high-grade dysplasia.
*Two mice were killed before the study end point (at 5.5 months of E2 treatment) because of morbidity.
In contrast to the marginal differences in tumor incidence, size, and multiplicity between the E2(6m) and E2(6m)/no antagonist(1m) groups, mice treated with ICI for 1 month after 6-month E2 treatment [E2(6m)/ICI(1m) group] displayed highly significant and much greater reductions in cervical cancer incidence (8%; P = 0.0003), median cancer size (0 mm2; P = 0.0003), and tumor multiplicity (0.2 ± 0.5; P = 0.003) compared with the E2(6m)/no antagonist(1m) group (Fig. 1B; Table 1). Cancer phenotypes in these mice were also significantly weaker than those in mice treated with estrogen for 6 months and aged for three additional months without treatment (15). These results indicate that endogenous estrogen contributes to persistence of cervical cancer.
It was also evident that in the entire epithelia of lower female reproductive tracts, the majority of ICI-treated mice no longer had cervical intraepithelial neoplasia (CIN) or vaginal intraepithelial neoplasia (VIN), which are precancerous dysplastic lesions that can progress to cervical and vaginal cancers, respectively (Fig. 1C). By comparison, CIN and VIN lesions were found throughout the cervical/vaginal epithelia of the E2(6m) and E2(6m)/no antagonist(1m) groups (Fig. 1C and Table 1). Absence of CIN and VIN was also observed in mice treated with ICI only for 2 weeks, although these mice still carried small cancers (Fig. 1Diii). Focal CIN/VIN lesions, however, remained in mice treated with ICI for only 1 week, and epithelia of these mice were thicker than those in mice treated with ICI for longer periods (Fig. 1C, compare iii to iv and v).
It has been proposed that ASM is the earliest identifiable precursor lesion to cervical cancer (14, 16). Whereas all mice in the E2(6m) and E2(6m)/no antagonist(1m) groups displayed ASM, none of the mice in the E2(6m)/ICI(1m) group had ASM (Fig. 1E ii–iv).
Effect of ICI on Vaginal Disease in the Same Mice.
We further analyzed the data to determine whether ICI has a beneficial effect on vaginal cancers arising in our experimental mice. Reductions were seen in the incidence and tumor multiplicity in the vagina of the E2(6m)/ICI(1m) group, but because the penetrance of the disease in this tissue in the control groups [E2(6m) and E2(6m)/no antagonist(1m)] is much less than that in the cervix, these reductions were not statistically significant (Table 1). We therefore compared the overall vaginal disease states in these mice by giving arbitrary scores from 1 to 5 for the worst-case disease state (no dysplasia, VIN 1–3, cancer) of each animal as described in Materials and Methods. Vaginal disease in the E2(6m)/ICI(1m) group was significantly reduced compared with that in the E2(6m)/no antagonist(1m) group (P = 0.0008). The E2(6m) and E2(6m)/no antagonist(1m) groups displayed vaginal disease to a similar degree (P = 0.6).
Efficacy of Raloxifene in Treating Cervical and Vaginal Diseases.
Our results described above indicate that a complete ER antagonist, ICI, may be an effective drug for treating and/or preventing cervical cancer in women. ICI, however, will induce menopausal symptoms because it inhibits ER function in all tissues/cells. Therefore, if one were to use ER antagonists to treat women with cervical cancer, then SERMs would be a more appropriate drug for most, and particularly premenopausal women. We sought, therefore, to test a SERM for its efficacy in treating cervical cancer in mice. Among the Food and Drug Administration-approved, commercially available SERMs, we chose raloxifene based on its ER antagonistic effect in the mouse uterus and its activity profiles in human tissues: ER agonistic effect in bone, antagonistic effect in breast, and neutral effect in endometrium (6, 21). Raloxifene is approved for the treatment of osteoporosis and the prevention of breast cancer, and it has no major common side effects in women (6).
K14E6/K14E7 double-transgenic mice were treated with exogenous estrogen for 6 months and then with raloxifene for an additional month [E2(6m)/Ral(1m) group; see Fig. 1A]. Histological analyses revealed that, similar to ICI, treatment of tumor-bearing mice with the SERM raloxifene for 1 month eradicated cancers and dysplasia in the cervix and vagina as well as ASM (Fig. 1Ev and Table 1). Cervical cancer incidence and cervical/vaginal disease status were highly significantly lower than those of E2(6m)/no antagonist(1m) group (P < 0.001).
So far, we demonstrated that two different ER antagonists, ICI and raloxifene, are effective in curing both cancer and dysplasia in the cervix and vagina in our transgenic mouse model (Table 1). The epithelia of lower reproductive tracts from mice treated with ICI or raloxifene for ≥2 weeks were thin, with only two to three layers of cells (Fig. 1C), and uterine wet weight (16.3 ± 5.1 mg) was significantly reduced compared with that of untreated mice (68.1 ± 2.5 mg). These phenotypes are identical to those observed in ERα-deficient mice and tissue recombinants (16, 22). These data strongly support the conclusion that ICI and raloxifene can eliminate the majority of both precancerous and cancerous lesions in both the cervix and the vagina by inhibiting ERα. This conclusion is also supported by the absence of ASM in ICI- or raloxifene-treated cervices because genetic deletion of ERα renders mice resistant to the development of ASM (16).
Some Cancers Remain After ER Antagonist Treatment.
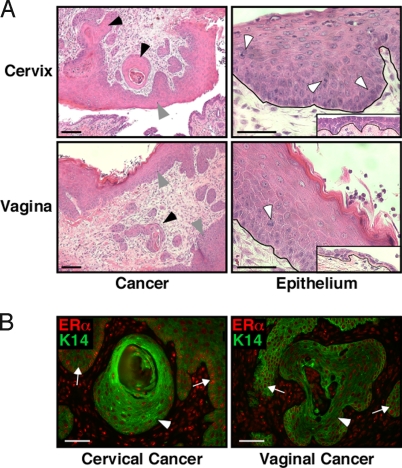
Although ICI was extremely efficient in clearing established cancers as described above, 2 of 13 mice in the E2(6m)/ICI(1m) group retained tumors (Table 1). We do not know whether these tumors were just more slowly regressing or are, in fact, ICI-resistant tumors. We predict that the latter possibility is likely because epithelia near the cancers remaining after ICI treatment were as thick as those in mice not treated with ICI, whereas the rest of epithelia in these mice were hypoplastic, identical to those in ICI-treated, cancer-free mice (Fig. 2A; see also Fig. 1C). One possibility is that the cancers retained in the ICI-treated mice and/or their associated stroma are producing their own estrogen, which leads to locally elevated estrogen concentrations sufficient to suppress antagonistic function of ICI. In this regard, some human cervical cancers overexpress aromatase, a rate-limiting enzyme in estrogen biosynthesis (23). Therefore, aromatase inhibitors may be effective in treating these potentially ICI-resistant cervical/vaginal cancers. Although these residual cancers still express ERα (Fig. 2B), it is also possible that these tumors are ERα-independent, as is found to be the case in a subset of tumors arising in the human breast, another estrogen-responsive tissue (6).
Fig. 2.
Cancers remaining after therapeutic ICI treatment are associated with nonhypoplastic epithelium. (A) (Left) Low-magnification images of the two cancers [one cervical (Upper) and the other vaginal (Lower)] that persisted in 2 of 13 mice treated with ICI for 1 month. Black and gray arrowheads point to cancers and cancer-associated epithelia, respectively. (Scale bar, 100 μm.) (Right) High-magnification images of cervical (Upper) or vaginal (Lower) epithelium that was immediately juxtaposed to these two cancers. Note the nonhypoplastic (i.e., thick) epithelium akin to what is seen in female mice in normal estrus (see Fig. 1Dv). This is in stark contrast to the hypoplastic epithelia found elsewhere in the reproductive tracts of these same ICI-treated mice (Insets), as well as in the other ICI-treated, cancer-free mice (see Fig. 1Cv). White arrowheads point to dysplastic cells. Black lines delineate the basement membrane. (Scale bar, 50 μm.) (B) Cancers remaining in ICI-treated mice express ERα. Tissues were stained for ERα (red) and K14 (green), an epithelial cell marker. Arrowheads and arrows point to cancers and cancer-associated epithelia, respectively. (Scale bar, 50 μm.)
Prevention of Cervical Cancer Development by ICI.
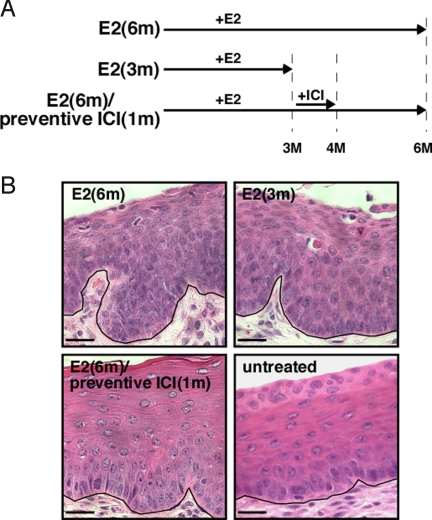
CIN and VIN are thought to be the precursor lesions to cervical and vaginal cancers, respectively. Because these lesions largely disappeared in ICI- or raloxifene-treated mice (Table 1), we performed an additional experiment to determine whether ER antagonists can prevent the onset of cervical/vaginal cancers. To address this, we began treating mice with ICI after only 3 months of E2 treatment (Fig. 3A), a time period at which CINs/VINs are present but no cancers have yet developed [Fig. 3B; Table 2, E2(3m) group]. Mice treated with ICI for 1 month at the 3-month time point [E2(6m)/preventive ICI(1m) group] showed neither CIN nor cervical cancer at the 6-month endpoint (Table 2). Cervical epithelium of the E2(6m)/preventive ICI(1m) group was almost identical to that of age-matched untreated K14E6/K14E7 mice in estrus (Fig. 3B). These phenotypes are significantly different from those of the E2(6m) group that had not received ICI (P = 0.002, cervical cancer incidence; P = 0.0009, cervical disease). Thus, inhibition of ERα not only promotes regression of cervical cancer but also prevents the development of this malignancy in mice. The E2(6m)/preventive ICI(1m) group also displayed a significantly lower degree of vaginal disease compared with the E2(6m) group (P = 0.002) (Table 2).
Fig. 3.
One-month ICI treatment prevents the onset of cervical cancer. (A) Treatment regimens for the preventive approach are depicted. See Materials and Methods for details. (B) Shown are high-magnification images of representative H&E-stained endocervical sections from indicated groups of mice. Unlike epithelia of mice in the E2(6m) or E2(3m) group, those in the E2(6m)/preventive ICI(1m) group are indistinguishable from normal epithelia of untreated mice in estrus. Black lines delineate the basement membrane. (Scale bar, 25 μm.)
Table 2.
Comparison of cervical/vaginal disease in E2-treated K14E6/K14E7 mice with or without preventive ICI treatment
| Treatment group | Group size, n | No disease |
Dysplasia only |
Cancer and dysplasia |
Cancer incidence, % |
||
|---|---|---|---|---|---|---|---|
| Cervix (vagina) | CIN1 (VIN1) | CIN2 (VIN2) | CIN3 (VIN3) | Cervix (vagina) | Cervix (vagina) | ||
| E2(3m) | 6 | 0 (2) | 1 (2) | 3 (2) | 2 (0) | 0 (0) | 0 (0) |
| E2(6m)/preventive ICI(1m) | 6 | 6 (6) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| E2(6m)* | 6 | 0 (0) | 0 (0) | 0 (1) | 0 (2) | 6 (3) | 100 (50) |
*Same group as described in Table 1.
A relevant question we also tried to answer in this study is whether cancers recur rapidly after ER antagonist treatment is ceased. To answer that question, we held a subset of mice in E2(6m)/ICI(1m) group for an additional 6 weeks after the 1-month treatment with ICI, and during this 6-week period we put them back on exogenous estrogen treatment. At the endpoint we observed neither cancers nor even CIN lesions. Rather, we found the cervical epithelia to remain hypoplastic, similar to that observed in the E2(6m)/ICI(1m) group. We interpret these data to indicate that ICI is sustained in the mouse system for a long time at levels high enough to inhibit ERα. Such sustained ICI activity prevents us from evaluating whether recurrence of disease is an issue. Thus, we are currently investigating other SERMs, including raloxifene, to identify an ER antagonist suitable for experiments to address this question.
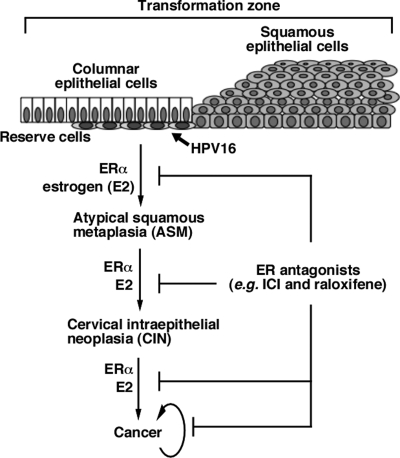
The findings from our preclinical studies are consistent with the hypothesis that ERα is necessary for the progression and maintenance of cervical cancer in mice. Therefore, we predict that other drugs that inhibit the estrogen-ERα pathway in the cervix should have similar therapeutic and preventive effects on cervical cancer. We have demonstrated that ERα is indeed necessary for the development of ASM, which is thought to be a precursor for cervical cancer and to originate from a specific cell type called reserve cells in the cervical transformation zone (14, 16). Taken together, we propose a model in which reserve cells are the precursor cell type for cervical carcinogenesis, and after HPV16 infection they slowly progress to ASM, CIN, and then cancer in the presence of estrogen and ERα (Fig. 4) These two factors are also required for the persistence of cervical cancer. ER antagonists can cause efficient regression of cancer, dysplasia, and ASM and prevent malignant progression. Requirement for HPV oncogenes in all steps of cervical carcinogenesis has been well-demonstrated in both cervical cancer-derived cell lines and transgenic mouse models (24–26). Although our transgenic mouse model for HPV-associated cervical cancer recapitulates most aspects of human cervical cancer (13, 14, 19), it is obvious that further study is needed to determine whether this proposed model is relevant to human cervical cancer. If it proves relevant, then it would have clear implications in the prevention and control of malignant cervical and vaginal disease in women, and it may provide alternatives to currently available modalities for treating cervical cancer and precursor lesions. It would also raise some concerns that the use of tamoxifen, a SERM with ER agonist activities in the human female reproductive tract, or estrogen (hormone) replacement therapy could lead to increases in cervical neoplasia in HPV-infected women.
Fig. 4.
A model for cervical carcinogenesis. See the text for details.
Materials and Methods
Mice.
K14E7 and K14E6 transgenic mice were designed to express HPV16 E7 and E6, respectively, in stratified squamous epithelia, natural HPV infection sites in humans (27, 28). Female progenies were genotyped by PCR, and a slow-releasing 17β-estradiol (E2) tablet (0.05 mg, 60 days; Innovative Research of America) was inserted s.c. under the dorsal skin every 2 months beginning at 4–6 weeks of age. After 6-month treatment with E2, one group of mice was killed immediately to evaluate cancer phenotypes [E2(6m) group], another group was maintained for another month without further treatment [E2(6m)/no antagonist(1m) group], and the third group was treated for a month with ICI [E2(6m)/ICI(1m) group] or raloxifene [E2(6m)/Ral(1m) group]. For the prevention study, mice were treated with E2 for 3 months and were either killed [E2(3m) group] or treated with ICI and E2 for a month and then E2 for two more months [E2(6m)/preventive ICI(1m) group]. Female reproductive tracts were harvested at each end point, fixed in paraformaldehyde, and embedded in paraffin. Every tenth 5-μm section was stained with H&E and histopathologically scored to identify the worst grade of cervical/vaginal disease present and size of cancers in each animal, as previously described (19). Mice were housed in McArdle Laboratory Animal Care Unit of the University of Wisconsin Medical School, approved by the Association for Assessment of Laboratory Animal Care. All procedures were carried out according to an animal protocol approved by the University of Wisconsin Medical School Institutional Animal Care and Use Committee.
Drug Treatment.
Faslodex (50 mg/mL; human formulation for intramuscular injection of ICI; Astra Zeneca) was purchased from the University of Wisconsin Hospital Pharmacy. Mice were s.c. injected with 0.15 mL (equivalent to 7.5 mg of ICI) of Faslodex twice a week for a month (total of nine injections). EVISTA tablets (Eli Lilly), which are a human formulation of raloxifene (60 mg per tablet) were also purchased from the University of Wisconsin Hospital Pharmacy. Tablets were resuspended in PBS at a final concentration of 10 mg/mL. Mice were injected i.p. with 1.5 mg of raloxifene for a month, 5 days a week. These treatments increased neither morbidity nor mortality.
Immunohistochemistry.
Paraffin-embedded female reproductive tracts were stained with anti-ERα and anti-keratin 14 antibodies as previously described (16).
Statistical Analyses.
Two-sided Fisher's exact test was used for statistical analyses of cancer incidence and two-sided Wilcoxon rank sum test for those of disease state, cancer size, and tumor multiplicity, respectively. Two-sided analyses were carried out with MSTAT software version 12.0.0 (www.mcardle.wisc.edu/mstat). For assessing overall disease states, arbitrary scores were given to each lesion as follows: no dysplasia = 1; CIN1 (VIN1) = 2; CIN2 (VIN2) = 3; CIN3 (VIN3) = 4; and cancer = 5; the datasets were compared by using the Wilcoxon rank sum test.
Acknowledgments.
We thank Drs. Jordan and Ariazi at Fox Chase for advising use of fulvestrant and raloxifene. This study was supported by National Institutes of Health Grants CA120847, CA098428, CA141583, and CA113297 (to P.F.L.).
Footnotes
The authors declare no conflict of interest.
References
- 1.zur Hausen H. Papillomaviruses and cancer: From basic studies to clinical application. Nat Rev Cancer. 2002;2:342–350. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- 2.Roden R, Wu TC. How will HPV vaccines affect cervical cancer? Nat Rev Cancer. 2006;6:753–763. doi: 10.1038/nrc1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cline JM. Neoplasms of the reproductive tract: The role of hormone exposure. ILAR J. 2004;45:179–188. doi: 10.1093/ilar.45.2.179. [DOI] [PubMed] [Google Scholar]
- 4.Moreno V, et al. Effect of oral contraceptives on risk of cervical cancer in women with human papillomavirus infection: The IARC multicentric case-control study. Lancet. 2002;359:1085–1092. doi: 10.1016/S0140-6736(02)08150-3. [DOI] [PubMed] [Google Scholar]
- 5.Munoz N, et al. Role of parity and human papillomavirus in cervical cancer: The IARC multicentric case-control study. Lancet. 2002;359:1093–1101. doi: 10.1016/S0140-6736(02)08151-5. [DOI] [PubMed] [Google Scholar]
- 6.Jordan VC. Chemoprevention of breast cancer with selective oestrogen-receptor modulators. Nat Rev Cancer. 2007;7:46–53. doi: 10.1038/nrc2048. [DOI] [PubMed] [Google Scholar]
- 7.Bigler LR, Tate Thigpen J, Blessing JA, Fiorica J, Monk BJ. Evaluation of tamoxifen in persistent or recurrent nonsquamous cell carcinoma of the cervix: A Gynecologic Oncology Group study. Int J Gynecol Cancer. 2004;14:871–874. doi: 10.1111/j.1048-891X.2004.14523.x. [DOI] [PubMed] [Google Scholar]
- 8.Persson I, Yuen J, Bergkvist L, Schairer C. Cancer incidence and mortality in women receiving estrogen and estrogen-progestin replacement therapy–long-term follow-up of a Swedish cohort. Int J Cancer. 1996;67:327–332. doi: 10.1002/(SICI)1097-0215(19960729)67:3<327::AID-IJC4>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 9.Friedrich M, Mink D, Villena-Heinsen C, Woll-Hermann A, Schmidt W. Tamoxifen and proliferation of vaginal and cervical epithelium in postmenopausal women with breast cancer. Eur J Obstet Gynecol Reprod Biol. 1998;80:221–225. doi: 10.1016/s0301-2115(98)00117-1. [DOI] [PubMed] [Google Scholar]
- 10.Aggarwal BB, Ichikawa H. Molecular targets and anticancer potential of indole-3-carbinol and its derivatives. Cell Cycle. 2005;4:1201–1215. doi: 10.4161/cc.4.9.1993. [DOI] [PubMed] [Google Scholar]
- 11.Jin L, et al. Indole-3-carbinol prevents cervical cancer in human papilloma virus type 16 (HPV16) transgenic mice. Cancer Res. 1999;59:3991–3997. [PubMed] [Google Scholar]
- 12.Bell MC, et al. Placebo-controlled trial of indole-3-carbinol in the treatment of CIN. Gynecol Oncol. 2000;78:123–129. doi: 10.1006/gyno.2000.5847. [DOI] [PubMed] [Google Scholar]
- 13.Brake T, Connor JP, Petereit DG, Lambert PF. Comparative analysis of cervical cancer in women and in a human papillomavirus-transgenic mouse model: Identification of minichromosome maintenance protein 7 as an informative biomarker for human cervical cancer. Cancer Res. 2003;63:8173–8180. [PubMed] [Google Scholar]
- 14.Elson DA, et al. Sensitivity of the cervical transformation zone to estrogen-induced squamous carcinogenesis. Cancer Res. 2000;60:1267–1275. [PubMed] [Google Scholar]
- 15.Brake T, Lambert PF. Estrogen contributes to the onset, persistence, and malignant progression of cervical cancer in a human papillomavirus-transgenic mouse model. Proc Natl Acad Sci USA. 2005;102:2490–2495. doi: 10.1073/pnas.0409883102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chung SH, Wiedmeyer K, Shai A, Korach KS, Lambert PF. Requirement for estrogen receptor alpha in a mouse model for human papillomavirus-associated cervical cancer. Cancer Res. 2008;68:9928–9934. doi: 10.1158/0008-5472.CAN-08-2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shai A, Brake T, Somoza C, Lambert PF. The human papillomavirus E6 oncogene dysregulates the cell cycle and contributes to cervical carcinogenesis through two independent activities. Cancer Res. 2007;67:1626–1635. doi: 10.1158/0008-5472.CAN-06-3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arbeit JM, Howley PM, Hanahan D. Chronic estrogen-induced cervical and vaginal squamous carcinogenesis in human papillomavirus type 16 transgenic mice. Proc Natl Acad Sci USA. 1996;93:2930–2935. doi: 10.1073/pnas.93.7.2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riley RR, et al. Dissection of human papillomavirus E6 and E7 function in transgenic mouse models of cervical carcinogenesis. Cancer Res. 2003;63:4862–4871. [PubMed] [Google Scholar]
- 20.Ariazi EA, Ariazi JL, Cordera F, Jordan VC. Estrogen receptors as therapeutic targets in breast cancer. Curr Top Med Chem. 2006;6:181–202. [PubMed] [Google Scholar]
- 21.Crabtree JS, et al. Activity of three selective estrogen receptor modulators on hormone-dependent responses in the mouse uterus and mammary gland. Mol Cell Endocrinol. 2008;287:40–46. doi: 10.1016/j.mce.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 22.Buchanan DL, et al. Role of stromal and epithelial estrogen receptors in vaginal epithelial proliferation, stratification, and cornification. Endocrinology. 1998;139:4345–4352. doi: 10.1210/endo.139.10.6241. [DOI] [PubMed] [Google Scholar]
- 23.Nair HB, et al. Induction of aromatase expression in cervical carcinomas: Effects of endogenous estrogen on cervical cancer cell proliferation. Cancer Res. 2005;65:11164–11173. doi: 10.1158/0008-5472.CAN-05-1087. [DOI] [PubMed] [Google Scholar]
- 24.DeFilippis RA, Goodwin EC, Wu L, DiMaio D. Endogenous human papillomavirus E6 and E7 proteins differentially regulate proliferation, senescence, and apoptosis in HeLa cervical carcinoma cells. J Virol. 2003;77:1551–1563. doi: 10.1128/JVI.77.2.1551-1563.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jabbar SF, Abrams L, Glick A, Lambert PF. Persistence of high-grade cervical dysplasia and cervical cancer requires the continuous expression of the human papillomavirus type 16 E7 oncogene. Cancer Res. 2009;69:4407–4414. doi: 10.1158/0008-5472.CAN-09-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wells SI, et al. Transcriptome signature of irreversible senescence in human papillomavirus-positive cervical cancer cells. Proc Natl Acad Sci USA. 2003;100:7093–7098. doi: 10.1073/pnas.1232309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herber R, Liem A, Pitot H, Lambert PF. Squamous epithelial hyperplasia and carcinoma in mice transgenic for the human papillomavirus type 16 E7 oncogene. J Virol. 1996;70:1873–1881. doi: 10.1128/jvi.70.3.1873-1881.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song S, Pitot HC, Lambert PF. The human papillomavirus type 16 E6 gene alone is sufficient to induce carcinomas in transgenic animals. J Virol. 1999;73:5887–5893. doi: 10.1128/jvi.73.7.5887-5893.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]