Abstract
How cancer cells bind to vascular surfaces and extravasate into target organs is an underappreciated, yet essential step in metastasis. We postulate that the metastatic process involves discrete adhesive interactions between circulating cancer cells and microvascular endothelial cells. Sialyl Lewis X (sLeX) on prostate cancer (PCa) cells is thought to promote metastasis by mediating PCa cell binding to microvascular endothelial (E)-selectin. Yet, regulation of sLeX and related E-selectin ligand expression in PCa cells is a poorly understood factor in PCa metastasis. Here, we describe a glycobiological mechanism regulating E-selectin-mediated adhesion and metastatic potential of PCa cells. We demonstrate that α1,3 fucosyltransferases (FT) 3, 6, and 7 are markedly elevated in bone- and liver-metastatic PCa and dictate synthesis of sLeX and E-selectin ligands on metastatic PCa cells. Upregulated FT3, FT6, or FT7 expression induced robust PCa PC-3 cell adhesion to bone marrow (BM) endothelium and to inflamed postcapillary venules in an E-selectin-dependent manner. Membrane proteins, CD44, carcinoembryonic antigen (CEA), podocalyxin-like protein (PCLP), and melanoma cell adhesion molecule (MCAM) were major scaffolds presenting E-selectin-binding determinants on FT-upregulated PC-3 cells. Furthermore, elevated FT7 expression promoted PC-3 cell trafficking to and retention in BM through an E-selectin dependent event. These results indicate that α1,3 FTs could enhance metastatic efficiency of PCa by triggering an E-selectin-dependent trafficking mechanism.
Keywords: metastasis, selecins, HCELL, homing, CTCs
Metastatic prostate cancer (PCa) claimed the lives of 28,660 American men in 2008 (1). Delineating mediators of PCa metastasis to target organs may lead to identification of prognostic biomarkers and anti-metastatic therapeutics. Recently, our lab has advanced a scenario to account for organ metastasis involving PCa cell adhesion to surface receptors expressed on microvascular endothelial cells of the target organ (2–4). Bone-metastatic PCa cells are known to attach more avidly to bone marrow endothelial cells (BMEC) compared with endothelial linings of nontarget organs (5, 6). For example, human bone-metastatic PCa MDA PCa 2b (MDA) cells roll and adhere on BMEC by binding endothelial (E)-selectin, raising the possibility that PCa metastasis could be conferred through E-selectin − E-selectin ligand adhesive interactions (2–4). In fact, BM microvessels express E-selectin constitutively, while E-selectin is inducible on endothelial linings of inflamed tissues and of bronchial mucosa, a common PCa target tissue (7–9). Mechanistically, an identical traffic control axis involving selectins is well-known in the extravasation of hematopoietic progenitor cells (HPC) into BM (10, 11). HPC rolling on E- and platelet (P)-selectin is regulated by sLeX-bearing glycoforms of CD44 (HCELL), PSGL-1 and glycolipids (10, 12, 13). Synthesis of sLeX in HPC is catalyzed in the Golgi compartment by members of the glycosyltransferase gene family (14). The final step involves the transfer of fucose to N-acetylglucosamine at the terminal α2,3 sialo-lactosamine unit by α1,3 fucosyltransferases (FT) 3, 4, 5, 6, and/or 7, depending on cell type (13, 15). That metastatic PCa cells traverse the vasculature through ‘hematopoietic mimicry’ is consistent with several observations, including the association of sLeX with PCa grade and progression (2, 16, 17), cancer cell E-selectin ligand activity is a direct correlate with metastatic potential (18, 19) and cancer cells can trigger E-selectin expression on liver sinusoidal microvasculature to steer circulating cancer cells to the liver (20, 21). Thus, mapping glyco-metabolic pathways regulating E-selectin ligand synthesis may be vital for understanding PCa metastasis.
In this report, we identify E-selectin ligand formation through α1,3 fucosylation as a critical event in triggering vascular adhesion of circulating PCa cells. We show corroborative evidence that native PCa lesions derived from bone and liver metastases are enriched in α1,3 FT3, FT6. and FT7 mRNA compared with normal or localized malignant prostate tissue. PCa metastases also expressed all glycosyltransferases involved in sLeX synthesis. Recapitulating FT enrichment through overexpression in sLeX (-) PCa PC-3 cells was sufficient for induction of sLeX and E-selectin-binding determinants on CD44, CEA, PCLP, MCAM, and, potentially, glycolipids. As a result, PC-3 FT cells rolled on BMEC and inflamed postcapillary venules via E-selectin; and elevated FT7 expression, in particular, promoted PC-3 cell trafficking to and retention in BM dependent on E-selectin. These findings demonstrate that α1,3 FTs are regulators of sLeX and E-selectin ligand biosynthesis in PCa PC-3 cells and may encourage PCa metastasis by inducing intravascular cell adhesion.
Results
Metastatic PCa Cells Overexpress α1,3 FT3, FT6, and FT7.
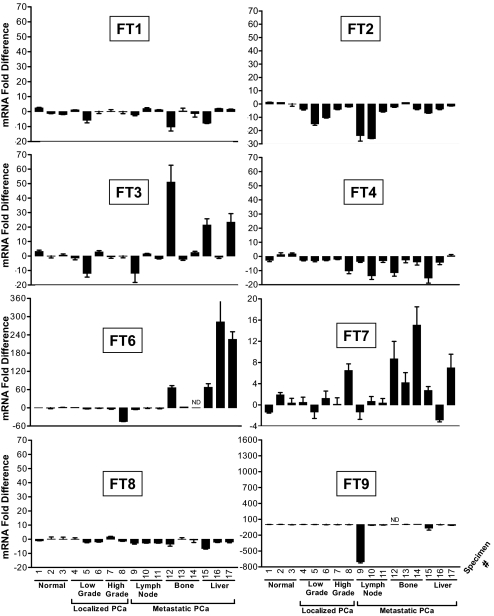
sLeX is associated with Gleason score and progression of PCa (2, 16, 17). To identify glycosyltransferases involved in sLeX expression, we analyzed relative mRNA amount of glycosyltransferases in normal prostate tissue and in localized and metastatic PCa tissue. Of six metastatic PCa specimens from bone and liver, all were enriched for at least one α1,3 FT, FT3, FT6, or FT7 (Fig. 1). In particular, a 2- to 283-fold elevation in α1,3 FT3, FT6, and FT7 was detected in three, four, and five of six metastatic specimens from bone and liver, respectively. In fact, metastatic specimens, 12, 15, and 17 each exhibited concurrent elevation of all three α1,3 FT. In addition, FT7 expression was elevated in one localized high grade sample (specimen 8). Two other α1,3 FTs, FT4, and FT9, were expressed in all prostate tissue, whereas α1,3 FT5 was not detected with two different primer pairs. FT4 expression, in general, was 3- to 15-fold lower in metastatic specimens, although was 40- to 2,900-fold lower than FT3, FT6, and FT7 in metastatic PCa tissue enriched for α1,3 FT. Prostate tissue also expressed α1,2 FT1 and FT2, as well as α1,6 FT8. With the exception of FT5, normal prostate tissue, as well as localized and metastatic PCa tissue expressed all other glycosyltransferases required for the synthesis of sLeX, including α1,4 galactosyl- (GalT), α2,3 sialyl- (ST), and α1,3 FTs (Fig. S1). Since sLeX is often displayed on core 2 branched O-glycans, core 2 ß1,6 N-acetylglucosaminyltransferases (C2GlcNAcT)-I, -II, and -III were also assayed, and C2GlcNAcT-I and not C2GlcNAcT-II and -III was mainly upregulated in PCa compared with normal prostate tissue.
Fig. 1.
α1,3 FT expression is elevated in metastatic PCa lesions. Real-time PCR of FT expression was performed on normal prostate tissue and on localized and metastatic PCa from LN, bone, and liver of different patients. Relative mRNA expression is shown after normalizing to expression level in normal prostate tissue in triplicate determinations on a single cDNA tissue sample (n = 3 ± SEM). ND, not determined.
FT3, FT6, and FT7 Induce sLeX Expression on Metastatic PCa PC-3 Cells.
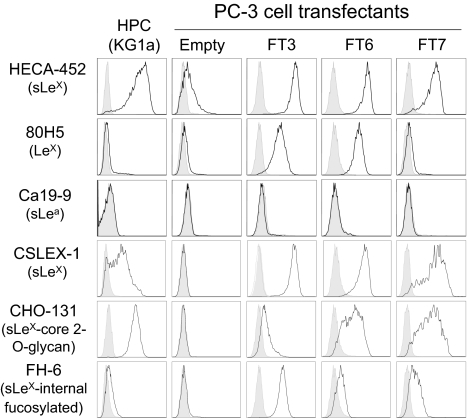
The upregulated α1,3 FT mRNA in metastatic PCa tissue prompted us to investigate whether FT3, FT6, or FT7 could be involved directly in the expression of sLeX on metastatic PCa tissue and on PCa cell lines (2–4, 16). To probe the glyco-metabolic pathway of sLeX, we overexpressed FT3, FT6, or FT7 in the PCa PC-3 cell line, aided by the fact that PC-3 cells are transfectable (17), express only a minimal amount of sLeX (4), do not bind E-selectin (2, 3), and do not display elevated levels of FT3, FT6, or FT7 (4). In addition, PC-3 cells express a similar repertoire of glycosyltransferases and membrane protein markers as normal and malignant prostate tissue, including C2GlcNAcTs, GalTs, STs, α1,3FTs, and CD44 (3, 4). Other PCa cell lines, namely MDA PCa 2b cells, were not used due to high native sLeX expression, slow growth rate and poor transfection potential (2). PC-3 clonal transfectants displaying high α1,3 FT expression were analyzed by flow cytometry with several mAbs that recognize different structural variants of sLeX and other Lewis antigens (Fig. 2). Overexpression of FT3, FT6, or FT7 in PC-3 cells induced sLeX synthesis (Fig. 2). FT6 and FT7 also generated sLeX on core 2 O-glycans as determined by reactivity with mAb CHO-131, a positive correlate of P-selectin ligand expression (22). Interestingly, FT3, and to a lesser extent FT6 and FT7, induced a sLeX variant containing internal fucosylation, presumably sialyl dimeric LeX and/or the VIM-2 determinant (23), based on binding of mAb FH-6. LeX was induced by FT3 and FT6, but not by FT7. Surprisingly, PC-3 FT3 cells were negative for sLea and Leb, two moieties dependent on α1,4 FT activity of FT3. In control experiments evaluating specificity of anti-Lewis antigen mAbs, PC-3 FT4 cells expressed sLeX and LeX (Fig. S2).
Fig. 2.
α1,3 FTs are potent inducers of sLeX synthesis in PCa PC-3 cells. KG1a (HPC) cells, PC-3 empty, FT3, FT6, and FT7 cells were assayed for sLeX variants, LeX, and sLea. (Open histograms) Staining with anti-Lewis carbohydrate mAb or with isotype control Ab (Shaded histograms); (n = 3). Shown are representative histograms from three experiments.
FT3, FT6, and FT7 Induce E-Selectin-Mediated Adhesion of Metastatic PCa PC-3 Cells.
We then investigated whether α1,3 FT might induce E-selectin-mediated PCa cell rolling and adhesion. PC-3 empty cells did not adhere to E-selectin under blood flow conditions in accord with negligible expression of sLeX, PC-3 FT3, FT6, and FT7 cells adhered to and rolled on E-selectin under static and flow conditions (Fig. 3 A–C). Strikingly, PC-3 FT7 cells rolled with a 3- to 4-fold slower velocity on E-selectin than PC-3 FT3 or FT6 cells (Fig. 3C). Slower rolling velocity of PC-3 FT7 cells was not due to better transfection efficiency, since three other PC-3 FT7 cell variants showed a similar slower cell rolling behavior compared with PC-3. FT3, and FT6 cell variants, despite equivalent sLeX levels and mRNA expression level of respective α1,3 FT (Fig. S3 A–C). We next explored PC-3 FT cell rolling on native E-selectin expressed by BMEC. Both PC-3 empty or FT cells did not roll on unstimulated BMEC, while PC-3 FT3, FT6, and FT7 cells, but not PC-3 empty cells, rolled avidly on IL-1β-stimulated BMEC (Fig. 3D). Pretreatment of PC-3 FT cells with neuraminidase, an enzyme that cleaves terminal sialic acid, completely blocked E-selectin-binding (Fig. 3A). PC-3 FT7 cell rolling was inhibited completely by anti-E-selectin mAb (Fig. 3D).
Fig. 3.
α1,3 FTs induce robust PCa PC-3 cell rolling and adhesion on E-selectin. PC-3 cell transfectants were examined for E-selectin ligand activity under (A) static and (B–D) flow conditions. (B–C) Shown is the number of cell rolling events and cell rolling velocities on E-selectin at 100× magnification. (D) Cell rolling on BMEC stimulated with IL-1β. Mean number of cell rolling events or rolling velocity recorded per field is shown (n = 3 ± SEM; **, P < 0.01, statistically significant compared with FT3 or FT6; ***, P < 0.001, statistically significant compared with untreated control; two-tailed Student t-test; ND, not detected).
FT3, FT6, and FT7 Trigger E-Selectin-Mediated Intravascular PCa PC-3 Cell Adhesion.
We used intravital microscopy of cremaster venules to analyze PCa cell adhesion to E-selectin expressed on inflamed microvascular endothelium. We first verified by immunoassay that E-selectin was upregulated on cremaster venules in mice pretreated with an intrascrotal injection of TNF-α. E-selectin was not detected on venules of untreated mice. We found that PC-3 empty and FT cell rolling activity was negligible on untreated control venules (Fig. 4 A and B and Movie S1). Likewise, PC-3 empty cell rolling was minor on TNF-α-induced inflamed venules (Fig. 4 A and B), although extensive rolling and adhesion of endogenous leukocytes was noted and consistent with induction of intravascular expression of E-selectin (Movie S2). PC-3 FT3, FT6, or FT7 cells rolled and adhered on TNF-α-inflamed venules (Fig. 4B and Movie S3). Preincubation of PC-3 FT cells with neuraminidase or bromelain inhibited adhesion (Fig. 4B). To validate the central role of E-selectin, anti-E-selectin mAb 16A (UZ4) was infused before injection of PC-3 FT cells (24). As a result, PC-3 FT cell binding to inflamed venules was blocked (Fig. 4B and Movie S4), indicating that E-selectin was the predominant receptor in PC-3 FT cell adhesion at shear stresses greater than 4 dynes/cm2. Indeed, prior observations of rolling leukocytes confirmed the dominance of E-selectin over P-selectin in the TNF-α-inflamed cremaster model (25).
Fig. 4.
α1,3 FTs trigger E-selectin-mediated intravascular PCa PC-3 cell adhesion and α1,3 FT7 promotes PCa PC-3 cell trafficking to and retention in BM. (A) Representative photomicrographs of TNF-α-inflamed cremaster muscle venules taken at specified time intervals (in seconds) (Scale bar, 10 μm.) Arrowheads denote EGFP expressing PC-3 FT7 cells bound to inflamed microvessels. (B) The number of PC-3 cells bound along a 1-mm section of the mouse cremaster venule over 2 min. These experiments were repeated a minimum of three times and represent mean number of cell binding events (SEM). EGFP+ PC-3 empty, FT3, FT6, and FT7 cells in BM and spleens were assayed by RT-PCR analysis of EGFP at 2, 8, and 24 h postinjection. Percent incidence of EGFP detection in BM (EGFP detection/total mouse number) at 24 h is represented in C. Mean BM-homing factor (SEM) for each time point is represented in D. (E) At 24 h, BM-homing factor (SEM) of PC-3 FT7 cells in mice treated with isotype control was compared with BM-homing factors of PCa FT7 cells in mice treated with anti-E-selectin mAb and of PCa empty vector cells. *, P = 0.0343 and Phi-coefficient = 0.46 compared with PC-3 empty cells; **, P = 0.0309 compared with PCa empty cells; ***, P < 0.01 compared with PC-3 FT7 cells incubated with isotype control mAb. Homing experiments or treatment group was repeated at least twice on 11 mice.
α1,3 FT7 Promotes PCa PC-3 Cell Trafficking to BM.
We then investigated whether α1,3 FT upregulation might regulate PCa cell trafficking to organs associated with metastatic PCa. PC-3 empty or FT cells stably expressing EGFP were injected into the left ventricle of Rag2/Janus kinase(Jak)-3 double null mice deficient in T, B, and NK cells (Fig. S4) (26, 27), and analyzed over a 24 h period for PCa cell retention in BM, lungs. and spleen. As expected, lung tissue tested positive in all mice injected with PC-3 empty, FT3, FT6, or FT7 cells. At 24 h, the incidence of PC-3 FT7 cells in BM was 92%, whereas PC-3 empty, FT3, and FT6 cells were retained in BM to a lesser degree (<50% incidence) (Fig. 4C). PC-3 empty, FT3, FT6, and FT7 cells were present in similar levels in splenic tissue at 2, 8, and 24 h time points. Thus, to ascertain whether FT expression promoted BM-trafficking, we normalized the threshold of EGFP amplification in BM to threshold EGFP amplification in splenic tissue to formulate a BM-homing factor. While PC-3 FT3 cells showed a slight predilection for BM-trafficking at 2 h, PC-3 FT7 cells accumulated considerably in BM over a 24-h period, as the BM-homing factor was significantly greater than all other cell lines (P = 0.03) (Fig. 4D). In fact, at 24 h, a different PC-3 FT7 cell clone also trafficked more efficiently to BM than did other PC-3 FT variants (Fig. S5). PC-3 FT7 BM-trafficking over 24 h was reduced 80% by preinjection with anti-E-selectin mAb (Fig. 4E) (P < 0.01).
CD44, CEA, PCLP, and MCAM Are Candidate E-Selectin Ligands on Metastatic PCa PC-3 Cells.
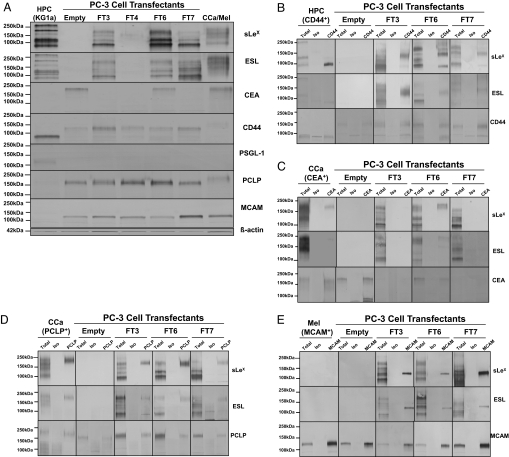
In Western blots, we noted that PC-3 FT3, FT6, and FT7 cells expressed several membrane proteins positive for sLeX that bound E-selectin (Fig. 5A). Considering the pattern of sLeX expression on blotted PCa FT proteins and prior data identifying E-selectin ligands on colon carcinoma (CCa) LS174T cells, we hypothesized that CD44, CEA, and PCLP were candidate proteins on PC-3 cells bearing sLeX moieties (28–30). To this end, immunoblotting and flow cytometry for candidate E-selectin ligands indicated that CD44 and PCLP proteins were expressed on PC-3 FT cells, whereas immunoblotting revealed that CEA was only expressed on PC-3 empty and FT6 cell transfectants, possibly due to loss of CEA mRNA from long-term cell culturing (Fig. 5A and Fig. S6 A and B). RT-PCR analysis indicated that PCLP mRNA levels in FT6 cells were higher compared with that found in FT3 or FT7 cell transfectants (Fig. S6B). Using MALDI-TOF mass spectrometry, an E-selectin-binding, sLeX-bearing protein, MCAM (≈130kDa), was identified at 130 kDa. In short, a 130-kDa gel fragment, which corresponded to the mAb HECA-452-stained band, was excised from 4–20% SDS/PAGE gels of PC-3 FT7 cell lysates affinity purified with Jacalin, a lectin recognizing O-glycans. Expression of MCAM, which is a cell adhesion molecule of the Ig superfamily in human melanomas (31), was then validated by immunoblot and flow cytometry (Fig. 5A and Fig. S6A). Another potential candidate, PSGL-1, was not detected in Western blots of PCa cell lysates or by flow cytometry (Fig. 5A and Fig. S6A). To directly determine whether CEA, CD44, PCLP, and MCAM were targets for α1,3 fucosylation, PC-3 cell immunoprecipitates were blotted with anti-sLeX or E-selectin/Fc (Fig. 5 B–E). As expected in PC-3 empty and FT4 cells (32), sLeX-bearing or E-selectin-binding CD44, CEA, PCLP, or MCAM were not detected (Fig. 5 B–E), whereas FT3, FT6, and FT7 cells expressed sLeX and E-selectin ligand on a variant form of CD44 (≈140—150 kDa) and, to a lesser degree, on a 98-kDa form of CD44, both known as HCELL (Fig. 5B) (10). Furthermore, sLeX and E-selectin ligand were expressed on CEA (≈180 kDa) of PC-3 FT6 cells (Fig. 5C), while PC-3 FT3, FT6, and FT7 cells expressed robust levels of sLeX and E-selectin ligand on PCLP (≈180 kDa) (Fig. 5D) and on MCAM (≈125 kDa) (Fig. 5E). PCLP, recently shown to function as an E-selectin ligand on CCa cells (30), is a sialomucin expressed on high endothelial venules and functions as an L-selectin ligand (33). FT upregulation did not alter surface expression of CD44, PCLP, MCAM, PSGL-1, β1, β2, β3, α4, or receptors for hyaluronic acid (Fig. S6A).
Fig. 5.
CD44, CEA, PCLP, and MCAM are candidate E-selectin ligands on PCa PC-3 cells. (A) Western blots of PC-3 cell transfectant protein were probed with anti-sLeX mAb HECA-452, E-selectin/Fc, anti-CEA, anti-CD44, anti-PSGL-1, anti-PCLP, anti-MCAM, or anti-β-actin. KG1a (HPC) and LS174T colon carcinoma (CCa) cell lysates were used as controls for CD44, CEA, and PCLP detection, and Malme-3M melanoma cells (Mel) cell lysate were used as control for MCAM detection. (B) Anti-CD44, (C) CEA, (D) PCLP, and (E) MCAM immunoprecipitates were blotted with HECA-452 and E-selectin/Fc, and with either anti-CD44, anti-CEA, anti-PCLP, or anti-MCAM. Immunoblotting with isotype controls did not show specific staining activity.
Discussion
Mediators of PCa metastasis have not been fully characterized despite clinical significance to patients. We have postulated that sLeX-bearing E-selectin ligands are important determinants. A major obstacle has been obtaining PCa metastases from patients for performance of complementary studies with PCa cell lines. PCa cell lines in culture tend to lose glycosyltransferase expression, thereby biasing results (4). Using native human PCa tissues, as well as a bone-metastatic PCa PC-3 cell line model, we demonstrate that α1,3 FT3, 6, and 7 regulate sLex synthesis on membrane glycoproteins, CD44, CEA, PCLP, and MCAM, and potentially on lipids concomitant with induction of E-selectin-mediated cell rolling and adhesion to inflamed microvessels. Furthermore, FT7 expression created the slowest rolling PC-3 cell and was critical for E-selectin-mediated PC-3 trafficking activity to BM. These findings implicate FT7 in BM colonization of PCa and underscore a key role for FT3, FT6, and/or FT7 in the synthesis of sLeX-bearing E-selectin-binding determinants on CD44, CEA, PCLP, and MCAM on PCa cells.
Our lab and others have argued that PCa cell trafficking may be conferred by co-opting an identical hematopoietic traffic control scenario involving α1,3 fucosylation of N-acetylglucosamine at the terminal α2,3 sialo-lactosamine unit on PCa cells (2, 13). To this end, we found that α1,3 FT elevation was alone sufficient to complete sLeX synthesis and convert a non-E-selectin-binding PC-3 cell to a cell that binds E-selectin. Others have shown that α1,3 FTs are associated with progression of several solid cancers of the colon (34–36), breast (37), lung (38, 39), liver (40), and pancreas (41, 42). The question is whether PCa cells expressing abnormally high levels of α1,3 FTs enter BM at specific microvascular domains where E-selectin and SDF-1α are coexpressed constitutively and regulate leukocytic infiltration (9).
That FT3, FT6, and FT7 are associated with PCa metastasis raises the mechanistic question as to their individual role. As noted here, three metastatic specimens from bone and liver displayed elevation of only one α1,3 FT, suggesting that a single upregulated FT may be sufficient for augmenting metastatic potential. However, five of six bone- and liver-metastatic lesions displayed elevated FT7 suggesting that, and in accord with the BM-homing results, FT7 may be a key regulator of the metastatic potential of PCa whereas FT3 and FT6 may be accessory. An intriguing question is whether synchronous upregulation of several different α1,3 FTs in a single PCa cell might influence the dissemination pattern and/or enhance metastatic potential. As an example, mice null for both FT4 and FT7 exhibit a greater impairment in selectin-dependent leukocyte rolling and trafficking than mice null for only FT7, indicating that α1,3 FTs may collaborate functionally (43–45). In this respect, we found that α1,3 FTs differentially synthesized internally-fucosylated sialyl dimeric LeX and LeX and also differentially targeted CEA, a known E-selectin ligand on CCa cells (28). Since LeX was produced by FT3 and FT6 and not FT7, LeX might occupy a sLeX-selectin binding site, thereby antagonizing α2,3 sialylation and trafficking activity to E-selectin-expressing vascular beds. LeX, in contrast to sLeX, is absent or only minimally expressed on PCa tissue, arguing that metastatic progression may selectively pressure against LeX while favoring sLeX expression (16).
We identified CD44, CEA, PCLP, and MCAM as candidate sLeX-bearing E-selectin-binding determinants in PCa PC-3 cells. Upon further analysis of these candidate ligands by real-time PCR, we found that CD44, CEA, PCLP, and MCAM mRNA were constitutively expressed in all PCa tissues, whereas CEA mRNA was elevated 10- to 80-fold in high grade PCa and in LN, bone and liver metastases (Fig. S7 A–D). To our surprise, however, PC-3 FT6 cells, which express the E-selectin-binding form of CEA, did not show enhanced bone-homing activity. These findings suggest that, while CEA expression may directly correspond to PCa progression and metastasis, it does not appear to augment early seeding events through vascular E-selectin-binding activity. Prior data suggest that leukocyte selectin ligand, PSGL-1, is also associated with PCa progression and metastasis; however, in this current study, we used PCa PC-3 cells, which inherently express nil levels of PSGL-1 (3), and, therefore, did not exploit PSGL-1 as an E-selectin-binding scaffold. Collectively, our data indicate that PCa cell membrane proteins, PSGL-1, CD44, CEA, PCLP, and MCAM, and even perhaps, glycolipids, are opportune scaffolds for α1,3 FT decorating enzymes. The combination of elevated FT3, FT6, and FT7, perhaps, increases metastatic potential and not necessarily a particular α1,3 fucosylated membrane glycoprotein. However, because our cell models do not include other upregulated posttranslational modifications, such as sulfotransferase activities that may complement α1,3 fucosylation, it is premature to discount the potential importance of individual protein scaffolds in conferring efficient metastatic activity. The fact that specific glycoproteins function as E-selectin ligands in certain PCa cell line models is suggestive of the heterogeneous nature of metastatic PCa and resultant E-selectin ligand repertoire.
In summary, we found that in vivo PCa PC-3 cell–endothelial cell interactions are influenced by E-selectin ligand expression under the control of α1,3 FT3, FT6, and FT7. α1,3 FTs may have additional prometastatic functions in as much as FT3 has been shown to regulate PCa cell growth (17). Considering the promise of isolating circulating PCa cells from patients (46, 47), we speculate that analysis of glyco-metabolic regulators of E-selectin ligand biosynthesis on these native PCa cells will help solidify our findings, supporting the notion that circulating PCa cells harness similar posttranslational mechanisms for controlling trafficking receptor function as hematopoietic cells. Additional tissue-specific trafficking factors likely involve integrin and chemokine receptors on native circulating PCa cells. Discovery of such tissue-specific homing markers will facilitate the development of more specific and potent therapeutics to prevent PCa metastasis.
Materials and Methods
Cell Lines, Native Tissues, and Mice.
All information for culturing cell lines (3), for acquiring native normal prostate and PCa tissues and for breeding of immunodeficient mice used in these studies are described in SI Materials and Methods.
Antibodies.
Please refer to SI Materials and Methods for the antibody list.
Real-Time PCR Analysis of Glycosyltransferase Gene Expression.
RNA was extracted from frozen prostate tissue, reverse transcribed and subjected to real-time PCR with SYBR Green, as described previously (4). Primer sequences used for real-time PCR analysis have been described previously (4).
Transfection of α1,3 FTs cDNA.
Human FT3, FT4, FT6, and FT7 cDNA were a kind gift from Dr. Robert Fuhlbrigge (Brigham and Women's Hospital, Boston, MA). PC-3 cells were transfected with pcDNA3 (Invitrogen) containing FT cDNA and selected with 400 μg/mL Geneticin (Invitrogen). Clonal populations were screened for high and univariate expression of sLeX or LeX by flow cytometry.
Flow Cytometry.
Flow cytometry was performed as previously described (4, 27).
PCa Cell Binding Assay.
Cell adhesion assays were performed as previously described (4).
Analysis of PCa Cell Rolling in Parallel-Plate Flow Chamber.
Cell rolling frequency/velocity assays were performed as previously described (2–4, 48).
Intravital Microscopic Analysis of PCa Cell Rolling on Inflamed Postcapillary Venules.
Six- to eight-week-old C57BL/6J mice were prepared for intravital microscopy, as described previously (49, 50). Please refer to SI Materials and Methods for complete experimental description.
Short-Term PCa Cell Trafficking Assay.
Please refer to SI Materials and Methods for a detailed description of our PCa cell homing assay method.
SDS/PAGE, Western Blotting, Immunoprecipitation, and Mass Spectrometry.
Cell membrane/total protein was prepared and quantified by Bradford method, as described previously (2, 10). SDS/PAGE, Western blotting, immunoprecipitation, and mass spectrometry were conducted as previously described (3). Please refer to SI Materials and Methods for complete experimental description.
Enzymatic Inhibitor Treatments.
Cells were pretreated with 0.2 U/mL Vibrio cholerae neuraminidase (sialidase) (Roche Applied Sciences) for 1 h at 37 °C. Alternatively, cells were pretreated with a broadly-active protease, bromelain (0.1%) (Sigma), for 1 h at 37 °C to digest all E-selectin glycoprotein ligands (2, 3).
Statistical Analysis.
Results were analyzed by two-tailed Student t-test or by contingency table on GraphPad Prism software .
Supplementary Material
Acknowledgments.
We thank Dr. Thomas S. Kupper (Brigham and Women's Hospital) and the Harvard Skin Disease Research Center for providing leukocyte migration core service. Supported by American Cancer Society Research Scholar Award, (06–024-01-CSM to C.J.D.), National Institutes of Health (NIH) National Cancer Institute (NCI) grant (RO1 CA118124 to C. Dimitroff), NIH National Center for Complementary and Alternative Medicine grant (RO1 AT004268 to C.J.D.), NIH National Institute of Arthritis and Musculoskeletal and Skin Diseases grant (P30 AR042689 to Dr. Thomas S. Kupper) and NIH NCI Specialized Programs of Research Excellence grant (P50 CA69568 to K.J.P.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0906074106/DCSupplemental.
References
- 1.Jemal A, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Dimitroff CJ, Lechpammer M, Long-Woodward D, Kutok JL. Rolling of human bone-metastatic prostate tumor cells on human bone marrow endothelium under shear flow is mediated by E-selectin. Cancer Res. 2004;64:5261–5269. doi: 10.1158/0008-5472.CAN-04-0691. [DOI] [PubMed] [Google Scholar]
- 3.Dimitroff CJ, et al. Identification of leukocyte E-selectin ligands, P-selectin glycoprotein ligand-1 and E-selectin ligand-1, on human metastatic prostate tumor cells. Cancer Res. 2005;65:5750–5760. doi: 10.1158/0008-5472.CAN-04-4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barthel SR, et al. Analysis of glycosyltransferase expression in metastatic prostate cancer cells capable of rolling activity on microvascular endothelial (E)-selectin. Glycobiology. 2008;18:806–817. doi: 10.1093/glycob/cwn070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lehr JE, Pienta KJ. Preferential adhesion of prostate cancer cells to a human bone marrow endothelial cell line. J Natl Cancer Inst. 1998;90:118–123. doi: 10.1093/jnci/90.2.118. [DOI] [PubMed] [Google Scholar]
- 6.Cooper CR, et al. Preferential adhesion of prostate cancer cells to bone is mediated by binding to bone marrow endothelial cells as compared to extracellular matrix components in vitro. Clin Cancer Res. 2000;6:4839–4847. [PubMed] [Google Scholar]
- 7.Schweitzer KM, et al. Constitutive expression of E-selectin and vascular cell adhesion molecule-1 on endothelial cells of hematopoietic tissues. Am J Pathol. 1996;148:165–175. [PMC free article] [PubMed] [Google Scholar]
- 8.Roche WR, Montefort S, Baker J, Holgate ST. Cell adhesion molecules and the bronchial epithelium. Am Rev Respir Dis. 1993;148:S79–82. doi: 10.1164/ajrccm/148.6_Pt_2.S79. [DOI] [PubMed] [Google Scholar]
- 9.Sipkins DA, et al. In vivo imaging of specialized bone marrow endothelial microdomains for tumour engraftment. Nature. 2005;435:969–973. doi: 10.1038/nature03703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dimitroff CJ, Lee JY, Rafii S, Fuhlbrigge RC, Sackstein R. CD44 is a major E-selectin ligand on human hematopoietic progenitor cells. J Cell Biol. 2001;153:1277–1286. doi: 10.1083/jcb.153.6.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rood PM, et al. E-selectin and very late activation antigen-4 mediate adhesion of hematopoietic progenitor cells to bone marrow endothelium. Ann Hematol. 2000;79:477–484. doi: 10.1007/s002770000182. [DOI] [PubMed] [Google Scholar]
- 12.Sackstein R, Dimitroff CJ. A hematopoietic cell L-selectin ligand that is distinct from PSGL-1 and displays N-glycan-dependent binding activity. Blood. 2000;96:2765–2774. [PubMed] [Google Scholar]
- 13.Barthel SR, Gavino JD, Descheny L, Dimitroff CJ. Targeting selectins and selectin ligands in inflammation and cancer. Expert Opin Ther Targets. 2007;11:1473–1491. doi: 10.1517/14728222.11.11.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lowe JB. Glycan-dependent leukocyte adhesion and recruitment in inflammation. Curr Opin Cell Biol. 2003;15:531–538. doi: 10.1016/j.ceb.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 15.de Vries T, Knegtel RM, Holmes EH, Macher BA. Fucosyltransferases: Structure/function studies. Glycobiology. 2001;11:119R–128R. doi: 10.1093/glycob/11.10.119r. [DOI] [PubMed] [Google Scholar]
- 16.Martensson S, et al. Sialyl-Lewis(x) and related carbohydrate antigens in the prostate. Hum Pathol. 1995;26:735–739. doi: 10.1016/0046-8177(95)90220-1. [DOI] [PubMed] [Google Scholar]
- 17.Inaba Y, et al. Gene transfer of alpha1,3-fucosyltransferase increases tumor growth of the PC-3 human prostate cancer cell line through enhanced adhesion to prostatic stromal cells. Int J Cancer. 2003;107:949–957. doi: 10.1002/ijc.11513. [DOI] [PubMed] [Google Scholar]
- 18.Sawada R, Tsuboi S, Fukuda M. Differential E-selectin-dependent adhesion efficiency in sublines of a human colon cancer exhibiting distinct metastatic potentials. J Biol Chem. 1994;269:1425–1431. [PubMed] [Google Scholar]
- 19.Mannori G, et al. Inhibition of colon carcinoma cell lung colony formation by a soluble form of E-selectin. Am J Pathol. 1997;151:233–243. [PMC free article] [PubMed] [Google Scholar]
- 20.Khatib AM, et al. Rapid induction of cytokine and E-selectin expression in the liver in response to metastatic tumor cells. Cancer Res. 1999;59:1356–1361. [PubMed] [Google Scholar]
- 21.Biancone L, Araki M, Araki K, Vassalli P, Stamenkovic I. Redirection of tumor metastasis by expression of E-selectin in vivo. J Exp Med. 1996;183:581–587. doi: 10.1084/jem.183.2.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walcheck B, et al. The monoclonal antibody CHO-131 binds to a core 2 O-glycan terminated with sialyl-Lewis x, which is a functional glycan ligand for P-selectin. Blood. 2002;99:4063–4069. doi: 10.1182/blood-2001-12-0265. [DOI] [PubMed] [Google Scholar]
- 23.Kannagi R, Hakomori S. A guide to monoclonal antibodies directed to glycotopes. Adv Exp Med Biol. 2001;491:587–630. doi: 10.1007/978-1-4615-1267-7_38. [DOI] [PubMed] [Google Scholar]
- 24.Hammel M, et al. Species-specific and conserved epitopes on mouse and human E-selectin important for leukocyte adhesion. Exp Cell Res. 2001;269:266–274. doi: 10.1006/excr.2001.5317. [DOI] [PubMed] [Google Scholar]
- 25.Ramos CL, et al. Differential effect of E-selectin antibodies on neutrophil rolling and recruitment to inflammatory sites. Blood. 1997;89:3009–3018. [PubMed] [Google Scholar]
- 26.Park SY, et al. Developmental defects of lymphoid cells in Jak3 kinase-deficient mice. Immunity. 1995;3:771–782. doi: 10.1016/1074-7613(95)90066-7. [DOI] [PubMed] [Google Scholar]
- 27.Gainers ME, et al. Skin-homing receptors on effector leukocytes are differentially sensitive to glyco-metabolic antagonism in allergic contact dermatitis. J Immunol. 2007;179:8509–8518. doi: 10.4049/jimmunol.179.12.8509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomas SN, Zhu F, Schnaar RL, Alves CS, Konstantopoulos K. Carcinoembryonic antigen and CD44 variant isoforms cooperate to mediate colon carcinoma cell adhesion to E- and L-selectin in shear flow. J Biol Chem. 2008;283:15647–15655. doi: 10.1074/jbc.M800543200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanley WD, Burdick MM, Konstantopoulos K, Sackstein R. CD44 on LS174T colon carcinoma cells possesses E-selectin ligand activity. Cancer Res. 2005;65:5812–5817. doi: 10.1158/0008-5472.CAN-04-4557. [DOI] [PubMed] [Google Scholar]
- 30.Thomas SN, Schnaar RL, Konstantopoulos K. Podocalyxin-like protein is an E-/L-selectin ligand on colon carcinoma cells: Comparative biochemical properties of selectin ligands in host and tumor cells. Am J Physiol Cell Physiol. 2008;296:C505–C513. doi: 10.1152/ajpcell.00472.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lehmann JM, Riethmuller G, Johnson JP. MUC18, a marker of tumor progression in human melanoma, shows sequence similarity to the neural cell adhesion molecules of the immunoglobulin superfamily. Proc Natl Acad Sci USA. 1989;86:9891–9895. doi: 10.1073/pnas.86.24.9891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang MC, Laskowska A, Vestweber D, Wild MK. The alpha (1,3)-fucosyltransferase Fuc-TIV, but not Fuc-TVII, generates sialyl Lewis X-like epitopes preferentially on glycolipids. J Biol Chem. 2002;277:47786–47795. doi: 10.1074/jbc.M208283200. [DOI] [PubMed] [Google Scholar]
- 33.Sassetti C, Tangemann K, Singer MS, Kershaw DB, Rosen SD. Identification of podocalyxin-like protein as a high endothelial venule ligand for L-selectin: Parallels to CD34. J Exp Med. 1998;187:1965–1975. doi: 10.1084/jem.187.12.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hiller KM, et al. Transfection of alpha(1,3)fucosyltransferase antisense sequences impairs the proliferative and tumorigenic ability of human colon carcinoma cells. Mol Carcinog. 2000;27:280–288. [PubMed] [Google Scholar]
- 35.Majuri ML, Niemela R, Tiisala S, Renkonen O, Renkonen R. Expression and function of alpha 2,3-sialyl- and alpha 1,3/1,4-fucosyltransferases in colon adenocarcinoma cell lines: Role in synthesis of E-selectin counter-receptors. Int J Cancer. 1995;63:551–559. doi: 10.1002/ijc.2910630416. [DOI] [PubMed] [Google Scholar]
- 36.Weston BW, et al. Expression of human alpha(1,3)fucosyltransferase antisense sequences inhibits selectin-mediated adhesion and liver metastasis of colon carcinoma cells. Cancer Res. 1999;59:2127–2135. [PubMed] [Google Scholar]
- 37.Matsuura N, et al. Gene expression of fucosyl- and sialyl-transferases, which synthesize sialyl Lewisx, the carbohydrate ligands for E-selectin, in human breast cancer. Int J Oncol. 1998;12:1157–1164. doi: 10.3892/ijo.12.5.1157. [DOI] [PubMed] [Google Scholar]
- 38.Ogawa J, Inoue H, Koide S. Expression of alpha-1,3-fucosyltransferase type IV and VII genes is related to poor prognosis in lung cancer. Cancer Res. 1996;56:325–329. [PubMed] [Google Scholar]
- 39.Martin-Satue M, de Castellarnau C, Blanco J. Overexpression of alpha(1,3)-fucosyltransferase VII is sufficient for the acquisition of lung colonization phenotype in human lung adenocarcinoma HAL-24Luc cells. Br J Cancer. 1999;80:1169–1174. doi: 10.1038/sj.bjc.6690482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang QY, Wu SL, Chen JH, Liu F, Chen HL. Expressions of Lewis antigens in human non-small cell pulmonary cancer and primary liver cancer with different pathological conditions. J Exp Clin Cancer Res. 2003;22:431–440. [PubMed] [Google Scholar]
- 41.Aubert M, et al. Peritoneal colonization by human pancreatic cancer cells is inhibited by antisense FUT3 sequence. Int J Cancer. 2000;88:558–565. doi: 10.1002/1097-0215(20001115)88:4<558::aid-ijc7>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 42.Mas E, et al. Fucosyltransferase activities in human pancreatic tissue: Comparative study between cancer tissues and established tumoral cell lines. Glycobiology. 1998;8:605–613. doi: 10.1093/glycob/8.6.605. [DOI] [PubMed] [Google Scholar]
- 43.Weninger W, et al. Specialized contributions by alpha(1,3)-fucosyltransferase-IV and FucT-VII during leukocyte rolling in dermal microvessels. Immunity. 2000;12:665–676. doi: 10.1016/s1074-7613(00)80217-4. [DOI] [PubMed] [Google Scholar]
- 44.Smithson G, et al. Fuc-TVII is required for T helper 1 and T cytotoxic 1 lymphocyte selectin ligand expression and recruitment in inflammation, and together with Fuc-TIV regulates naive T cell trafficking to lymph nodes. J Exp Med. 2001;194:601–614. doi: 10.1084/jem.194.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Homeister JW, et al. The alpha(1,3)fucosyltransferases FucT-IV and FucT-VII exert collaborative control over selectin-dependent leukocyte recruitment and lymphocyte homing. Immunity. 2001;15:115–126. doi: 10.1016/s1074-7613(01)00166-2. [DOI] [PubMed] [Google Scholar]
- 46.Nagrath S, et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature. 2007;450:1235–1239. doi: 10.1038/nature06385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alix-Panabieres C, Riethdorf S, Pantel K. Circulating tumor cells and bone marrow micrometastasis. Clin Cancer Res. 2008;14:5013–5021. doi: 10.1158/1078-0432.CCR-07-5125. [DOI] [PubMed] [Google Scholar]
- 48.Wiese G, Barthel SR, Dimitroff CJ. Analysis of physiologic E-selectin-mediated leukocyte rolling on microvascular endothelium. J Vis Exp. 2009;11:pii 1009. doi: 10.3791/1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang J, et al. Targeted gene disruption demonstrates that P-selectin glycoprotein ligand 1 (PSGL-1) is required for P-selectin-mediated but not E-selectin-mediated neutrophil rolling and migration. J Exp Med. 1999;190:1769–1782. doi: 10.1084/jem.190.12.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Falati S, Gross P, Merrill-Skoloff G, Furie BC, Furie B. Real-time in vivo imaging of platelets, tissue factor and fibrin during arterial thrombus formation in the mouse. Nat Med. 2002;8:1175–1181. doi: 10.1038/nm782. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.