Abstract
Dysbindin has been implicated in the pathogenesis of schizophrenia, but little is known about how dysbindin affects neuronal function in the circuitry underlying psychosis and related behaviors. Using a dysbindin knockout line (dys−/−) derived from the natural dysbindin mutant Sandy mice, we have explored the role of dysbindin in dopamine signaling and neuronal function in the prefrontal cortex (PFC). Combined cell imaging and biochemical experiments revealed a robust increase in the dopamine receptor D2, but not D1, on cell surface of neurons from dys−/− cortex. This was due to an enhanced recycling and insertion, rather than reduced endocytosis, of D2. Disruption of dysbindin gene resulted in a marked decrease in the excitability of fast-spiking (FS) GABAergic interneurons in both PFC and striatum. Dys−/− mice also exhibited a decreased inhibitory input to pyramidal neurons in layer V of PFC. The increased D2 signaling in dys−/− FS interneurons was associated with a more pronounced increase in neuronal firing in response to D2 agonist, compared to that in wild-type interneurons. Taken together, these results suggest that dysbindin regulates PFC function by facilitating D2-mediated modulation of GABAergic function.
Keywords: dopamine D2 receptor, schizophrenia, prefrontal cortex
Genetic variants in a gene on 6p22.3, dysbindin (DTNBP1), have been shown to be one of the several genes that are associated with schizophrenia (1). Schizophrenia patients have significantly reduced expression of dysbindin mRNA and protein in prefrontal cortex and hippocampus (2, 3). While it remains unclear how changes in dysbindin expression could contribute to the pathogenesis of schizophrenia, cell biological studies have begun to address the physiological function of dysbindin in neurons. Downregulation of dysbindin by siRNA in cultured neurons leads to decreases in the expression of SNAP25 and levels of extracellular glutamate or dopamine (4, 5). Dysbindin contributes to normal biogenesis of lysosome-related organelles (LROs) by binding to proteins in the BLOC-1 complex (6, 7), which regulates trafficking of LROs. The Sandy mouse (Sdy), which harbors an in-frame deletion of two exons of the dysbindin gene (8), exhibits a reduced readily releasable pool of synaptic vesicles and larger vesicle size (9). Although dysbindin protein is localized both pre- and postsynaptically (7), little is known about its postsynaptic function. Recently, downregulation of dysbindin has been shown to increase cell surface expression of dopamine receptor D2 (D2), but not dopamine receptor D1 (D1), in human SH-SY5Y neuroblastoma cells and in cultured cortical neurons (10).
Dopamine receptor internalization (or endocytosis) is a general mechanism to adjust neuronal responses to dopamine stimulation. Both D1 and D2 are G protein coupled receptors (GPCRs) that undergo constitutive and ligand-induced internalization. Unlike D1, which is recycled back to the plasma membrane after endocytosis, D2 is generally trafficked to the lysosomal pathway and degraded (11–14). Thus, downregulation of dysbindin might be expected to impact D2 function but not D1 function, as reported in cell cultures (10). An increase in cell surface D2 after dysbindin knockdown could be due to an enhanced expression of D2 protein, a reduced D2 internalization, or an increased insertion of D2 to cell surface. It is also unclear whether manipulation of dysbindin expression in vivo could alter surface expression of endogenous D2. Most importantly, the role of dysbindin in neuronal function has not been rigorously studied.
In this study, we have investigated the kinetics of D2 endocytosis and postendocytotic trafficking in cortical neurons from wild-type and dysbindin null (dys−/−) mice. Using a combination of biochemical and immunocytochemical approaches, we show that there is a significant increase in cell surface expression of D2, but not D1, in cortical neurons derived from dys−/− mice. D2 undergoes normal constitutive and dopamine-induced internalization, but reinserts itself to the plasma membrane much faster following endocytosis in dys−/− neurons as compared to wild-type neurons. Consistent with an elevated D2 signaling, GABAergic inputs to layer V pyramidal neurons are reduced in PFC slices. In parallel, the excitability of fast-spiking (FS) interneurons is decreased in both PFC and striatum slices derived from dys−/− mice. Consistent with the selective enhancement of D2 signaling, application of D2 agonist quinpirole elicits a more pronounced increase in the firing frequency of FS interneurons in PFC from dys−/− mice, as compared to that of wild-type mice. Taken together, these results have identified a physiological function of dysbindin in PFC neurons and its underlying mechanism.
Results
Increased Cell Surface D2 in Cortical Neurons from Dys−/− Mice.
Dys−/− mice were derived from Sandy mice (8) by backcrossing to C57BL/6J background for more than 10 generations. Pure neuronal cultures were prepared from cortex from wild-type and dys−/− mice. All membrane proteins on neuronal surface were labeled by biotinylation, followed by precipitation with ImmunoPure Streptavidin and Western blot using various antibodies (Fig. 1A). Neurons from dys−/− mice exhibited a fourfold increase in the steady-state expression level of D2 on their surface, whereas total levels of D2 in these neurons were not changed (Fig. 1A). Surface expression of D1, a similar GPCR with distinct function, was not changed (Fig. 1B). N-Cadherin (N-Cad) is a cell surface adhesion molecule and transferrin receptor (TfR) is a receptor that undergoes constitutive recycling. Surface expression of N-Cad and TfR were also unchanged (Fig. 1A). Thus, dysbindin mutation affects the surface expression of D2 but not D1. To confirm this phenomenon by another independent method, we cotransfected N-terminal FLAG-D2 and eGFP into cultured neurons from wild-type and dys−/− mice, and quantified the steady-state level of surface D2 by surface immunofluorescence intensity using an antibody against the extracellular N-terminal FLAG (Fig. 1C). Lack of dysbindin protein increased surface D2 by approximately 136%. Furthermore, introduction of dysbindin back to dys−/− neurons rescued the cellular phenotype: cells expressing dysbindin-GFP exhibited normal D2 surface expression as compared to nontransfected cells in the same dys−/− cultures (Fig. 1D).
Fig. 1.
Elevated Expression of Surface D2 in Dys−/− Cortical Neurons. (A) Biotinylation assay of cell surface receptors. Cultured cortical neurons from wild-type and dys−/− mice were treated with biotin to label all surface proteins. Biotinylated proteins were precipitated by streptavidin and immunoblotted for D2, D1, TfR, and N-Cad. The amount of Tubulin in the lysate was used as a loading control for whole cell lysates. For quantification of surface D2, the biotinylated D2 was normalized to total D2. In this and all other figures, the data are presented as mean ± SEM. Student t-test was used to compare data from dys−/− mice and those from their wild-type controls. *, P < 0.05. (B) Quantification of cell surface D1 in dys−/− neurons and their wild-type controls. Surface D1 was normalized to total D1, which does not differ between genotypes. (C) Representative images showing steady-state levels of surface D2. Wild-type or dys−/− neurons were transfected with eGFP and FLAG-D2 (shown in inset). Surface D2 was detected by anti-FLAG antibody in non-permeable conditions. (Scale bar, 20 μm.) Quantification of surface FLAG-D2 immunofluorescence is shown on the right. *, P < 0.05. (D) Rescue of surface D2 phenotype in dys−/− neurons by transfection of dysbindin. Note that in neuron transfected with GFP-dysbindin (green arrow), surface D2 levels are much lower than the nearby untransfected neuron (white arrow). Antibody against amino-terminal sequence of D2 was used to detect endogenous D2. (Scale bar, 20 μm.) Quantification of surface D2 immunofluorescence is shown on the right. *, P < 0.05.
Normal Spontaneous and Dopamine-Induced Endocytosis of D2.
Normal total D2 expression (Fig. 1A) suggests normal synthesis and degradation of D2 in dys−/− neurons. An increased surface D2 therefore could be due to facilitation of membrane insertion and/or inhibition of endocytosis of D2. To discriminate between these possibilities, we used a previously established assay to determine D2 endocytosis (15). For spontaneous endocytosis, neuronal surface proteins, labeled by cleavable biotin, underwent endocytosis in the absence of dopamine for 30 or 60 min. The remaining surface biotin was cleaved, and the intracellular biotinylated proteins were precipitated by streptavidin and detected by various antibodies. The ligand-independent D2 endocytosis in wild-type neurons occurred in a modest rate at 37 °C, and reached the plateau at 60 min (Fig. S1 A and C) (40.84 ± 3.15% of total surface D2). Dysbindin knockout did not affect the endocytosis rate of D2 (Fig. S1 B and C) (42.97 ± 4.67% of total surface D2). Under our experimental conditions, the internalized D2 was metabolically stable and showed little degradation up to 60 min after endocytosis. As controls, we also measured the endocytosis rate of N-Cad and TfR. The internalization of N-Cad was slow and barely detectable over 60 min (around 25%), whereas that of TfR was rapid and robust (76% at 60 min). Dysbindin mutation affected the endocytosis of neither N-Cad nor TfR (Fig. S1C).
We next addressed whether dopamine-induced D2 endocytosis could be regulated by dysbindin. The endocytosis assay was performed the same way except dopamine was added to the culture medium to trigger receptor internalization. Application of dopamine (10 μM) induced a rapid and time-dependent endocytosis of D2 in wild-type and dys−/− neurons (Fig. S2 A and B). Approximately 43% of surface D2 were internalized within 30 min, and by 60 min, the endocytosis of D2 reached more than 80% (Fig. S2C). The total amounts of D2 in neuronal lysates remained constant during the experimental period, which suggested that dopamine stimulated D2 endocytosis without altering the level of total D2 protein in neurons. No difference in dopamine-induced endocytosis was found between the genotypes (Fig. S2C). Further, dopamine had no effect on the endocytosis of the control proteins N-Cad and TfR (Fig. S2C).
Imaging experiments were performed to verify the biochemical results. Neurons were transfected with eGFP (green) and FLAG-D2, and surface D2 was labeled with monoclonal antibody (M1) against FLAG under nonpermeable condition. Cells were incubated with or without dopamine for 60 min to induce D2 endocytosis, followed by removal of remaining M1 bound to “unendocytosed” D2 on cell surface (Fig. S3A). Immunofluorescence staining revealed specifically the endocytosed D2. In the absence of dopamine, D2 remained primarily on the plasma membrane (Fig. S3B Left), and little if any D2 was detectable in the cytoplasm of soma (data not shown). Surface FLAG staining was eliminated by EDTA treatment and few very faint surface D2 puncta were observed, because M1 binding to D2 is Ca2+ sensitive (Fig. S4A). After 60 min of dopamine exposure and EDTA stripping, large amounts of D2 were present in the intracellular vesicular structures (Fig. S3B Right). Dys−/− neurons behaved identically as wild-type neurons, and exhibited no difference in dopamine-induced endocytosis of D2 (Fig. S3 B and C). These results support the biochemical findings that D2 undergoes normal basal and agonist-induced internalization in dys−/− mouse.
Increased Rate and Extent of D2 Membrane Reinsertion.
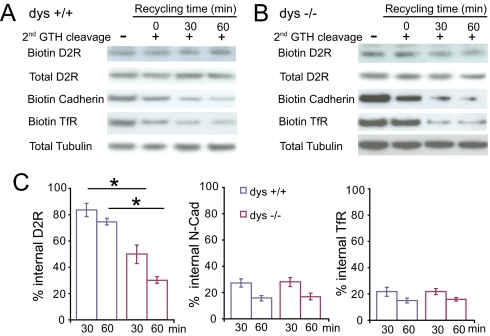
To test whether the increase in surface expression of D2 in dys−/− neurons results from an enhancement of insertion of D2 on the plasma membrane, we measured the reappearance of biotinylated D2 on the cell surface after endocytosis. Neurons were stimulated with dopamine (10 μM) for 60 min to induce endocytosis, chilled to 4 °C to arrest membrane trafficking, and then incubated at 37 °C in the absence of dopamine to allow recycling of D2. In wild-type neurons, internalized D2 was hardly reinserted into the plasma membrane, and 83.68 ± 4.96% and 74.63 ± 2.56% of the internalized D2 were still retained in the cytoplasm at 30 and 60 min after recycling was initiated (Fig. 2A). In contrast, in dys−/− neurons, a significant amount of D2 recycled back to the plasma membrane, and only 49.87 ± 6.95% and 30.07 ± 2.45% of the internalized D2 were retained in the cytoplasm at 30 and 60 min, respectively (Fig. 2B). The decrease in cytoplasmic D2 was not due to an enhanced D2 degradation because protease inhibitor (Leupeptin, 100 μg/mL) was included throughout the experiments, and the expression levels of total D2 were not changed under these conditions (Fig. 2 A and B).
Fig. 2.
Increased Recycling of Internalized D2 Shown by Biochemical Assay. (A and B) Biotinylation assay of D2 recycling in wild-type (A) and dys−/− (B) cortical neurons. Surface proteins were labeled with cleavable biotin, treated with dopamine (10 μM) at 37 °C for 60 min (internalization), and the residual surface biotin was removed by a first round glutathione treatment. The internalized membrane proteins were then allowed to recycle back to cell surface at 37 °C for 30 or 60 min. A second round of glutathione was applied to cleave any appearing surface biotin. Representative immunoblots show remaining intracellular D2, N-Cad, and TfR. A control experiment (left lane) was performed at 4 °C to prevent recycling after endocytosis and internalized proteins remained inside the cells. The loss of biotinylated D2 after a second biotin cleavage provides a measure method of receptor recycling. (C) Quantitative analysis of D2 recycling. The remaining internalized D2 was normalized to that presented at time 0. *, P < 0.05.
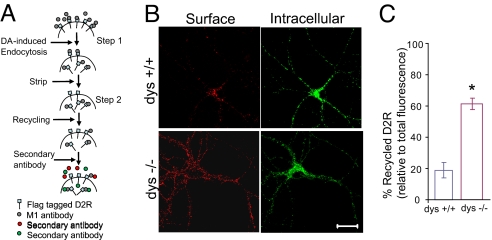
Using live-cell antibody feeding, we visualized the recycling of FLAG-D2. Surface FLAG-D2 was labeled with M1 antibody, and D2 endocytosis was triggered by dopamine. After 60 min, cells were chilled to arrest membrane trafficking, and washed extensively with EDTA-containing PBS to remove dopamine as well as M1 attached to D2 remaining on the plasma membrane. Cells were then incubated at 37 °C for 60 min in the presence of D2 antagonist, haloperidol, and the recycling of intracellular M1-labeled D2 back to cell surface was monitored by fluorescence microscopy (Fig. 3A). Recycled surface D2 exhibited a punctate distribution over cell body and dendritic branches (Fig. 3B Upper). In wild-type neurons, the amount of recycled (reinserted) D2 was 19.97 ± 7.17% of total endocytosed D2 (Fig. 3 B Left and C). This selective decrease in surface D2 was accompanied by a corresponding increase in intracellular D2 in the same neuron (Fig. 3B Right). In marked contrast, 61.20 ± 3.63% of endocytosed D2 were reinserted to the plasma membrane of dys−/− neurons, (Fig. 3 B Lower and C). In parallel, less D2 remained intracellular (Fig. 3B Right). Taken together, our biochemical and imaging experiments suggest that an increase in the rate of D2 recycling is the main factor for the elevated surface expression of D2 in dys−/− neurons.
Fig. 3.
Increased Reinsertion of Internalized FLAG-D2 Shown by Imaging. (A) Schematic of recycling assay. Neurons expressing FLAG-D2 were incubated at 37 °C with M1 antibody (Ca2+ sensitive) for 20 min, followed by dopamine for 60 min. The surface M1 antibody was removed by stripping, and neurons were returned to incubator for receptor recycling. Neurons were fixed and stained with Alexa-546 conjugated secondary antibody to label surface D2 under nonpermeable conditions (red), or Alexa-488 conjugated secondary antibody (green) to label intracellular D2 under permeable conditions. (B) Representative images from recycling experiment showing recycled (Left, Surface) and intracellular (Right) FLAG-D2 after 60 min of recycling. Immunofluorescence staining of D2 was performed under non-permeable (red) and permeable (green) conditions. (Scale bar, 20 μm.) (C) Quantification of D2 reinsertion measured as the ratio of surface (red)/total (red + green) fluorescence. *, P < 0.05.
Functional Deficits in GABAergic Interneurons in PFC and Striatum.
A major target of dopaminergic regulation in the brain is PFC, a brain structure known to play a critical role in executive functions (16), and prominently implicated in the pathophysiology of schizophrenia (17). The increase in cell surface expression of D2 in dys−/− neurons prompted us to investigate the physiological role of dysbindin in PFC. Whole-cell recordings were performed on layer V neurons in PFC slices. The identification of layer V pyramidal cells was based on their large soma size and low input resistance. A 1.2-s long, 150 pA depolarization step induced approximately 15 action potentials in current-clamp mode (Fig. 4A). Whereas the firing frequencies increased in parallel with the increase in the current injection, there was no difference between wild-type and dys−/− neurons in the spike frequency in a range of depolarizing currents (Fig. 4B). Thus, a null mutation in dysbindin gene does not appear to alter the excitability of layer V pyramidal neurons.
Fig. 4.
Decreased FS Interneurons Excitability and GABAergic Transmission in Dys−/− Mice. (A–F) Neuronal excitability. Repetitive firings were evoked by various depolarizing steps, and action potential numbers were plotted against the depolarizing currents injected into neurons. Representative traces are shown on the left and quantifications on the right. (A and B) The excitability of layer V pyramidal neurons in PFC. (C and D) The excitability of layer V FS interneurons in PFC. FS interneurons were identified by their shape, location and fast-spiking (FS) characters. Note the firing frequency from dys−/− neurons was much lower than that from wild-type neurons. *, P < 0.05, **, P < 0.01. (E and F) The excitability of striatal FS interneurons. FS interneurons were identified by their shape, location and electrophysiological characteristics. *, P < 0.05. (G) Sample traces and quantification of sEPSCs recorded from layer V pyramidal neurons from wild-type and dys−/− mice. (H) Sample traces and quantification of sIPSCs recorded from layer V pyramidal neurons from wild-type and dys−/− mice. Note the significant decreases in both the frequency and amplitude of sIPSCs in dys−/− neurons. *, P < 0.01.
We next performed similar recording on interneurons in PFC slices. There are three types of interneurons in PFC: fast-spiking (FS), regular spiking (RS), and low-threshold spiking (LTS) (18–21). RS and LTS interneurons are also called Non-FS interneurons. The GABAergic interneurons were identified by their size and shape, as well as the fast-spiking nature (Fig. S5). (i) FS cells exhibit fast-spiking, nonadaptive firing patterns in response to depolarization. In contrast, both RS and LTS cells exhibit profound adapting firing. (ii) The duration of action potentials (half-width 0.75 ± 0.07 ms) of FS cells is shorter than that of Non-FS cells (half-width 1.50 ± 0.04 ms). The after hyperpolarization (19.72 ± 0.84 mV) is large, compared to that of Non-FS cells (12.25 ± 0.12 mV). (iii) Unlike RS and LTS cells, FS cells also exhibit characteristic spontaneous postsynaptic potentials (sPSPs) with high frequency and large amplitude. In the subsequent studies, we focused on FS interneurons.
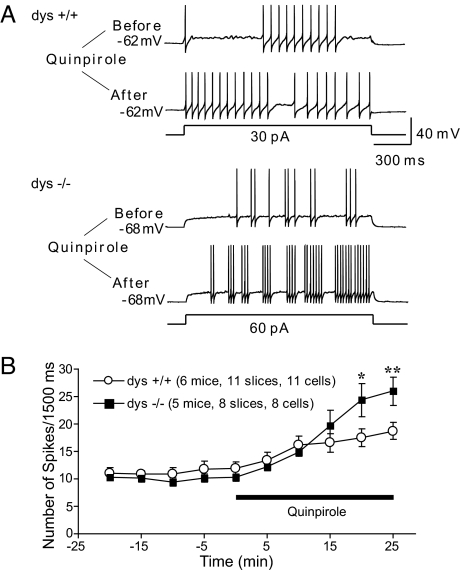
Application of a depolarizing step (1.2 s, 150 pA) induced more than 100 action potentials in wild-type FS interneurons (Fig. 4C Upper). In contrast, the same depolarization applied to a dys−/− FS interneuron induced significantly fewer spikes (Fig. 4C Lower). A systematic analysis showed a general decrease in spike frequencies in dys−/− neurons in a wide range of depolarizing steps (Fig. 4D). These results suggest that mutation of dysbindin gene selectively inhibits the excitability of layer V FS interneurons, without affecting the excitability of pyramidal neurons in the same layer.
D2 receptor mechanisms are also known to play a powerful role in the functions of striatum (22). We therefore examined whether mutation of dysbindin gene also affects the excitability of striatal FS interneurons. A depolarizing step (1.5 s, 200 pA) induced 109 ± 22 action potentials in wild-type, but only 45 ± 14 spikes in dys−/−, striatal interneurons (Fig. 4E). There was a general decrease in spike frequencies in dys−/− neurons in a wide range of depolarizing steps (Fig. 4F). Thus, regulation of excitability by dysbindin is not limited to FS interneurons in PFC.
The change in interneuron excitability further prompted us to examine GABAergic inputs to layer V pyramidal cells in PFC slices. In the presence of glutamatergic antagonists (CNQX, 50 μM and APV, 50 μM), spontaneous inhibitory postsynaptic currents (sIPSCs) appeared inward currents at the holding potential of −70 mV (Fig. 4H, upper trace). The average frequency and amplitude of sIPSCs were 3.3 ± 0.7 Hz and 18.0 ± 3.1 pA, respectively, in wild-type neurons. Mutation in dysbindin gene dramatically reduced the strength of inhibitory synaptic currents recorded from dys−/− pyramidal cells. The average frequency and amplitude of sIPSCs were 1.4 ± 0.2 Hz and 11.1 ± 0.9 pA, respectively, in dys−/− neurons (Fig. 4H, lower trace). In contrast, spontaneous excitatory synaptic currents (sEPSCs), recorded at the holding potential of −65 mV in the presence of GABAA antagonist bicuculine, were not changed in recordings from dys−/− pyramidal cells as compared to those recorded from wild-type pyramidal cells (Fig. 4G). Taken together, dysbindin appears to regulate layer V interneuron excitability and transmission.
Effect of Quinpirole on Excitability of Prefrontal Layer V FS Interneuron.
Activation of D2 leads to an increase in the excitability of FS interneurons in PFC (23). Paradoxically, dys−/− FS interneuron with increased surface expression of D2 exhibited a decreased excitability (Fig. 4 C–F), suggesting a compensatory or homeostasis regulation of excitability. To determine the effect of dysbindin mutation on D2 responses of FS interneurons, we applied D2 receptor selective agonist quinpirole (10 μM) to PFC slices, and measured changes in neuronal excitability, as reflected by the number of action potentials induced by a fixed step depolarization. Consistent with previous reports, application of quinpirole resulted in an increase in firing frequency in FS interneurons derived from wild-type PFC slices (Fig. 5A Upper). In dys−/− FS interneurons, quinpirole elicited a much greater effect on firing frequency (Fig. 5A Lower). Time course experiments indicated that the quinpirole-induced increase in excitability occurred relatively fast (Fig. 5B). Thus, while dys−/− neurons have lower baseline excitability, they respond better to D2 activation, compared to wild-type FS interneurons.
Fig. 5.
Effect of Quinpirole on the Excitability of FS Interneurons. (A) Sample traces showing the changes in firing rates recorded in layer V FS interneurons in PFC upon application of quinpirole. Currant clamp recordings were performed by holding the membrane potentials at their resting potentials. A depolarizing pulse (1.5 s) was applied to evoke typically 8–13 spikes in the baseline every 5 min. Note the increase in firing frequency after treatment with quinpirole in both wild-type and dys−/− neurons. (B) Time course of quinpirole effect. The quinpirole-induced increase in spike frequency in dys−/− FS interneurons is more pronounced than that from wild-type neurons. *, P < 0.05; **, P < 0.01.
Higher Locomotor Activity in Dys−/− Mice.
Hyperactivity is widely used and considered as a rodent correlate of schizophrenia-like positive symptoms (24, 25). To test if dysbindin genetic modification may affect locomotor activity, we tested 14 dys−/− and 13 dys+/+ male littermates in an open field arena. A genotype difference was detected (F1,25 = 14.38, P < 0.001). Dys−/− mice were more active than dys+/+ littermates in the novel environment (P < 0.001) (Fig. S6). These results are consistent with the results from a recent study of sandy mice on a C57BL/6J genetic background (26).
Discussion
We have studied the role of dysbindin in dopamine signaling and neuronal functions, using a dysbindin mutant line. There are three major findings: 1) Mutation of dysbindin resulted in an accumulation of D2 on the plasma membrane of cortical neuron, and this is due to an enhanced insertion of D2 to the neuronal membrane, rather than an increased expression of D2 protein or a decreased endocytosis. 2) Dys−/− mice exhibited a decreased excitability of FS interneurons in PFC and striatum, and reduced inhibitory inputs to pyramidal neurons in layer V of PFC. 3) As a consequence of increased surface expression of D2, dys−/− FS interneuron had a more pronounced increase in neuronal firing in response to D2 agonist quinpirole. These results may help clarify the physiological function of dysbindin in brain in vivo, and shed light on how alterations in dysbindin expression in schizophrenia could contribute to the pathophysiologic mechanisms of the disorder.
Upon agonist stimulation, GPCRs undergo endocytosis followed by trafficking to early endosomes. Subsequently, some GPCRs, such as D1, recycle back to the plasma membrane (11), while others, such as D2, enter late endosomes and then lysosomes for degradation (12, 13). Sorting of individual GPCRs between recycling and degradation fates is therefore a critical factor that determines neurotransmitter responsiveness. As a key member of the BLOC-1 complex (27, 28), dysbindin is implicated in intracellular protein trafficking involving lysosomes or LROs (29). Consistent with this idea, we find that downregulation of dysbindin in mice affects surface expression of D2 but not D1 in cortical neurons. We further show that in dys−/− mice, the recycling but not endocytosis of D2 is increased, resulting in increased D2 expression on the plasma membrane. It is conceivable that a blockade of D2 transport from early endosomes to late endosomes and lysosomes would lead to an increased incorporation of D2 in the default recycling pathway. Thus, our results suggest a possible role of dysbindin in controlling the trafficking of D2 from early endosomes to late endosomes and lysosomes.
Consistent with the above interpretation, recent studies have shown that dysbindin and other three subunits of BLOC-1 (pallidin, snapin, and muted) interact with adaptor protein complex 3 (AP-3) and present in AP-3 microvesicles (27, 30). The heterotetrameric protein AP-3, a coat component, is present in endosomes, where it participates in biogenesis of vesicles that deliver proteins to late endosomes and lysosomes (30). In addition, AP-3 interacts with VAMP7-TI, an R-SNARE protein involved in vesicle fusion with late endosomes/lysosomes. The cellular levels of VAMP7-TI are selectively decreased in BLOC-1 or AP-3 deficient cells (28). Thus, it is tempting to speculate that when dysbindin is downregulated, the function of BLOC-1-Ap-3 complex is disrupted. This, in turn, would divert D2 trafficking from the lysosome pathway to the default recycling pathway, leading to an increase in the incorporation of D2 into the plasma membrane.
Dopamine signaling plays an important role in prefrontal functions (31, 32). A previous study showed that acute activation of D2 increases PFC interneuron excitability (23). An increased expression of cell surface D2 in dys−/− PFC FS interneurons is therefore likely to enhance the ability to respond to D2 by increasing their excitability. Indeed, dys−/− FS interneuron exhibited a much larger increase in neuronal firing than wild-type FS interneuron upon acute exposure to quinpirole (Fig. 5). Unexpectedly, however, mutation of dysbindin gene reduces the baseline excitability of FS interneurons in the absence of D2 agonist quinpirole (Fig. 4 C and D). Neuronal homeostasis theory suggests that neurons have the ability to adjust synaptic or intrinsic excitability in a homeostatic manner to keep their total firing rates relatively constant, thus to prevent neural circuits from becoming hyper- or hypoactive (33). Upon acute exposure to D2 agonists, dys−/− FS interneuron may exhibit a more pronounced increase in firing rate due to an increased expression of cell surface D2. During development, however, homeostatic mechanisms may be engaged to reduce the baseline excitability in dys−/− PFC. As a consequence, dys−/− neurons might have a bigger dynamic range or signal to noise ratio in their D2 responses, compared to their wild-type counterparts. Interestingly, we found that the D2-mediated decrease in excitability is relatively restricted to FS interneurons but not pyramidal neurons. One explanation is that most of the D2 in layer V of PFC are associated with GABAergic interneurons (34). The significance of this change in PFC-mediated executive functions or schizophrenia represents an important subject for future study.
GABAergic interneurons in cortex are important for the interactions between inhibitory and excitatory circuitries and are crucially involved in gamma-band (30–80 Hz) oscillation activity (35–37). Disruption of gamma-band oscillation and synchronization, especially in the 40-Hz range, has been shown to be associated with psychiatric diseases, such as schizophrenia (36, 38). In our present study, mutation of dysbindin gene decreased interneuron excitability and local circuit inhibition from interneurons to pyramidal cells, which might result in impaired cortical circuitry. We showed that both frequency and amplitude of spontaneous IPSCs were reduced in layer V pyramidal cells from dys−/− PFC. Under such disinhibition, gamma-band oscillation and synchronization might be impaired, leading to perceptual distortions and failures of cognitive integration that characterize schizophrenia. The abnormal surface expression of D2 and decreased excitability of interneurons in this dys−/− animal model represents a unique opportunity to examine the neural mechanisms underlying an important clinical phenomenon. It should be pointed out that we do not know whether the attenuation of GABAergic inputs to PFC pyramidal neurons results from the alterations of D2 signaling. In fact, the decrease in FS excitability and IPSCs could also be due to changes in other receptors (such as the NMDA receptor subunit NR2A) involved in the lysosomal pathways, as a consequence of dysbindin mutation. Resolution of these issues awaits future investigation.
Locomotor activity assay is frequently used to model positive schizophrenia symptoms in rodents. We found that dys−/− mice on the C57BL/6J background are hyperactive in the open field, which is consistent with the recent results of sandy mice on the same genetic background (26). The increased locomotor activity might be caused by enhanced D2 signals in dys−/− mice. The D2 family of dopamine receptors has long been considered as a key therapeutic target for schizophrenia (39). D2 actions in prefrontal neurons are associated with saccade-related (response-related) activity (40). A more likely explanation is that the phasic saccadic responses modulated by D2 may be a corollary discharge that informs the prefrontal network that a motor command has been completed (40). Recent studies suggest that imprecision of corollary discharge, a mechanism for distinguishing self-generated thoughts from externally generated percepts, is related to positive symptoms of schizophrenia, including auditory hallucinations (41). Auditory hallucinations are the most common symptoms in schizophrenia and common side effects of D2 agonist. High dose quinpirole has been shown to induce hallucinatory-like behaviors in young adult monkeys (42), suggesting that excess D2 stimulation might impair corollary discharge. Here we showed that mutation of dysbindin gene resulted in increased surface D2. Thus, enhanced D2 actions might disrupt corollary discharge emanating from PFC and contribute to hallucinations. However, a previous study did not find an association between dysbindin high-risk haplotypes and hallucination symptom in schizophrenia (43).
Materials and Methods
Primary Neuron Cultures and Transfection.
Cortical neuron cultures were prepared from either E18 wild-type or dys−/− mouse embryos for biochemical and immunostaining experiments. The cultures were maintained for 15–21 days in vitro. Neurons were transfected with either N-terminal FLAG-D2 or FLAG-D1 or Dysbindin-GFP construct, or cotransfected with FLAG-D2 and eGFP at DIV7 using the calcium phosphate method.
Surface Biotinylation and Receptor Recycling Experiments.
Membranes were prepared from primary neuron cultures after the biotinylation reaction. Briefly, the cell surface was biotinylated with noncleavable sulfo-NHS-biotin in PBS (30 mg/mL, 2 h, 4 °C). Unreacted biotin was quenched and removed by three successive washes of biotinylated cells in PBS containing 10 mM Glycine. Biotinylated receptors were isolated by streptavidin precipitation and detected by immunoblotting. The extent of receptor recycling was visualized by a previously described method (44). Detailed procedure is described in SI Materials and Methods.
Physiological Recording.
Coronal slices from wild-type and dys−/− mice (15–35 days old) were prepared as described in SI Materials and Methods.
Supplementary Material
Acknowledgments.
We thank Dr. David R. Sibley (National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda) for N-terminal FLAG-D2 human cDNA and Dr. Richard T. Swank (Roswell Park Cancer Institute, Buffalo, NY) for genotyping information of dys−/− mice. This work is supported by the Intramural Research Programs of the National Institute of Child Health and Human Development and the National Institute of Mental Health.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0904289106/DCSupplemental.
References
- 1.Straub RE, et al. Genetic variation in the 6p22.3 gene DTNBP1, the human ortholog of the mouse dysbindin gene, is associated with schizophrenia. Am J Hum Genet. 2002;71:337–348. doi: 10.1086/341750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Talbot K, et al. Dysbindin-1 is reduced in intrinsic, glutamatergic terminals of the hippocampal formation in schizophrenia. J Clin Invest. 2004;113:1353–1363. doi: 10.1172/JCI20425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weickert CS, et al. Human dysbindin (DTNBP1) gene expression in normal brain and in schizophrenic prefrontal cortex and midbrain. Arch Gen Psychiatry. 2004;61:544–555. doi: 10.1001/archpsyc.61.6.544. [DOI] [PubMed] [Google Scholar]
- 4.Kumamoto N, et al. Hyperactivation of midbrain dopaminergic system in schizophrenia could be attributed to the down-regulation of dysbindin. Biochem Biophys Res Commun. 2006;345:904–909. doi: 10.1016/j.bbrc.2006.04.163. [DOI] [PubMed] [Google Scholar]
- 5.Numakawa T, et al. Evidence of novel neuronal functions of dysbindin, a susceptibility gene for schizophrenia. Hum Mol Genet. 2004;13:2699–2708. doi: 10.1093/hmg/ddh280. [DOI] [PubMed] [Google Scholar]
- 6.Falcon-Perez JM, Starcevic M, Gautam R, Dell'Angelica EC. BLOC-1, a novel complex containing the pallidin and muted proteins involved in the biogenesis of melanosomes and platelet-dense granules. J Biol Chem. 2002;277:28191–28199. doi: 10.1074/jbc.M204011200. [DOI] [PubMed] [Google Scholar]
- 7.Talbot K, et al. Dysbindin-1 is a synaptic and microtubular protein that binds brain snapin. Hum Mol Genet. 2006;15:3041–3054. doi: 10.1093/hmg/ddl246. [DOI] [PubMed] [Google Scholar]
- 8.Li W, et al. Hermansky-Pudlak syndrome type 7 (HPS-7) results from mutant dysbindin, a member of the biogenesis of lysosome-related organelles complex 1 (BLOC-1) Nat Genet. 2003;35:84–89. doi: 10.1038/ng1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen XW, et al. DTNBP1, a schizophrenia susceptibility gene, affects kinetics of transmitter release. J Cell Biol. 2008;181:791–801. [Google Scholar]
- 10.Iizuka Y, Sei Y, Weinberger DR, Straub RE. Evidence that the BLOC-1 protein dysbindin modulates dopamine D2 receptor internalization and signaling but not D1 internalization. J Neurosci. 2007;27:12390–12395. doi: 10.1523/JNEUROSCI.1689-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ariano MA, et al. Agonist-induced morphologic decrease in cellular D1A dopamine receptor staining. Synapse. 1997;27:313–321. doi: 10.1002/(SICI)1098-2396(199712)27:4<313::AID-SYN5>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 12.Bartlett SE, et al. Dopamine responsiveness is regulated by targeted sorting of D2 receptors. Proc Natl Acad Sci USA. 2005;102:11521–11526. doi: 10.1073/pnas.0502418102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeziorski M, White FJ. Dopamine agonists at repeated “autoreceptor-selective” doses: Effects upon the sensitivity of A10 dopamine autoreceptors. Synapse. 1989;4:267–280. doi: 10.1002/syn.890040403. [DOI] [PubMed] [Google Scholar]
- 14.von Zastrow M. Mechanisms regulating membrane trafficking of G protein-coupled receptors in the endocytic pathway. Life Sci. 2003;74:217–224. doi: 10.1016/j.lfs.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 15.Vickery RG, von Zastrow M. Distinct dynamin-dependent and -independent mechanisms target structurally homologous dopamine receptors to different endocytic membranes. J Cell Biol. 1999;144:31–43. doi: 10.1083/jcb.144.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chudasama Y, Robbins TW. Functions of frontostriatal systems in cognition: Comparative neuropsychopharmacological studies in rats, monkeys and humans. Biol Psychol. 2006;73:19–38. doi: 10.1016/j.biopsycho.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 17.Weinberger DR, et al. Prefrontal neurons and the genetics of schizophrenia. Biological psychiatry. 2001;50:825–844. doi: 10.1016/s0006-3223(01)01252-5. [DOI] [PubMed] [Google Scholar]
- 18.Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature. 1999;402:75–79. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- 19.Kawaguchi Y, Wilson CJ, Augood SJ, Emson PC. Striatal interneurones: Chemical, physiological and morphological characterization. Trends Neurosci. 1995;18:527–535. doi: 10.1016/0166-2236(95)98374-8. [DOI] [PubMed] [Google Scholar]
- 20.Wang HX, Gao WJ. Cell type-specific development of NMDA receptors in the interneurons of rat prefrontal cortex. Neuropsychopharmacology. 2009;34:2028–2040. doi: 10.1038/npp.2009.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiang Z, Huguenard JR, Prince DA. GABAA receptor-mediated currents in interneurons and pyramidal cells of rat visual cortex. J Physiol. 1998;506:715–730. doi: 10.1111/j.1469-7793.1998.715bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kellendonk C, et al. Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning. Neuron. 2006;49:603–615. doi: 10.1016/j.neuron.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 23.Tseng KY, O'Donnell P. Dopamine-glutamate interactions controlling prefrontal cortical pyramidal cell excitability involve multiple signaling mechanisms. J Neurosci. 2004;24:5131–5139. doi: 10.1523/JNEUROSCI.1021-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arguello PA, Gogos JA. Modeling madness in mice: One piece at a time. Neuron. 2006;52:179–196. doi: 10.1016/j.neuron.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 25.Powell CM, Miyakawa T. Schizophrenia-relevant behavioral testing in rodent models: A uniquely human disorder? Biol Psychiatry. 2006;59:1198–1207. doi: 10.1016/j.biopsych.2006.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cox MM, et al. Neurobehavioral abnormalities in the dysbindin-1 mutant, sandy, on a C57BL/6J genetic background. Genes Brain Behav. 2009;8:390–397. doi: 10.1111/j.1601-183X.2009.00477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Di Pietro SM, et al. BLOC-1 interacts with BLOC-2 and the AP-3 complex to facilitate protein trafficking on endosomes. Mol Biol Cell. 2006;17:4027–4038. doi: 10.1091/mbc.E06-05-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salazar G, et al. BLOC-1 complex deficiency alters the targeting of adaptor protein complex-3 cargoes. Mol Biol Cell. 2006;17:4014–4026. doi: 10.1091/mbc.E06-02-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonifacino JS. Insights into the biogenesis of lysosome-related organelles from the study of the Hermansky-Pudlak syndrome. Ann N Y Acad Sci. 2004;1038:103–114. doi: 10.1196/annals.1315.018. [DOI] [PubMed] [Google Scholar]
- 30.Newell-Litwa K, Seong E, Burmeister M, Faundez V. Neuronal and non-neuronal functions of the AP-3 sorting machinery. J Cell Sci. 2007;120:531–541. doi: 10.1242/jcs.03365. [DOI] [PubMed] [Google Scholar]
- 31.Goldman-Rakic PS, Lidow MS, Smiley JF, Williams MS. The anatomy of dopamine in monkey and human prefrontal cortex. J Neural Transm Suppl. 1992;36:163–177. doi: 10.1007/978-3-7091-9211-5_8. [DOI] [PubMed] [Google Scholar]
- 32.Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74:1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 33.Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- 34.Khan ZU, et al. Differential regional and cellular distribution of dopamine D2-like receptors: An immunocytochemical study of subtype-specific antibodies in rat and human brain. J Comp Neurol. 1998;402:353–371. doi: 10.1002/(sici)1096-9861(19981221)402:3<353::aid-cne5>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 35.McBain CJ, Fisahn A. Interneurons unbound. Nat Rev Neurosci. 2001;2:11–23. doi: 10.1038/35049047. [DOI] [PubMed] [Google Scholar]
- 36.Spencer KM, et al. Abnormal neural synchrony in schizophrenia. J Neurosci. 2003;23:7407–7411. doi: 10.1523/JNEUROSCI.23-19-07407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whittington MA, Traub RD. Interneuron diversity series: Inhibitory interneurons and network oscillations in vitro. Trends Neurosci. 2003;26:676–682. doi: 10.1016/j.tins.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 38.Lee KH, Williams LM, Breakspear M, Gordon E. Synchronous gamma activity: A review and contribution to an integrative neuroscience model of schizophrenia. Brain Res Brain Res Rev. 2003;41:57–78. doi: 10.1016/s0165-0173(02)00220-5. [DOI] [PubMed] [Google Scholar]
- 39.Seeman P, Lee T. Antipsychotic drugs: Direct correlation between clinical potency and presynaptic action on dopamine neurons. Science. 1975;188:1217–1219. doi: 10.1126/science.1145194. [DOI] [PubMed] [Google Scholar]
- 40.Wang M, Vijayraghavan S, Goldman-Rakic PS. Selective D2 receptor actions on the functional circuitry of working memory. Science. 2004;303:853–856. doi: 10.1126/science.1091162. [DOI] [PubMed] [Google Scholar]
- 41.Ford JM, Gray M, Faustman WO, Roach BJ, Mathalon DH. Dissecting corollary discharge dysfunction in schizophrenia. Psychophysiology. 2007;44:522–529. doi: 10.1111/j.1469-8986.2007.00533.x. [DOI] [PubMed] [Google Scholar]
- 42.Arnsten AF, Cai JX, Steere JC, Goldman-Rakic PS. Dopamine D2 receptor mechanisms contribute to age-related cognitive decline: The effects of quinpirole on memory and motor performance in monkeys. J Neurosci. 1995;15:3429–3439. doi: 10.1523/JNEUROSCI.15-05-03429.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fanous AH, et al. Relationship between a high-risk haplotype in the DTNBP1 (dysbindin) gene and clinical features of schizophrenia. Am J Psychiatry. 2005;162:1824–1832. doi: 10.1176/appi.ajp.162.10.1824. [DOI] [PubMed] [Google Scholar]
- 44.Tanowitz M, von Zastrow M. A novel endocytic recycling signal that distinguishes the membrane trafficking of naturally occurring opioid receptors. J Biol Chem. 2003;278:45978–45986. doi: 10.1074/jbc.M304504200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.