Abstract
The 5-HT2A serotonin receptor is the most abundant serotonin receptor subtype in the cortex and is predominantly expressed in pyramidal neurons. The 5-HT2A receptor is a target of several hallucinogens, antipsychotics, anxiolytics, and antidepressants, and it has been associated with several psychiatric disorders, conditions that are also associated with aberrations in dendritic spine morphogenesis. However, the role of 5-HT2A receptors in regulating dendritic spine morphogenesis in cortical neurons is unknown. Here we show that the 5-HT2A receptor is present in a subset of spines, in addition to dendritic shafts. It colocalizes with PSD-95 and with multiple PDZ protein-1 (MUPP1) in a subset of dendritic spines of rat cortical pyramidal neurons. MUPP1 is enriched in postsynaptic density (PSD) fractions, is targeted to spines in pyramidal neurons, and enhances the localization of 5-HT2A receptors to the cell periphery. 5-HT2A receptor activation by the 5-HT2 receptor agonist DOI induced a transient increase in dendritic spine size, as well as phosphorylation of p21-activated kinase (PAK) in cultured cortical neurons. PAK is a downstream target of the neuronal Rac guanine nucleotide exchange factor (RacGEF) kalirin-7 that is important for spine remodeling. Kalirin-7 regulates dendritic spine morphogenesis in neurons but its role in neuromodulator signaling has not been investigated. We show that peptide interference that prevents the localization of kalirin-7 to the postsynaptic density disrupts DOI-induced PAK phosphorylation and spine morphogenesis. These results suggest a potential role for serotonin signaling in modulating spine morphology and kalirin-7's function at cortical synapses.
Keywords: GPCR, neuromodulator, PAK, Rac, synapse
Dendritic spine morphogenesis is an important component of structural plasticity in the mammalian forebrain. Changes in dendritic spine shape, size, and number underlie synaptic functional changes and accompany neuronal development, learning and memory, and behavior (1). Additionally, abnormal spine morphogenesis has been associated with many neurodevelopmental, neuropsychiatric, and neurodegenerative diseases, suggesting the importance of appropriate development, plasticity, and maintenance of these structures to neuronal function (2). Spine morphogenesis relies on alterations in the actin cytoskeleton, but the molecular mechanisms that regulate this process are just beginning to be uncovered. The role of neuromodulators, in particular of serotonin, in spine remodeling is not well understood.
The 5-HT2A receptor is the most abundantly expressed serotonin receptor subtype in the cortex, and it is predominantly expressed in pyramidal neurons (3–5). It is a target of several hallucinogens, antipsychotics, anxiolytics, and antidepressants, and it has been functionally and genetically associated with schizophrenia, autism, attention-deficit/hyperactivity disorder, and affective disorders (6–11). 5-HT2A receptors are expressed late in development, coinciding with the period of synaptogenesis (12), and localize to dendrites, dendritic shafts, and dendritic spines (4, 13, 14). Serotonin (5-hydroxytryptamine; 5-HT) regulates spine density in the hippocampus in both developing and adult animals (15), and treatment with the selective serotonin reuptake inhibitor fluoxetine increases spine density in the CA1 and CA3 regions of the hippocampus (16), suggesting a role for 5-HT signaling in spiny synapse morphogenesis. There is some evidence suggesting that the 5-HT2A receptor may play a role in regulating synapse number in embryonic chick spinal cord (17), but whether 5-HT exerts control over synaptic structural plasticity in mammalian cortical neurons through 5-HT2A receptors is unknown.
Kalirin-7, a brain-specific guanine nucleotide exchange factor (GEF) for the small GTPase Rac, is a major regulator of dendritic spine morphogenesis in pyramidal neurons (18). Kalirin-7 is enriched in the postsynaptic density of excitatory synapses in the mammalian forebrain, and its activation enhances Rac activation, p21-activated kinase (PAK) phosphorylation, dendritic spine maintenance and morphogenesis, and AMPA receptor trafficking (19–21). Kalirin-7 interacts with several synaptic protein complexes in neurons, including complexes associated with EphB receptors (21), NMDA receptors (20), and N-cadherin (22). The PDZ-binding motif located on the C terminus of kalirin-7 mediates its interaction with PSD-95 (20), and may allow it to interact with other PDZ-domain-containing proteins (19). However, the regulation of kalirin-7 activity by protein complexes associated with neuromodulators such as 5-HT is unexplored.
In this study we tested the hypothesis that 5-HT2A receptor signaling influences synaptic structural plasticity in the cortex. We show that short-term activation of the 5-HT2A receptor modulates dendritic spine morphogenesis in cortical pyramidal neurons via a kalirin-7-dependent mechanism.
Results
5-HT2A Receptors Are Present in a Subset of Spines and Colocalize with Synaptic Proteins.
Ultrastructural studies have found 5-HT2A receptors in dendrites, dendritic shafts, and dendritic spines of cortical and hippocampal neurons in rats and in primates (4, 14). In cultured rat cortical pyramidal neurons, both endogenous (Fig. 1A) and exogenous flag-tagged 5-HT2A receptors (f-5-HT2A) (Fig. 1B) localized to the somato-dendritic compartment, as previously reported (23). In individual cortical pyramidal neurons, both endogenous 5-HT2A and exogenous f-5-HT2A were targeted to intracellular endomembrane structures in the soma (ie), and were present in distinct clusters in spines (sp) and dendritic shafts (ds) along the apical dendrite (Fig. 1 A and B). Enrichment of f-5-HT2A in spine heads was substantiated by line scans of f-5-HT2A immunofluorescence across a spine and adjacent shaft (Fig. 1C). Localization of f-5-HT2A to dendritic shaft (ds) and spines (sp) was confirmed with co-expression of GFP (Fig. 1D). Endogenous 5-HT2A receptors also partially colocalized with the presynaptic marker bassoon (Fig. 1E and Fig. S1A), indicating a synaptic localization.
Fig. 1.
5-HT2A receptors are present in spines and colocalize with synaptic proteins in cultured cortical neurons. (A) Endogenous 5-HT2A receptors localize to the soma and dendrites of mature cultured cortical neurons (DIV 24). (B) Flag-tagged 5-HT2A receptors localize to intracellular endomembranes (“ie”), the dendritic shaft (“ds”), and spine-like structures (“sp”) in the dendrites of mature cultured cortical neurons. (C) Higher magnification of spine-like structures in B. A line scan through the spine and shaft shows increased fluorescence intensity in the head compared to the shaft. (D) Co-expression of flag-5-HT2A and GFP reveal localization of 5-HT2A receptors to dendritic spines (“sp”) and the dendritic shaft (“ds”). (E) Endogenous 5-HT2A receptors colocalize with the presynaptic marker bassoon in the dendrites of cultured cortical neurons (arrowheads). (F) Endogenous 5-HT2A receptors colocalize with PSD-95 in the dendrites of cultured cortical neurons (arrowheads). (G) Histogram of PSD-95 puncta that colocalize with 5-HT2A receptors. (H) 5-HT2A receptors localize to smaller spines. GFP-transfected cells were co-stained for endogenous 5-HT2A receptors. A larger proportion of spines that contained 5-HT2A had small-to-medium area (< 1 μm2) (arrowheads), while fewer large spines (> 1 μm2) contained 5-HT2A signal (open arrowhead). (I) Histogram of the areas of spines that contain 5-HT2A receptors. Insets show typical dendritic spine shapes for each range of spine area: small (area <1 μm2), medium (area = 1 μm2), and large (area >1 μm2). [Scale bar, 5 μm (E, F, and H); 20 μm (A).]
The PDZ binding motif (X-Ser/Thr-X-Val/Ile/Leu-COOH) of the 5-HT2A receptor is critical for its interaction with PSD-95 in hEK293 cells and neurons (23, 24). In agreement with recent studies, endogenous 5-HT2A receptors partially colocalized with PSD-95 in cortical pyramidal neurons (25) (Fig. 1E and Fig. S1B). As PSD-95 content has been show to be proportional to spine size (26), we quantified synapse content of 5-HT2A as a function of PSD-95 puncta area (Fig. 1 F and G). The majority of synapses that contained 5-HT2A were smaller than the mean PSD-95 puncta area (57.5% of synapses were smaller than 0.485 μm2) (Fig. 1G). In a similar manner, we classified dendritic spines that contained 5-HT2A according to dendritic spine area, designating spines smaller than 1.0 μm2 in area as “small” spines, and spines larger than 1.0 μm2 as “large” spines. Of the spines that contained 5-HT2A receptors, a larger proportion (69.8%) of these spines were small, with areas less than 1.0 μm2 (Fig. 1 H and I). These data indicate that in cultured cortical pyramidal neurons, 5-HT2A receptors are present in synapses of a subset of dendritic spines that are small in size.
MUPP1 Is Enriched in Cortical PSD Fractions and Localizes to Synapses in Pyramidal Neurons.
In pyramidal neurons MUPP1 has been shown to be present in spines and to interact with synaptic proteins (27); however, its localization has not been explored in cortical neurons. To further examine MUPP1's synaptic localization, we performed a subcellular fractionation of rat cortex, and found that MUPP1 was enriched in the membranous and cytoskeletal P1 and P2 fractions, as well as in crude synaptosomes (Fig. S2A). In mature cultured cortical neurons, endogenous MUPP1 colocalized extensively with PSD-95 (Fig. S2B), with bassoon (Fig. S2C), and with kalirin-7 in a subset of spines (Fig. S2 C and D).
MUPP1 Enhances the Localization of the 5-HT2A Receptor to the Cell Periphery.
MUPP1 could be detected in the dendritic shaft and spines of cultured cortical neurons (Fig. S2 B and C), and has been shown to interact with 5-HT2A and 5-HT2C receptors (28). Indeed, in cultured rat cortical neurons, MUPP1 and 5-HT2A receptors colocalized in the dendritic shaft, and in a subset of spines (arrowheads) (Fig. 2A and Fig. S1C). Since a number of G protein-coupled receptors (GPCRs) need an interacting partner for membrane expression (29, 30), we hypothesized that MUPP1 may fulfill a similar role and may act as a scaffold for 5-HT2A receptors and affect their trafficking. To test this, we transfected COS-7 cells with myc-tagged MUPP1 (myc-MUPP1) and f-5-HT2A, individually or together, and examined their localization in fixed cells (Fig. 2 B–E). Myc-MUPP1 expressed alone was associated with the cell periphery (p), along with Golgi-like structures (G) (Fig. 2B). When expressed alone, most f-5-HT2A immunofluorescence was detected in vesicular-like, intracellular structures (v) throughout the cell and in Golgi-like structures (G), and little was detectable at the cell periphery (p) (Fig. 2C). However, when f-5-HT2A was coexpressed with myc-MUPP1, enrichment of f-5-HT2A immunoreactivity was detected at the cell periphery, likely at the plasma membrane, as illustrated by a line scan of f-5-HT2A immunofluorescence across the plasma membrane and nearby cytoplasm (Fig. 2 D and E). In addition, we tested the importance of the carboxy-terminal PDZ binding motif in 5-HT2A for MUPP1-induced cell periphery targeting. COS-7 cells were co-transfected with MUPP1 and the wildtype fluorescent receptor (5-HT2A-GFP-Ct) or a mutant receptor lacking the PDZ binding motif (5-HT2A-GFP-AAA). Co-expression of MUPP1 increased the targeting of a portion of 5-HT2A-GFP-Ct to the membrane periphery where both proteins colocalized (Fig. S3 A-C). However, mutating the PDZ binding motif to all alanine in 5-HT2A-GFP-AAA significantly reduced this colocalization. These findings suggest the receptor PDZ-binding motif is required for MUPP1 and 5-HT2A colocalization at the cell periphery.
Fig. 2.
MUPP1 promotes 5-HT2A receptor membrane trafficking in COS-7 cells. (A) Endogenous 5-HT2A receptors and MUPP1 partially colocalize in mature cultured cortical neurons (DIV 24). (B) MUPP1 overexpressed in COS-7 cells localizes to the cell periphery (“p”), intracellular endomembranes (“ie”), and vesicular-like structures (“v”). (C) 5-HT2A receptors overexpressed in COS-7 cells localize to intracellular endomembranes (“ie”) and vesicular-like structures (“v”). (D) Co-expression of the 5-HT2A receptor and MUPP1 results in increased localization of 5-HT2A receptors to the cell periphery compared to 5-HT2A expression alone. (E) Line scans show increased fluorescence intensity of 5-HT2A at the cell periphery when co-expressed with MUPP1. [Scale bar, 5 μm (A).]
5-HT2A Receptor Activation by DOI Causes a Transient Increase in Spine Size.
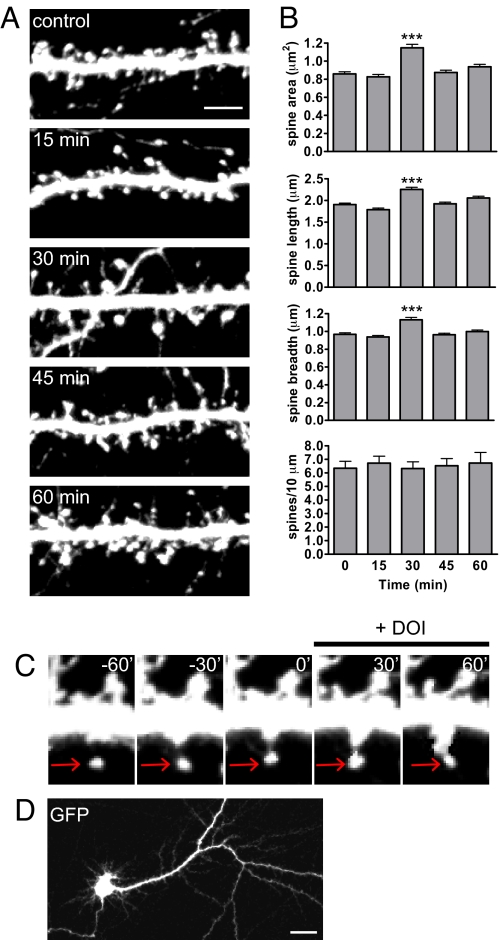
Previous studies have shown that 5-HT may influence synapse number and size; we therefore hypothesized that 5-HT2A receptor activation influences dendritic spine morphology in cortical pyramidal neurons. To test this, we incubated GFP-expressing cultured cortical neurons (DIV 21) with the 5-HT2 receptor-specific agonist (±)-2,5-dimethoxy-4-iodoamphetamine hydrochloride (DOI) (1 μM) for a maximum of 60 min (Fig. 3A). DOI treatment induced a transient increase in spine area, length, and breadth at 30 min, followed by a recovery to control values (area: 0 min, 0.86 ± 0.02 μm2; 15 min, 0.83 ± 0.03 μm2; 30 min, 1.15 ± 0.04 μm2; 45 min, 0.87 ± 0.02 μm2; 60 min, 0.93 ± 0.03 μm2; length: 0 min, 1.91 ± 0.03 μm; 15 min, 1.79 ± 0.04 μm; 30 min, 2.25 ± 0.05 μm; 45 min, 1.92 ± 0.04 μm; 60 min, 2.06 ± 0.04 μm; breadth: 0 min, 0.97 ± 0.02 μm; 15 min, 0.94 ± 0.02 μm; 30 min, 1.13 ± 0.03 μm; 45 min, 0.97 ± 0.02 μm; 60 min, 1.00 ± 0.02 μm) (Fig. 3B). There was no change in dendritic spine linear density in response to DOI (spine density: 0 min, 6.34 ± 1.4 spines per 10 μm; 15 min, 6.72 ± 1.1 spines per 10 μm; 30 min, 6.32 ± 1.1 spines per 10 μm; 45 min, 6.53 ± 1.3 spines per 10 μm; 60 min, 6.73 ± 1.9 spines per 10 μm) (Fig. 3B), or in the percentage of spines associated with bassoon puncta (Fig. S4 A and B). Time-lapse imaging of neurons undergoing DOI perfusion (1 μM) for up to 60 min showed a transient increase in spine size after 30 min, with a recovery to control size by 60 min (Fig. 3C). A wide-field image of the cell after the 2-h imaging session showed that the cell did not undergo photodamage (Fig. 3D).
Fig. 3.
Activation of 5-HT2A receptors with DOI results in dendritic spine remodeling. (A) Time course of dendritic spine morphogenesis in response to 1 μM DOI. Mature cultured cortical neurons (DIV 21) expressing GFP were treated with 1 μM DOI in ACSF + APV for 0–60 min, and fixed immediately. (B) Quantification of dendritic spine morphogenesis in A. Spine area, length, and breadth are increased after 30 min DOI, while spine density remains unchanged. (C) Time-lapse imaging of a dendritic spine in response to DOI. Mature GFP-expressing cortical neurons (DIV 20) were imaged live for 60 min in aCSF + APV, and during 60-min DOI perfusion (1 μM). Spine area increases after 30 min of DOI perfusion, but decreases by 60 min. (D) Image of a neuron after undergoing 2 h of live imaging to confirm cell health. ***, P < 0.001. [Scale bar, 5 μm (A); 20 μm (D).]
5-HT2A Receptor Activation by the Agonist DOI Enhances Phosphorylation of PAK.
PAK is a molecular target of activated Rac, and is an important regulator of the actin cytoskeletal rearrangements that underlie spine remodeling. Activation of a Rac/PAK pathway induces larger spines (20–22); thus, we hypothesized that activation of 5-HT2A receptors influences PAK signaling. We incubated cultured cortical neurons with DOI and examined phosphorylation of PAK on residue T423; phosphorylation at this site is indicative of activation by GTP-bound Rac1 or Cdc42 (31). DOI treatment for 30 min resulted in a dose-dependent increase in PAK phosphorylation in cortical neuron homogenates, with maximal phosphorylation with 1 μM (P < 0.05) (Fig. 4 A and B). A time course of DOI treatment (1 μM) resulted in a transient increase p-PAK immunofluorescence signal in dendrites and dendritic spines at 30 min, with a return of p-PAK signal to control levels by 60 min (30 min, P < 0.001) (Fig. 4 C and D).
Fig. 4.
5-HT2A receptor activation by DOI induces PAK phosphorylation in a concentration- and time-dependent manner. (A) Representative Western blot of dose response of PAK phosphorylation with increasing concentrations of DOI. Mature cortical neurons (DIV 24) were treated for 30 min with 0–1 μM DOI in aCSF + APV, lysed in RIPA buffer, and analyzed by SDS/PAGE. (B) Quantification of PAK phosphorylation in response to increasing concentrations of DOI. (C) Time course of PAK phosphorylation in response to 1 μM DOI. PAK phosphorylation in the dendrites of cultured cortical neurons peaks at 30 min of DOI treatment (1 μM). Insets show p-PAK average intensity in spine-like structures. (D) Quantification of average intensity of p-PAK in cultured cortical neurons treated with DOI (1 μM) for the indicated times. (E) Endogenous 5-HT2A receptors and kalirin-7 partially colocalize in mature cultured cortical neurons. *, P < 0.05; ***, P < 0.001. [Scale bar, 5 μm (C and E); 1 μm (C and E, insets).]
DOI-Dependent Spine Remodeling and PAK Phosphorylation Is Kalirin-7-Dependent.
Kalirin-7 is a major regulator of Rac activity and PAK phosphorylation in mature cortical synapses. We hypothesized that kalirin-7 may be mediating the effects of 5-HT2A receptor activation on PAK phosphorylation and spine morphology. 5-HT2A receptors colocalized with kalirin-7 in a subset of spines of mature cortical neurons (Fig. 4E and Fig. S5A). To test whether the transient increase in dendritic spine area and PAK phosphorylation were kalirin-dependent, we incubated neurons with a kalirin-interfering peptide (K7 int), which mimics the kalirin-7 C-terminal PDZ-binding motif and displaces kalirin-7 from the PSD, or a mutated control peptide (K7 mut) (Fig. S5B). We have previously characterized these peptides: neither peptide affects basal spine area 24 h after a 2-h incubation, but K7 int effectively blocks kalirin-7-mediated spine morphogenesis (20). Pretreatment with K7 int prevented the DOI-induced increase in spine size, while K7 mut did not (area: control, 0.67 ± 0.03 μm2; 30 min DOI, 0.82 ± 0.04 μm2; K7 int + DOI, 0.65 ± 0.02 μm2; K7 mut + DOI, 0.85 ± 0.04 μm2; length: control, 2.14 ± 0.05 μm; 30 min DOI, 2.19 ± 0.07 μm; K7 int + DOI, 2.00 ± 0.05 μm; K7 mut + DOI, 2.21 ± 0.06 μm; breadth: control, 0.82 ± 0.02 μm; 30 min DOI, 0.91 ± 0.02 μm; K7 int + DOI, 0.81 ± 0.02 μm; K7 mut + DOI, 0.90 ± 0.01 μm) (Fig. 5 A and B). Spine density remained unchanged with the pretreatment of either peptide compared to control (control, 5.85 ± 0.34 spines per 10 μm; 30 min DOI, 4.84 ± 0.28 spines per 10 μm; K7 int + DOI, 6.53 ± 0.67 spines per 10 μm; K7 mut + DOI, 6.87 ± 0.50 spines per 10 μm). Similarly, incubation with the interfering peptide prevented the DOI-induced increase in p-PAK immunofluorescence signal, while the mutant peptide did not (P < 0.001) (Fig. 5 C and D).
Fig. 5.
Kalirin-7 is required for DOI-induced dendritic spine remodeling. (A) Preincubation of mature cortical neurons (DIV 24) with a peptide against kalirin-7 (K7 int) prevents dendritic spine remodeling after DOI treatment (1 μM, 30 min), but a mutant peptide (K7 mut) has no effect on DOI-induced spine morphogenesis. Neurons were incubated with either K7 int or K7 mut for 2 h in feeding media 1 day before DOI treatment. Neurons were then treated with DOI (1 μM, 30 min) in ACSF + APV and fixed immediately. (B) Quantification of spine remodeling in response to peptide interference and DOI. (C) Peptide interference with kalirin-7 function (K7 int) prevents DOI-dependent PAK phosphorylation, while a mutant peptide has no effect. Neurons were pretreated with peptide for 2 h, allowed to incubate overnight, treated with DOI (1 μM, 30 min) in aCSF + APV, fixed immediately, and immunostained with a p-PAK-T423 antibody. Insets show spine-like structures in higher magnification. (D) Quantification of p-PAK fluorescence intensity. (E) Model of the regulation of dendritic spine remodeling by 5-HT2A receptors. 5-HT2A receptors are present in spiny synapses on cortical pyramidal neurons accompanied by scaffold proteins such as PSD-95 and MUPP1. Activation of the 5-HT2A receptor induces kalirin-7-dependent PAK phosphorylation and dendritic spine remodeling. *, P < 0.05; ***, P < 0.001. [Scale bar, 5 μm (A and C); 1 μm (C, insets).]
In conclusion, 5-HT2A receptor activation by the agonist DOI resulted in a transient increase in spine average area in cultured cortical pyramidal neurons, dependent on kalirin-7 association with the PSD.
Discussion
Evidence for the regulation of dendritic spine morphogenesis by neuromodulators is very limited. Recent studies suggest roles in modulating dendritic spine morphology for serotonin through 5-HT4 receptors (32), dopamine through D1 dopamine receptors (33), and estradiol through an unknown receptor (34). Our data reveal a function for 5-HT2A receptors in regulating spine morphology of mature cortical pyramidal neurons. Activation of 5-HT2A receptors by DOI induced a kalirin-dependent, transient increase in spine size and enhances phosphorylation of PAK, suggesting a role for 5-HT in the regulation of spine plasticity in cortical pyramidal neurons and extending the list of receptors for neuromodulators that may play a role in regulating structural plasticity in the cortex (Fig. 5E).
Our data are consistent with previous findings of 5-HT2A receptor expression and localization in the brain. We found that 5-HT2A receptors localized to the apical dendrites of cultured cortical pyramidal neurons and to a subset of dendritic spines and synapses. The majority of spines that contained 5-HT2A receptors were classified as “small” (i.e., area less than 1.0 μm2). This population of 5-HT2A receptor-containing small spines would be well prepared to make the most substantial contribution to the DOI-induced increase in spine size. Although it is most likely that the effects of DOI on dendritic spine remodeling and PAK activation are mediated by 5-HT2A receptors based on their enriched expression in neocortical neurons (5, 25), DOI may also activate 5-HT2C receptors, which may contribute to the effects of DOI on spines.
The effect of DOI treatment on cortical spines was transient, with a peak in spine size at 30 min. This transient effect may be due to the nature of 5-HT2A receptor signaling properties, including internalization or desensitization (35), a phenomenon observed for both agonists and antagonists of the 5-HT2A receptor. Other factors that might contribute to the transient effect on spines are agonist metabolism or reuptake by monoamine transporters, feedback signaling that alters the GPCR activation state, termination of G protein signaling, termination of small GTPase signaling, or requirement of a secondary signal to stabilize the structural changes as a result of the signaling.
The role of serotonin in modulating excitatory synapses in pyramidal neurons is just beginning to be defined. Colocalization of 5-HT2A with GluR2 and NR1 in the dentate gyrus has been detected by ultrastructural studies (13), and in the prefrontal cortex, 5-HT2A receptor activity may function to modulate glutamatergic neurotransmission in pyramidal neurons (36, 37). Importantly, kalirin-7 plays a role in the regulation of glutamatergic signaling in neurons by mediating AMPAR insertion at spiny synapses upon NMDAR activation (20). Thus, several pathways downstream of the 5-HT2A receptor may interact to regulate trafficking of both NMDAR and AMPAR, as well as kalirin-mediated structural changes. The spatial and temporal relationships between each of these pathways remain to be determined.
5-HT2A receptor signaling rapidly modulates dendritic spine morphogenesis in cortical neurons in a kalirin-dependent manner. Kalirin-7 interacts with and is regulated by signaling complexes associated with NMDA receptors, N-cadherin, and EphB receptors via different PDZ scaffold proteins, such as PSD-95 and afadin/AF-6, and it regulates dendritic spine morphogenesis and AMPA receptor trafficking in neurons (18). Thus, kalirin-7 may act as a postsynaptic signaling hub by integrating multiple pathways and producing an output signal to regulators of the actin cytoskeleton in spines. However, kalirin-7's regulation by neuromodulators such as 5-HT has not been investigated. Here we show colocalization of kalirin-7 with both 5-HT2A receptors and MUPP1 in dendritic spines of mature cortical neurons, where kalirin-7 is enriched. Additionally, we show that kalirin targeting at the postsynaptic density is required for DOI-induced dendritic spine remodeling and PAK phosphorylation. Our data suggest that 5-HT2A receptors and kalirin-7 may be functionally linked, and that 5-HT signaling may modulate kalirin-7 activity at mature synapses and modulate synapse size in vivo. 5-HT may thus be another upstream regulator of kalirin-7, providing a neuromodulatory component to the signaling network that kalirin-7 integrates in spines.
The interaction of GPCRs with PDZ proteins is an important regulatory mechanism for the trafficking and signaling of these receptors (24, 25, 30). Both 5-HT2A and 5-HT2C receptors interact with distinct PDZ proteins, determined by non-conserved residues just upstream of their canonical PDZ-binding motifs (38). The C terminus of the 5-HT2A receptor has been shown to interact with MUPP1 in vitro (28), and several reports have demonstrated that the C-terminal PDZ binding motif of the 5-HT2A receptor is required for its dendritic targeting, and interaction with PSD-95 is important for receptor trafficking and signal transduction (23–25). In agreement with these previous studies, here we found that in cultured cortical neurons, 5-HT2A receptors colocalize with both PSD-95 and MUPP1 in the dendrites and dendritic spines of cortical pyramidal neurons, and when coexpressed in COS-7 cells, MUPP1 facilitates the membrane targeting of 5-HT2A receptors which can be prevented by mutating the receptor PDZ binding motif. Importantly, MUPP1 is a scaffold for a growing list of synaptic proteins that are involved in GPCR and small GTPase signaling (27, 39–41). Our data, along with previous studies, are consistent with the roles of both MUPP1 and PSD-95 as protein scaffolds that may integrate signals through GPCRs at the synapse, provide avenues for crosstalk between signaling pathways, and function in the membrane localization of some receptors and signaling proteins. The interaction of 5-HT2A receptors with two different PDZ proteins may reflect different functions of each interaction or distinct pools of the receptor within the dendritic compartment. Preferential binding of one PDZ protein over another may thus have profound effects on the trafficking or signaling patterns of 5-HT2A receptors, diversifying the effects of 5-HT on a neuron.
The proteins in this pathway linking 5-HT to spine remodeling have been implicated in neuropsychiatric disorders by multiple lines of genetic, pharmacological, and neuropathological evidence. Disruption of 5-HT2A receptor signaling is implicated in several prevalent psychiatric disorders (6–11), and the underlying effect of these alterations of serotonergic signaling may involve structural changes in synapses, which may alter neural circuits. Additionally, kalirin has reduced mRNA expression in the cortex of schizophrenia patients (42), and MUPP1 has been genetically associated with drug withdrawal seizures (43). Thus, the signaling pathway we describe here may be important in the pathogenesis of a number of psychiatric disorders.
Taken together, we report that 5-HT2A receptor signaling influences synaptic structural plasticity in cortical pyramidal neurons via a kalirin-7-dependent mechanism. This signaling pathway may provide a link between the serotonergic system and the regulation of dendritic spine morphology, and may provide insight into neuropsychiatric disorders in which members of this pathway are implicated.
Methods
Reagents.
The plasmid cDNA constructs encoding flag-tagged 5-HT2A (pCMV-Tag2B), 5-HT2A-GFP-Ct, and 5-HT2A-GFP-AAA were generated and validated in the laboratory of Dr. Bryan Roth as previously described (23). The plasmid encoding myc-MUPP1 (pME18S-MUPP1–7myc) was a gift from Drs. Shoichiro Tsukita and Yoko Hamazaki (Kyoto University). We also used pEGFP-N2 (Clontech). The polyclonal antibody recognizing kalirin-7 was described previously (19). GFP polyclonal antibody was a gift from Dr. Richard L. Huganir (Johns Hopkins University). The polyclonal antibody for 5-HT2A (Ab51) was generated by Dr. Bryan Roth (University of North Carolina) (44); and for MUPP1, by Dr. David Clapham (Harvard University) (27). The following antibodies were purchased: PSD-95 monoclonal (Upstate), GFP monoclonal (Chemicon), myc monoclonal (University of Iowa Hybridoma Bank, Santa Cruz), Flag monoclonal (Sigma), phospho-PAK-T423 antibody (Cell Signaling), MUPP1 monoclonal (BD Transduction), 5-HT2A monoclonal (PharMingen/BD Biosciences), actin monoclonal (Sigma), total PAK1 (Zymed), bassoon monoclonal (Stressgen).
Cell Cultures and Transfections.
Dissociated cultures of primary cortical neurons were prepared from E18 Sprague-Dawley rat embryos as described previously (20). See SI Methods for more details.
Agonist Treatments.
Neuron cultures on coverslips or 60-mm dishes were maintained in presence of 200 μM D,L-APV. Cells were then preincubated in ACSF (in mM: 125 NaCl, 2.5 KCl, 26.2 NaHCO3, 1 NaH2PO4, 11 glucose, 5 HEPES, 2.5 CaCl2, and 1.25 MgCl2) with 200 μM APV for 30 min at 37 °C, and then incubated in ACSF + APV + 1 μM DOI (Sigma) for 0, 15, 30, 45, or 60 min at 37 °C, or with 0 nM, 10 nM, 100 nM, 500 nM, or 1 μM DOI for 30 min. For peptide treatments, cells were incubated with 10 μM of either K7 int or K7 mut in Neurobasal + APV for 2 h, followed by a wash in peptide-free media, and incubated in culture medium overnight before DOI treatment. Coverslips or dishes were fixed and processed for immunostaining or lysed in RIPA buffer (in mM: 150 NaCl, 10 Tris-HCl pH 7.2, 5 EDTA, 0.1% SDS, 1% Triton X-100, 1% deoxycholate, plus protease and phosphatase inhibitors) for SDS/PAGE and Western blotting. Intensities of Western blot bands were quantified by densitometry using ImageJ (rsb.info.nih.gov/ij/).
Immunocytochemistry.
Neurons were fixed in either 4% formaldehyde/4% sucrose PBS for 20 min, or in 100% methanol prechilled to -20 °C for 15 min, as previously described (20). See SI Methods for details.
Additional methods can be found in the SI Text.
Supplementary Material
Acknowledgments.
We thank Kevin Woolfrey and Jon VanLeeuwen for editing and Noah Sciaky for assistance with imaging analyses. This work was supported by National Institutes of Health Grant R01MH 071316, the Alzheimer's Association, the National Alliance for Research on Schizophrenia and Depression, and the National Alliance for Autism Research (to P.P.); National Institutes of Health Grant 1F31MH085362 (to K.A.J.); an American Heart Association postdoctoral fellowship (to D.P.S.); National Institutes of Health Grant T32HD040127 and the University of North Carolina Neurodevelopmental Disorders Research Center (to J.A.A); and National Institutes of Health grants R01MH61887, N01MH32004, and U19MH82441 (to B.L.R.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0905884106/DCSupplemental.
References
- 1.Alvarez VA, Sabatini BL. Anatomical and physiological plasticity of dendritic spines. Annu Rev Neurosci. 2007;30:79–97. doi: 10.1146/annurev.neuro.30.051606.094222. [DOI] [PubMed] [Google Scholar]
- 2.Blanpied TA, Ehlers MD. Microanatomy of dendritic spines: Emerging principles of synaptic pathology in psychiatric and neurological disease. Biol Psychiatry. 2004;55:1121–1127. doi: 10.1016/j.biopsych.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Allen JA, Yadav PN, Roth BL. Insights into the regulation of 5-HT2A serotonin receptors by scaffolding proteins and kinases. Neuropharmacology. 2008;55:961–968. doi: 10.1016/j.neuropharm.2008.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miner LA, Backstrom JR, Sanders-Bush E, Sesack SR. Ultrastructural localization of serotonin2A receptors in the middle layers of the rat prelimbic prefrontal cortex. Neuroscience. 2003;116:107–117. doi: 10.1016/s0306-4522(02)00580-8. [DOI] [PubMed] [Google Scholar]
- 5.Pompeiano M, Palacios JM, Mengod G. Distribution of the serotonin 5-HT2 receptor family mRNAs: Comparison between 5-HT2A and 5-HT2C receptors. Brain Res Mol Brain Res. 1994;23:163–178. doi: 10.1016/0169-328x(94)90223-2. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez-Maeso J, et al. Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron. 2007;53:439–452. doi: 10.1016/j.neuron.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 7.Gray JA, Roth BL. Molecular targets for treating cognitive dysfunction in schizophrenia. Schizophr Bull. 2007;33:1100–1119. doi: 10.1093/schbul/sbm074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ribases M, et al. Exploration of 19 serotoninergic candidate genes in adults and children with attention-deficit/hyperactivity disorder identifies association for 5HT2A, DDC and MAOB. Mol Psychiatry. 2009;14:71–85. doi: 10.1038/sj.mp.4002100. [DOI] [PubMed] [Google Scholar]
- 9.Veenstra-VanderWeele J, et al. Transmission disequilibrium studies of the serotonin 5-HT2A receptor gene (HTR2A) in autism. Am J Med Genet. 2002;114:277–283. doi: 10.1002/ajmg.10192. [DOI] [PubMed] [Google Scholar]
- 10.Weisstaub NV, et al. Cortical 5-HT2A receptor signaling modulates anxiety-like behaviors in mice. Science. 2006;313:536–540. doi: 10.1126/science.1123432. [DOI] [PubMed] [Google Scholar]
- 11.Berg KA, Harvey JA, Spampinato U, Clarke WP. Physiological and therapeutic relevance of constitutive activity of 5-HT 2A and 5-HT 2C receptors for the treatment of depression. Prog Brain Res. 2008;172:287–305. doi: 10.1016/S0079-6123(08)00914-X. [DOI] [PubMed] [Google Scholar]
- 12.Roth BL, Hamblin MW, Ciaranello RD. Developmental regulation of 5-HT2 and 5-HT1c mRNA and receptor levels. Brain Res Dev Brain Res. 1991;58:51–58. doi: 10.1016/0165-3806(91)90236-c. [DOI] [PubMed] [Google Scholar]
- 13.Peddie CJ, Davies HA, Colyer FM, Stewart MG, Rodriguez JJ. Colocalisation of serotonin2A receptors with the glutamate receptor subunits NR1 and GluR2 in the dentate gyrus: An ultrastructural study of a modulatory role. Exp Neurol. 2008;211:561–573. doi: 10.1016/j.expneurol.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Jakab RL, Goldman-Rakic PS. 5-Hydroxytryptamine2A serotonin receptors in the primate cerebral cortex: Possible site of action of hallucinogenic and antipsychotic drugs in pyramidal cell apical dendrites. Proc Natl Acad Sci USA. 1998;95:735–740. doi: 10.1073/pnas.95.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feria-Velasco A, del Angel AR, Gonzalez-Burgos I. Modification of dendritic development. Prog Brain Res. 2002;136:135–143. doi: 10.1016/s0079-6123(02)36013-8. [DOI] [PubMed] [Google Scholar]
- 16.Hajszan T, MacLusky NJ, Leranth C. Short-term treatment with the antidepressant fluoxetine triggers pyramidal dendritic spine synapse formation in rat hippocampus. Eur J Neurosci. 2005;21:1299–1303. doi: 10.1111/j.1460-9568.2005.03968.x. [DOI] [PubMed] [Google Scholar]
- 17.Niitsu Y, Hamada S, Hamaguchi K, Mikuni M, Okado N. Regulation of synapse density by 5-HT2A receptor agonist and antagonist in the spinal cord of chicken embryo. Neurosci Lett. 1995;195:159–162. doi: 10.1016/0304-3940(95)11805-7. [DOI] [PubMed] [Google Scholar]
- 18.Penzes P, Jones KA. Dendritic spine dynamics–a key role for kalirin-7. Trends Neurosci. 2008;31:419–427. doi: 10.1016/j.tins.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Penzes P, et al. The neuronal Rho-GEF Kalirin-7 interacts with PDZ domain-containing proteins and regulates dendritic morphogenesis. Neuron. 2001;29:229–242. doi: 10.1016/s0896-6273(01)00193-3. [DOI] [PubMed] [Google Scholar]
- 20.Xie Z, Srivastava DP, et al. Kalirin-7 controls activity-dependent structural and functional plasticity of dendritic spines. Neuron. 2007;56:640–656. doi: 10.1016/j.neuron.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Penzes P, et al. Rapid induction of dendritic spine morphogenesis by trans-synaptic ephrinB-EphB receptor activation of the Rho-GEF kalirin. Neuron. 2003;37:263–274. doi: 10.1016/s0896-6273(02)01168-6. [DOI] [PubMed] [Google Scholar]
- 22.Xie Z, et al. Coordination of synaptic adhesion with dendritic spine remodeling by AF-6 and kalirin-7. J Neurosci. 2008;28:6079–6091. doi: 10.1523/JNEUROSCI.1170-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xia Z, Hufeisen SJ, Gray JA, Roth BL. The PDZ-binding domain is essential for the dendritic targeting of 5-HT2A serotonin receptors in cortical pyramidal neurons in vitro. Neuroscience. 2003;122:907–920. doi: 10.1016/s0306-4522(03)00589-x. [DOI] [PubMed] [Google Scholar]
- 24.Xia Z, Gray JA, Compton-Toth BA, Roth BL. A direct interaction of PSD-95 with 5-HT2A serotonin receptors regulates receptor trafficking and signal transduction. J Biol Chem. 2003;278:21901–21908. doi: 10.1074/jbc.M301905200. [DOI] [PubMed] [Google Scholar]
- 25.Abbas AI, et al. PSD-95 is essential for hallucinogen and atypical antipsychotic drug actions at serotonin receptors. J Neurosci. 2009;29:7124–136. doi: 10.1523/JNEUROSCI.1090-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bourne JN, Harris KM. Balancing structure and function at hippocampal dendritic spines. Annu Rev Neurosci. 2008;31:47–67. doi: 10.1146/annurev.neuro.31.060407.125646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krapivinsky G, Medina I, Krapivinsky L, Gapon S, Clapham DE. SynGAP-MUPP1-CaMKII synaptic complexes regulate p38 MAP kinase activity and NMDA receptor-dependent synaptic AMPA receptor potentiation. Neuron. 2004;43:563–574. doi: 10.1016/j.neuron.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 28.Becamel C, et al. Interaction of serotonin 5-hydroxytryptamine type 2C receptors with PDZ10 of the multi-PDZ domain protein MUPP1. J Biol Chem. 2001;276:12974–12982. doi: 10.1074/jbc.M008089200. [DOI] [PubMed] [Google Scholar]
- 29.Parameswaran N, Spielman WS. RAMPs: The past, present and future. Trends Biochem Sci. 2006;31:631–638. doi: 10.1016/j.tibs.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 30.Bockaert J, et al. GPCR-interacting proteins (GIPs): Nature and functions. Biochem Soc Trans. 2004;32:851–855. doi: 10.1042/BST0320851. [DOI] [PubMed] [Google Scholar]
- 31.Sells MA, Pfaff A, Chernoff J. Temporal and spatial distribution of activated Pak1 in fibroblasts. J Cell Biol. 2000;151:1449–1458. doi: 10.1083/jcb.151.7.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Restivo L, Roman F, Dumuis A, Bockaert J, Marchetti E, Ammassari-Teule M. The promnesic effect of G-protein-coupled 5-HT4 receptors activation is mediated by a potentiation of learning-induced spine growth in the mouse hippocampus. Neuropsychopharmacology. 2008;33:2427–2434. doi: 10.1038/sj.npp.1301644. [DOI] [PubMed] [Google Scholar]
- 33.Wang HD, Deutch AY. Dopamine depletion of the prefrontal cortex induces dendritic spine loss: Reversal by atypical antipsychotic drug treatment. Neuropsychopharmacology. 2008;33:1276–1286. doi: 10.1038/sj.npp.1301521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Srivastava DP, et al. Rapid enhancement of two-step wiring plasticity by estrogen and NMDA receptor activity. Proc Natl Acad Sci USA. 2008;105:14650–14655. doi: 10.1073/pnas.0801581105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gray JA, Roth BL. Paradoxical trafficking and regulation of 5-HT(2A) receptors by agonists and antagonists. Brain Res Bull. 2001;56:441–451. doi: 10.1016/s0361-9230(01)00623-2. [DOI] [PubMed] [Google Scholar]
- 36.Marek GJ, Aghajanian GK. 5-HT2A receptor or alpha1-adrenoceptor activation induces excitatory postsynaptic currents in layer V pyramidal cells of the medial prefrontal cortex. Eur J Pharmacol. 1999;367:197–206. doi: 10.1016/s0014-2999(98)00945-5. [DOI] [PubMed] [Google Scholar]
- 37.Zhou FM, Hablitz JJ. Activation of serotonin receptors modulates synaptic transmission in rat cerebral cortex. J Neurophysiol. 1999;82:2989–2999. doi: 10.1152/jn.1999.82.6.2989. [DOI] [PubMed] [Google Scholar]
- 38.Becamel C, et al. The serotonin 5-HT2A and 5-HT2C receptors interact with specific sets of PDZ proteins. J Biol Chem. 2004;279:20257–20266. doi: 10.1074/jbc.M312106200. [DOI] [PubMed] [Google Scholar]
- 39.Estevez MA, et al. The neuronal RhoA GEF, Tech, interacts with the synaptic multi-PDZ-domain-containing protein, MUPP1. J Neurochem. 2008;106:1287–1297. doi: 10.1111/j.1471-4159.2008.05472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Balasubramanian S, Fam SR, Hall RA. GABAB receptor association with the PDZ scaffold Mupp1 alters receptor stability and function. J Biol Chem. 2007;282:4162–4171. doi: 10.1074/jbc.M607695200. [DOI] [PubMed] [Google Scholar]
- 41.Guillaume JL, et al. The PDZ protein mupp1 promotes Gi coupling and signaling of the Mt1 melatonin receptor. J Biol Chem. 2008;283:16762–16771. doi: 10.1074/jbc.M802069200. [DOI] [PubMed] [Google Scholar]
- 42.Hill JJ, Hashimoto T, Lewis DA. Molecular mechanisms contributing to dendritic spine alterations in the prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2006;11:557–566. doi: 10.1038/sj.mp.4001792. [DOI] [PubMed] [Google Scholar]
- 43.Shirley RL, Walter NA, Reilly MT, Fehr C, Buck KJ. Mpdz is a quantitative trait gene for drug withdrawal seizures. Nat Neurosci. 2004;7:699–700. doi: 10.1038/nn1271. [DOI] [PubMed] [Google Scholar]
- 44.Roth BL, et al. 5-Hydroxytryptamine2A (5-HT2A) receptor desensitization can occur without down-regulation. J Pharmacol Exp Ther. 1995;275:1638–1646. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.