Abstract
With Manduca sexta as a model system, we analyzed how natural odor mixtures that are most effective in eliciting flight and foraging behaviors are encoded in the primary olfactory center in the brain, the antennal lobe. We used gas chromatography coupled with multiunit neural-ensemble recording to identify key odorants from flowers of two important nectar resources, the desert plants Datura wrightii and Agave palmeri, that elicited responses from individual antennal-lobe neurons. Neural-ensemble responses to the A. palmeri floral scent, comprising >60 odorants, could be reproduced by stimulation with a mixture of six of its constituents that had behavioral effectiveness equivalent to that of the complete scent. Likewise, a mixture of three floral volatiles from D. wrightii elicited normal flight and feeding behaviors. By recording responses of neural ensembles to mixtures of varying behavioral effectiveness, we analyzed the coding of behaviorally “meaningful” odors. We considered four possible ensemble-coding mechanisms—mean firing rate, mean instantaneous firing rate, pattern of synchronous ensemble firing, and total net synchrony of firing—and found that mean firing rate and the pattern of ensemble synchrony were best correlated with behavior (R = 41% and 43%, respectively). Stepwise regression analysis showed that net synchrony and mean instantaneous firing rate contributed little to the variation in the behavioral results. We conclude that a combination of mean-rate coding and synchrony of firing of antennal-lobe neurons underlies generalization among related, behaviorally effective floral mixtures while maintaining sufficient contrast for discrimination of distinct scents.
Keywords: floral scent, insect behavior, neural codes, neural synchrony, olfaction
Natural stimuli elicit diverse responses from central neurons in the brain, which confounds efforts to determine the patterns of neural activity underlying natural behavioral responses. This is true in particular for the early stages of processing of olfactory information, for which it has been suggested that different neural codes play similar or overlapping roles (1–4). For example, spatially defined codes based on neural firing rate in the olfactory bulb (OB) of mammals and the antennal lobe (AL) of insects are thought to define stimulus identity and concentration (3, 5–7). Time-related response features have been reported to carry information about odorant identity as well (8–10). Although evidence for the importance of these neural responses is compelling, the relative contribution of different coding mechanisms and how well each correlates with behavior remain poorly understood.
The predominant use of olfactory stimuli, usually monomolecular odorants, that have no behavioral significance for the animal under study has limited analysis of neural coding mechanisms that underlie natural behavior. Natural olfactory stimuli typically are mixtures of which the identities, concentrations, and ratios of chemical constituents are important for many odor-mediated behaviors. Examples of the behavioral effectiveness of complex odor stimuli in both invertebrates and vertebrates are numerous. For example, elephants and mice respond to gender-specific pheromone mixtures (11–13), and insects respond to multicomponent conspecific pheromones, food odors, and alarm cues (14–17).
Despite abundant behavioral examples, links between odor-evoked behavior and the processing and discrimination of complex olfactory stimuli remain unclear. One rigorous approach to this problem is to determine directly which neural codes correlate with an animal's behavior, which requires establishing the relationships among natural stimuli, different formats of neural representation, and well-defined behavioral responses. For example, coding of odor identity through a chemotopic distribution of activated glomeruli has been hypothesized for the insect AL, although the overlap of activity patterns evoked by mixtures with those in response to single odorants of little behavioral relevance can be substantial. Only a few studies have demonstrated behavioral correlates (18–21). Alternatively, the temporal domain is thought to be important through either a rate code or a spike-timing code, but how the population of responsive neurons encodes information about mixtures remains uncertain (8, 22, 23). The contribution of different coding mechanisms, and the manner by which the olfactory system generalizes among different stimuli that are behaviorally effective in contrast to odors that are not effective, have yet to be clarified.
Odor generalization, as we use the concept here, is a measure of the similarity of behavioral and neural responses to a given odor and test odors. This kind of generalization reveals subtle similarities and establishes graded response patterns with changing features (e.g., constituents) of the tested mixtures (24). Thus, generalization (equal response to given mixture A and test mixture B) means that the mixtures are perceptually very similar and may share features used for predicting the behavioral responses, but not necessarily that they cannot be discriminated.
To analyze how an olfactory system discriminates between and generalizes among behaviorally effective and ineffective stimuli, we previously focused on floral scents that are important indicators of nectar resources for the sphinx moth Manduca sexta (25). In the Southwestern United States, M. sexta prefers to forage for nectar from flowers of its Solanaceous hostplant Datura wrightii (14, 26–28). By means of gas chromatography with coupled mass spectrometry (GC-MS) and GC in tandem with multiunit recording (GC-MR, in which AL neural-ensemble responses to fractionated odorants are recorded as they are eluted from the GC column), we identified nine critical odorants in the floral scent of D. wrightii that, together as a mixture, elicit innate flight and foraging behavior in M. sexta (25). That mixture was represented in the neural circuitry of the AL through odor-evoked, intensity-invariant, synchronous firing of neurons, and the spatiotemporal activity pattern accurately discriminated between the mixture and its constituents.
In the present study we used closely related, behaviorally effective and ineffective mixtures of odorants from the complete floral scents of two natural nectar sources to demonstrate that ensembles of AL neurons efficiently encode complex mixture stimuli through a subset of odorants rather than the complete bouquet. Furthermore, the behavioral responses to these different mixtures of varying behavioral effectiveness allowed correlation among the different neural codes with behavior. Together, these results indicate that mixture processing in the moth's olfactory system can be determined by a bounded odor stimulus and that the combination of rate- and time-based neural codes provides a means for complex odor generalization and discrimination.
Results
Behavioral Responses to Mixture Stimuli.
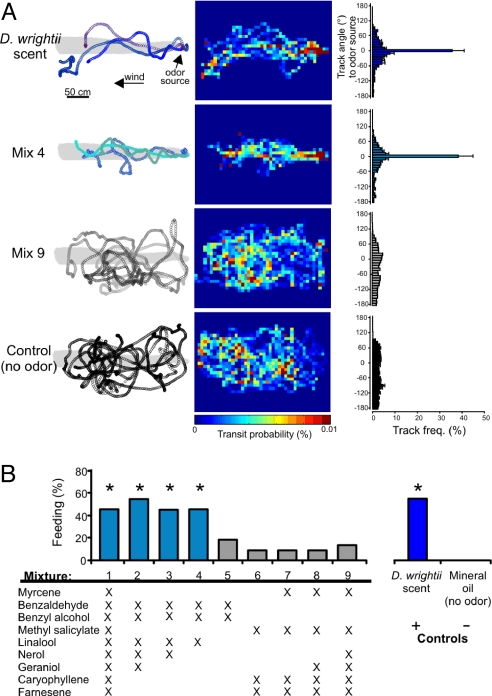
Our earlier study (25) showed that a reduced mixture of D. wrightii floral volatiles was sufficient, but it was not clear whether an even smaller mixture might be necessary and sufficient, to elicit normal flight and foraging behavior. Behavioral experiments conducted in a laboratory wind tunnel to address this question revealed that only three of the nine components previously identified, benzaldehyde (Bea), benzyl alcohol (Bol), and linalool (Lin), were necessary and sufficient to elicit behavioral responses similar to those in response to the complete the floral scent (Fig. 1A; G test: P > 0.50). If these three components were removed from the floral mixture, then behavioral responses were depressed to levels not significantly different from those observed with the mineral oil control (Fig. 1B; G test: P > 0.05). This result suggests that the moths were attracted and responded to this small subset of volatiles even when they were accompanied by the rest of the components of the complete floral scent, and that as long as these three components were present in tested mixtures moths could generalize among mixture stimuli.
Fig. 1.
2D flight tracks of moths in response to the mixture stimuli. (A Left) Flight tracks in response to D. wrightii floral scent, mixture 4, mixture 9, and odorless mineral-oil control. Three representative tracks were chosen using random numbers from among the digitized treatment groups. Circles correspond to 16-ms intervals. (A Center) The cumulative transit probabilities for all moths in the treatment groups (n = 22 moths per treatment). (A Right) The track angles for all moths in each treatment group. Note that the D. wrightii floral scent and mixture 4 elicited anemotactic behavior in the moths. In contrast, mixture 9 elicited random flight as did the odorless (mineral oil) control. (B) The percentage of moths that fed from the paper flowers emitting the different mixtures. Odorants within each partial mixture are shown below the histogram (n = 22 moths per mixture treatment). Asterisks denote a significant difference from the mineral-oil control (G test: P < 0.05).
Analysis of Floral Volatiles by GC-MR and Behavioral Effectiveness.
Like the scent of D. wrightii flowers, that of flowers in Agave palmeri (Agavaceae) umbels can attract foraging adult M. sexta (14, 29). A. palmeri is not a host plant for oviposition but is an abundant nectar resource for M. sexta and thus provides another olfactory stimulus with which to explore the neural bases of foraging behavior. Building on our earlier study of the floral scent D. wrightii, we conducted GC-MS and GC-MR analyses of A. palmeri floral volatiles. As in the case of D. wrightii, the floral scent of A. palmeri comprises >60 compounds, but they are very different from those emitted by D. wrightii flowers. A. palmeri floral scent includes carboxylic-acid esters (≈50% in terms of mass per unit volume), monoterpenes (≈30%), and aromatic compounds (≈15%). AL neurons, however, did not respond to a majority of the compounds in the A. palmeri floral mixture at their natural concentrations. Data from this system revealed variability in odor-evoked responses among AL neurons but also consistent patterns of activity in response to certain odorants across multiple units in a neural ensemble (Fig. 2A).
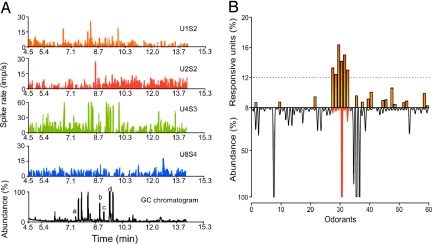
Fig. 2.
Responses of AL neurons to GC-fractionated scent from A. palmeri flowers. (A) Rate histograms (bin size, 100 ms) of single-unit responses to the eluting compounds from the A. palmeri headspace extract (1-μL injection) (bottom trace). Each unit was recorded from one of the four shanks on the electrode array (11 units monitored in the ensemble). Peaks a–d in the chromatogram (bottom record) correspond to ethyl tiglate, propyl valerate, myrcene, and ethyl sorbate, respectively. Certain odorants [e.g., ethyl tiglate (a) and ethyl sorbate (d)] evoked significant responses in units on different shanks. (B) The percentage of responsive units in each ensemble (threshold RI ≥ ± 2.0) was determined for each odorant in the floral headspace and plotted for each preparation (n = 6). Odorant numbers correspond to retention times, except for those odorants that gave robust responses (odorants 28–33), which were rearranged for clarity. A threshold of 12% (dotted line) of the entire dataset was used to identify the odorants that evoked the greatest activity: Bbu, Myr, Etg, Esb, Bea, and Pvl (odorants 28–33, respectively).
Analysis of responses of populations of AL neurons demonstrated strong ensemble selectivity for six A. palmeri volatiles; the remaining odorants evoked little or no activity in most units (Fig. 2B). To examine odorant-evoked responses among preparations (n = 6 moths; 78 units), we calculated the percentage of units in each ensemble that were significantly activated by each odorant [response index (RI) ≥2.0 SD] (Fig. 2B). This analysis confirmed that many units were activated by one or more of these six odorants: butyl butyrate (Bbu), myrcene (Myr), ethyl tiglate (Etg), ethyl sorbate (Esb), benzaldehyde (Bea), and propyl valerate (Pvl) (odorants 28–33 in Fig. 2B, respectively). To verify the identities of these odorants and their effectiveness in activating AL neurons, we tested synthetic standards (Table S1). Unit and ensemble responses to the synthetic and headspace odorants were similar (Fig. 3 A–C) and not statistically different (repeated measures ANOVA with Fisher's posthoc test: P > 0.54).
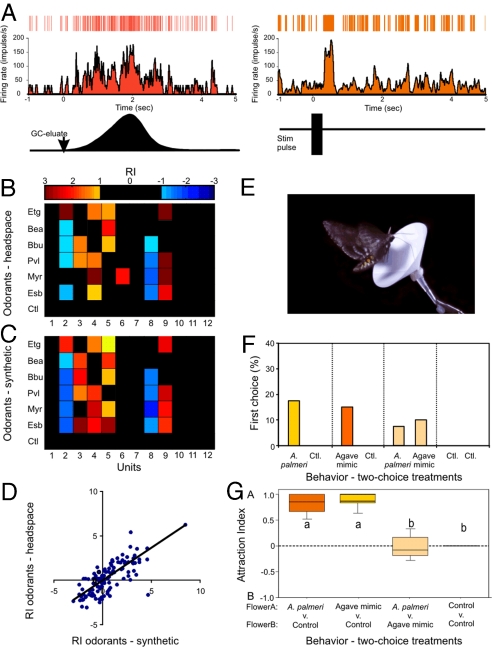
Fig. 3.
Comparison between active GC-fractionated odorants from A. palmeri flowers and equivalent synthetic compounds. (A) Poststimulus time histograms and raster plots of a unit that showed significant responses (based on CUMSUM test; see SI Text) to both floral headspace and synthetic ethyl sorbate. Lower plots (in black) are the GC peak of the compound (Left) and the synthetic stimulus pulse (Right). The arrow on the GC peak indicates stimulus onset. A 200-ms stimulus pulse of synthetic ethyl sorbate, at a concentration equal to that in the GC eluate, led to a brief increase in spiking rate that closely followed the stimulus duration. (B and C) Responses of one 12-unit ensemble to headspace (B) and synthetic (C) floral odorants, plotted as color-coded response matrices across all units (columns 1–12) and odorants (rows). Tested odorants were those evoking the strongest ensemble responses: Etg, Bea, Bbu, Pvl, Myr, and Esb. In addition, odorless mineral oil was tested as a negative control (Ctl). Excitatory (RI ≥1.0 SD) or inhibitory (RI ≤ −1.0 SD) responses are shown; for clarity, weak or null responses are not shown. (D) Relationship between unit responses (all preparations, n = 6) to synthetic vs. headspace odorants. Only units that elicited a significant response (excitatory, RI ≥2.0; inhibitory, RI ≤2.0) to one of the six odorants are shown. Also plotted are the responses of those units to the other odorants. There was a significant correlation between unit responses to synthetic and headspace odorants (solid line; mixed effects regression: P < 0.001). (E and F) Behavioral two-choice wind-tunnel tests examining upwind flight and feeding behaviors of moths to paper flowers (E) emitting the synthetic mixture of odorants determined through GC-MR and the natural floral scent composed of ≈60 odorants. The percentages of moths feeding from paper-flower treatments in two-choice tests are presented in F. Moths significantly chose paper flowers emitting the synthetic mixture and natural floral scent over the unscented control flowers (G test: P < 0.02), but when in the presence of both, moths did not distinguish between them (the A. palmeri mixture mimic and the natural bouquet) (G test: P = 0.99). (G) The attraction index between flower treatments in the two-choice tests. Box plots are the 25th and 75th percentile, error bars are the 5th and 95th percentile, and the horizontal line is the mean. Letters denote a significant difference between odor stimuli (two-tailed t-test: P < 0.05). n = 40 moths for each two-choice wind-tunnel experiment.
To examine the behavioral effectiveness of the six odorants, we conducted two-choice tests in a laboratory wind tunnel to compare behavioral responses to the synthetic mixture and the natural floral scent. Compared with an unscented paper-flower control, moths always selected either a paper flower treated with the synthetic mixture or one emitting the complete scent from a live A. palmeri umbel (Fig. 3 E–G; G test: P < 0.05). When moths were exposed to two paper flowers, one emitting the natural A. palmeri scent and the other emitting the synthetic mixture, moths fed from both flowers at equal frequencies (Fig. 3 F and G; G test: P > 0.50). Thus, the six-component synthetic mixture was an effective mimic of the natural A. palmeri floral scent.
Taken together, the results of our behavioral testing of reduced mixtures of volatiles from the flowers of D. wrightii and A. palmeri provide a foundation for examination of neural mechanisms underlying representation of mixture stimuli of different behavioral effectiveness.
Neural Processing for Odor Discrimination and Generalization.
To investigate the mechanisms by which the olfactory system encodes stimuli that are behaviorally effective, relative to those that are ineffective, we recorded responses of single neurons and populations in the AL with a 16-channel silicon multiprobe. Some individual neurons responded similarly to behaviorally effective and ineffective mixtures (e.g., unit 8 in Fig. S1A), whereas others distinguished between these two types of stimuli (e.g., units 2 and 3 in Fig. S1A) but also responded to single constituents that were behaviorally inactive (25). A majority (≈83%) of responsive neurons responded to behaviorally effective and ineffective mixtures, single odorants, or a combination of all three (Fig. S1B). Only one unit responded specifically to behaviorally effective mixtures, but it did not respond at a 10-fold lower concentration that still evoked behavior (Fig. S1C). Thus, it is unlikely that individual neurons are responsible for discriminating between behaviorally effective and ineffective odor mixtures.
We next examined how the neural population of the AL might encode a behaviorally effective stimulus and whether a time- or rate-based code best corresponds with the behavioral results. Previous work suggested that both of these coding strategies might operate to discriminate among odors in the moth AL (8, 30–32) and thus served as a starting point for this effort. Moreover, given the importance of three floral odorants from D. wrightii (Bea, Bol, and Lin) for behavior of M. sexta, we asked whether these components evoked neural responses that were more similar to those evoked by the behaviorally effective mixtures.
We examined the mean firing rate and pattern of synchronous firing, here defined as spikes occurring within 5 ms, of AL neurons (n = 14 preparations) in response to the behaviorally effective (mixtures 1–4) and ineffective (mixtures 5–9) stimuli and the single odorants Bea, Bol, and Lin for 400 ms after stimulus onset. The mean firing rate of individual neurons and the pattern of ensemble synchrony were calculated, respectively, on the basis of the number of spikes produced and the percentage of synchronous spikes occurring in pairs of neurons in that time window (see SI Text for details). In a single ensemble, the response patterns based on firing rate and firing synchrony were significantly correlated between the behaviorally effective mixtures 1 and 4 (Fig. 4A; r ≥ 0.87, P < 0.0001) but less so between mixture 4 and the single odorants or between mixture 4 and behaviorally ineffective mixture 6 (r = 0.55 ± 0.20 SEM).
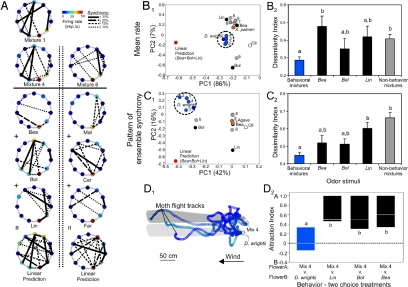
Fig. 4.
Determination of the neural representation of mixture discrimination and generalization and the contribution of single odorants to mixture processing and behavior. (A) Unit mean firing rate and the pattern of ensemble synchrony. The firing-rate response for the 11-unit ensemble is represented as a circular matrix in which individual units are ordered in a clockwise direction starting from the 12:00 position (neuron 1, N-1). Each unit is represented as a circle in the perimeter of the matrix, and its firing rate is represented by its color (see color scale). Also shown are the synchrony patterns (solid, dashed, and dotted lines connecting pairs of units) that underlie the ensemble response to each stimulus, where each connecting line represents the level of synchronous firing (after shuffle correction) of specific pairs. Ensemble responses to two behaviorally effective mixtures, mixture 1 (nine odorants) and mixture 4 (three odorants), a nonbehavioral mixture (mixture 6), the three single odorants of mixture 4 (Bea, Bol, and Lin), and the three single odorants of mixture 6 (Car, Far, and Mal). Also shown is the linear sum of the spatiotemporal responses to the single odorants (linear prediction). (B1 and B2) Multivariate analysis of the mean firing rate of ensembles to behaviorally effective and ineffective mixtures and single odorants. (B1) PCA of the ensemble (shown in A) mean firing rate responses to all of the mixtures and the single odorants Bea, Bol, and Lin. Blue circles correspond to behaviorally effective mixtures, gray to behaviorally ineffective mixtures, orange to A. palmeri, black to single odorants, and red to the linear sum of the single odorants making up mixture 4. Note the clustering of the behaviorally effective mixtures (denoted by dashed circle). (B2) Dissimilarity indices in the ensemble firing rates in response to mixture 4 and the other behaviorally effective mixtures (blue bar), the single odorant constituents of mixture 4 (Bea, Bol, and Lin; black bars), and behaviorally ineffective mixtures (gray bar) (n = 14 preparations). (C1 and C2) Multivariate analysis of the pattern of ensemble synchrony to behaviorally effective and ineffective mixtures and single odorants. (C1) PCA of the ensemble synchrony coefficients shown in A. Note the clustering of the behaviorally effective mixtures (blue circles, dashed circle) relative to the single odorants and behaviorally ineffective mixtures. (C2) Dissimilarity indices in the ensemble synchrony coefficients in response to mixture 4 and the other behaviorally effective and ineffective mixtures (blue and gray bars, respectively) and the single odorants of Mixture 4 (Bea, Bol, and Lin; black bars) (n = 14 preparations). (D1 and D2) Behavioral responses of moths to the behaviorally effective mixtures compared with the constituents. (D1) Three moth flight tracks to two floral odor sources; one emitting the D. wrightii scent and the other emitting mixture 4. Moths were tested individually, and the tracks of individual moths are represented by different shades of blue. The dimensions of the odor plume are represented by the gray shading. Circles correspond to 16-ms intervals. (D2) Behavioral attraction index of mixture 4 relative to the D. wrightii floral scent and the single odorants Bea, Bol, and Lin. Letters denote significant different between odor stimuli (P < 0.05).
To investigate further the relationship between the single odorants and mixtures, we examined the population responses in multivariate space [principal components analysis (PCA)] for both the firing-rate-based (11 dimensions) and synchrony-based (55 dimensions) responses (see SI Text for details). For a single preparation, this analysis revealed that the firing-rate and synchrony codes distinctly separated the behaviorally effective and ineffective mixtures and the behaviorally important odorants Bea, Bol, and Lin (Fig. 4 B1 and C1). Moreover, whereas the single odorants Bea and Lin were clustered near the behaviorally ineffective mixtures (mixtures 6–9), the single odorant Bol, mixture 5 (containing Bea and Bol), and the arithmetic summation of the single odorant-evoked responses (Bea + Bol + Lin) occupied different regions of the olfactory space and were well separated from the behaviorally effective mixtures (Fig. 4 A, B1, and C1). Similarly, arithmetic summation of the behaviorally ineffective constituent-evoked responses [methyl salicylate (Mal) + caryophyllene (Car) + farnesene (Far)] produced a pattern in the ensemble different from that evoked by mixture 6 (Fig. 4A). These results suggest that the tight clustering of behaviorally effective mixtures was caused by the similar population responses they produced in an intact network and that simple arithmetic summation of the single odorant-evoked responses did not mimic the network pattern for mixtures. Examining the normalized Euclidean distances (dissimilarity indices) between the D. wrightii odor stimulus and all other mixture stimuli for all preparations (n = 14 moths; 189 units) revealed a similar trend for the mean firing rate and pattern of ensemble synchrony codes, where the behaviorally effective mixtures were significantly dissimilar to the single odorants and behaviorally ineffective mixtures (Fig. 4B2 and C2; Kruskal-Wallis test: χ4,131 ≥ 16.11, P < 0.01). Finally, we assessed the behavioral potency of the mixtures in comparison with the three key odorants (Bea, Bol, and Lin) by means of two-choice assays in the wind tunnel. The results revealed that moths significantly preferred the mixture over than the single odorants (Fig. 4 D1 and D2; Kruskal-Wallis test: χ1,24 = 10.24, P = 0.01) and could not discriminate mixture 4 from the D. wrightii floral scent (mean attraction index = −0.004, ± 0.18) (see Materials and Methods for calculation of the attraction index). Thus, although these three odorants were effective when mixed, individually the ensemble responses they evoked did not resemble those evoked by the mixture, suggesting that mixtures were perceived qualitatively differently from single odorants. This finding was supported by the behavioral results.
A confounding factor in these results is that each mixture in our odor panel was tested at a different total intensity, because mixture constituents were maintained at the same concentrations and proportions as those in the floral headspace, so that the neural representations might reflect differences in concentration rather than behavioral effectiveness. We therefore examined the effects on discrimination of behaviorally effective and ineffective odor mixtures when the intensities of all stimuli were equalized. Preparations (n = 4) were stimulated with mixtures 2–9 at the same intensity as mixture 1 (7.0 × 10−4 mm Hg). The response patterns derived from the mean firing rate and the dissimilarity indices from these preparations showed that regardless of intensity, behaviorally effective mixtures were represented similarly in the AL (Fig. S2 A1 and A2; Kruskal-Wallis test with multiple comparisons: P > 0.05) and differently from the behaviorally ineffective mixtures (Kruskal-Wallis test with multiple comparisons: P < 0.05). Moreover, the synchrony patterns evoked by behaviorally effective stimuli were similar (Fig. S2B1), and the patterns in response to behaviorally effective and ineffective stimuli were dissimilar to one another (≈15%) (Fig. S2B2; Kruskal-Wallis test: P = 0.08). Thus, the representation of behaviorally effective mixtures in the moth's AL was independent of stimulus intensity.
Ensemble Representation at Short (<1 s) Time Scales.
Our previous results suggested that both rate- and synchrony-based neural codes may distinguish behaviorally effective from ineffective odor stimuli, but given the relatively long time window in which these codes were examined (400 ms) and the fact that flying moths must resolve the dynamics of an olfactory stimulus under much greater temporal constraint (≈200 ms) (33–36), it remains uncertain how quickly AL ensembles can discriminate among odor stimuli.
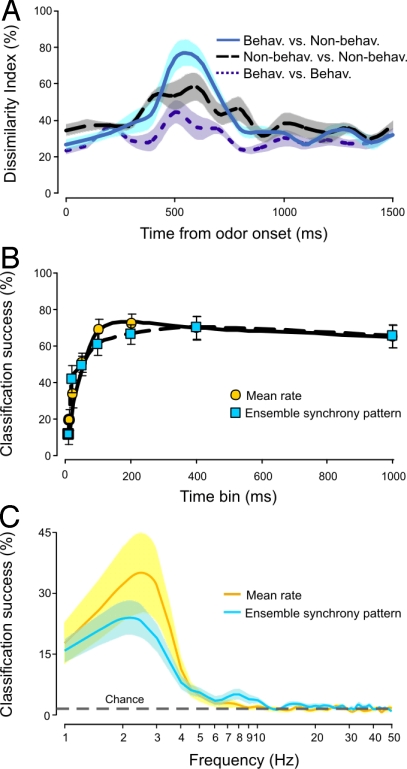
To estimate the minimum time needed by the neural population of the AL to resolve stimulus quality, we first tested when the behaviorally effective and ineffective mixtures were maximally distinguished in the ensemble responses. This was accomplished through a population-vector paradigm to represent ensemble activity as in previous studies of insect and mammalian olfactory systems (37–41). The ensemble responses were organized into two vectors, one for the mean firing rate of evoked responses of the ensemble and the other for the pattern of ensemble synchrony. The population vectors for each odor stimulus were determined in consecutive 100-ms time intervals, thereby creating a vector time series that allowed examination of the evolution of ensemble activity over a total period of 1,500 ms (1–4, 8, 22, 23). The ensemble response to each odor stimulus could be compared with another by considering their normalized Euclidean distances (dissimilarity indices) relative to the D. wrightii floral scent (6, 37), thereby allowing a comparison in ensemble activity before, during, and after olfactory stimulation. Results of this analysis for the mean-firing-rate responses of a single ensemble demonstrated that the maximum discrimination of behaviorally effective and ineffective mixtures occurred ≈600 ms after stimulus onset, which in these experiments was approximately when responses to odor stimuli peaked (Fig. 5A). Similar results were found for the pattern of ensemble synchrony (Fig. S3).
Fig. 5.
Classification success of ensemble responses to behaviorally effective and ineffective odors at varying timescales. (A) Dissimilarity indices (calculated from the mean firing-rate responses of the ensemble) between odor stimuli through time from odor onset (200-ms odor pulse at time = 0 ms) for one preparation. Dissimilarity indices between behaviorally effective and ineffective mixtures (light blue solid line), ineffective mixtures to one another (dashed black line), and effective mixtures to one another (dotted dark blue line) are shown. Shaded areas denote the ± SEM. (B) Mean classification success measured during response to odor when time-bin durations (10–1,000 ms) were varied for the pattern of ensemble synchrony (blue squares) and mean rate (orange circles). Symbols are means ± SEM for n = 11 moths. (C) Percentage of success for classifications based on Fourier analysis of the population activity at different time scales during a 1,000-ms interval (sampled at 100 Hz) after a 200-ms odor pulse. Mean rate (orange line) and the pattern of ensemble synchrony (blue line) were determined (shaded area denotes ± SEM; n = 11 moths).
Next, at that 600-ms time point, we examined how different time scales could affect the efficiency of discrimination. Population vectors were constructed in such a way that the ensemble's mean firing rate or the pattern of ensemble synchrony was integrated over increasing time periods, ranging from 10 to 1,000 ms. These population vectors were then used to classify odor stimuli. The results demonstrated that the time period was important for successful classification of the neural representation of behaviorally effective and ineffective odor stimuli (Fig. 5B). The rate of successful classification reached its maximum (≈70%) at 100 ms, and time periods >200 ms did not improve the classification of odor stimuli (Fig. 5B). Below 20 ms, the ability of the neural responses to classify the different stimuli as behaviorally effective or ineffective was low (≈20%) (Fig. 5B). This pattern of successful classification was reflected similarly in the Fourier transformation of the 10-ms time series through stimulus onset, where the most successful classification occurred at ≈2.5 Hz, which corresponded to the 400-ms cycle time (Fig. 5 A and C). Ensemble representation of odor information thus occurred quickly and accurately in the moth's AL.
Behavioral Correlates of Different Neural Codes.
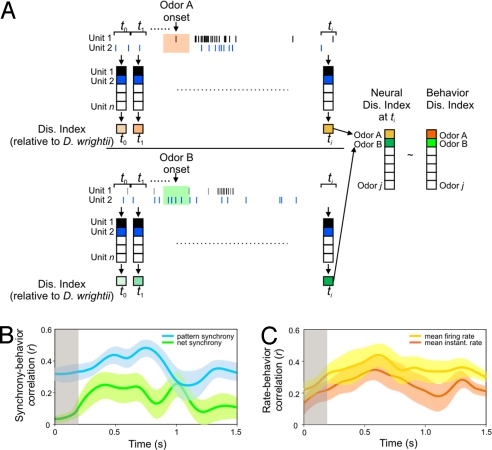
The ability of the moth's olfactory system to respond quickly to odor mixtures (≈200 ms) (33–35) and classify them by using both rate- and time-related neural codes (refs. 25, 32, and 36 and results of this study) presented an opportunity to inquire how transient odor responses are processed by the AL and may correlate with behavior. The mean firing rate and the pattern of ensemble synchrony are only two time- and rate-based codes, therefore we examined additional neural codes in these dimensions (mean instantaneous firing rate and net ensemble synchrony), which also might contain information for odor discrimination (41). Again we used population vectors to represent ensemble activity before, during, and after odor stimulation (37–41). In these studies, however, we specifically examined how the different neural rate and synchrony codes might correlate with behavior. As a function of these analyses, the n-unit or n-unit-pair activity within each ensemble was organized into four distinct n-dimensional vectors, with each of the four vectors representing a different neural code. The first two vectors were rate codes (mean firing rate and mean instantaneous firing rate), and the second pair were synchrony codes (the pattern of ensemble synchrony and total net synchrony). In addition, the behavioral response vector, defined by a set of parameters such as upwind flight, odor-source location, and proboscis extension (Figs. 2 and 3 and Table S2; see SI Text for details), for each odor stimulus could be transformed into dissimilarity indices, thereby allowing direct correlation between various types of neural codes for a panel of odor stimuli and the behavioral responses to the same set of odor stimuli over time (Fig. 6A). A higher correlation would indicate that a given neural code may better account for behavioral effectiveness.
Fig. 6.
Ensemble population responses and correlation of neural codes with behavior. (A) Population-vector responses constructed from any time bin (t1 to ti) where the ith dimension corresponded to the mean firing rate, the mean instantaneous firing rate, the pattern of ensemble synchrony, or the net synchrony composed of unit 1 to unit n (rate codes), or unit-pair 1 to unit-pair n (synchrony codes). The resulting dissimilarity index of each odor stimulus (OdorA to Odorj) relative to D. wrightii ensemble responses was then correlated with the dissimilarity indices of the behavioral response to the mixture stimuli (OdorA to Odorj). (B) Correlation of behavior and synchrony codes through time (0–1,500 ms) for all preparations (n = 14). The blue line corresponds to the mean correlation for the pattern of ensemble synchrony and behavior, and the green line is for the net synchrony. (C) Correlation of behavior with the rate codes through time for all preparations (n = 14). The orange line is the mean correlation for the mean firing rate, and the red line marks the mean instantaneous rate. Shaded areas around the lines are ± SEM. The gray shaded areas denote the time course (200 ms) of the odor stimulation.
Results of this analysis demonstrated that each neural code was correlated with behavior to a different degree, but all exhibited a dynamical increase in correlation upon odor stimulation. For example, for all preparations (n = 14) the correlation between behavior and the two synchrony codes (pattern of ensemble synchrony and net synchrony) increased significantly upon odor stimulation (Fig. 6A, gray shading; 200-ms pulse) before decreasing again (one-way ANOVA for time: F1,42 ≥ 4.43, P < 0.05). The pattern of ensemble synchrony, however, was more highly correlated than net synchrony (43% and 21%, respectively) (Fig. 6B and Figs. S 4 A and B and S5). Likewise, examination of the correlation between rate codes and behavior revealed a dynamical increase in correlation upon odor stimulation (one-way ANOVA for time: F1,42 ≤ 3.54, P < 0.07) and that the mean firing rate was more highly correlated with behavior than instantaneous firing rate (41% and 34%, respectively) (Fig. 6C and Figs. S 4 C and D and S5). We used a stepwise regression model to provide information about the neural coding mechanisms that best predict behavior. Results of the stepwise regression revealed that much of the variance in the behavioral results (R = 46 of 48%) could be explained first by the pattern of ensemble synchrony (Table S3; stepwise regression, step 1: R = 0.39, P < 0.0001) and then by the mean firing rate (Table S3; stepwise regression, step 2: R = 0.46, P < 0.0001). By contrast, net synchrony and the mean instantaneous rate contributed less (∼2%) to the variance in the behavioral results (Table S3). Thus, both the pattern of ensemble synchrony and mean firing rate accurately coded for the behavioral effectiveness of mixture stimuli.
Discussion
Investigations of neural correlates of odor-modulated behaviors in insects have been limited by inadequate knowledge about natural stimuli, which typically are mixtures of odorants. In addition, how the AL processes such mixtures has remained unclear. In the present studies, we used the moth M. sexta and the scents of two natural resources for nectar-feeding, flowers of D. wrightii and A. palmeri, to inquire how the floral bouquets are encoded in the moth's AL. Tandem GC-MR of neural responses to the components of A. palmeri floral scent demonstrated that unit activity in the AL was strongly affected by only six of the >60 volatile compounds in the A. palmeri floral headspace. In addition, the mixture of these six components elicited the same behavior as the floral scent, and moths did not distinguish between the natural scent and the six-component mixture.
A key question is whether a moth requires all of the odorants within the mixture or if certain odorants are more important than others and sufficient for behavior and the neural representation in the AL. We pursued this question through behavioral experiments with a nine-component mixture that mimics the scent of a D. wrightii flower. Moths required only three components of this mixture, namely Bol, Bea, and Lin, to elicit flight behavior that was indistinguishable from that in response to the nine-component mixture or the complete D. wrightii floral scent [which comprises >60 odorants at 15-fold higher concentration, as determined by GC- flame ionization detection (FID)] (Table S4). Furthermore, removal of these three key odorants from the mixture resulted in failure to elicit the behavior, whereas addition of the same three critical odorants to any combination of other floral-scent components restored effectiveness in eliciting the behavior. From the behavioral responses to the two floral scents, one mimicked by a six-component mixture (A. palmeri) and the other by a three-component mixture (D. wrightii), it appeared that the moth's olfactory system had evolved a strategy of processing minimal subsets of odorants to encode complex stimuli. A combinatorial coding scheme has been proposed as a means by which the AL could simultaneously encode the critical odorants within a complex bouquet (42), but our study, and related research on the oriental fruit moth Cydia molesta (43), point to an additional strategy through which moths use only a subset of odorants to represent a complete bouquet of hostplant volatiles. In a similar manner, sensory information about just two of the eight components of the sex pheromone of M. sexta, processed in a pair of glomeruli in the male moth's AL, is necessary and sufficient to elicit and sustain upwind flight toward the source of the mixture (16, 19, 35, 36). Thus there may be a common strategy—in essence, chemosensory parsimony—for processing behaviorally effective odors that simplifies that task.
Selective Neural Representation of Complex Stimuli.
A key requisite for investigating the relationship between multiple neural representations and behavior resides in a hierarchical behavioral output resulting from a panel of stimuli with differentiated behavioral potencies. That is, an efficient neural code should preserve the rank order of stimulus potencies derived from behavioral observation. Use of A. palmeri floral scent and a panel of behaviorally effective and ineffective mixtures of components from that complex odor allowed us to examine which neural coding mechanisms best correlate with behavioral effectiveness. Our findings demonstrated that both the pattern of ensemble firing synchrony and the mean firing rate of neurons in the AL could predict the behavioral effectiveness of the mixtures in our panel, suggesting that available information is embedded in both representations to allow a moth to make a behavioral decision. By contrast, the mean instantaneous firing rate and net synchrony of firing have less predictive value. Moreover, the correlation between neural representation and behavior is transient. That is, before and after the stimulus, AL ensemble synchrony and mean firing rate were not associated with behavior, but when the stimulus was presented, those neural-coding mechanisms quickly became correlated with behavior before decreasing again.
The relative contributions of neural codes based on rate and synchrony for vision have been debated (44, 45), and it remains uncertain how meaningful these codes might be for olfaction. Evidence of transient odor-evoked synchrony of neuronal firing has been obtained in the ALs of moths and OBs of rats, particularly for output neurons from individual glomeruli (22, 32, 46). Interglomerular synchrony of neuronal activity, by contrast, may serve to bind features of a complex stimulus, and synchronized activity of certain units in an ensemble has been correlated with behavior (30, 31). Recent theoretical and experimental work in the visual cortex has suggested that when both neural codes operate in parallel, the stimulus is processed more quickly, minimizing behavioral reaction times (47). The arrival of synchronized spikes through inputs to a downstream neuron provides more concentrated input per unit time and thus increases the probability that the postsynaptic neuron (PN) will fire (48, 49). For brief (≈100 ms) and variable stimuli that are changing in time and space, such as those occurring in a turbulent odor plume (27, 36, 50), the nervous system must accurately encode the stimuli quickly (51). Synchronized activity, in parallel with increased firing rate, may serve this need because the information provided by the synchronous spikes is maximal in the shortest time (47, 49).
Mixture Processing and the Diminished Importance of Single Odorants.
Behavioral and electrophysiological studies have demonstrated repeatedly that mixtures are discriminated from their single constituents (52–54) and that certain components of a mixture are more salient than others (54–56). Indeed, our findings showed that the odorants Bea, Lin, and especially Bol are important, although individually they do not evoke the behavior of interest. Neural-ensemble responses to these odorants, either individually or their mathematical sum, do not resemble the ensemble responses elicited by a mixture of the three compounds. This observation raises the question: what interactions might take place in the AL so that the mixture is uniquely represented by neuronal activity?
Glomeruli in the moth's AL are innervated by diverse local interneurons (LNs) that modulate PN responses (32, 57). In the OB and AL, many LNs are responsible for interglomerular GABAergic inhibition that serves to enhance contrast in odor representation (58), influence PN responses (59), and mediate intraglomerular and interglomerular synchrony of neural activity (9, 30–32, 60). Moreover, recent work has demonstrated that GABAB-mediated inhibition acting directly on primary-afferent inputs can shape responses of PNs differentially, thus serving as a gain-control mechanism (61), although how this presynaptic inhibition might affect mixture representations remains unclear. Nevertheless in the AL, inhibitory networks function to shape glomerular responses to mixtures in a manner not predictable from responses to the mixture constituents or from the afferent input (62, 63).
Floral Odor Coding and Stimulus Generalization.
Spatiotemporal patterns of activity in AL neural ensembles provide a means by which behaviorally effective and ineffective odor mixtures can be discriminated and related stimuli can be generalized. Many flowers that are pollinated by hawkmoths emit similar scents, dominated by aromatic compounds and oxygenated monoterpenes, and that can be encoded similarly and generalized by the moth's olfactory system and activate the same olfactory information channels underlying innate odor biases (14, 28). By contrast, other odor mixtures that evoke a different AL network response may be perceived differently from the floral odors to which the moth is innately biased, thereby allowing those other odors to become associated with a reward and learned. M. sexta is biased toward flowers that share scent features, yet also feeds from flowers that do not exhibit those “hawkmoth-pollinated” traits. Future work may show whether coding generalization occurs in response to scents of other flowers visited by M. sexta and how information transferred by two different olfactory channels, one involving innate biases and the other through olfactory conditioning, may be represented differentially by the olfactory system.
Materials and Methods
Procedures for rearing and preparing moths, behavioral wind tunnel experiments, odor collection and analysis, formulating and delivering olfactory stimuli, and recording and analyzing electrophysiological data are detailed in SI Text.
Behavioral Experiments.
The effectiveness with which complete floral scents and synthetic mixtures elicited oriented flight and foraging behavior of moths to an upwind odor source was tested in a laboratory wind tunnel (Plexiglas, length × width × height = 4.0 × 1.5 × 1.5 m). For each stimulus, a paper flower bearing a 10-μL aliquot of odor solution was positioned near the upwind end of the wind tunnel. Males were tested individually to each odor stimulus. Mixtures were tested in random order, and the wind tunnel was cleaned with 70% ethanol before and after testing. In each day of testing, two to four moths per stimulus were exposed to the positive (D. wrightii floral scent emitted from paper flower) or negative (odorless mineral oil) controls. Only moths that initiated flight were used in the analyses, and 20–40 moths were used for each stimulus group.
Electrophysiological Recording.
Laboratory-reared male M. sexta moths were prepared for electrophysiological recording as described (25). Neural population responses to olfactory stimuli were recorded by using a 16-channel multiunit recording system with silicon-based multielectrode arrays (NeuralNexus Technologies) feeding into twin eight-channel amplifiers (Neuralynx; ref. 8). Captured spikes were sorted by standard methods (8, 25, 30) (Fig. S6), and spikes arising from the same neural unit were time-stamped and used to create raster plots and peri-stimulus time histograms (see Figs. 2 and 3). The odor-evoked population (ensemble) activity of the captured units was analyzed further according to their firing rate and the spike synchrony (within a 5-ms window after shuffle subtraction) between pairs of units in the ensemble (8, 25). For full details on the experimental preparation, odor stimulation, data analysis (including rate and synchrony analyses), and histological identification of the locations of the recording probes, see SI Text.
Supplementary Material
Acknowledgments.
We thank L. Abrell, R. Alarcón, T. A. Christensen, J. P. Martin, and C. E. Reisenman for help with this project; J. Fellous and B. Smith for useful discussions; P. Jansma and S. Mackzum for technical assistance; R. Raguso, G. Galizia, and R. Gutierrez-Osuna for helpful suggestions and critical reading of the manuscript; and C. Hedgcock for the M. sexta photo. This work was supported by National Institutes of Health Grant R01-DC02751 (to J.G.H.), National Science Foundation Grant IOS 01-082270 (to J.A.R.), National Institutes of Health Postdoctoral Training Grant K12 GM000708 (to J.A.R.), and a seed grant from the University of Arizona's Center for Insect Science (to J.G.H. and J.A.R.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0910592106/DCSupplemental.
References
- 1.Christensen T, Waldrop BR, Hildebrand J. Multitasking in the olfactory system: Context-dependent responses to odors reveal dual GABA-regulated coding mechanisms in single olfactory projection neurons. J Neurosci. 1998;18:5999–6008. doi: 10.1523/JNEUROSCI.18-15-05999.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedrich RW. Mechanisms of odor discrimination: Neurophysiological and behavioral approaches. Trends Neurosci. 2006;29:40–47. doi: 10.1016/j.tins.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Galizia CG, Menzel R. The role of glomeruli in the neural representation of odours: Results from optical recording studies. J Insect Physiol. 2001;47:115–130. doi: 10.1016/s0022-1910(00)00106-2. [DOI] [PubMed] [Google Scholar]
- 4.Laurent G, et al. Odor encoding as an active, dynamical process: Experiments, computation, and theory. Annu Rev Neurosci. 2001;24:263–297. doi: 10.1146/annurev.neuro.24.1.263. [DOI] [PubMed] [Google Scholar]
- 5.Christensen TA, Hildebrand JG. Coincident stimulation with pheromone components improves temporal pattern resolution in central olfactory neurons. J Neurophysiol. 1997;77:775–781. doi: 10.1152/jn.1997.77.2.775. [DOI] [PubMed] [Google Scholar]
- 6.Cleland TA, Johnson BA, Leon M, Linster C. Relational representation in the olfactory system. Proc Natl Acad Sci USA. 2007;104:1953–1958. doi: 10.1073/pnas.0608564104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sachse S, Galizia CG. The coding of odor intensity in the honeybee antennal lobe: Local computation optimizes odor representation. Eur J Neurosci. 2003;18:2119–2132. doi: 10.1046/j.1460-9568.2003.02931.x. [DOI] [PubMed] [Google Scholar]
- 8.Lei H, Christensen TA, Hildebrand JG. Spatial and temporal organization of ensemble representations for different odor classes in the moth antennal lobe. J Neurosci. 2004;24:11108–11119. doi: 10.1523/JNEUROSCI.3677-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schoppa NE, Westbrook GL. Glomerulus-specific synchronization of mitral cells in the olfactory bulb. Neuron. 2001;31:639–651. doi: 10.1016/s0896-6273(01)00389-0. [DOI] [PubMed] [Google Scholar]
- 10.Stopfer M, Bhagavan S, Smith BH, Laurent G. Impaired odor discrimination on desynchronization of odour-encoding neural assemblies. Nature. 1997;390:70–74. doi: 10.1038/36335. [DOI] [PubMed] [Google Scholar]
- 11.Luo M, Fee MS, Katz LC. Encoding pheromonal signals in the accessory olfactory bulb of behaving mice. Science. 2003;299:1196–1201. doi: 10.1126/science.1082133. [DOI] [PubMed] [Google Scholar]
- 12.Rasmussen LE, Lazar J, Greenwood DR. Olfactory adventures of elephantine pheromones. Biochem Soc Trans. 2003;31:137–141. doi: 10.1042/bst0310137. [DOI] [PubMed] [Google Scholar]
- 13.Wang Z, et al. Pheromone detection in male mice depends on signaling through the type 3 adenylyl cyclase in the main olfactory epithelium. J Neurosci. 2006;26:7375–7379. doi: 10.1523/JNEUROSCI.1967-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riffell JA, et al. Behavioral consequences of innate preferences and olfactory learning in hawkmoth–flower interactions. Proc Natl Acad Sci USA. 2008;105:3404–3409. doi: 10.1073/pnas.0709811105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stowe MK, Turlings TC, Loughrin JH, Lewis WJ, Tumlinson JH. The chemistry of eavesdropping, alarm, and deceit. Proc Natl Acad Sci USA. 1995;92:23–28. doi: 10.1073/pnas.92.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tumlinson JH, et al. Identification of a pheromone blend attractive to Manduca sexta (L.) males in a wind tunnel. Arch Insect Biochem Physiol. 1989;10:255–271. [Google Scholar]
- 17.Wright GA, Lutmerding A, Dudareva N, Smith BH. Intensity and the ratios of compounds in the scent of snapdragon flowers affect scent discrimination by honeybees (Apis mellifera) J Comp Physiol A. 2005;191:105–114. doi: 10.1007/s00359-004-0576-6. [DOI] [PubMed] [Google Scholar]
- 18.Carlsson MA, Chong KY, Daniels W, Hansson BS, Pearce TC. Component information is preserved in glomerular responses to binary odor mixtures in the moth Spodoptera littoralis. Chem Senses. 2007;32:433–443. doi: 10.1093/chemse/bjm009. [DOI] [PubMed] [Google Scholar]
- 19.Lei H, Vickers N. Central processing of natural odor mixtures in insects. J Chem Ecol. 2008;34:915–927. doi: 10.1007/s10886-008-9487-2. [DOI] [PubMed] [Google Scholar]
- 20.Guerrieri F, Schubert M, Sandoz JC, Giurfa M. Perceptual and neural olfactory similarity in honeybees. PLoS Biol. 2005;3:e60. doi: 10.1371/journal.pbio.0030060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guerrieri F, Lachnit H, Gerber B, Giurfa M. Olfactory blocking and odorant similarity in the honeybee. Learn Mem. 2005;12:86–95. doi: 10.1101/lm.79305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kashiwadani H, Sasaki YF, Uchida N, Mori K. Synchronized oscillatory discharges of mitral/tufted cells with different molecular receptive ranges in the rabbit olfactory bulb. J Neurophysiol. 1999;82:1786–1792. doi: 10.1152/jn.1999.82.4.1786. [DOI] [PubMed] [Google Scholar]
- 23.Friedrich RW, Habermann CJ, Laurent G. Multiplexing using synchrony in the zebrafish olfactory bulb. Nat Neurosci. 2004;7:862–871. doi: 10.1038/nn1292. [DOI] [PubMed] [Google Scholar]
- 24.Smith BH, Wright GA, Daly KC. In: Biology of Floral Scent. Dudareva N, Pichersky E, editors. Boca Raton, FL: CRC; pp. 263–296. [Google Scholar]
- 25.Riffell JA, Lei H, Christensen TA, Hildebrand JG. Characterization and coding of behaviorally significant odor mixtures. Curr Biol. 2009;19:335–340. doi: 10.1016/j.cub.2009.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alarcon R, Davidowitz G, Bronstein JL. Nectar usage in a southern Arizona hawkmoth community. Ecol Entomol. 2008;33:1–7. [Google Scholar]
- 27.Riffell JA, Abrell L, Hildebrand JG. Physical processes and real-time chemical measurement of the insect olfactory environment. J Chem Ecol. 2008;34:837–853. doi: 10.1007/s10886-008-9490-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raguso RA, Henzel C, Buchman SL, Nabhan GP. Trumpet flowers of the Sonoran Desert: Floral biology of Peniocereus cacti and sacred Datura. Int J Plant Sci. 2003;164:877–892. [Google Scholar]
- 29.Raguso RA. Why are some floral nectars scented? Ecology. 2004;85:1486–1494. [Google Scholar]
- 30.Christensen TA, Pawlowski VM, Lei H, Hildebrand JG. Multi-unit recordings reveal context-dependent modulation of synchrony in odor-specific neural ensembles. Nat Neurosci. 2000;3:927–931. doi: 10.1038/78840. [DOI] [PubMed] [Google Scholar]
- 31.Christensen TA, Lei H, Hildebrand JG. Coordination of central odor representations through transient, nonoscillatory synchronization of glomerular output neurons. Proc Natl Acad Sci USA. 2003;100:11076–11081. doi: 10.1073/pnas.1934001100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lei H, Christensen TA, Hildebrand JG. Local inhibition modulates odor-evoked synchronization of glomerulus-specific output neurons. Nat Neurosci. 2002;5:557–565. doi: 10.1038/nn0602-859. [DOI] [PubMed] [Google Scholar]
- 33.Mafra-Neto A, Cardé RT. Fine-scale structure of pheromone plumes modulates upwind orientation of flying moths. Nature. 1994;369:142–144. [Google Scholar]
- 34.Vickers NJ, Baker TC. Reiterative responses to single strands of odor promote sustained upwind flight and odor source location by moths. Proc Natl Acad Sci USA. 1994;91:5756–5760. doi: 10.1073/pnas.91.13.5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vickers NJ, Christensen TA, Baker TC, Hildebrand JG. Odor-plume dynamics influence the brain's olfactory code. Nature. 2001;410:466–470. doi: 10.1038/35068559. [DOI] [PubMed] [Google Scholar]
- 36.Lei H, Riffell J, Gage S, Hildebrand J. Contrast enhancement of stimulus intermittency in a primary olfactory network and its behavioral significance. J Biol. 2009;8:1–16. doi: 10.1186/jbiol120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bathellier B, Buhl DL, Accolla R, Carleton A. Dynamic ensemble odor coding in the mammalian olfactory bulb: Sensory information at different time scales. Neuron. 2008;57:586–598. doi: 10.1016/j.neuron.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 38.Brown SL, Joseph J, Stopfer M. Encoding a temporally structured stimulus with a temporally structured neural representation. Nat Neurosci. 2005;8:1568–1576. doi: 10.1038/nn1559. [DOI] [PubMed] [Google Scholar]
- 39.Mazor O, Laurent G. Transient dynamics versus fixed points in odor representations by locust antennal lobe projection neurons. Neuron. 2005;48:661–673. doi: 10.1016/j.neuron.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 40.Stopfer M, Jayaraman V, Laurent G. Intensity versus identity coding in an olfactory system. Neuron. 2003;39:991–1004. doi: 10.1016/j.neuron.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 41.Laurent G. A systems perspective on early olfactory coding. Science. 1999;286:723–728. doi: 10.1126/science.286.5440.723. [DOI] [PubMed] [Google Scholar]
- 42.Vickers NJ, Christensen TA, Hildebrand JG. Combinatorial odor discrimination in the brain: Attractive and antagonistic odor blends are represented in distinct combinations of uniquely identifiable glomeruli. J Comp Neurol. 1998;400:35–56. [PubMed] [Google Scholar]
- 43.Pinero JC, Giovanni Galizia C, Dorn S. Synergistic behavioral responses of female oriental fruit moths (Lepidoptera: Tortricidae) to synthetic host plant-derived mixtures are mirrored by odor-evoked calcium activity in their antennal lobes. J Insect Physiol. 2008;54:333–343. doi: 10.1016/j.jinsphys.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 44.Shadlen MN, Movshon JA. Synchrony unbound: A critical evaluation of the temporal binding hypothesis. Neuron. 1999;24:67–77. doi: 10.1016/s0896-6273(00)80822-3. [DOI] [PubMed] [Google Scholar]
- 45.Singer W. Neuronal synchrony: A versatile code for the definition of relations. Neuron. 1999;24:49–65. doi: 10.1016/s0896-6273(00)80821-1. [DOI] [PubMed] [Google Scholar]
- 46.Chen TW, Lin BJ, Schild D. Odor coding by modules of coherent mitral/tufted cells in the vertebrate olfactory bulb. Proc Natl Acad Sci USA. 2009;106:2401–2406. doi: 10.1073/pnas.0810151106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buehlmann A, Deco G. The neuronal basis of attention: Rate versus synchronization modulation. J Neurosci. 2008;28:7679–7686. doi: 10.1523/JNEUROSCI.5640-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abeles M. Role of the cortical neuron: Integrator or coincidence detector? Isr J Med Sci. 1982;18:83–92. [PubMed] [Google Scholar]
- 49.Salinas E, Sejnowski TJ. Correlated neuronal activity and the flow of neural information. Nat Rev Neurosci. 2001;2:539–550. doi: 10.1038/35086012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Murlis J, Jones CD. Fine-scale structure of odor plumes in relation to insect orientation to distant pheromone and other attractant sources. Physiol Entomol. 1981;6:71–86. [Google Scholar]
- 51.Vickers NJ. Winging it: Moth flight behavior and responses of olfactory neurons are shaped by pheromone plume dynamics. Chem Senses. 2006;31:155–166. doi: 10.1093/chemse/bjj011. [DOI] [PubMed] [Google Scholar]
- 52.Dekker T, Steib B, Cardé RT, Geier M. l- lactic acid: A human-signifying host cue for the anthropophilic mosquito Anopheles gambiae. Med Vet Entomol. 2002;16:91–98. doi: 10.1046/j.0269-283x.2002.00345.x. [DOI] [PubMed] [Google Scholar]
- 53.Hopkins TL, Young H. Attraction of the grasshopper, Melanoplus sanguinipes, to host plant odors and volatile components. Entomol Exp Appl. 1990;56:249–258. [Google Scholar]
- 54.Laska M, Hudson R. A comparison of the detection thresholds of odor mixtures and their components. Chem Senses. 1991;16:651–662. [Google Scholar]
- 55.Deisig N, Giurfa M, Lachnit H, Sandoz JC. Neural representation of olfactory mixtures in the honeybee antennal lobe. Eur J Neurosci. 2006;24:1161–1174. doi: 10.1111/j.1460-9568.2006.04959.x. [DOI] [PubMed] [Google Scholar]
- 56.Wright GA, Smith BH. Variation in complex olfactory stimuli and its influence on odor recognition. Proc R Soc London Ser B. 2004;271:147–152. doi: 10.1098/rspb.2003.2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reisenman CE, Heinbockel T, Hildebrand JG. Inhibitory interactions among olfactory glomeruli do not necessarily reflect spatial proximity. J Neurophysiol. 2008;100:554–564. doi: 10.1152/jn.90231.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sachse S, Galizia CG. Role of inhibition for temporal and spatial odor representation in olfactory output neurons: A calcium imaging study. J Neurophysiol. 2002;87:1106–1117. doi: 10.1152/jn.00325.2001. [DOI] [PubMed] [Google Scholar]
- 59.Wilson RI, Laurent G. Role of GABAergic inhibition in shaping odor-evoked spatiotemporal patterns in the Drosophila antennal lobe. J Neurosci. 2005;25:9069–9079. doi: 10.1523/JNEUROSCI.2070-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schoppa NE. Synchronization of olfactory bulb mitral cells by precisely timed inhibitory inputs. Neuron. 2006;49:271–283. doi: 10.1016/j.neuron.2005.11.038. [DOI] [PubMed] [Google Scholar]
- 61.Root CM, et al. A presynaptic gain control mechanism fine-tunes olfactory behavior. Neuron. 2008;59:311–321. doi: 10.1016/j.neuron.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Silbering AF, Galizia CG. Processing of odor mixtures in the Drosophila antennal lobe reveals both global inhibition and glomerulus-specific interactions. J Neurosci. 2007;27:11966–11977. doi: 10.1523/JNEUROSCI.3099-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schlief ML, Wilson RI. Olfactory processing and behavior downstream from highly selective receptor neurons. Nat Neurosci. 2007;10:623–630. doi: 10.1038/nn1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.