Abstract
A handoff model has been proposed to explain the egress from lysosomes of cholesterol derived from receptor-mediated endocytosis of LDL. Cholesterol is first bound by soluble Niemann-Pick C2 (NPC2) protein, which hands off the cholesterol to the N-terminal domain of membrane-bound NPC1. Cells lacking NPC1 or NPC2 accumulate LDL-derived cholesterol in lysosomes and fail to deliver LDL cholesterol to the endoplasmic reticulum (ER) for esterification by acyl-CoA acyltransferase (ACAT) and for inhibition of sterol regulatory element-binding protein cleavage. Here, we support this model by showing that the cholesterol transport defect in NPC1 mutant cells is restricted to lysosomal export. Other cholesterol transport pathways appear normal, including the movement of cholesterol from the plasma membrane to the ER after treatment of cells with 25-hydroxycholesterol or sphingomyelinase. The NPC1 or NPC2 block in cholesterol delivery to the ER can be overcome by 2-hydroxypropyl-β-cyclodextrin, which leads to a marked increase in ACAT-mediated cholesterol esterification. The buildup of cholesteryl esters in the cytosol is expected to be much less toxic than the buildup of free cholesterol in the lysosomes of patients with mutations in NPC1 or NPC2.
Keywords: cholesterol esterification, NPC1 and NPC2 proteins, sphingomyelinase
Two cholesterol-binding proteins, NPC1 and NPC2, act in concert to export lipoprotein-derived cholesterol from lysosomes (1, 2). Cholesterol enters lysosomes when cholesteryl ester (CE)-rich LDL enters cells by receptor-mediated endocytosis. Within lysosomes, the CEs of LDL are hydrolyzed by acid lipase, and the liberated cholesterol exits the lysosome en route to the endoplasmic reticulum (ER) and the plasma membrane (3). The dual requirement for NPC1 and NPC2 was discovered through studies of cells from patients with Niemann-Pick type C disease (2). These patients harbor two loss-of-function alleles at one or the other of these loci. As a result, cholesterol is trapped in lysosomes, and this excess lysosomal storage causes death through liver, lung, and brain failure (1). In a mouse model of this disease, toxicity was ameliorated and lifespan was extended by treatment with 2-hydroxypropyl-β-cyclodextrin (HPCD), which binds to cholesterol and facilitates its exit from lysosomes even in the absence of NPC1 (4). Within 24 h, HPCD treatment led to a decline in free cholesterol and a marked increase in CE in the liver and brain of these mutant mice. The mechanism of this change is unknown.
Recent studies have begun to clarify the separate roles of NPC1 and NPC2 in the lysosomal export process. NPC2, a soluble 132-aa protein, binds to cholesterol by attaching to the ring structure and the hydrophobic isooctyl side chain (5–7). In contrast, NPC1 is an intrinsic membrane protein containing 1,278 aa and 13 putative membrane-spanning helices. Its N-terminal domain, referred to as NPC1(NTD), projects into the lysosomal lumen (8, 9). Through recombinant DNA technology, NPC1(NTD) can be prepared as a water-soluble fragment of 240 aa in length. Infante, et al. (6) demonstrated that NPC1(NTD) binds to cholesterol in an orientation opposite to that of NPC2 (10). In NPC1, the 3β-hydroxyl group of cholesterol is buried, and the isooctyl side chain is exposed. Because of this difference in binding orientation, NPC1(NTD), but not NPC2, can bind to oxysterols such as 25-hydroxycholesterol (25-HC) that have a polar hydroxyl attached to the side chain (6).
The above in vitro findings led to the following working model for the exit of LDL-derived cholesterol from the lysosomes (10). After liberation from LDL, cholesterol is bound immediately by NPC2. This binding shields cholesterol from water and prevents its precipitation. NPC2 carries the cholesterol to the lysosomal membrane, where it transfers cholesterol to NPC1(NTD) without the sterol ever passing through the aqueous phase.
The working model predicts that the actions of NPC1 and NPC2 are restricted to late endosomes and lysosomes, the only locations in which both required proteins coexist at high levels. In earlier studies, Lange, et al. (11) suggested that the movement of cholesterol from endosomes and lysosomes to the plasma membrane is not defective in cells lacking functional NPC1. Moreover, Cruz, et al. (12) reported that the initial movement of LDL-derived cholesterol from the lysosomes to the plasma membrane does not require NPC1, but the subsequent internalization of plasma membrane cholesterol and its recycling back to the plasma membrane do. Neither of these conclusions could be reconciled easily with the above working model for NPC2/NPC1 interaction in lysosomes.
To address some of these questions, we here report a series of studies in which we measured the effects of lipoprotein-derived cholesterol and cellular cholesterol on ER regulatory processes in cells with mutations in NPC1 and NPC2. By using acyl-CoA acyltransferase (ACAT) activity as a measure of ER cholesterol delivery, we show that the movement of cholesterol from the lysosomes to the ER is blocked in NPC1- and NPC2-deficient cells and that this block can be alleviated by the treatment of the cells with HPCD.
Results
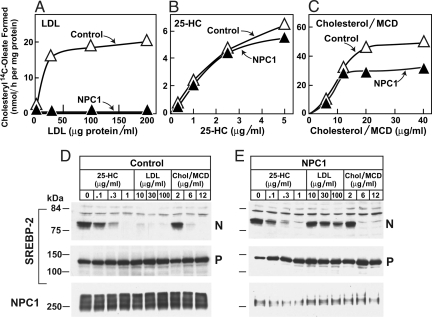
After uptake by receptor-mediated endocytosis, LDL-derived cholesterol is liberated in lysosomes, and a portion is transported to the ER, where it binds to Scap and blocks the proteolytic cleavage of sterol regulatory element-binding proteins (SREBPs) (13). Within the ER, some of this cholesterol is esterified to form CE. The esterification reaction can be monitored by incubating cells with [14C]oleate and determining the incorporation of radioactivity into CE (14). Fig. 1 A–C shows an experiment in which we compared esterification in control human fibroblasts and cells from a subject who is a compound heterozygote for mutations at the NPC1 locus (P237S substitution in one allele and I1061T in the other). The cells were depleted of cholesterol by prior incubation in medium containing lipoprotein-deficient serum. Thereafter, the cells were incubated for 5 h with a source of sterol, after which they were pulse-labeled for 2 h with [14C]oleate and incorporation into cholesteryl [14C]oleate was measured. Whereas cholesterol ester synthesis increased markedly in control cells treated with LDL, no such increase was seen in the NPC1 mutant cells (Fig. 1A). This result is similar to that reported by others (15, 16).
Fig. 1.
Role of NPC1 in sterol-mediated cholesteryl ester formation (A–C) and inhibition of SREBP-2 processing (D and E) in control and NPC1 mutant human fibroblasts. On day 0, nontransformed fibroblasts were set up in medium A with 10% FCS at 2 × 104 cells per 60-mm dish. On day 3, cells were refed with the same medium, and on day 5, cells were switched to medium A with 10% human lipoprotein-deficient serum (LPDS). (A–C) Cholesterol esterification. On day 7, each dish received medium A containing 10% LPDS, 50 μM compactin, 50 μM sodium mevalonate, and the indicated concentration of LDL (A), 25-hydroxycholesterol (25-HC) (B), or cholesterol/methyl-β-cyclodextrin (MCD) complex (C). After 5 h at 37 °C, each monolayer was pulse-labeled for 2 h with 0.2 mM sodium [14C]oleate-albumin (7,966 dpm/nmol). The cells were harvested for measurement of the content of cholesteryl [14C]oleate. Each value is the average of duplicate incubations. (D and E) Immunoblot analysis of SREBP-2 processing. On day 7, after incubation for 5 h with the indicated concentration of LDL, 25-HC, or cholesterol/MCD, N-acetyl-leucinal-leucinal-norleucinal was added at a final concentration of 25 μg/mL. After 1 h at 37 °C, six dishes were harvested and pooled for preparation of nuclear extract and 100,000 × g membrane fractions, which were analyzed by immunoblotting as described in SI Materials and Methods. P, precursor form of SREBP-2; N, cleaved nuclear form of SREBP-2.
In contrast to the difference with LDL, both cell lines responded equally when cholesterol was added in complex with methyl-β-cyclodextrin (MCD), which likely delivers cholesterol to cells without passing through lysosomes (Fig. 1C). As shown by Pentchev et al. (15) and Liscum and Faust (16), both cell lines also responded when incubated with 25-HC, which stimulates cholesterol esterification by causing cholesterol to translocate from the plasma membrane to ER (17, 18). A similar set of responses was seen when we measured the inhibition of SREBP-2 cleavage (Fig. 1 D and E). In control cells, but not NPC1 mutant cells, LDL blocked SREBP-2 cleavage, resulting in a decline in the nuclear form of SREBP-2. In contrast, cholesterol/MCD and 25-HC blocked SREBP-2 cleavage equally in the two cell lines.
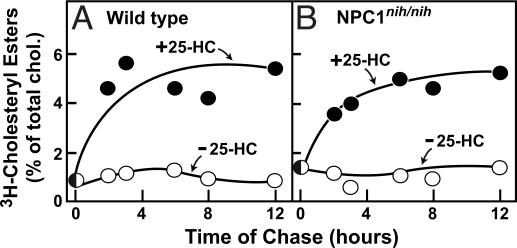
We next tested whether 25-HC can stimulate the esterification of total cell cholesterol in peritoneal macrophages from mice with a homozygous defect in NPC1 (npcnih/nih mice) (Fig. 2 A and B). To measure the esterification of total cholesterol, we preincubated wild-type and NPC1 mutant cells with [3H]cholesterol for 24 h to radiolabel all cholesterol pools. The [3H]cholesterol equilibrates with cellular cholesterol, the vast majority of which is located in the plasma membrane (19, 20). We then washed the cells and incubated them with 25-HC for various times, after which we measured the proportion of cellular [3H]cholesterol that had been converted to [3H]CE. As shown in Fig. 2 A and B, neither cell line esterified its cholesterol in the absence of 25-HC. In the presence of 25-HC, ≈5% of total cell cholesterol was esterified within 8 h in both wild-type and NPC1 mutant macrophages. Similar results were obtained in NPC1-deficient hamster cells by Wojtanik and Liscum (21).
Fig. 2.
25-Hydroxycholesterol (25-HC) stimulated esterification of total cellular cholesterol in macrophages from wild-type and NPC1 mutant mice. On day 0, peritoneal macrophages from wild-type (A) or npcnih/nih mice (B) were set up in medium B with 10% FCS as described in SI Materials and Methods. On day 1, the cells were incubated with medium B containing 10% FCS and 8.3 nM [3H]cholesterol (132,000 dpm/pmol; added in ethanol at a final concentration of 0.05%). After 24 h at 37 °C, each dish was washed with 5 mL of PBS with 0.2% BSA, after which the cells received fresh medium B with 0.2% BSA. After 12 h at 37 °C, each monolayer received medium B with 0.2% BSA in the absence or presence of 5 μg/mL 25-HC. After incubation for the indicated time, the cells were harvested for measurement of the content of [3H]cholesteryl ester (CE) and [3H]cholesterol, as described in SI Materials and Methods. Each value is the average of duplicate incubations and represents the percentage of [3H]cholesterol incorporated into [3H]CE relative to [3H]cholesterol content at zero time. Zero time values for [3H]cholesterol were 10.0 and 14.5 pmol/mg of protein for wild-type and npcnih/nih macrophages, respectively.
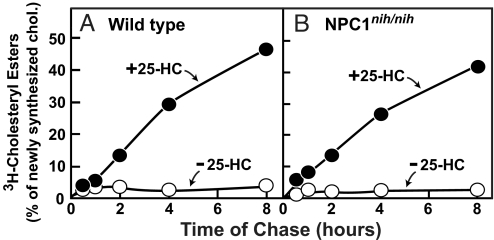
To determine whether 25-HC could stimulate the esterification of newly synthesized cholesterol in NPC1 mutant cells, we preincubated cultured fibroblasts from wild-type and npcnih/nih mice with [3H]acetate at 14 °C (Fig. 3 A and B). At this temperature the [3H]acetate is incorporated into [3H]cholesterol in the ER, but exit from the ER is slowed, leading to a buildup of newly synthesized [3H]cholesterol in the ER (22). We then warmed the cells to 37 °C and measured the esterification of this newly synthesized [3H]cholesterol. In the absence of 25-HC, none of the [3H]cholesterol was esterified in either cell line. In the presence of 25-HC, both cells lines converted >40% of the newly synthesized [3H]cholesterol to [3H]CE within 8 h (Fig. 3 A and B).
Fig. 3.
25-Hydroxycholesterol (25-HC) stimulated esterification of newly synthesized cholesterol in fibroblasts from wild-type and NPC1 mutant mice. On day 0, fibroblasts from wild-type (A) and npcnih/nih (B) mice were set up in medium B with 10% FCS and 4 × 104 cells per 60-mm dish. On day 3, cells were switched to medium B with 5% newborn calf lipoprotein-deficient serum (LPDS). On day 4, cells were incubated in medium C with 5% LPDS and pulsed-labeled with 167 μM [3H]acetate (333 dpm/pmol) for 3 h at 14 °C to label newly synthesized cholesterol. After the pulse, each monolayer was washed twice with 3 mL of PBS with 0.2% BSA and then chased at 37 °C with medium B containing 5% LPDS, 50 μM compactin (to inhibit cholesterol synthesis), 50 μM sodium mevalonate, and 0.1 mM sodium oleate-albumin in the absence or presence of 3 μg/mL 25-HC. At the indicated time of chase, the cells were harvested for measurement of [3H]cholesteryl ester (CE) and [3H]cholesterol. Each value is the average of duplicate incubations and represents the percentage of [3H]cholesterol incorporated into [3H]CE relative to [3H]cholesterol content at zero time. Zero time values for cellular [3H]cholesterol were 160 and 192 pmol/mg of protein in wild-type and npcnih/nih fibroblasts, respectively.
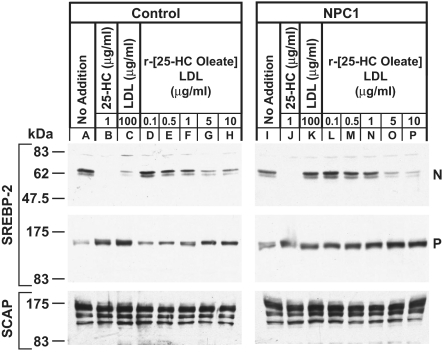
In previous studies, we demonstrated that NPC1(NTD) binds both cholesterol and 25-HC (6). Studies of NPC1 mutants confirmed that cholesterol binding to NPC1(NTD) is necessary for NPC1 to transport cholesterol from the lysosomes to the ER (10). In Fig. 4, we show an experiment designed to determine whether NPC1 is necessary to transport 25-HC from the lysosomes to the ER. We studied fibroblasts from a control subject and a subject with NPC1(P237S/I1061T) that had been immortalized by transfection with a cDNA encoding the catalytic subunit of telomerase, hTERT (23). We extracted all of the cholesterol of LDL and reconstituted the hydrophobic core of the particle with 25-HC oleate. This reconstituted LDL enters cells through the LDL receptor, whereupon the 25-HC oleate is cleaved in lysosomes and the liberated 25-HC suppresses cholesterol synthesis (24). Fig. 4 shows that native LDL failed to block SREBP-2 cleavage in the mutant NPC1 mutant fibroblasts (lane K). In contrast, LDL reconstituted with 25-HC oleate functioned in the mutant cells as well as it did in the control cells (compare lanes D–H and L–P). Thus, 25-HC can exit lysosomes without NPC1. This result is consistent with the findings of Dahl et al. (25), who showed that LDL reconstituted with 25-HC oleate could suppress cholesterol synthesis in NPC1-deficient hamster cells.
Fig. 4.
Inhibition of SREBP-2 processing by LDL-derived 25-hydroxycholesterol (25-HC), but not LDL-derived cholesterol, in NPC1 mutant human fibroblasts. On day 0, hTERT-control and hTERT-NPC1 human fibroblasts were set up in medium A with 10% FCS and 7 × 104 cells per 60-mm dish. On day 2, the medium was switched to medium A with 5% human lipoprotein-deficient serum (LPDS). On day 4, the cells were incubated for 5 h in medium A containing 5% LPDS, 50 μM compactin, 50 μM sodium mevalonate, and one of the following additions: 1 μg/mL 25-HC (lanes B, J), 100 μg of protein per milliliter of LDL (lanes C, K), or the indicated concentration of LDL reconstituted with 25-HC oleate (r-[25-HC oleate]LDL) (lanes D–H, L–P). Cells then received N-acetyl-leucinal-leucinal-norleucinal at a final concentration of 25 μg/mL. After a further 1 h at 37 °C, triplicate dishes were harvested and pooled for preparation of nuclear extract and 100,000 × g membrane fractions, which were analyzed by immunoblotting for SREBP-2 and Scap as described in SI Materials and Methods. P, precursor form of SREBP-2; N, cleaved nuclear form of SREBP-2.
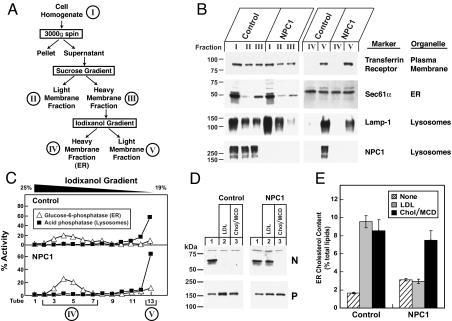
Studies of cholesterol esterification and inhibition of SREBP-2 processing are indirect measures of the concentration of cholesterol in the ER. To measure the concentration of cholesterol in the ER directly, we took advantage of a newly described technique for isolating pure ER membranes from cultured cells (20). In this procedure, cell homogenates are subjected to a two-step gradient ultracentrifugation (Fig. 5A). The heavy membrane fraction from the second gradient (iodixanol fraction IV) contains pure ER membranes as shown by its content of the ER marker Sec61α and its lack of markers for the plasma membrane (transferrin receptor) and lysosomes (Lamp-1 and NPC1) (Fig. 5B). Fig. 5C shows individual fractions from the iodixanol gradient, illustrating the wide separation between the ER marker glucose-6-phosphatase and the lysosomal marker acid phosphatase. To perform the experiment, hTERT-transformed control human fibroblasts and NPC1 mutant cells were incubated with LDL or with cholesterol complexed to MCD. Fig. 5D shows that both forms of cholesterol blocked the production of nuclear SREBP-2 in control cells, but only the cholesterol/MCD complex had this effect in the NPC1 mutant cells. When control cells were incubated with either LDL or cholesterol/MCD, the cholesterol content of ER membranes rose above the 5% threshold that we showed (20) is necessary to block SREBP-2 processing (Fig. 5E). In the NPC1 mutant cells, LDL failed to increase the ER cholesterol content, whereas cholesterol/MCD caused the normal increase (Fig. 5E).
Fig. 5.
Cholesterol content of purified endoplasmic reticulum (ER) membranes from control and NPC1 mutant human fibroblasts. (A) Endoplasmic reticulum membrane fractionation scheme. (B and C) Immunoblot and enzymatic analysis of membrane fractions. On day 0, 13 dishes of hTERT-control and hTERT-NPC1 fibroblasts were set up in medium A with 10% FCS and 4 × 105 cells per 100-mm dish. On day 3, the cells were harvested, and the ER membranes were prepared as described in SI Materials and Methods and shown in A. Equal volumes of each membrane fraction were subjected to immunoblot analysis for the indicated organelle markers (B) or assayed for the indicated enzyme activity (C). (D and E) Analysis of SREBP-2 cleavage (D) and cholesterol content of purified ER membranes (E). hTERT-control and hTERT-NPC1 fibroblasts (13 dishes for each condition) were set up as described above. On day 2, cells were switched to medium A with 5% human lipoprotein-deficient serum (LPDS). On day 3, cells were switched to medium A with 5% LPDS, 5 μM compactin, and 50 μM sodium mevalonate. On day 4, the cells received the same medium with 50 μM compactin and supplemented with either 100 μg of protein per milliliter of LDL or 20 μg/mL cholesterol/methyl-β-cyclodextrin (MCD) complex as indicated. After incubation for 6 h at 37 °C, the cells were harvested. (D) A portion of the harvested cells (5% of total) was fractionated and subjected to immunoblot analysis for SREBP-2. P, precursor form of SREBP-2; N, cleaved nuclear form of SREBP-2. (E) The remaining 95% of the harvested cells were used to purify the ER membranes. Lipids were extracted, and the amount of cholesterol and phospholipids was quantified as described in ref. 20. Cholesterol content in E is expressed as the molar percentage of total lipids (phospholipids + cholesterol). Each bar represents the mean ± SEM from five experiments.
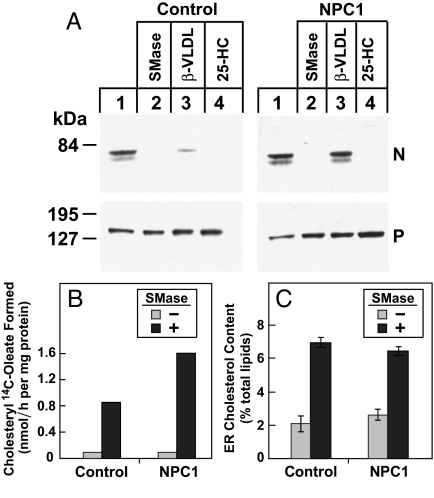
In earlier studies, we showed that the treatment of cultured cells with sphingomyelinase (SMase) leads to inhibition of SREBP processing and an increase in CE synthesis (26). We postulated that this was caused by the disruption of cholesterol/sphingomyelin complexes in the plasma membrane, with the resultant translocation of the plasma membrane cholesterol to the ER. To measure the concentration of cholesterol in the ER directly and to determine whether NPC1 is necessary for this transport, we performed the experiment shown in Fig. 6. Control hTERT-transformed human fibroblasts or NPC1 mutant cells were incubated at 37 °C for 2 h with SMase. We restricted the incubation to 2 h to guard against any toxicity from the SMase treatment. In the dishes used to measure SREBP-2 cleavage, we included an ACAT inhibitor to block the esterification of cholesterol in the ER. We used β-migrating very low density lipoprotein (β-VLDL) to deliver cholesterol to lysosomes, because this lipoprotein is richer in cholesterol than LDL. The SMase treatment blocked SREBP-2 cleavage in both the control and the NPC1 mutant cells (Fig. 6A). 25-Hydroxycholesterol also blocked cleavage in both cell lines, but β-VLDL failed to block cleavage in NPC1 mutant cells. As shown in Fig. 6B, SMase treatment increased CE synthesis similarly in control and NPC1 mutant fibroblasts. The SMase elicited the accumulation of similar amounts of cholesterol in the ER in both cell lines (Fig. 6C), a result consistent with previous findings in NPC1-deficient CHO cells (27). These data indicate that NPC1 is not required for the movement of cholesterol from the plasma membrane to the ER in response to SMase treatment.
Fig. 6.
Sphingomyelinase (SMase) treatment of control and NPC1 mutant fibroblasts stimulates cholesteryl ester (CE) synthesis. On day 0, hTERT-control and hTERT-NPC1 human fibroblasts were set up in medium A with 10% FCS at either 7 × 104 cells per 60-mm dish (A and B) or 4 × 105 cells per 100-mm dish (C). On day 2, cells were switched to medium A with 5% human lipoprotein-deficient serum (LPDS). (A) Immunoblot analysis of SREBP-2 cleavage. On day 4, the cells were incubated with medium A containing 5% LPDS, 50 μM compactin, 50 μM sodium mevalonate, 0.1% DMSO, and 10 μg/mL ACAT inhibitor Sandoz 58-035. After 30 min at 37 °C, cells received SMase (200 milliunits per milliliter), β-migrating very low density lipoprotein (β-VLDL) (10 μg of protein per milliliter), or 25-hydroxycholesterol (25-HC) (1 μg/mL) as indicated. After 2 h at 37 °C, triplicate dishes were harvested and pooled for preparation of nuclear extract and 100,000 × g membrane fractions, which were analyzed by immunoblotting for SREBP-2. P, precursor form of SREBP-2; N, cleaved nuclear form of SREBP-2. (B) Cholesterol esterification assay. On day 4, each dish received medium A with 5% LPDS, 50 μM compactin, and 50 μM sodium mevalonate in the absence or presence of 200 milliunits per milliliter of SMase. After 1 h at 37 °C, each monolayer was pulse-labeled for 1 h with 0.2 mM sodium [14C]oleate-albumin (32,433 dpm/nmol). Cells were harvested for measurement of cholesteryl [14C]oleate. Each value is the average of duplicate incubations. (C) Analysis of the cholesterol content of purified endoplasmic reticulum (ER) membranes. On day 3, cells from 13 dishes (100 mm) for each condition were switched to medium A containing 5% LPDS, 5 μM compactin, and 50 μM sodium mevalonate. On day 4, cells received the same medium supplemented with 50 μM rather than 5 μM compactin in the absence or presence of 200 milliunits per milliliter of SMase. After 2 h at 37 °C, the cells were harvested, and the ER membranes were purified as described in SI Materials and Methods and Fig. 5. Cholesterol and phospholipids were quantified as described in Fig. 5E. Cholesterol content is expressed as the molar percentage of total lipids (phospholipids + cholesterol). Each bar represents the mean ± SEM from three experiments.
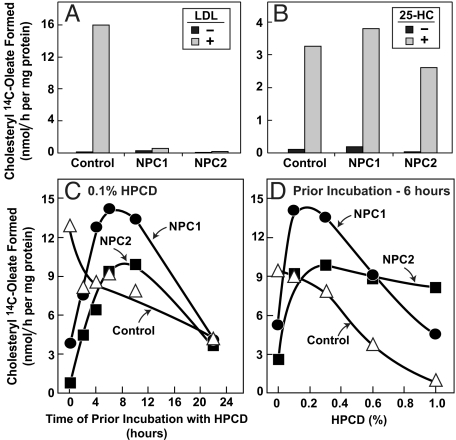
As mentioned in the Introduction, the treatment of NPC1-deficient mice with cyclodextrin increased CE content in a variety of tissues (4). To determine whether this increase is attributable to ACAT, we studied cells from patients with defects in either NPC1 or NPC2 (Fig. 7). Both of the mutant cell lines failed to show an increase in ACAT-mediated CE synthesis in the presence of LDL (Fig. 7A), but both showed a normal increase in this rate when incubated with 25-HC (Fig. 7B). To measure the effect of HPCD, we preincubated the cells with LDL to load the lysosomes with cholesterol. The cells then were switched to medium with or without 0.1% HPCD. After varying times, the cells were pulse-labeled for 2 h with [14C]oleate, and incorporation into CE was measured. In control cells, CE synthesis declined after HPCD treatment (open triangles in Fig. 7 C and D), likely because HPCD removes cholesterol from cells. In striking contrast, HPCD caused a burst of CE synthesis in both NPC1 and NPC2 mutant cells. The rate of synthesis increased for 10 h and then declined, presumably as the cholesterol from the lysosomes was depleted. Fig. 7D shows the rate of cholesterol esterification after prior incubation for 6 h with varying concentrations of HPCD. In the NPC1 and NPC2 cells, this rate was maximal at 0.1–0.3% HPCD. Cholesteryl ester synthesis was blocked by the ACAT inhibitor Sandoz 58-035, confirming that it was catalyzed by the ACAT in the ER (Table S1).
Fig. 7.
Cholesteryl ester formation in control, NPC1 mutant, and NPC2 mutant human fibroblasts treated with 2-hydroxypropyl-β-cyclodextrin (HPCD). (A–D) On day 0, nontransformed control fibroblasts, NPC1 mutant fibroblasts, and NPC2 mutant fibroblasts were set up in medium A with 10% FCS and 2 × 104, 2 × 104, and 7 × 104 cells per 60-mm dish, respectively. On day 3, cells were refed with the same medium. (A and B) On day 5, cells were switched to medium A with 10% human lipoprotein-deficient serum (LPDS). On day 7, each dish received medium A containing 10% LPDS, 50 μM compactin, and 50 μM sodium mevalonate in the absence or presence of 100 μg of protein per milliliter of LDL (A) or 5 μg/mL 25-hydroxycholesterol (25-HC) (B). After 5 h at 37 °C, each monolayer was pulse-labeled for 2 h with 0.2 mM sodium [14C]oleate-albumin (8,187 dpm/nmol). The cells then were harvested for measurement of cholesteryl [14C]oleate. (C and D) On day 6, cells were incubated with medium A with 10% FCS and 20 μg of protein per milliliter of LDL. After 16 h, each dish received medium A containing 10% FCS and 0.1% (wt/vol) HPCD (C) or the indicated concentration of HPCD (D). After incubation for the indicated time in C or after 6 h in D, each monolayer was pulse-labeled for 2 h with 0.2 mM sodium [14C]oleate-albumin (8,187 dpm/nmol). The cells then were harvested for measurement of cholesteryl [14C]oleate. (A–D) Each value is the average of duplicate incubations.
Discussion
The current studies establish several points about the function of NPC1 in human and animal cells: (i) Although NPC1 is essential for egress of lipoprotein-derived cholesterol from lysosomes, it is not required for the egress of lipoprotein-derived 25-HC (Fig. 4); (ii) NPC1 is not required for 25-HC to stimulate the esterification of cellular cholesterol, an event that requires the translocation of cholesterol from the plasma membrane to the ACAT enzyme (Figs. 2 and 3); (iii) NPC1 is not required for the delivery of cholesterol to ER membranes when the cholesterol is added to cells in complex with MCD (Figs. 1 and 5); (iv) NPC1 is not required for the translocation of cholesterol from the plasma membrane to the ER membranes upon treatment with SMase (Fig. 6); and (v) HPCD releases stored cholesterol from the lysosomes of cells with NPC1 or NPC2 mutations, whereupon a portion of the liberated cholesterol is transferred to the ER for esterification (Fig. 7). The latter findings provide a mechanism to explain the finding by Liu et al. (4) that HPCD treatment increased the content of CE in the liver and brain of NPC1 mutant mice.
The ability of LDL-derived 25-HC, but not LDL-derived cholesterol, to exit lysosomes in the absence of NPC1 is likely attributable to the greater water solubility of 25-HC, which allows the 25-HC to diffuse to and through the lysosomal membrane after liberation from the LDL by lysosomal acid lipase. NPC2 cannot be involved in this transfer, because 25-HC does not bind to the sterol-binding site on NPC2 (5–7). However, 25-HC does bind to the sterol-binding site on NPC1(NTD) (23). We believe that 25-HC binding to NPC1(NTD) is simply a consequence of the fact that the side chains of bound sterols are exposed on the surface of this protein, thereby permitting hydrophilic substitutions (10). There is no evidence to indicate that the 25-HC binding reflects a functional activity of NPC1.
Although the pathways by which cholesterol moves retrograde from the plasma membrane to the ER have not been established, the current data suggest that this movement does not require NPC1. Evidence comes from the observation in NPC1 mutant macrophages that 25-HC can stimulate the esterification of 5% of total cell cholesterol, most of which must have come from the plasma membrane (Fig. 2). Moreover, in NPC1 mutant fibroblasts, 25-HC can stimulate the esterification of 40% of newly synthesized cholesterol (Fig. 3). Further evidence for NPC1-independent transport comes from the experiment in which cholesterol was esterified after addition to cells in complex with MCD (Fig. 1C). This cholesterol likely partitions into the plasma membrane and must be transported to the ER for esterification by ACAT. This transport occurs normally in the NPC1-deficient cells. Finally, SMase treatment results in the translocation of cholesterol from the plasma membrane to the ER (Fig. 6C) and an increase in cholesterol esterification (Fig. 6B). This transport occurs equally in control and NPC1-deficient cells.
In a previous study of purified ER membranes, we found that 25-HC addition caused only a minor increase in the free cholesterol content of the ER (20). However, we and others have consistently demonstrated an increase in ACAT-mediated cholesterol esterification in cells treated with 25-HC (Figs. 1–3). A likely explanation lies in work from the laboratory of Chang and colleagues who showed that 25-HC directly activates ACAT when added to ACAT-containing membranes in vitro (28). Thus, we believe that cholesterol is esterified immediately when it reaches the ER under the influence of 25-HC, thereby avoiding an increase in the levels of unesterified cholesterol in the ER.
As mentioned in the Introduction, several earlier studies suggested that NPC1 is not required for the movement of cholesterol from the lysosomes to the plasma membrane. These studies used cyclodextrins to release cholesterol from the plasma membranes of NPC1-deficient cells. The studies were conducted before the recognition that cyclodextrins release cholesterol from NPC1-deficient lysosomes, and their conclusions thus require reexamination.
Underwood et al. (29) supplied evidence for separate pathways that direct lysosome-derived cholesterol to either the ER or the plasma membrane. The current studies are consistent with this thesis and suggest that HPCD can at least replace NPC1 in directing lysosomal cholesterol to the ER for esterification. Further studies are necessary to delineate the conditions, if any, in which HPCD can direct lysosome-derived cholesterol to the plasma membrane. How HPCD acts at a molecular level remains to be determined.
The ability of HPCD to release cholesterol from NPC1-deficient lysosomes has been exploited already in animal models to show a slowing of organ damage and prolongation of life (4, 30). The data in Fig. 7 suggest that this treatment also will be beneficial in animals that have mutations in NPC2.
Materials and Methods
Culture Media.
Medium A is DMEM containing 100 units per milliliter of penicillin and 100 μg/mL streptomycin sulfate. Medium B is DMEM (high glucose, 4.5 g/L) containing 100 units/mL penicillin and 100 μg/mL streptomycin sulfate. Medium C is medium B without sodium bicarbonate. Where indicated, sterols were added to the medium in an ethanolic solution in which the final ethanol concentration was <0.3%. In Fig. 7, HPCD was dissolved in culture media at the indicated concentration. The ACAT inhibitor Sandoz 58-035 was dissolved in DMSO and added to the medium at a final concentration of 0.7% (vol/vol).
Cell Culture.
Nontransformed skin fibroblasts from control subjects, from a patient with NPC1 disease (obtained from American Type Culture Collection, No. GM3123; compound heterozygote for mutations P237S and I1061T), and from a patient with NPC2 disease (obtained from Coriell Cell Repositories, No. GM18455; compound heterozygote for mutations E20X and C47F) were grown in a monolayer at 37 °C in 5% CO2 and maintained in medium A with 10% (vol/vol) FCS.
Control and NPC1(P237S/I1061T) human fibroblasts were immortalized with the catalytic subunit of human telomerase (hTERT) as described in ref. 23. These cells, designated hTERT-control and hTERT-NPC1, were grown in a monolayer at 37 °C in 5% CO2 in medium A with 10% (vol/vol) FCS.
Primary skin fibroblast cultures from a wild-type BALB/c mouse and a mutant BALB/c npcnih/nih mouse were established as described in ref. 23. The cells were grown in a monolayer at 37 °C in 5% CO2 in medium B with 10% (vol/vol) FCS.
Peritoneal macrophages were prepared from littermate wild-type BALB/c and mutant homozygous BALB/c npcnih/nih mice (31). Heterozygotes for breeding were provided by Stephen Turley and John Dietschy (University of Texas Southwestern Medical Center, Dallas, TX). Macrophages were harvested from the peritoneum of mice 3 days after i.p. injection of 2.5 mL of a 3% (wt/vol) thioglycollate solution and cultured as described in ref. 32. Cells were resuspended in medium B with 10% (vol/vol) FCS at a final density of 2 × 106 cells per 35-mm dish and grown in a monolayer at 37 °C in 5% CO2.
Other Methods.
Additional information is described in SI Materials and Methods.
Supplementary Material
Acknowledgments.
We thank Dorothy Goddard and Lisa Beatty for excellent technical assistance, Ijeoma Onwuneme for invaluable help with tissue culture, and Dr. Jeff McDonald for HPLC measurements. This work was supported by grants from the National Institutes of Health (HL20948) and the Perot Family Foundation. L.A.-M. and R.E.I. are supported by the Ara Parsegian Medical Foundation. R.E.I. also is supported by Medical Scientist Training Program Grant 5T32GM08014.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0910916106/DCSupplemental.
References
- 1.Pentchev PG, Vanier MT, Suzuki K, Patterson MC. In: The Metabolic and Molecular Bases of Inherited Disease. 7th Ed. Scriver CR, Beaudet AL, Sly WS, Valle D, editors. Vol II. New York: McGraw-Hill; 1995. pp. 2625–2639. [Google Scholar]
- 2.Liscum L, Sturley SL. Intracellular trafficking of Niemann-Pick C proteins 1 and 2: Obligate components of subcellular lipid transport. Biochim Biophys Acta. 2004;1685:22–27. doi: 10.1016/j.bbalip.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986;232:34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- 4.Liu B, et al. Reversal of defective lysosomal transport in NPC disease ameliorates liver dysfunction and neurodegeneration in the npc1−/− mouse. Proc Natl Acad Sci USA. 2009;106:2377–2382. doi: 10.1073/pnas.0810895106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liou H-L, et al. NPC2, the protein deficient in Niemann-Pick C2 disease, consists of multiple glycoforms that bind a variety of sterols. J Biol Chem. 2006;281:36710–36723. doi: 10.1074/jbc.M608743200. [DOI] [PubMed] [Google Scholar]
- 6.Infante RE, et al. Purified NPC1 protein: II. Localization of sterol binding to a 240-amino acid soluble luminal loop. J Biol Chem. 2008;283:1064–1075. doi: 10.1074/jbc.M707944200. [DOI] [PubMed] [Google Scholar]
- 7.Friedland N, Liou H-L, Lobel P, Stock AM. Structure of a cholesterol-binding protein deficient in Niemann-Pick type C2 disease. Proc Natl Acad Sci USA. 2003;100:2512–2517. doi: 10.1073/pnas.0437840100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carstea ED, et al. Niemann-Pick C1 disease gene: Homology to mediators of cholesterol homeostasis. Science. 1997;277:228–231. doi: 10.1126/science.277.5323.228. [DOI] [PubMed] [Google Scholar]
- 9.Davies JP, Ioannou YA. Topological analysis of Niemann–Pick C1 protein reveals that the membrane orientation of the putative sterol-sensing domain is identical to those of 3-hydroxy-3-methylglutaryl-CoA reductase and sterol regulatory element binding protein cleavage-activating protein. J Biol Chem. 2000;275:24367–24374. doi: 10.1074/jbc.M002184200. [DOI] [PubMed] [Google Scholar]
- 10.Kwon HJ, et al. Structure of N-terminal domain of NPC1 reveals distinct subdomains for binding and transfer of cholesterol. Cell. 2009;137:1213–1224. doi: 10.1016/j.cell.2009.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lange Y, Ye J, Rigney M, Steck TL. Dynamics of lysosomal cholesterol in Niemann-Pick type C and normal human fibroblasts. J Lipid Res. 2002;43:198–204. [PubMed] [Google Scholar]
- 12.Cruz JC, Sugii S, Yu C, Chang T-Y. Role of Niemann-Pick type C1 protein in intracellular trafficking of low density lipoprotein-derived cholesterol. J Biol Chem. 2000;275:4013–4021. doi: 10.1074/jbc.275.6.4013. [DOI] [PubMed] [Google Scholar]
- 13.Brown MS, Goldstein JL. The SREBP pathway: Regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89:331–340. doi: 10.1016/s0092-8674(00)80213-5. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein JL, Basu SK, Brown MS. Receptor-mediated endocytosis of low-density lipoprotein in cultured cells. Methods Enzymol. 1983;98:241–260. doi: 10.1016/0076-6879(83)98152-1. [DOI] [PubMed] [Google Scholar]
- 15.Pentchev PG, et al. The cholesterol storage disorder of the mutant BALB/c mouse. A primary genetic lesion closely linked to defective esterification of exogenously derived cholesterol and its relationship to human type C Neimann–Pick disease. J Biol Chem. 1986;261:2772–2777. [PubMed] [Google Scholar]
- 16.Liscum L, Faust JR. Low density lipoprotein (LDL)-mediated suppression of cholesterol synthesis and LDL uptake is defective in Niemann-Pick type C fibroblasts. J Biol Chem. 1987;262:17002–17008. [PubMed] [Google Scholar]
- 17.Brown MS, Dana SE, Goldstein JL. Cholesteryl ester formation in cultured human fibroblasts: Stimulation by oxygenated sterols. J Biol Chem. 1975;250:4025–4027. [PubMed] [Google Scholar]
- 18.Lange Y, Steck TL. Quantitation of the pool of cholesterol associated with acyl-CoA: Cholesterol acyltransferase in human fibroblasts. J Biol Chem. 1997;272:13103–13108. doi: 10.1074/jbc.272.20.13103. [DOI] [PubMed] [Google Scholar]
- 19.Zambrano F, Fleischer S, Fleischer B. Lipid composition of the Golgi apparatus of rat kidney and liver in comparison with other subcellular organelles. Biochim Biophys Acta. 1975;380:357–369. doi: 10.1016/0005-2760(75)90104-6. [DOI] [PubMed] [Google Scholar]
- 20.Radhakrishnan A, Goldstein JL, McDonald JG, Brown MS. Switch-like control of SREBP-2 transport triggered by small changes in ER cholesterol: A delicate balance. Cell Metab. 2008;8:512–521. doi: 10.1016/j.cmet.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wojtanik KM, Liscum L. The transport of low density lipoprotein-derived cholesterol to the plasma membrane is defective in NPC1 cells. J Biol Chem. 2003;278:14850–14856. doi: 10.1074/jbc.M300488200. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan MR, Simoni RD. Transport of cholesterol from the endoplasmic reticulum to the plasma membrane. J Cell Biol. 1985;101:446–453. doi: 10.1083/jcb.101.2.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Infante RE, et al. Purified NPC1 protein: I. Binding of cholesterol and oxysterols to a 1278-amino acid membrane protein. J Biol Chem. 2008;283:1052–1063. doi: 10.1074/jbc.M707943200. [DOI] [PubMed] [Google Scholar]
- 24.Krieger M, Goldstein JL, Brown MS. Receptor-mediated uptake of low density lipoprotein reconstituted with 25-hydroxycholesteryl oleate suppresses 3-hydroxy-3-methylglutaryl-coenzyme A reductase and inhibits growth of human fibroblasts. Proc Natl Acad Sci USA. 1978;75:5052–5056. doi: 10.1073/pnas.75.10.5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dahl NK, Reed KL, Daunais MA, Faust JR, Liscum L. Isolation and characterization of Chinese hamster ovary cells defective in the intracellular metabolism of low density lipoprotein-derived cholesterol. J Biol Chem. 1992;267:4889–4896. [PubMed] [Google Scholar]
- 26.Scheek S, Brown MS, Goldstein JL. Sphingomyelin depletion in cultured cells blocks proteolysis of sterol regulatory element binding proteins at site 1. Proc Natl Acad Sci USA. 1997;94:11179–11183. doi: 10.1073/pnas.94.21.11179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacobs NL, et al. Analysis of a Chinese hamster ovary cell mutant with defective mobilization of cholesterol from the plasma membrane to the endoplasmic reticulum. J Lipid Res. 1997;38:1973–1987. [PubMed] [Google Scholar]
- 28.Cheng D, Chang CCY, Qu X-M, Chang T-Y. Activation of acyl-coenzyme A: Cholesterol acyltransferase by cholesterol or by oxysterol in a cell-free system. J Biol Chem. 1995;270:685–695. doi: 10.1074/jbc.270.2.685. [DOI] [PubMed] [Google Scholar]
- 29.Underwood KW, Jacobs NL, Howley A, Liscum L. Evidence for a cholesterol transport pathway from lysosomes to endoplasmic reticulum that is independent of the plasma membrane. J Biol Chem. 1998;273:4266–4274. doi: 10.1074/jbc.273.7.4266. [DOI] [PubMed] [Google Scholar]
- 30.Camargo F, et al. Cyclodextrins in the treatment of a mouse model of Niemann-Pick C disease. Life Sci. 2001;70:131–142. doi: 10.1016/s0024-3205(01)01384-4. [DOI] [PubMed] [Google Scholar]
- 31.Loftus SK, et al. Murine model of Niemann-Pick C disease: Mutation in a cholesterol homeostasis gene. Science. 1997;277:232–235. doi: 10.1126/science.277.5323.232. [DOI] [PubMed] [Google Scholar]
- 32.Basu SK, et al. Biochemical and genetic studies of the apoprotein E secreted by mouse macrophages and human monocytes. J Biol Chem. 1982;257:9788–9795. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.