Abstract
Central nervous system (CNS) trauma can result in tissue disruption, neuronal and axonal degeneration, and neurological dysfunction. The limited spontaneous CNS repair in adulthood and aging is often insufficient to overcome disability. Several investigations have demonstrated that targeting HDAC activity can protect neurons and glia and improve outcomes in CNS injury and disease models. However, the enthusiasm for pan-HDAC inhibition in treating neurological conditions is tempered by their toxicity toward a host of CNS cell types –a biological extension of their anticancer properties. Identification of the HDAC isoform, or isoforms, that specifically mediate the beneficial effects of pan-HDAC inhibition could overcome this concern. Here, we show that pan-HDAC inhibition not only promotes neuronal protection against oxidative stress, a common mediator of injury in many neurological conditions, but also promotes neurite growth on myelin-associated glycoprotein and chondroitin sulfate proteoglycan substrates. Real-time PCR revealed a robust and selective increase in HDAC6 expression due to injury in neurons. Accordingly, we have used pharmacological and genetic approaches to demonstrate that inhibition of HDAC6 can promote survival and regeneration of neurons. Consistent with a cytoplasmic localization, the biological effects of HDAC6 inhibition appear transcription-independent. Notably, we find that selective inhibition of HDAC6 avoids cell death associated with pan-HDAC inhibition. Together, these findings define HDAC6 as a potential nontoxic therapeutic target for ameliorating CNS injury characterized by oxidative stress-induced neurodegeneration and insufficient axonal regeneration.
Keywords: HDAC inhibitor, histone deacetylase, neuroprotection
Injury secondary to oxidative stress in the central nervous system (CNS) is an important clinical problem affecting millions worldwide. In most CNS injury and degeneration paradigms an initial insult, which may be mechanical, ischemic, toxic, or genetic, is followed by a delayed and prolonged period of secondary damage that involves a number of destructive pathophysiological and biochemical events that culminate in cell body and axonal degeneration and dysfunction (1, 2). Given that damage continues to evolve days and weeks after acute injury, and years in chronic neurodegeneration, with functional impairments corresponding to the location and severity of the initial insult, a temporal window of opportunity exists to limit the spread of damage (neuroprotection) and improve recovery (3). Indeed, it has been demonstrated that the rescue of as few as 10% of spinal cord axons during a spinal cord injury equates to significant functional recovery (4, 5).
In addition to cell body and axonal loss, the spontaneous ability of surviving but damaged CNS axons to regenerate back to appropriate targets after injury is often insufficient for the restoration of function (6). A major impediment for regeneration of axons is the CNS environment. Factors, such as myelin-associated glycoprotein (MAG), oligodendrocyte glycoprotein (OMgp), and the extracellular domain of Nogo-A (Nogo-66) exert neurite growth inhibitory effects through a host of receptors that are expressed on axons (7). Signaling via Nogo receptor (NogoR) and the p75 neurotrophin receptor (p75NTR), for example, leads to activation of the small GTPase, RhoA, (8, 9) and subsequent activation of Rho kinase (ROCK), and downstream signaling that negatively modulate actin filament dynamics, growth cone stability, and axonal growth (6, 10, 11). Overcoming this extrinsic inhibition in the adult CNS is central to the process of axon regeneration and CNS repair. In addition to myelin, axons may also be confronted by proteoglycan-rich territories at the site of injury, which form physical and chemical barriers to regrowth (12). Thus, strategies that overcome environmental barriers to regeneration are also imperative for functional recovery. In developing neuroprotective strategies, it is critical these interventions, aimed at enhancing survival of cell bodies and axons, do not impede subsequent efforts to promote regeneration.
Considerable research activity has focused on histone deacetylase (HDAC) inhibitors as neuroprotective agents for a number of neurodegenerative diseases and CNS injuries (13, 14). In addition to neuroprotection, there is growing evidence that HDAC inhibitors can also enhance learning and memory in the CNS (15, 16). HDAC inhibitors selectively alter gene transcription, in part, by permitting chromatin remodeling and by changing the composition of multiprotein complexes bound to specific gene promoters (17). Further, HDAC inhibitors increase the acetylation of many nonhistone proteins such as transcription factors, hormone receptors, chaperone proteins, and cytoskeletal proteins, modifying their function or activity (13). While HDAC2 has recently been identified as the specific HDAC family member involved in memory formation (16), studies have yet to define specific family member(s) involved in neuronal degeneration. Furthermore, whether HDAC inhibition promotes, encumbers, or is neutral to axonal regeneration after injury is unknown.
In this manuscript we provide evidence that inhibition of HDAC activity in neurons, in vitro, cannot only promote protection against oxidative stress-induced death but also promote neurite/axonal growth on nonpermissive substrates, such as MAG. We show that HDAC6 may play a role in the pathological process downstream of neuronal oxidative stress as its upregulation coincides with neuronal commitment to death. Targeting HDAC6 with selective pharmacological inhibitors or small interfering RNAs promotes both neuroprotection and axonal regeneration, implicating it as the critical HDAC whose inhibition mediates both of these effects. Furthermore, targeting HDAC6 abrogates all toxicity associated with pan-HDAC inhibition (18, 19). Consistent with HDAC6 being predominantly localized to the cytoplasm and acetylating a range of cytoplasmic target proteins, our findings suggest that the effects of HDAC6 inhibition occurs through a local mechanism that is independent of transcription.
Results
Pan-HDAC Inhibition Protects Against Oxidative Stress-Induced Neurodegeneration.
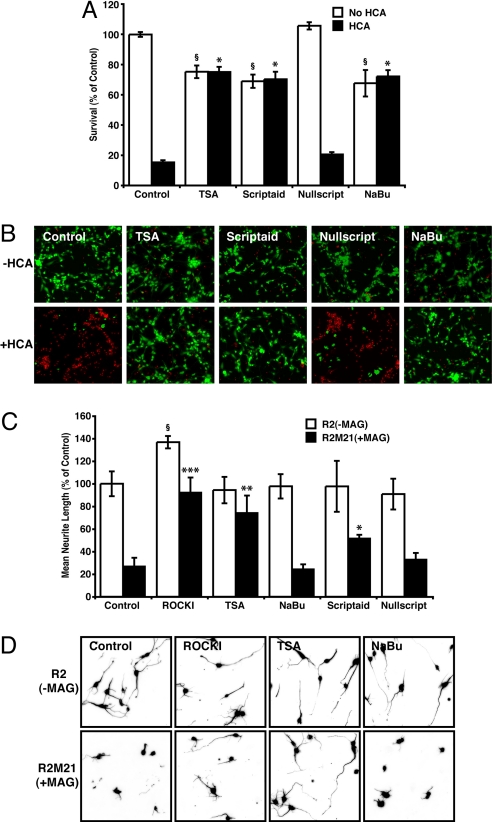
Oxidative stress is well characterized to be an underlying mediator of neurodegeneration that is common to CNS disease and injury (20). Previously, we have demonstrated that the pan-HDAC inhibitors, TSA, and Scriptaid, or the Class I HDAC inhibitor, sodium buyrate, can protect against oxidative stress-induced death in neurons, in vitro and in permanent and transient focal brain ischemia in rodents (19). To confirm the neuroprotective capacity of pan-HDAC inhibitors, primary cortical neurons were treated with TSA, Scriptaid, and sodium butyrate in the presence or absence of oxidative stress and the extent of neuron survival examined. Despite the observation that pan-HDAC inhibition alone caused some toxicity (≈20–30% >24 h), all of the HDAC inhibitors protected neurons from the oxidative insult (Fig. 1 A and B). Furthermore, Nullscript, a structural analog of Scriptaid that lacks HDAC inhibitory activity, showed no protection, suggesting that protection depends on HDAC inhibition and not an off-target effect of the hydroxamic acid moiety. These experiments support a well-established body of literature that targeting HDAC activity might be a valuable strategy for protecting neurons in the CNS (13, 19).
Fig. 1.
Pharmacological HDAC inhibition protects primary neurons from oxidative stress and promotes neurite outgrowth in the presence of MAG. (A) Neuronal viability after treatment with homocysteic acid (HCA; 5 mM) with or without pan-HDAC inhibitors TSA (0.66 μM), Scriptaid (6.13 μM), Nullscript (6.13 μM), and sodium butyrate (NaBu; 1 mM) for 24 h. *, Significant protection compared to HCA treatment alone P < 0.001. §, Significant death compared to non-HCA control P < 0.001. (B) Live/Dead staining of treated rat primary cortical cultures. Live cells are identified by green fluorescence, whereas dead cells identified by red fluorescence. (C and D) Mean neurite length measured after 24 h coculture with either R2 or R2M21+MAG CHO cells in the presence or absence of the RhoA kinase Inhibitor (ROCKI), Y27632 (10 μM), or the HDAC inhibitors, TSA, NaBu, Scriptaid, or Nullscript. Neurites were identified by neuron-specific β-III tubulin immunostaining and fluorescence microscopy (D), and measured by using Metamorph (C). Significant increase in neurite length relative to nontreated R2M21+MAG denoted by *, P < 0.05, **, P < 0.01, or ***, P < 0.001. Significant increase in neurite length relative to nontreated R2 denoted by §, P < 0.05.
Pan-HDAC Inhibition Promotes Neurite Outgrowth in Cortical Neurons Cocultured with CHO-MAG Cells.
After traumatic injury or neurodegeneration, the adult CNS has some intrinsic ability to regenerate. However, extrinsic growth inhibitory factors in the injury territory, such as components of myelin, actively block axonal growth (6). Because it is important that any strategy adopted in preserving neural tissue does not impede subsequent spontaneous regeneration or strategies to promote regeneration, we examined the effect of HDAC inhibition on neurite growth of neurons cocultured with either control chinese hamster ovary (CHO) cells or CHO cells that express MAG. Primary cortical neurons were grown on a CHO monolayer in the presence or absence of a pan-HDAC inhibitor for 24 h, fixed, and immunostained for βIII-tubulin, a neuron-specific marker present in axons and dendrites of neurons. The average neurite lengths after treatment with TSA, Scriptaid, or sodium butyrate were not different to nontreated controls, suggesting that HDAC inhibition has no effect on basal neurite outgrowth (Fig. 1 C and D). To investigate the effect of HDAC inhibition on neurite outgrowth in a context relevant to CNS injury, we examined their effect on neurons exposed to MAG. Consistent with previous reports, neurite outgrowth of rat primary cortical neurons cocultured with CHO cells expressing MAG was significantly attenuated compared to neurons cocultured with control CHO cells (Fig. 1 C and D) (21). By contrast, treatment with the HDAC inhibitors, TSA or Scriptaid promoted efficient neurite outgrowth in neurons cocultured with CHO-MAG cells (Fig. 1 C and D). Surprisingly, neurite length was comparable to that of neurons cocultured with control CHO cells, or cocultured with CHO-MAG cells in the presence of the selective ROCK inhibitor, Y27632 (Fig. 1 C and D), or dibutyryl cAMP; two agents known to promote neurite outgrowth in this model (21). Nullscript, the analog of Scriptaid that lacks HDAC inhibitory activity, did not promote neurite outgrowth. Importantly, neither TSA nor Scriptaid treatment decreased CHO cell expression of MAG (Fig. S1). Interestingly, unlike TSA and Scriptaid, the Class I HDAC inhibitor sodium butyrate did not promote neurite outgrowth (Fig. 1 C and D), suggesting that TSA and Scriptaid are likely acting via class II HDAC (HDAC4, 5, 6, 7, and 10) inhibition.
HDAC6 Expression Is Upregulated in a Time-Dependent Manner with Oxidative Stress.
The above observations support the conclusion that HDAC enzymes may represent a promising target that will protect neurons, as well as promote neurite outgrowth and repair in the CNS after injury. However, it is unknown which HDAC isoform or isoforms are likely targets contributing to neuroprotection or neurite outgrowth. Furthermore, several recent studies point to the necessity of certain HDACs for neuronal development and survival (18, 22), correlating with observations that pan-HDAC inhibition, while broadly neuroprotective against stress associated with disease and injury, is toxic (Fig. 1 A and B) (19). Thus, we rationalized that it might be possible to target a single HDAC isoform to solicit the beneficial effects of this strategy without the toxicity associated with global HDAC inhibition.
To identify which HDACs might be bona fide targets for neuroprotection, we examined the expression levels of each HDAC isoform during an oxidative insult or during MAG inhibition. Because sodium butyrate, a Class I HDAC inhibitor, displayed similar toxicity to pan-HDAC inhibitors such as TSA and Scriptaid, but did not promote neurite outgrowth, we limited our investigations to Class II HDACs. Quantitative PCR studies revealed that the expression levels of the Class II HDACs, HDAC4, 5, 7, and 10 were not appreciably increased with either oxidative stress or neurite growth inhibition (Fig. S2). In contrast to this, the expression of HDAC6 was found to increase in a time-dependent manner with oxidative stress (Fig. S2), suggesting that HDAC6 may be oxidatively regulated or play a role in pathological signaling. Furthermore, its inhibition may contribute to the neuroprotective effect observed with global HDAC inhibition.
Selective HDAC6 Inhibition Protects Against Oxidative Stress-Induced Neurodegeneration and Promotes Neurite Outgrowth in Cortical Neurons Cocultured with CHO-MAG Cells.
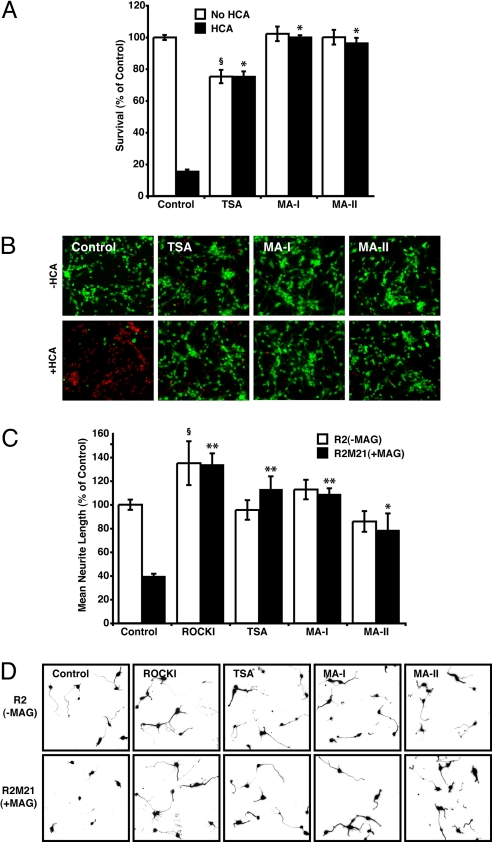
HDAC6 is a cytoplasmic enzyme that interacts with and deacetylates substrates such as α-tubulin, Hsp90, and cortactin (23–25). Through these interactions, HDAC6 regulates many important biological processes, including cell migration, microtubule stability, intracellular trafficking, immune synapse formation, and the fate of misfolded proteins (26, 27). We surmised that if HDAC6 is important for mediating the pathological effects of oxidative stress and the inhibition of neurite extension by MAG, compounds that selectively interfere with HDAC6 activity should be neuroprotective and promote neurite growth. To investigate this prospect, we examined the efficacy of two mercaptoacetamide-based HDAC6-selective inhibitors, 2-Mercapto-N-[6-(3-phenylureido)hexyl] acetamide (MA-I) and N-{5-[3-(4-Dimethylaminophenyl)ureido]pentyl}-2-mercaptoacetamide (MA-II) [Fig. S3 and (28)], for their ability to protect primary cortical neurons and overcome neurite outgrowth inhibition. As predicted, both of the pharmacological HDAC6-selective inhibitors, MA-I and MA-II, fully protected neurons from the oxidative insult with no toxicity (Fig. 2 A and B) as well as promoted efficient neurite outgrowth in neurons cultured on CHO-MAG cells (Fig. 2 C and D). Moreover, neurite length was comparable to that of neurons treated with TSA, the selective ROCK inhibitor Y27632, or cocultured with control CHO cells (Fig. 2 C and D). To confirm that these compounds were inhibiting HDAC6 activity, MA-I and MA-II were examined for their ability to increase α-tubulin acetylation levels. Both inhibitors enhanced tubulin acetylation to levels that were comparable to treating with the pan-HDAC inhibitor, TSA (Fig. S4). However, while TSA treatment increased histone H4 acetylation, treatment with MA-I or MA-II did not increase acetylation of histone H4 (Fig. S4).
Fig. 2.
Pharmacological inhibitors selective for HDAC6 are protective in oxidatively stressed neurons, and promote neurite outgrowth in the presence of MAG. (A) Neuronal viability after treatment with homocysteic acid (HCA; 5 mM) with or without the pan-HDAC inhibitor TSA (0.66 μM), or the HDAC6-selective inhibitors MA-I (10 μM), and MA-II (10 μM) for 24 h. Viability was measured by using MTT assay. *, Significant protection compared to HCA treatment alone P < 0.001. §, Significant death compared to non-HCA control P < 0.001. (B) Live/Dead staining of treated rat primary cortical cultures. (C and D) Mean neurite length of cortical neurons measured after 24 h coculture with either R2 and R2M21+MAG cells in the presence or absence of the RhoA kinase Inhibitor (ROCKI), Y27632 (10 μM), the pan-HDAC inhibitor, TSA, or the HDAC6-selective inhibitors MA-I, and MA-II. Neurites were identified by neuron-specific β-III tubulin immunostaining and fluorescence microscopy (D), and measured by using Metamorph (C). Significant increase in neurite length relative to nontreated R2M21+MAG denoted by *, P < 0.05 or **, P < 0.001. Significant increase in neurite length relative to nontreated R2 denoted by §, P < 0.05.
HDAC6 Knockdown by Small Interfering RNAs Protects Against Oxidative Stress-Induced Neurodegeneration and Promotes Neurite Outgrowth in Cortical Neurons Cocultured with CHO-MAG Cells.
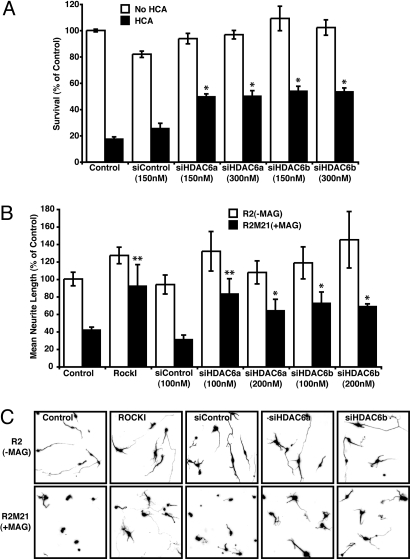
All of our results to this point have used pharmacological inhibitors of HDAC and HDAC6-specific activity. To confirm our observations that HDAC6 inhibition can promote neuroprotection and overcome the inhibitory effects of MAG, we used a small interfering RNA (siRNA) approach to specifically knockdown HDAC6. Two siRNAs targeting different regions of HDAC6 were synthesized and linked to the penetratin-1 peptide via a disulphide bond (29). Transduction of cortical neurons with either HDAC6 siRNA resulted in the knockdown of HDAC6 mRNA (Fig. S5), and the concomitant increase in tubulin acetylation, but not histone H4 acetylation (Fig. S4). In contrast to these observations, a scrambled siRNA control neither knocked down HDAC6 expression nor increased tubulin acetylation (Figs. S4 and S5). Having functionally validated the efficacy of our siRNAs, we examined their capacity to protect primary cortical neurons and overcome neurite outgrowth inhibition. Consistent with the pharmacological data in Fig. 2 A and B, a decrease in HDAC6 activity by siRNA knockdown conferred resistance to neurons against oxidative death (Fig. 3A). Similarly, HDAC6 knockdown by either of the HDAC6 siRNAs promoted neurite outgrowth in neurons cultured on CHO cells expressing MAG (Fig. 3 B and C). In contrast to this, the scrambled siRNA control neither protected nor promoted neurite outgrowth (Fig. 3 A–C).
Fig. 3.
Targeted knockdown of HDAC6 using RNAi is protective and promotes neurite outgrowth. (A) Rat primary cortical neuron viability after treatment with homocysteic acid (HCA; 5 mM) with or without a nonspecific 21-nt duplex (siControl), or HDAC6 specific siRNAs (siHDAC6a and siHDAC6b) for 24 h. Viability was measured by using MTT assay. *, Significant protection compared to HCA treatment alone P < 0.001. (B and C) Mean neurite length of cortical neurons measured 24 h after coculture with either R2 and R2M21+MAG cells in the presence or absence of the RhoA kinase Inhibitor (ROCKI), Y27632 (10 μM), or nonspecific 21-nt duplex (siControl), or HDAC6 specific siRNAs (siHDAC6a and siHDAC6b). Neurites were identified by neuron-specific β-III tubulin immunostaining and fluorescence microscopy (C), and measured by using Metamorph (B). Significant increase in neurite length relative to nontreated R2M21+MAG denoted by *, P < 0.05 or **, P < 0.001.
Selective HDAC6 Inhibition Promotes Neurite Outgrowth in Dorsal Root Ganglion Neurons Exposed to MAG or CSPGs.
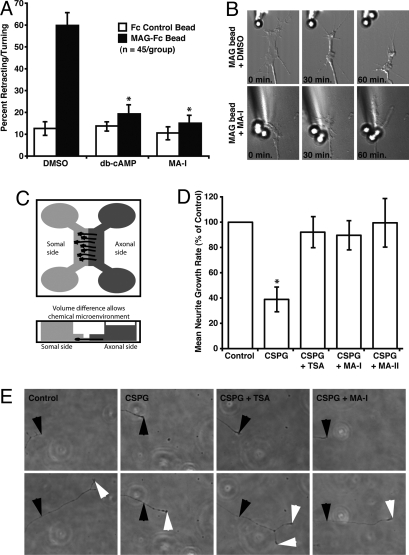
To further validate our neurite outgrowth studies we examined the effect of HDAC6 inhibition on a different neuronal cell type, and in a different MAG inhibition assay. Dorsal root ganglion (DRG) neurons from L4–6 of adult male rats were treated with vehicle (DMSO) alone, dibutyryl cAMP, or MA-I, and presented to MAG-Fc immobilized to carboxylate microspheres. In cultures treated with vehicle, MAG-Fc presented to the DRG axon terminal caused approximately 60% of axons to retract or turn away, consistent with the ability of acute MAG exposure to induce axonal growth cone collapse (Fig. 4 A and B and Movie S1) (30). By contrast, the acute exposure of either dibutyryl cAMP or MA-I-treated DRG axons to immobilized MAG resulted in a degree of turning and retraction that was not different to exposure to control microspheres immobilized with Fc (Fig. 4 A and B and Movie S2). Importantly, these experiments were performed in the presence of the RNA synthesis inhibitor DRB, which inhibits >90% of RNA synthesis (30), suggesting that the effect of HDAC6 inhibition on axon growth is independent of transcription. These results are also consistent with our observations that histone H4 acetylation is unchanged in the presence of either MA-I or MA-II (Fig. S4).
Fig. 4.
Pharmacological inhibitors selective for HDAC6 promote dorsal root ganglion neuron axonal outgrowth in the presence of MAG and CSPGs. (A) Percent axonal retraction of rat adult dorsal root ganglion neurons when presented to Fc control or MAG-Fc coated microspheres in the presence of vehicle control (DMSO), dibutyryl cAMP or the HDAC6-selective inhibitor, MA-I. *, Significant decrease in neurite retraction/turning relative to nontreated control P < 0.001. (B) Representative micrographs at 0, 30, and 60 min after the presentation of MAG to the growth cone of a DRG axon. (C) Schematic of microfluidic-based culture platform adapted from Taylor et al. (31). The culture chamber consists of somal and axonal compartments connected by microgrooves. Rat DRG neurons are added to the somal side and axonal growth is guided into the axonal side through the microgrooves. Volume difference between the somal side and axonal side allows chemical microenvironments to be isolated. (D) Growth rate of rat adult dorsal root ganglion axons exposed to laminin (Control) or CSPG/laminin in the presence of DMSO vehicle, TSA, or the HDAC6-selective inhibitors, MA-I and MA-II. Growth rates of axons exposed to CSPG/laminin in the presence HDAC inhibitors were not significantly different to growth rates of axons exposed to laminin-only. *, Significant decrease in neurite growth relative to laminin-only controls P < 0.05 by. (E) Representative micrographs of axons in the CSPG- containing axonal compartment, at 2 h (Top) and 5 h (Bottom) after treatment. Black arrows show axonal growth cones at 2 h. White arrows show axonal growth cones at 5 h.
In addition to the axon growth-inhibitory effects of MAG and other myelin-derived substrates, chondroitin sulfate proteoglycans (CSPGs) are upregulated around the damaged site during injury and act intracellularly via Rho GTPase and cytoskeletal modifications to prevent axonal regeneration (12). To determine whether our findings extend to promoting axon regrowth in the presence of other inhibitory substances, we examined the effect of HDAC6 inhibition on DRG axonal growth on CSPGs. Because we were also interested in determining whether the promotion of axonal regrowth by HDAC6 inhibition requires treatment of the DRG cell body or whether treatment of the axon alone is sufficient, we used a microfluidic culture platform. Microfluidic platforms have been used to isolate and direct the growth of CNS axons into a fluidically isolated environment without the use of targeting neurotrophins (31). DRGs from P4 rats were grown in the chambers for 72 h and the axons growing into the isolated CSPG-containing compartment were treated with either vehicle (DMSO), TSA, MA-I or MA-II for 2 h before axonal rate measurements (Fig. 4C). In chambers treated with vehicle alone, the presence of CSPGs significantly inhibited DRG axon growth, consistent with the ability of CSPGs to induce axonal growth cone collapse (Fig. 4 D and E) (6). By contrast, treatment of the fluidically isolated axons with either TSA or the HDAC6 selective inhibitors, MA-I and MA-II, resulted in an axonal growth rate on CSPGs that was indistinguishable to laminin controls (Fig. 4 D and E). These experiments, which enable an isolated axonal treatment environment, imply that the effect of HDAC6 inhibition on axonal growth inhibition may occur locally and supports our previous assertion that this is a transcriptionally independent process.
Discussion
During CNS injury, neural degeneration and regeneration failure are extensively overlapping processes. Thus, the identification of molecular targets whose manipulation can enhance both of these aspects would be advantageous for the mutually enforcing goal of reducing dysfunction and disability. We have shown that targeting global HDAC activity is sufficient to protect neurons from oxidative stress-induced death (Fig. 1 A and B) as well as counteract MAG- and CSPG-mediated neurite growth inhibition (Figs. 1 C and D and 4 D and E). Our findings point to HDAC6 as the precise HDAC target for mediating both of these actions as its specific inhibition by two different pharmacological inhibitors, or its knockdown with two different siRNAs, is sufficient for complete protection and restoration of neurite growth (Figs. 2–4). While we cannot rule out the possibility that other HDAC family members may also be targets for neuroprotection, our findings suggest that the promotion of neurite outgrowth in the presence of inhibitory substrates, if not exclusively HDAC6, is confined to Class II HDACs (Fig. 1 C and D). A notable finding with targeting HDAC6 is that it also overcomes all of the neuronal toxicity associated with the use of pan-HDAC inhibitors (Fig. 2 A and B), suggesting its specific inhibition may eliminate a range of untoward effects seen with the clinical application of pan-HDAC inhibitors in cancer (32). This finding is also consistent with the recent demonstration that mice lacking HDAC6 are viable and develop normally (33).
HDAC6 was identified based on homology to yeast Hda-1 (34). However, its identified physiological substrate was the cytoplasmic protein α-tubulin (23), consistent with the earlier functional studies showing that HDAC6 is actively maintained and primarily functions in the cytoplasm (34). In agreement with this, our findings show that the selective inhibition of HDAC6, either by pharmacological inhibitors or siRNA knockdown selectively increases α-tubulin acetylation, without altering histone H4 acetylation (Fig. S4) and that the effects of HDAC6 inhibition on neurite outgrowth are independent of transcription (Fig. 4).
While HDAC6 inhibition mediates both neuroprotection and regeneration actions, it remains to be determined whether the targets of HDAC6 activity in these disparate processes overlap or are independent. Recent findings show that HDAC6 inhibition can increase cellular antioxidant activity, suggesting precedence for protection against oxidative insults. For instance, HDAC6 has been identified as a specific deacetylase of peroxiredoxin-1 and -2, whose main function is peroxide reduction (35). Acetylation of peroxiredoxin-1 and -2 increases their reducing activity, their resistance to superoxidation, and their resistance to transition to high-molecular-mass complexes (35). In addition to peroxiredoxins, HDAC6 is a specific deacetylase of the abundant, homodimeric molecular chaperone, Hsp90, that sequesters HSF-1 and acts to chaperone steroid hormone receptors and certain protein kinases (24). A number of reports suggest that Hsp90 may be a viable target for neuroprotection as HSP90 inhibitors have been found to promote HSF-1 release and augment the heat-shock response, which protects against neurotoxic insults in a variety of models of neurodegenerative disease (36, 37). Acetylation of HSP90 at lysine 294 has been shown to modulate its activity by regulating client protein and cochaperone binding (24).
In contrast to neuroprotection, acetylation of the HDAC6 target α-tubulin may underlie neurite outgrowth on nonpermissive substrates. Recently, the multisubunit histone acetyltransferase Elongator complex, which contributes to transcript elongation, was found to acetylate α-tubulin and to be critical for the regulation of migration, projection length and branching of developing cortical neurons (38). Silencing of the scaffold subunit (Elp1) or catalytic subunit (Elp3) of Elongator was found to reduce levels of acetylated α-tubulin and significantly decrease axonal length and impair branching of projection neurons. Supporting this, the expression of a nonacetylatable α-tubulin mutant in cortical neurons leads to comparable defects (38). Another target demonstrated to be negatively regulated by HDAC6 that may promote neurite outgrowth is the F-actin binding protein, cortactin (25). Indeed, cortactin has been implicated in neurite outgrowth based on its role in cytoskeletal reorganization and its presence in the growth cones of developing hippocampal neurons (39). Studies have shown that HDAC6 binds cortactin directly and induces cortactin deacetylation (25). Deacetylation of cortactin alters its ability to bind F-actin by modulating a charge patch in its repeat region. Introduction of charge-preserving or charge-neutralizing mutations in this repeat region correlates with the gain or loss of F-actin binding ability, respectively (25). F-actin polymerization in the region of the growth cone that includes filopodia- and lamellipodia-like veils (P domain) drives protrusion of the leading edge membrane and thus growth cone mobility and branching (40).
Irrespective of the precise mechanisms involved, our findings are congruent with a model in which oxidative stress subsequent to ischemia, trauma, toxins, or genetic mutation, leads to an induction of HDAC6. In this setting HDAC6 deacetylates one or more as yet undefined targets in the cell body or axon, respectively, to either induce death or impede regeneration. Selective inhibition of HDAC6 not only enhances resistance to death but also alters the intrinsic growth state of the neuron so that it is no longer sensitive to extant growth inhibitory substrates. The findings suggest that the low-molecular-weight HDAC6 inhibitors described herein are candidate therapeutics for a host of acute and chronic neurodegenerative conditions with large therapeutic windows after the initial injury. Studies are underway to define analogs of these inhibitors that will possess the optimal chemical characteristics to penetrate the blood brain barrier for testing in animal models of stroke, spinal cord injury, and Alzheimer's disease.
Materials and Methods
HDAC Inhibitors: Trichostatin A (TSA), Scriptaid, and Nullscript (Biomol International); sodium butyrate (Sigma–Aldrich); MA-I and MA-II were prepared in the laboratories of Dr. Alan Kozikowski (University of Illinois) (28); Homocysteic acid (HCA; Sigma–Aldrich); MAG expressing (R2M21) and control (R2) Chinese hamster ovary cells (CHO cells) were a kind gift from Marie Filbin (Hunter College). Embryonic day 17 pregnant and adult Sprague–Dawley rats were obtained from Harlan Sprague–Dawley.
Primary Neurons and Cell Culture.
All animal surgeries and euthanasia were performed according to institutional Animal Care and Use Committee guidelines under approved protocols. Rat primary neuronal cultures and DRGs were obtained as described in refs. 26 and 30.
HDAC inhibitor and cytotoxicity studies were performed as described in ref. 19. Penetratin-1-linked siRNAs were generated according to the method by Davidson et al. (29). HDAC6-specific and scrambled control sequences are as follows: siControl: aactaaatgtgcgcgagcctc; siHDAC6a: aagccgtgctttcaggagagg; siHDAC6b: aaagcaaagacagctaaggca. Viability was assessed as described in ref. 19. For CHO-MAG cocultures and neurite outgrowth assays, MAG expressing (R2M21) and control (R2) CHO cells were cultured according to (21). CHO cells were plated at 5 × 104 cells per well overnight in 96-well culture plates coated with 1 mg/mL poly d-lysine and 100 μg/mL fibronectin. Primary rat cortical neurons were plated on the CHO monolayer at a cell density of 1 × 103 cells per well. Neurons were treated with the respective inhibitor or penetratin-1-linked siRNA at the time of plating and stained and visualized after 24 h.
For localized treatment of DRG axons with MAG-Fc experiments, MAG-Fc (R&D Systems) was covalently coupled to 4.5 μm Carboxylate Microspheres per manufacturers instructions (Polysciences Inc.). Microspheres loaded with Fc region of human IgG were used as controls. Microspheres and or HDAC inhibitor were added to DRG cultures 18 h after plating, in the presence of RNA synthesis inhibitor 5,6-dichloro-1-ß-d-ribobenzimidazole (DRB; 80 μM; Sigma–Aldrich). DRGs were imaged (Nikon Eclipse TS100 microscope with QICAM 12-bit Fast1394 camera) at 10 min and 2 h after addition of microspheres and the turning angle at 2 h relative to the direction of the axon shaft at 10 min, and percentage of collapse were determined. For time-lapse imaging, microspheres were presented to axons using a pulled borosilicate micropipette (outer diameter, 1.0 mm; inner diameter, 0.5 mm; length, 10 cm) fitted to an automated micromanipulator (Xeno Works). Growth was monitored by DIC imaging >60 min by confocal microscopy (Leica TCS/SP2 confocal system on an inverted Leica DMIRE2 microscope with environmental chamber; Life Imaging Services).
For CSPG treatment of DRG axons in microfluidic culture platforms, microfluidic chambers were prepared according to the method of Taylor et al. (31). Rat DRG cultures were grown in the chambers for 72 h before treatment. Growth of fluidically isolated axons was monitored by phase imaging (Nikon Eclipse TE-2000-E) at 2 and 5 h after treatment.
Immunocytochemistry, Western Blot Analysis, and Quantitative RT-PCR.
Cocultures were washed with PBS then fixed with 4% paraformaldehyde in PBS for 15 min. Cells were blocked for 1 h at room temperature in 3% normal goat serum and 1% TTX-100. Cells were incubated overnight at 4 °C with β-III tubulin (1:1000; Millipore) primary antibody diluted in blocking buffer, then washed in 0.1% TTX-100 in PBS and incubated for 1 h at RT with rhodamine conjugated anti-mouse IgG. Cells were washed with PBS and visualized by using a Zeiss Axiovert 200M microscope (Zeiss). Images of random fields were captured with the Axiocam MRm CCD camera (Zeiss). Neurite length was measured and analyzed by using Metamorph (Molecular Devices). Significant neurite outgrowth threshold was set at 50% that of the cell soma diameter.
Immunoblot analysis was performed as described in ref. 19. The following primary antibodies: Acetylated α-tubulin (Sigma–Aldrich), total α-tubulin (Sigma–Aldrich), histone H4 (Millipore), acetyl histone H4 (Millipore), and MAG (Millipore).
Total RNA preparation and quantitation was performed as described in ref. 19. Duplex reactions were performed by using FAM-labeled HDAC4, 5, 6, 7a, and 10 gene expression assays, and a VIC-labeled β-actin gene expression assay (Applied Biosystems).
Statistics.
All data are representative of at least three independent experiments performed under blinded conditions. Statistical significance was determined by two-way ANOVA followed by Bonferroni posttests, except where noted otherwise.
Supplementary Material
Acknowledgments.
We are indebted to Dr. Carol Troy for assistance with Penetratin-1-linked siRNAs, Drs. Wilfredo Mellado and Marie Filbin for R2 and R2M21 cell lines and protocols, and Dr. Noo Li Jeon for assistance with microfluidic devices. This work was funded by the Adelson Medical Research Foundation (to B.L., R.R.R., and J.L.T.), the Christopher Reeve Paralysis Foundation (to S.R.J.), Burke Medical Research Foundation (to B.L.), and the National Institutes of Health Grants R01-NS049041 (to J.L.T.) and R01-NS056306 (to S.R.J.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0907935106/DCSupplemental.
References
- 1.Anderson MF, Sims NR. The effects of focal ischemia and reperfusion on the glutathione content of mitochondria from rat brain subregions. J Neurochem. 2002;81:541–549. doi: 10.1046/j.1471-4159.2002.00836.x. [DOI] [PubMed] [Google Scholar]
- 2.Young W. Secondary injury mechanisms in acute spinal cord injury. J Emerg Med. 1993;11(Suppl 1):13–22. [PubMed] [Google Scholar]
- 3.Springer JE. Apoptotic cell death following traumatic injury to the central nervous system. J Biochem Mol Biol. 2002;35:94–105. doi: 10.5483/bmbrep.2002.35.1.094. [DOI] [PubMed] [Google Scholar]
- 4.Blight AR. Cellular morphology of chronic spinal cord injury in the cat: Analysis of myelinated axons by line-sampling. Neuroscience. 1983;10:521–543. doi: 10.1016/0306-4522(83)90150-1. [DOI] [PubMed] [Google Scholar]
- 5.Fehlings MG, Tator CH. The relationships among the severity of spinal cord injury, residual neurological function, axon counts, and counts of retrogradely labeled neurons after experimental spinal cord injury. Exp Neurol. 1995;132:220–228. doi: 10.1016/0014-4886(95)90027-6. [DOI] [PubMed] [Google Scholar]
- 6.Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci. 2006;7:617–627. doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunt D, Mason MR, Campbell G, Coffin R, Anderson PN. Nogo receptor mRNA expression in intact and regenerating CNS neurons. Mol Cell Neurosci. 2002;20:537–552. doi: 10.1006/mcne.2002.1153. [DOI] [PubMed] [Google Scholar]
- 8.GrandPre T, Nakamura F, Vartanian T, Strittmatter SM. Identification of the Nogo inhibitor of axon regeneration as a Reticulon protein. Nature. 2000;403:439–444. doi: 10.1038/35000226. [DOI] [PubMed] [Google Scholar]
- 9.Wang KC, et al. Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature. 2002;417:941–944. doi: 10.1038/nature00867. [DOI] [PubMed] [Google Scholar]
- 10.Ng J, Luo L. Rho GTPases regulate axon growth through convergent and divergent signaling pathways. Neuron. 2004;44:779–793. doi: 10.1016/j.neuron.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 11.Yang N, et al. Cofilin phosphorylation by LIM-kinase 1 and its role in Rac-mediated actin reorganization. Nature. 1998;393:809–812. doi: 10.1038/31735. [DOI] [PubMed] [Google Scholar]
- 12.Monnier PP, Sierra A, Schwab JM, Henke-Fahle S, Mueller BK. The Rho/ROCK pathway mediates neurite growth-inhibitory activity associated with the chondroitin sulfate proteoglycans of the CNS glial scar. Mol Cell Neurosci. 2003;22:319–330. doi: 10.1016/s1044-7431(02)00035-0. [DOI] [PubMed] [Google Scholar]
- 13.Kazantsev AG, Thompson LM. Therapeutic application of histone deacetylase inhibitors for central nervous system disorders. Nat Rev Drug Discov. 2008;7:854–868. doi: 10.1038/nrd2681. [DOI] [PubMed] [Google Scholar]
- 14.Langley B, Brochier C, Rivieccio MA. Targeting histone deacetylases as a multifaceted approach to treat the diverse outcomes of stroke. Stroke. 2009;40:2899. doi: 10.1161/STROKEAHA.108.540229. [DOI] [PubMed] [Google Scholar]
- 15.Fischer A, Sananbenesi F, Wang X, Dobbin M, Tsai LH. Recovery of learning and memory is associated with chromatin remodelling. Nature. 2007;447:178–182. doi: 10.1038/nature05772. [DOI] [PubMed] [Google Scholar]
- 16.Guan JS, et al. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature. 2009;459:55–60. doi: 10.1038/nature07925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gui CY, Ngo L, Xu WS, Richon VM, Marks PA. Histone deacetylase (HDAC) inhibitor activation of p21WAF1 involves changes in promoter-associated proteins, including HDAC1. Proc Natl Acad Sci USA. 2004;101:1241–1246. doi: 10.1073/pnas.0307708100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim D, et al. Deregulation of HDAC1 by p25/Cdk5 in neurotoxicity. Neuron. 2008;60:803–817. doi: 10.1016/j.neuron.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Langley B, et al. Pulse inhibition of histone deacetylases induces complete resistance to oxidative death in cortical neurons without toxicity and reveals a role for cytoplasmic p21(waf1/cip1) in cell cycle-independent neuroprotection. J Neurosci. 2008;28:163–176. doi: 10.1523/JNEUROSCI.3200-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bossy-Wetzel E, Schwarzenbacher R, Lipton SA. Molecular pathways to neurodegeneration. Nat Med. 2004;10(Suppl):S2–S9. doi: 10.1038/nm1067. [DOI] [PubMed] [Google Scholar]
- 21.Domeniconi M, Filbin MT. Overcoming inhibitors in myelin to promote axonal regeneration. J Neurol Sci. 2005;233:43–47. doi: 10.1016/j.jns.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 22.Majdzadeh N, et al. HDAC4 inhibits cell-cycle progression and protects neurons from cell death. Dev Neurobiol. 2008;68:1076–1092. doi: 10.1002/dneu.20637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hubbert C, et al. HDAC6 is a microtubule-associated deacetylase. Nature. 2002;417:455–458. doi: 10.1038/417455a. [DOI] [PubMed] [Google Scholar]
- 24.Scroggins BT, et al. An acetylation site in the middle domain of Hsp90 regulates chaperone function. Mol Cell. 2007;25:151–159. doi: 10.1016/j.molcel.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang X, et al. HDAC6 modulates cell motility by altering the acetylation level of cortactin. Mol Cell. 2007;27:197–213. doi: 10.1016/j.molcel.2007.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawaguchi Y, et al. The deacetylase HDAC6 regulates aggresome formation and cell viability in response to misfolded protein stress. Cell. 2003;115:727–738. doi: 10.1016/s0092-8674(03)00939-5. [DOI] [PubMed] [Google Scholar]
- 27.Serrador JM, et al. HDAC6 deacetylase activity links the tubulin cytoskeleton with immune synapse organization. Immunity. 2004;20:417–428. doi: 10.1016/s1074-7613(04)00078-0. [DOI] [PubMed] [Google Scholar]
- 28.Kozikowski AP, et al. Functional differences in epigenetic modulators-superiority of mercaptoacetamide-based histone deacetylase inhibitors relative to hydroxamates in cortical neuron neuroprotection studies. J Med Chem. 2007;50:3054–3061. doi: 10.1021/jm070178x. [DOI] [PubMed] [Google Scholar]
- 29.Davidson TJ, et al. Highly efficient small interfering RNA delivery to primary mammalian neurons induces MicroRNA-like effects before mRNA degradation. J Neurosci. 2004;24:10040–10046. doi: 10.1523/JNEUROSCI.3643-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Willis DE, et al. Extracellular stimuli specifically regulate localized levels of individual neuronal mRNAs. J Cell Biol. 2007;178:965–980. doi: 10.1083/jcb.200703209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taylor AM, et al. A microfluidic culture platform for CNS axonal injury, regeneration and transport. Nat Methods. 2005;2:599–605. doi: 10.1038/nmeth777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bruserud O, Stapnes C, Ersvaer E, Gjertsen BT, Ryningen A. Histone deacetylase inhibitors in cancer treatment: A review of the clinical toxicity and the modulation of gene expression in cancer cell. Curr Pharm Biotechnol. 2007;8:388–400. doi: 10.2174/138920107783018417. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, et al. Mice lacking histone deacetylase 6 have hyperacetylated tubulin but are viable and develop normally. Mol Cell Biol. 2008;28:1688–1701. doi: 10.1128/MCB.01154-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verdel A, Khochbin S. Identification of a new family of higher eukaryotic histone deacetylases. Coordinate expression of differentiation-dependent chromatin modifiers. J Biol Chem. 1999;274:2440–2445. doi: 10.1074/jbc.274.4.2440. [DOI] [PubMed] [Google Scholar]
- 35.Parmigiani RB, et al. HDAC6 is a specific deacetylase of peroxiredoxins and is involved in redox regulation. Proc Natl Acad Sci USA. 2008;105:9633–9638. doi: 10.1073/pnas.0803749105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fujimoto M, et al. Active HSF1 significantly suppresses polyglutamine aggregate formation in cellular and mouse models. J Biol Chem. 2005;280:34908–34916. doi: 10.1074/jbc.M506288200. [DOI] [PubMed] [Google Scholar]
- 37.Waza M, et al. 17-AAG, an Hsp90 inhibitor, ameliorates polyglutamine-mediated motor neuron degeneration. Nat Med. 2005;11:1088–1095. doi: 10.1038/nm1298. [DOI] [PubMed] [Google Scholar]
- 38.Creppe C, et al. Elongator controls the migration and differentiation of cortical neurons through acetylation of alpha-tubulin. Cell. 2009;136:551–564. doi: 10.1016/j.cell.2008.11.043. [DOI] [PubMed] [Google Scholar]
- 39.Du Y, Weed SA, Xiong WC, Marshall TD, Parsons JT. Identification of a novel cortactin SH3 domain-binding protein and its localization to growth cones of cultured neurons. Mol Cell Biol. 1998;18:5838–5851. doi: 10.1128/mcb.18.10.5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lowery LA, Van Vactor D. The trip of the tip: Understanding the growth cone machinery. Nat Rev Mol Cell Biol. 2009;10:332–343. doi: 10.1038/nrm2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.