Abstract
Sodium (Na) is uncommon in plants but essential to the metabolism of plant consumers, both decomposers and herbivores. One consequence, previously unexplored, is that as Na supplies decrease (e.g., from coastal to inland forests), ecosystem carbon should accumulate as detritus. Here, we show that adding NaCl solution to the leaf litter of an inland Amazon forest enhanced mass loss by 41%, decreased lignin concentrations by 7%, and enhanced decomposition of pure cellulose by up to 50%, compared with stream water alone. These effects emerged after 13–18 days. Termites, a common decomposer, increased 7-fold on +NaCl plots, suggesting an agent for the litter loss. Ants, a common predator, increased 2-fold, suggesting that NaCl effects cascade upward through the food web. Sodium, not chloride, was likely the driver of these patterns for two reasons: two compounds of Na (NaCl and NaPO4) resulted in equivalent cellulose loss, and ants in choice experiments underused Cl (as KCl, MgCl2, and CaCl2) relative to NaCl and three other Na compounds (NaNO3, Na3PO4, and Na2SO4). We provide experimental evidence that Na shortage slows the carbon cycle. Because 80% of global landmass lies >100 km inland, carbon stocks and consumer activity may frequently be regulated via Na limitation.
Keywords: biogeochemistry, biogeography, decomposition, fungi, termites
Models of the carbon cycle start with the coavailability of water and solar energy as key constraints to photosynthesis and respiration (1–4). Lowland tropical forests, however, have ample sunshine, often ample moisture (5), and, frequently, weathered soils (6). In such forests, nutrient shortages, most notably the shortage of P, have been shown by experiment and comparative study to constrain both net primary productivity (NPP; g C/m2 per y) and decomposition (7–13). Moreover, recent theory and experiment have suggested that the rate of decomposition, a collaborative process involving thousands of species, is unlikely to be constrained, Liebig style, by a single element (14, 15). Here, we build on those studies and those of Chadwick et al. (16) to suggest that Na plays a key role in regulating decomposition in inland ecosystems.
Of the 25 or so elements required for life (17), Na is unique. Most terrestrial plants have little need of Na (18). Herbivores and decomposers, in contrast, must amass Na in concentrations 100- to 1,000-fold over the plants they consume (19). In animals, costly sodium pumps maintain gradients of cell concentration and membrane voltage (>1% deviations of whole body sodium are signs of pathology; ref. 17). In plants, K, not Na, performs this function (20). This biogeochemical disconnect between plants and those that eat them suggests that consumers, but not plants, should suffer when Na inputs to ecosystems decline.
Decomposers metabolize >90% of terrestrial plant biomass (21). Decomposition, the breakdown of necromass into CO2 and inorganic compounds, is largely performed by microbes. Some are free living (e.g., basidiomycete and ascomycete fungi), and others live in the guts of animal decomposers (detritivores) like termites and isopods. Both fungi and animals have high Na requirements relative to plants they consume (22, 23). Both obtain energy by breaking down abundant carbon-rich macromolecules like cellulose. Yet little is known about how the activity of an ecosystem's decomposers is constrained by the shortage of sodium.
Sodium has a geography. It accumulates locally where ground water is mined for agriculture (19) and where roads are salted to prevent icing (24). Regionally, extensive rainfall promotes leaching (25), and the Na content of that rainfall decreases exponentially as one travels inland from sources of oceanic aerosols (26, 27). This decline in aerosol deposition has consequences for ecosystem levels of sodium: coastal forests in Panama have higher concentrations of Na than the Peruvian Amazon both in freshwater streams and rivers [149 vs. 20–60 μmol/L (26, 28)] and soil [0.008 vs. 0.005 ppm (29)]. Consistent with the hypothesis of Na limitation, here we show that litter decomposition rates, and the abundance of decomposers and their predators, increase with NaCl fertilization in an inland Amazon forest.
Results
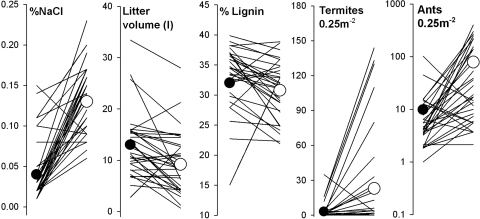
We tested the hypothesis that Na shortage limits decomposers in a forest near Iquitos Peru, 2,000 km from its ocean source of aerosols (26). The first experiment compared decomposition and decomposer densities across 35 paired 0.5 × 0.5-m plots through old-growth forest. One of each pair was fertilized with a 0.5% solution of NaCl and stream water every other day, whereas the other received only stream water. After ≈18 days litter concentrations of Na were 3-fold higher on +NaCl plots (paired t32 = −9.1, P < 0.0001; Fig. 1).* Litter volume on +NaCl plots, in contrast, was 71% lower than controls (paired t34 = 3.99, P = 0.0003; Fig. 1); the concentration of recalcitrant lignin was 7% lower (paired t32 = −9.1, P < 0.0001; Fig. 1).
Fig. 1.
Effects of Na treatments on soil salinity, litter decomposition (volume and litter content), and the abundance of two common litter taxa in an Amazon forest. Lines from left to right denote differences between control and +NaCl treatment in 35 paired plots. Circles are treatment means: black = control (stream water), white = 0.5% NaCl solution made from stream water.
There was marked increase of termites on +NaCl plots (Fig. 1). Termites that nest in high densities within the forest floor (30) emerged from the soil and were observed consuming litter. Systematic scans over the course of the experiment revealed termites on the surface of six +NaCl plots but no control plots (Kruskal Wallis X2 = 6.5, P = 0.011). At harvest, 7-fold more termites were extracted from +NaCl plots than controls (paired t34 = −4.13, P = 0.0002; Fig. 1). Monitoring revealed 8-fold more ants on +NaCl plots (paired t34 = −3.8, P = 0.0006) and 2-fold more ants when the plots were harvested (paired t34 = −2.2, P = 0.03; Fig. 1). Thus, both a key decomposer and a key predator of tropical brown food webs accumulated on +NaCl plots.
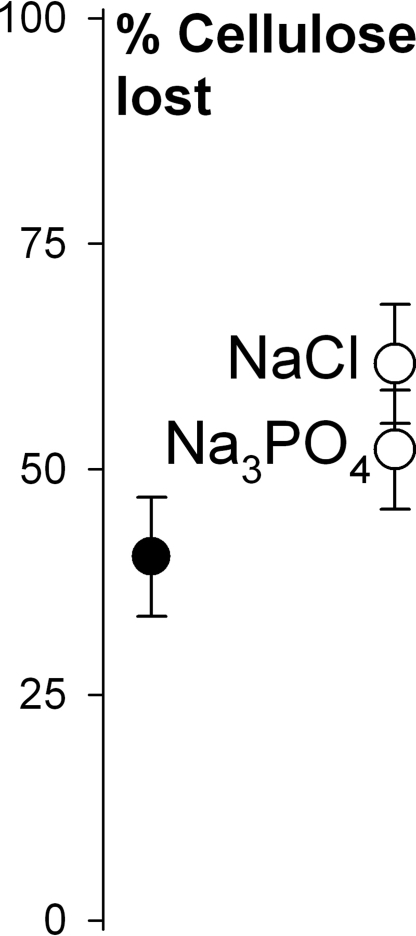
A second experiment confirmed higher mass loss of cellulose with increased access to Na (Fig. 2). After 13 days, mass loss varied across the landscape (F19,60 = 12.6, P < 0.0001) and was 53% higher on NaCl-treated cellulose than water-treated controls; mass loss of Na3PO4-treated cellulose was 29% higher (F2,60 = 10.3, P < 0.0003, Tukey minimum significant difference test: NaCl = Na3PO4 > H2O). Cellulose disappeared from the forest floor more quickly in the presence of Na, whether coupled with chloride or phosphate.
Fig. 2.
Average mass loss of cellulose treated with stream water versus two forms of sodium (least square means ± 2 SEMs). Circles are treatment means: black = control (stream water), white = 0.1 M Na solution made from stream water.
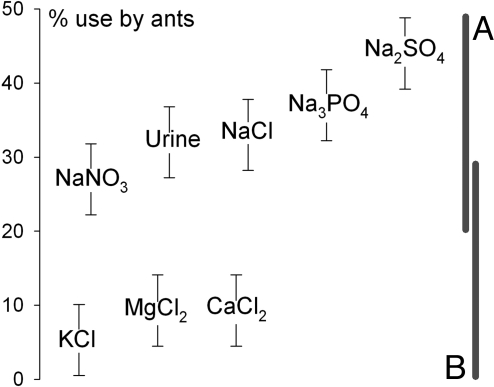
A third experiment verified that the Na in NaCl was the primary attractant to ants. Vials with solutions of seven compounds (plus urine) were presented along five transects across the forest floor (Fig. 3). Both solution type (F7,39 = 9.22, P < 0.0001) and transect (F4,39 = 7.47, P = 0.0003) accounted for variation in ant use of vials. Ants used all but the lowest-ranked Na solution (NaNO3), more than three solutions with Cl but no Na (KCl, MgCl2, and CaCl2; Fig. 3). Urine is likely the most common Na supplement to the brown food web, attracting a variety of arthropods. NaCl and urine vials received similar numbers of visits.
Fig. 3.
Ant use (least square means percentage of 15 baits ± SEMs) of eight solutions offered along five transects across the forest floor. Gray bars represent higher (A) and lower (B) use of baits using Tukey criteria of minimum differences (P < 0.05).
Discussion
In tropical rainforests, where solar energy and precipitation are often in ample supply, biogeochemical gradients of elements like phosphorus can limit both NPP and decomposition (11) and may account for the deeper litter in the Amazon interior compared with more coastal forests (13). Here, we provide experimental evidence that shortage of another element, sodium, slows the degradation of cellulose and lignin and promotes carbon storage, all with likely no direct impact on the construction of new plant tissue.
Sodium has long been suspected of regulating inland populations of herbivores, from North American meadow voles (31) to elephants of the African savannah (32). We found that termites, which occupy 68% of Earth's land area representing 77% of terrestrial NPP (33, 34), increased 7-fold on plots that averaged 3-fold more Na. Termites transform carbon from soil, leaf litter, and coarse woody debris (34) into significant quantities of methane and carbon dioxide (33). They also apparently crave sodium: termites of an African savannah 250 km inland increase Na concentrations 6 m down into the soil (35); pest control agents in North America are taught to add Na-rich sports drinks to termite baits (Edward Vargo, personal communication). Our experimental evidence shows behavior consistent with Na limitation (i.e., increased densities and activity with added Na) in an animal decomposer (i.e., detritivore).
Furthermore, the concentration of lignin, a recalcitrant macromolecule that inhibits cellulolysis, also decreased on +NaCl plots, implicating the action of powerful peroxidase systems used by a subset of free-living microbes and termite symbionts (34, 36–38). At present, we do not have strong evidence that fungal activity increased on +NaCl plots. However, in this inland Amazon forest we observed none of the dense mycelial mats characteristic of coastal tropical forests (39), suggesting some form of fungal inhibition.
Decomposition is the conversion of detritus to microbial and animal food (29) that in turn supports a large fraction of the forest fauna (40). The increase of ants on +NaCl plots suggests that ant populations in the inland Amazon are Na-limited directly (through metabolic requirements) and/or indirectly via the increase in prey on Na-rich patches. Our observations suggest both are true. The gradient of increasing Na preference from coastal to inland ant communities (41), combined with intense recruitment in this study to Na (but not Cl) by herbivorous ants like Cephalotes atratus, supports the role of Na deficits in directly influencing ant activity. Moreover, predators like the trap-jaw ants of the genera Pyramica and Odontomachus, which are less constrained by Na deficits because of their high-sodium diets (41), also increased on the +NaCl plots. The increased decomposer activity brought about by added Na cascaded up the brown food web.
In forest ecosystems, leaf litter and coarse woody debris can represent a significant carbon sink (42, 43) although much uncertainty remains regarding the dynamics and standing stocks of both (44). If the local experiments reported here scale up, then as one moves inland away from ocean inputs of sodium (16) stores of leaf litter and coarse woody debris should increase, and the release of CO2 from forests should decrease. The resulting imprint on the global carbon cycle would not be trivial as >80% of Earth's terrestrial surface is >100 km inland (the distance where ant communities begin to show signs of Na deficit; Table 1 and ref. 41). The Earth's vast inland boreal forests (45) and fire-maintained grasslands (46) might also be particular candidates for Na limitation of decomposers. Furthermore, road salts (which collectively exceed oceanic inputs of Na by 4-fold in the United States; ref. 24) and hurricanes (that can transport the equivalent of 0.3 mm of seawater inland; ref. 47)† may leave their own Na-based imprint on consumer communities and carbon stocks.
Table 1.
Area of terrestrial landmass (km2) found at four zones representing distances to the ocean and potential oceanic aerosols
| Location | Distance |
% >100 km inland | |||
|---|---|---|---|---|---|
| <10 km | 10–100 km | 100–1,000 km | > 1,000 km | ||
| Continent | |||||
| Africa | 1.66E + 05 | 2.85E + 06 | 2.11E + 07 | 5.40E + 06 | 89.8 |
| Asia | 6.12E + 05 | 6.83E + 06 | 3.00E + 07 | 5.58E + 06 | 82.7 |
| Australia | 6.99E + 04 | 1.13E + 06 | 6.30E + 06 | 0.00E + 00 | 84.0 |
| North America | 9.67E + 05 | 6.40E + 06 | 1.47E + 07 | 6.09E + 05 | 67.5 |
| Oceania | 2.92E + 04 | 2.25E + 05 | 7.05E + 03 | 0.00E + 00 | 2.7 |
| South America | 1.42E + 05 | 1.87E + 06 | 1.23E + 07 | 3.14E + 06 | 88.5 |
| Antarctica | 6.79E + 05 | 3.74E + 06 | 7.81E + 06 | 0.00E + 00 | 63.9 |
| Europe | 3.19E + 05 | 2.71E + 06 | 6.31E + 06 | 0.00E + 00 | 67.6 |
| Ecoregion domain | |||||
| Dry | 3.87E + 05 | 3.92E + 06 | 3.33E + 07 | 9.12E + 06 | 90.8 |
| Humid temperate | 8.92E + 05 | 6.03E + 06 | 1.48E + 07 | 7.23E + 05 | 69.1 |
| Humid tropical | 9.26E + 05 | 7.08E + 06 | 2.60E + 07 | 4.75E + 06 | 79.4 |
| Polar | 2.40E + 06 | 1.14E + 07 | 2.44E + 07 | 1.30E + 05 | 64.1 |
| Ecoregion divisions with largest inland regions | |||||
| Temperate desert regime mountains | 0.00E + 00 | 0.00E + 00 | 6.15E + 04 | 5.53E + 05 | 100.0 |
| Temperate steppe regime mountains | 0.00E + 00 | 1.10E + 03 | 6.65E + 05 | 4.02E + 05 | 99.9 |
| Temperate steppe | 2.21E + 04 | 2.09E + 05 | 4.03E + 06 | 5.33E + 05 | 95.2 |
| Tropical/subtropical desert regime mountains | 2.55E + 04 | 2.06E + 05 | 1.59E + 06 | 1.37E + 06 | 92.7 |
| Tropical/subtropical desert | 1.32E + 05 | 1.35E + 06 | 1.32E + 07 | 2.58E + 06 | 91.4 |
| Temperate desert | 4.43E + 04 | 4.33E + 05 | 3.32E + 06 | 1.69E + 06 | 91.3 |
| Prairie regime mountains | 1.12E + 04 | 1.04E + 05 | 8.50E + 05 | 2.93E + 05 | 90.9 |
| Prairie | 3.61E + 04 | 4.03E + 05 | 3.82E + 06 | 1.67E + 05 | 90.1 |
| Tropical/subtropical steppe regime mountains | 4.87E + 04 | 4.68E + 05 | 3.33E + 06 | 6.96E + 05 | 88.6 |
| Savanna | 2.83E + 05 | 2.49E + 06 | 1.49E + 07 | 2.92E + 06 | 86.5 |
| Tropical/subtropical steppe | 1.15E + 05 | 1.25E + 06 | 7.16E + 06 | 1.29E + 06 | 86.1 |
| Subtropical regime mountains | 3.25E + 04 | 2.19E + 05 | 1.05E + 06 | 2.42E + 05 | 83.7 |
| Sub-Arctic division | 1.90E + 05 | 2.09E + 06 | 1.00E + 07 | 7.78E + 03 | 81.4 |
Materials and Methods
The experiments took place December 16, 2008 to January 6, 2009 at the Amazon Center for Tropical Studies (ACTS) field station 67 km NE of Iquitos, in Loreto Province, Peru (3.25°S, 72.91° W). ACTS is embedded in a humid tropical lowland forest of clay oxisols and ultisols (49) that receives ≈3,000 mm of rainfall spread relatively evenly throughout the year (50). ACTS was chosen as a likely candidate for decomposer limitation as it received low concentrations of Na in its rainfall (26) and among the highest use of NaCl baits in a 17-site survey of New World ant assemblages (41).
In all three experiments we made solutions from the water of a small (≈2 m wide, 50 cm deep) low-flow, low-gradient, sandy-bottom tributary of the Rio Sucusari that abutted our study site. Analysis of this water with an inductively coupled plasma-optical emission spectrophotometer revealed a Na concentration of 81 μmol/L.
First Experiment.
In the first experiment, NaCl solution was added directly to the litter on 0.25-m2 (0.5 × 0.5m) plots. Thirty-five 2 × 2-m blocks were flagged on either side of an ACTS trail running along and 4 m above the stream. Blocks were arrayed on both sides of the trail. Blocks were separated ≥3 m from those on the same side of the trail and ≥5 m from those on the opposite side of the trail. All blocks were located on flat expanses of open litter. Two 0.25-m2 plots were positioned in each block so as to avoid coarse woody debris (branches >10 cm in diameter). These plots were flagged and assigned to control or +NaCl treatments.
Beginning December 18, 2008 control plots received 250 mL of stream water on alternate days or 250 mL of 0.5% (by weight) NaCl solution dribbled slowly over the litter. Before watering, each plot was monitored for ants and termites on the surface of the litter. Abundance was classified on a log10 scale (i.e., 1 = 1–9 individuals, 2 = 10–99, 3 > 100–999).
Plots were harvested to quantify litter volume, percentage of lignin, percentage of NaCl, and the abundance of termites and ants. Five blocks were harvested on December 25; the other 30 blocks were harvested, in batches of five blocks per day, January 1–6. At harvest, litter depth was measured within each corner of a plot by inserting a wire flag pressed downward to mineral soil. All litter was harvested down to mineral soil and shaken for 30 s through 1-cm2 mesh. The siftate was stored in a cloth bag and placed for 24 h in a miniwinkler (51) to extract invertebrates. This siftate was further hand-sorted to remove any remaining invertebrates ≥2 mm. The samples were sorted in M.K.'s lab to quantify the abundance of ants and termites. To determine Na and fiber composition, samples were then dried at 50 °C and a 10-g subsample analyzed by the Oklahoma State Soil, Water, and Forage Analytical Laboratory. Here, we report results for percentage of Na (via a Spectro CirOs ICP spectrometer) and percentage of lignin (estimated by digesting cellulose + lignin in 72% sulfuric acid). See ref. 52 for further details.
Pairwise t tests were used to compare changes in litter volume, chemistry, and termite and ant abundance with +NaCl. We pooled the five plots harvested on December 25 with those harvested from January 1–6; thus we refer to harvest results after ≈18 days.
Second Experiment.
We also examined the effect of increased sodium on the decomposition of cellulose, the world's most abundant biomolecule (21). Cellulose filter paper (9-cm disks, 100% cellulose; Fisherbrand) was saturated in one of two Na compounds (0.1 M Na3PO4 or 0.1 M NaCl made with stream water) or a control treatment (stream water). On December 23, 2008, disks were arranged in 20 replicate rows along a 40-m east–west transect in the forest understory 100 m NE of the ACTS station. Rows were established every 1–2 m along the transect; each included a control disk, a sodium phosphate disk, and a sodium chloride disk, 0.5 m apart. For each disk, a small patch of litter was cleared down to mineral soil, and the disk (folded twice to form a four-layered quarter circle) was secured to the soil with a surveyor flag and recovered with litter. Cellulose disks were recovered after 13 days and dried at 50 °C to stable mass. The percentage mass loss was used as an estimate of decomposition rate and analyzed by two-way ANOVA, with treatment and block effects.
Third Experiment.
We explored which chemical elements were attractive to consumers by presenting the local ant community with a choice of eight compounds in solution, patterned after a similar choice experiment in a North American butterfly population (53). In addition to NaCl, three solutions contained Cl (CaCl2, MgCl2, and KCl) and three contained Na (NaNO3, Na2SO4, and Na3PO4). All such solutions were made by dissolving compounds in stream water. The eighth solution was human urine contributed the morning of the experiment by S.P.Y. and N.A.C. (equal portions subsequently homogenized). Ants were offered these solutions in transects of labeled 2 mL of Eppendorf vials. Each vial was half-stuffed with cotton saturated with one of eight solutions. For each of five replicate transects, 15 vials of each solution were snapped shut and thoroughly mixed in plastic bag. Each transect was run on one of five different trails. Every 1 m along each transect, a vial was randomly selected from the bag, uncapped, and placed on the litter surface 1 m off trail. We collected the vials after 1 h, snapping the cap shut and capturing the ants by using the baits within. For each transect, we calculated the percentage of vials for each solution visited by ants. These percentages were compared with ANOVA, using transect as a block.
How Much of Continents Are Inland?
To estimate the potential importance of sodium shortage in oceanic aerosols at the global scale, we quantified the fraction of the terrestrial surface at different distances from the ocean. We used the Digital Chart of the World (54), developed from the Operational Navigational Chart at 1:1,000,000 scale from the U.S. Defense Mapping Agency (reorganized into the National Geospatial-Intelligence Agency) and national mapping agencies from Australia, Canada, and the United Kingdom.
Acknowledgments.
We thank P. Bucur, P. Jensen, and S. Madigosky for logistical support; the Peruvian Instituto Nacional de Recursos Naturales for permits; the Amazon Conservatory for Tropical Studies and Amazon Explorama Lodges for access to the field site; B. Nairn for analysis of stream water; E. Vargo, B. Thorne, and R. Constantino for consultations on termite ecology; and J. Gillooly, J. Powers, and T. Valone for comments on the manuscript. This work was supported by a grant from the National Geographic Society.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
The litter chemistry of two +NaCl plots was unknown as termites had eaten all of the litter.
Murphy SF, Stallard RF, The Third Interagency Conference on Research in the Watersheds, September 8–11, 2008, Estes Park, CO.
References
- 1.Meetemeyer V. Macroclimate and lignin control of litter decomposition rates. Ecology. 1978;59:465–472. [Google Scholar]
- 2.Gholz HL, Wedin DA, Smitherman SM, Harmon ME, Parton WJ. Long-term dynamics of pine and hardwood litter in contrasting environments: Toward a global model of decomposition. Global Change Biol. 2000;6:751–765. [Google Scholar]
- 3.Allen AP, Gillooly JF, Brown JH. Linking the global carbon cycle to individual metabolism. Funct Ecol. 2005;19:202–213. [Google Scholar]
- 4.Cox PM, Betts RA, Jones CD, Spall SA, Totterdell IJ. Acceleration of global warming due to carbon-cycle feedbacks in a coupled climate model. Nature. 2000;408:184–187. doi: 10.1038/35041539. [DOI] [PubMed] [Google Scholar]
- 5.Phillips OL, et al. Drought sensitivity of the Amazon rainforest. Science. 2009;323:1344–1347. doi: 10.1126/science.1164033. [DOI] [PubMed] [Google Scholar]
- 6.Walker TW, Syers JK. The fate of phosphorus during pedogenesis. Geoderma. 1976;15:1–19. [Google Scholar]
- 7.Elser JJ, et al. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol Lett. 2007;10:1135–1142. doi: 10.1111/j.1461-0248.2007.01113.x. [DOI] [PubMed] [Google Scholar]
- 8.Reich PB, Oleksyn J. Global patterns of plant leaf N and P in relation to temperature and latitude. Proc Natl Acad Sci USA. 2004;101:11001–11006. doi: 10.1073/pnas.0403588101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wardle DA, Walker LR, Bardgett RD. Ecosystem properties and forest decline in contrasting long-term chronosequences. Science. 2004;305:509–513. doi: 10.1126/science.1098778. [DOI] [PubMed] [Google Scholar]
- 10.Thingstad TF, et al. Nature of phosphorus limitation in the ultraoligotrophic eastern Mediterranean. Science. 2005;309:1068–1071. doi: 10.1126/science.1112632. [DOI] [PubMed] [Google Scholar]
- 11.Vitousek PM. Nutrient Cycling and Limitation: Hawai'i as a Model System. Princeton: Princeton Univ Press; 2004. [Google Scholar]
- 12.Cleveland CC, Reed SC, Townsend AR. Nutrient regulation of organic matter decomposition in a tropical rain forest. Ecology. 2006;87:492–503. doi: 10.1890/05-0525. [DOI] [PubMed] [Google Scholar]
- 13.Kaspari M, Yanoviak S. The biogeography of litter depth in tropical forests: Evaluating the phosphorus growth rate hypothesis. Funct Ecol. 2008;22:919–923. [Google Scholar]
- 14.Kaspari M, et al. Multiple nutrients regulate litterfall and decomposition in a tropical forest. Ecol Lett. 2008;11:35–43. doi: 10.1111/j.1461-0248.2007.01124.x. [DOI] [PubMed] [Google Scholar]
- 15.Saito MA, Goepfert TJ, Ritt JT. Some thoughts on the concept of colimitation: Three definitions and the importance of bioavailability. Limnol Oceanogr. 2008;53:276–290. [Google Scholar]
- 16.Chadwick OA, Derry LA, Vitousek PM, Huebert BJ, Hedin LO. Changing sources of nutrients during four millions years of ecosystem development. Nature. 1999;397:491–497. [Google Scholar]
- 17.Frausto da Silva JJR, Williams RJP. The Biological Chemistry of the Elements: The Inorganic Chemistry of Life. Oxford: Oxford Univ Press; 2001. [Google Scholar]
- 18.Marschner H. Mineral Nutrition in Higher Plants. San Diego: Academic; 1995. [Google Scholar]
- 19.National Research Council. Mineral Tolerance of Animals. Washington, DC: Natl Acad Press; 2005. [Google Scholar]
- 20.Taiz L, Zeiger E. Plant Physiology. 2nd Ed. Sunderland, MA: Sinauer; 1998. [Google Scholar]
- 21.Chapin FS, Matson PA, Mooney HA. Principles of Ecosystem Ecology. New York: Springer; 2002. [Google Scholar]
- 22.Cromack FJ, et al. In: The Role of Arthropods in Forest Ecosystems. Mattson WJ, editor. New York: Springer; 1977. pp. 78–84. [Google Scholar]
- 23.Stark N. Nutrient cycling pathways and litter fungi. Bioscience. 1972;22:355–360. [Google Scholar]
- 24.Jackson RB, Jobbgy EG. From icy roads to salty streams. Proc Natl Acad Sci USA. 2005;102:14487–14488. doi: 10.1073/pnas.0507389102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vitousek PM, Sanford RL. Nutrient cycling in moist tropical forest. Annu Rev Ecol Syst. 1986;17:137–167. [Google Scholar]
- 26.Stallard RF, Edmond JM. Geochemistry of the Amazon 1. Precipitation chemistry and the marine contribution to the dissolved load at the time of peak discharge. J Geophys Res. 1981;86:9844–9858. [Google Scholar]
- 27.National Atmospheric Deposition Program. Sodium Ion Wet Deposition. IL: Champaign; 2006. [Google Scholar]
- 28.Stallard RF. Tropical Forests, Past, Present, Future: Abstract Volume of The Association for Tropical Biology Annual Meeting. Balboa: Smithsonian Tropical Research Institute; 2002. Comparative biogeochemistry of catchments with steep and gentle slopes, Barro Colorado Island, Panama; p. 111. [Google Scholar]
- 29.Kaspari M, Yanoviak SP. Biogeochemistry and the structure of tropical brown food webs. Ecology. 2009 doi: 10.1890/08-1795.1. in press. [DOI] [PubMed] [Google Scholar]
- 30.Vargo EL, Husseneder C. Biology of subterranean termites: Insights from molecular studies of Reticulitermes and Coptotermes. Annu Rev Entomol. 2009;54:379–403. doi: 10.1146/annurev.ento.54.110807.090443. [DOI] [PubMed] [Google Scholar]
- 31.Aumann GD, Emlen JT. Relation of population density to sodium availability and sodium selection by microtine rodents. Nature. 1965;208:198–199. doi: 10.1038/208198a0. [DOI] [PubMed] [Google Scholar]
- 32.Weir JS. Spatial distribution of elephants in an African national park in relation to environmental sodium. Oikos. 1972;23:1–13. [Google Scholar]
- 33.Zimmerman PR, Greenberg JP, Wandiga SO, Crutzen PJ. Termites: A potentially large source of atmospheric methane, carbon dioxide, and molecular hydrogen. Science. 1982;218:563–565. doi: 10.1126/science.218.4572.563. [DOI] [PubMed] [Google Scholar]
- 34.Wood TG. In: The Role of Terrestrial and Aquatic Organisms in Decomposition Processes. Anderson JM, Macfayden A, editors. Oxford: Blackwell Scientific; 1976. pp. 145–168. [Google Scholar]
- 35.Watson JP. The soil below a termite mound. Eur J Soil Sci. 1962;13:46–59. [Google Scholar]
- 36.Kirk TK, Farrell RL. Enzymatic “combustion”: The microbial degradation of lignin. Annu Rev Microbiol. 1987;41:465–501. doi: 10.1146/annurev.mi.41.100187.002341. [DOI] [PubMed] [Google Scholar]
- 37.Kato K, Kozaki S, Sakuranaga M. Degradation of lignin compounds by bacteria from termite guts. Biotechnol Lett. 1998;20:459–462. [Google Scholar]
- 38.Pasti MB, Pometto AL, Nuti MP, Crawford DL. Lignin-solubilizing ability of actinomycetes isolated from termite (Termitidae) gut. Appl Environ Microbiol. 1990;56:2213–2218. doi: 10.1128/aem.56.7.2213-2218.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lodge DJ. In: The Food Web of a Tropical Rain Forest. Reagan DP, Waide RB, editors. Chicago: Univ Chicago Press; 1996. pp. 53–108. [Google Scholar]
- 40.Fittkau EJ, Klinge H. On biomass and trophic structure of the central Amazonian rain forest ecosystem. Biotropica. 1973;5:2–14. [Google Scholar]
- 41.Kaspari M, Yanoviak S, Dudley R. On the biogeography of salt limitation: A study of ant communities. Proc Natl Acad Sci USA. 2008;105:17848–17851. doi: 10.1073/pnas.0804528105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keller M, Palace M, Asner GP, Pereira R, Silva JNM. Coarse woody debris in undisturbed and logged forests in the eastern Brazilian Amazon. Global Change Biol. 2004;10:784–795. [Google Scholar]
- 43.Chambers JQ, Higuchi N, Schimel JP, Ferreira LV, Melack JM. Decomposition and carbon cycling of dead trees in tropical forests of the central Amazon. Oecologia. 2000;122:380–388. doi: 10.1007/s004420050044. [DOI] [PubMed] [Google Scholar]
- 44.Clark DB, Clark DA, Brown S, Oberbauer SF, Veldkamp E. Stocks and flows of coarse woody debris across a tropical rain forest nutrient and topography gradient. Forest Ecol Manage. 2002;164:237–248. [Google Scholar]
- 45.Dixon RK, et al. Carbon pools and flux of global forest ecosystems. Science. 1994;263:185–190. doi: 10.1126/science.263.5144.185. [DOI] [PubMed] [Google Scholar]
- 46.Stephenson NL. Climatic control of vegetation distribution: The role of water balance. Am Nat. 1990;135:649–670. [Google Scholar]
- 47.Wentz FJ, Ricciardulli L, Hilburn K, Mears C. How much more rain will global warming bring? Science. 2007;317:233–235. doi: 10.1126/science.1140746. [DOI] [PubMed] [Google Scholar]
- 48.Bailey RG, Ropes L. Ecoregions: The Ecosystem Geography of the Oceans and Continents. Berlin: Springer; 1998. [Google Scholar]
- 49.Holdridge LR, Grenke WC, Hatheway WH, Liang T, Tosi JAJ. Forest Environments in Tropical Life Zones: A Pilot Study. New York: Pergamon; 1971. [Google Scholar]
- 50.Madigosky SR, Vatnick I. Microclimatic characteristics of a primary tropical Amazonian rain forest, ACEER, Iquitos, Peru. Selbyana. 2000;21:165–172. [Google Scholar]
- 51.Agosti D, Majer JD, Alonso LE, Schultz TR. Measuring and Monitoring Biological Diversity: Standard Methods for Ground-Living Ants. Washington DC: Smithsonian Press; 2000. [Google Scholar]
- 52.Oklahoma State University Soil, Water, and Forage Analytical Laboratory. 2006. [Accessed February 1, 2009]. Available at www.soiltesting.okstate.edu/
- 53.Arms K, Feeny P, Lederhouse RC. Sodium: Stimulus for puddling behavior by tiger swallowtail butterflies, Papilio glaucus. Science. 1974;185:372–374. doi: 10.1126/science.185.4148.372. [DOI] [PubMed] [Google Scholar]
- 54.Department of Defense. Digital Chart of the Worlds. Washington, DC: Department of Defense; 1992. [Google Scholar]