Abstract
Ecological speciation is the promotion of reproductive isolation via the divergent adaptation of populations to alternative environments. A prediction peculiar to ecological speciation is that hybrids between such populations should be adapted poorly to parental environments, yielding reduced fitness and postmating isolation. However, F1 analyses alone cannot demonstrate that ecological (“extrinsic”) factors contribute to such isolation. Rather, this requires documenting a “switch” in the relative fitnesses of reciprocal backcrosses between environments. Specifically, each backcross should exhibit higher fitness in the environment of its pure parent, with which it shares the most genes, including environment-specific ones. In contrast, because genetic proportions are expected to be similar for all backcrosses (≈¾ from one parental type and ≈¼ from the other), the more general genetic incompatibilities responsible for “intrinsic” isolation predict no such environment-specific fitness switches. Thus, although intrinsic isolation may contribute to the fitness reduction and variation underlying such patterns, it offers an insufficient explanation for them. Here, we present a quantitative genetic “backcross” analysis of sympatric Neochlamisus bebbianae leaf beetle populations adapted to maple versus willow host plants. Results statistically supported ecological speciation predictions, notably the switch in relative fitness for backcross types, the expected rank order of cross type fitnesses, and appreciable extrinsic isolation. We additionally documented genetic variation in host-associated fitness, ruled out nongenetic maternal effects, and discuss the maintenance of ecological differentiation in sympatry. In summary, our study provides a rare and strongly supported demonstration of genetically based, ecologically dependent postmating isolation during ecological speciation.
Keywords: divergent adaptation, ecological speciation, host races, hybrid fitness, reproductive isolation
Understanding the mechanisms of speciation is a fundamental problem in evolutionary biology (1). “Ecological speciation” refers to the evolution of reproductive isolation as an incidental consequence of the divergent adaptation of populations to alternative environments (2–4). Such divergent natural selection long has been thought to play a part in the speciation process (5), but case studies have begun only recently to accumulate (3, 6, 7). Nonetheless, a broad comparative analysis suggests that ecological divergence plays a taxonomically general role in speciation (8), whereas the isolation of ecological contributions via such comparative approaches now is being applied fruitfully to individual study systems (4, 9–12). More generally, well developed model systems for evaluating ecological speciation have been developed in taxa as disparate as stickleback fishes (9, 13), Rhagoletis fruit flies (14), and Mimulus monkey flowers (15).
Many advances have been made in the evaluation of ecologically associated premating reproductive barriers (6). Premating barriers often are associated clearly with ecological divergence and contribute to reproductive isolation, for example, via habitat isolation (4, 14), temporal isolation (16), and premating immigrant inviability (17). Sexual isolation also has been shown to be influenced by ecological factors (4, 18). However, the potential ecological contributions to postmating barriers have been studied much less (7). Moreover, because multiple reproductive barriers have been investigated in relatively few study systems (17, 19, 20), the relative roles of postmating versus premating barriers in speciation are not well understood.
Two aspects of postmating isolation have been distinguished in the literature (1, 7, 21). First, “intrinsic” postmating isolation (hereafter referred to as “intrinsic isolation” for simplicity) reflects low hybrid fitness owing to general genetic incompatibilities between the genomes of divergent populations. For example, such intrinsic isolation may reflect negative epistatic interactions between the alternative alleles that have become fixed across loci between populations. Many studies have demonstrated intrinsic isolation (1). Second, “extrinsic” or ecologically dependent postmating isolation (hereafter referred to as “extrinsic isolation”) specifically refers to reduced hybrid fitness due to the maladaptive intermediacy of their ecologically relevant genotypes and phenotypes in parental environments (22). Thus, extrinsic isolation arises as populations divergently adapting to alternative environments climb different adaptive peaks, producing hybrids that fall into fitness valleys (23). Extrinsic isolation provides strong support for ecological speciation.
Despite belief in its importance and support from theoretical models (24), few studies have evaluated ecological contributions to postmating isolation, perhaps because of the labor-intensive nature of such research. Indeed, as pointed out by Coyne and Orr (1), “It has become fashionable to suggest that extrinsic, and especially ecological, postzygotic isolation is more common or more important than intrinsic in nature. This might well be true. However, at present, such assertions rest more on intuition than data” (p 255). Important examples invoking such ecological factors include an investigation of F1 hybrids between the benthic and the limnetic forms of three-spine stickleback (22). These F1 hybrids were anatomically intermediate between parental morphologies and grew more poorly in the parental environments than each parent. Similarly, studies of three herbivorous insect species, each with populations specialized on one of two host plants, found F1 hybrids to perform more poorly than parental types on one (25) or both parental hosts (26, 27).
These patterns could be explained by contributions from extrinsic isolation if (i) F1 hybrids inherited the alleles underlying the (divergent) local adaptation of each parent population and (ii) the combination of these alleles in hybrids yielded phenotypes unsuitable to either parental environment. However, inferring extrinsic isolation from such results has been criticized (ref. 1, p 250) on the grounds that they cannot rule out intrinsic factors as a sufficient explanation for observed postmating isolation. That is, F1 fitness reduction simply could reflect general genetic incompatibilities between alleles at loci that do not contribute to ecological adaptation. Even when F1 hybrid fitness is lower in parental environments (where they are exposed to natural ecological factors) than in the laboratory (where they are not) (e.g., ref. 22), extrinsic isolation may not be the cause. This is because such results could instead reflect a general tendency for organisms to perform better in “benign” lab environments as compared with “harsher” natural ones (28, 29).
A solution to the problem of rigorously documenting extrinsic isolation was provided by Rundle and Whitlock (30), who extended a quantitative genetic model of population crosses (31) to include two environments. The extended model demonstrates that whereas the analysis of F1 hybrids alone cannot distinguish the contributions of intrinsic versus extrinsic gene effects, extending the analysis to the next generation can. Specifically, evaluating both reciprocal backcross hybrids in each parental environment allows the additive-by-environment interaction (α1ε) to be isolated and evaluated.
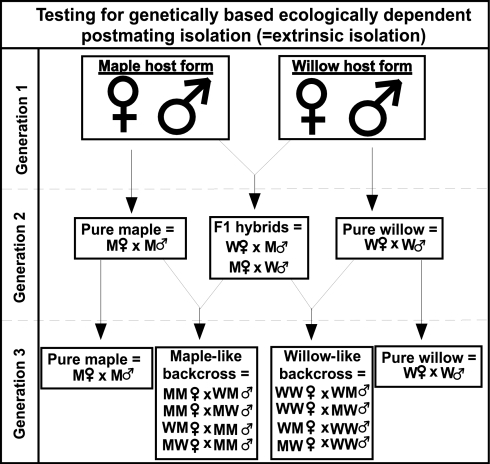
The “backcross approach” developed by Rundle and Whitlock on the basis of this model provides an experimental crossing design (Fig. 1) that allows potential extrinsic contributions to postmating isolation to be addressed empirically. This approach relies on three points. First, for each reciprocal backcross (i.e., crosses between one or the other “pure” parental type and an F1 hybrid), ¾ of its genes ultimately derive from one pure parental type, and ¼ derive from the other. Second, if and only if extrinsic factors are contributing, environment-specific fitnesses are predicted to differ between the two reciprocal backcross types, depending on which pure parental type produced the backcross. This is because backcross type A will possess ¾ of the genes involved in ecological adaptation to the environment of its pure type A parent and only ¼ of the genes involved in adaptation to the alternative environment of pure parental type B. The analogous pattern holds with respect to backcross type B. These observations underlie the inference of Rundle and Whitlock (30) that extrinsic isolation is demonstrated when the relative fitnesses of the two backcross types vary (switch) between parental environments. That is, each backcross type should exhibit relatively higher fitness in the environment of the parent to which it is most genetically similar. This result is indicated statistically by an interaction between backcross type and parental environment. Third, because each backcross type necessarily exhibits the same degree of hybridity, the two backcross types, in contrast, are not predicted inherently to exhibit considerable differences in environment-specific fitness if caused by intrinsic genetic incompatibilities alone.
Fig. 1.
Experimental crossing design to create F1 and backcross hybrids, along with pure parental (i.e., within-host-form) crosses, for tests of extrinsic isolation. Generation 1 was collected from the field as immature animals and reared to maturity in the laboratory, whereas generations 2 and 3 were propagated entirely in the laboratory or greenhouse.
In the present study, we adopt and extend the backcross approach in an analysis of sympatric populations of Neochlamisus bebbianae leaf beetles at a site in Vermont, where their respective host plants intermingle in the same microhabitat. These study populations represented the “maple host form” and the “willow host form” of N. bebbianae (4), which specialize on red maple (Acer rubrum, Aceraceae) and Bebb's willow (Salix bebbiana, Salicaceae) host plants, respectively. These host forms are differentiated partially ecologically in host preference and performance traits and exhibit partial premating reproductive isolation, apparently as a consequence of divergent host adaptation (4, 6, 32, 33). They thus have provided an informative system for investigating ecological speciation.
Our experiments involved two generations of mating, yielding parental and hybrid offspring representing various cross types (Fig. 1). For each beetle family in our experiments, an equal number of offspring were reared on each of the two host/test plants representing the parental environments in our study. This provided relative growth rate (RGR) data (34). Faster insect development (greater RGR), for example, may increase the likelihood of survival to maturation in the face of predators, high reproductive success reflecting longer access to mates, or reaching the life history stage required for diapause when the growing season is short (35). Thus, we used RGR as our measure of relative viability or fitness (35), following Rundle (36). Our primary goal was (i) to use the backcross approach to rigorously document whether extrinsic isolation exists between study populations. Complementary objectives included (ii) evaluating genetic variation in host performance via the examination of family-level variation, (iii) investigating possible nongenetic maternal effects (37), (iv) comparing the contributions of various reproductive barriers to reproductive isolation, and (v) considering the relevance of these and prior findings with respect to the sympatric status of these populations.
Results
Generation 2: Performance of F1 Hybrid and Pure Parental Crosses.
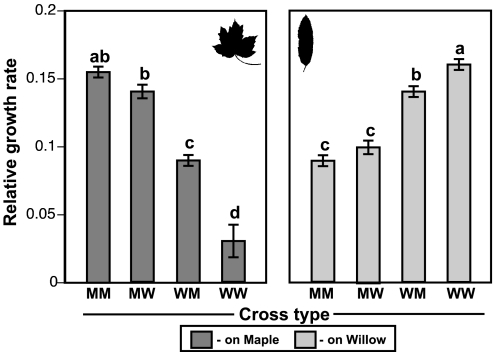
These four cross types demonstrated a significant Cross Type by Host Environment interaction (Table 1), and most pairwise cross type comparisons proved significant for each host (Fig. 2). Fitness was the greatest for pure parental types on their native host plant, followed by both hybrid crosses, with pure parental types on the foreign host doing the most poorly. Both reciprocal hybrids performed best on their maternal host (see below). The reduced fitness of hybrids compared with that of pure parental types on their native host, and the switching of relative performance of cross types across hosts (i.e., the interaction term mentioned above) were consistent with the possibility of extrinsic postmating isolation, if not proving it.
Table 1.
ANOVA on relative growth rate demonstrating extrinsic postmating isolation
| Effects | df | MS | F | P |
|---|---|---|---|---|
| Generation 2: F1 hybrid and pure crosses | ||||
| Cross type | 3 | 0.00194 | 1.29 | 0.3022 |
| Host environment | 1 | 0.01515 | 30.51 | <0.0001 |
| Cross type by host environment | 3 | 0.08559 | 172.40 | <0.0001 |
| Family (cross type) | 22 | 0.00151 | 1.28 | 0.1869 |
| Family (cross type) by environment | 16 | 0.00050 | 0.42 | 0.9764 |
| Residual | 249 | 0.00118 | ||
| Generation 3: Backcross hybrids | ||||
| Backcross type | 1 | 0.00098 | 2.19 | 0.1469 |
| Host environment | 1 | 0.00065 | 0.43 | 0.5171 |
| Backcross type by host environment | 1 | 0.03999 | 26.55 | <0.0001 |
| Family (cross type) | 38 | 0.00045 | 0.58 | 0.9794 |
| Family (cross type) by environment | 35 | 0.00151 | 1.94 | 0.0015 |
| Residual | 348 | 0.00078 |
Fig. 2.
Mean ± SEM of the relative growth rate to day 14 for F1 hybrid and pure parental cross types on red maple (dark gray) and Bebb's willow (light gray) foliage. Different letters over bars indicate significant differences between cross types, based on a Tukey HSD test at P < 0.05. Abbreviations applied to offspring of each cross type: MM = maple female × maple male; MW = maple female × willow male; WM = willow female × maple male; WW = willow female × willow male.
Generation 3: Performance of Backcross Hybrid and Pure Parental Crosses.
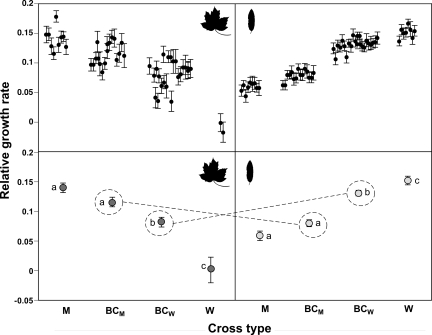
As in the generation 2 results, Cross Type fitness varied significantly on both maple (F3,46 = 20.5, P = 0.0004) and willow (F3,51 = 58.8, P < 0.0001), with backcrosses consistently exhibiting lower fitnesses than pure parental types on their native hosts and thus a degree of postmating isolation. Most central to this study, these patterns were exactly as predicted by the ecological speciation hypothesis and documented extrinsic isolation between these maple and willow host form populations. Two results support this conclusion. First, the relative fitnesses of these reciprocal backcrosses switched order across test plants (Fig. 3), as also indicated by a highly significant Backcross Type by Host Environment interaction term (Table 1). Specifically, the relative fitnesses of each backcross type on a given test plant corresponded to its genetic similarity to the pure parental type natively associated with that plant. A genetic basis for these results was supported further by a significant Family by Host Environment term (Table 1), indicating genetic variation in host-specific performance. For the sake of completeness, we also evaluated possible variation among the four unique crosses that comprise each backcross type (Fig. 1) via a hierarchical ANOVA. However, this factor did not affect the Backcross Type by Host Environment interaction that is the focus of our study, so the simpler analysis is presented. Second, the rank order of the cross type fitnesses is precisely as expected in each host environment (Fig. 3), and six of eight adjacent cross type fitnesses differed significantly (Fig. 3). The likelihood of this ranking pattern being observed by chance is P = (1/4!)2 = 0.0017.
Fig. 3.
Mean ± SEM of the relative growth rate to day 14 for backcross hybrid and pure parental cross types on red maple (Left) and Bebb's willow (Right) foliage. Individual family means are presented (Upper) as well as the means of these family means (Lower). Each backcross type is circled and connected between panels by a dashed line to illustrate the ecologically dependent fitness of backcross hybrids (i.e., switching). This pattern documents extrinsic postmating isolation, a critical prediction of ecological speciation. The small number of families representing the pure parental willow cross type on maple reflects the general incapacity of this host form to survive on maple foliage. Different letters show significant differences from a Tukey HSD test at P < 0.05.
Generation 3: Evaluation of Possible Maternal Effects.
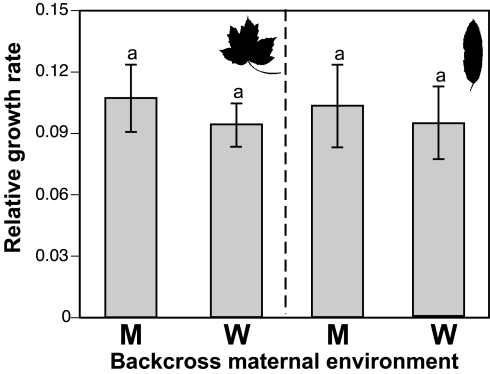
To evaluate the possibility that maternal rearing environment influenced offspring fitness, we added Maternal Host Plant as a fixed effect to the ANOVA model. No such influences were observed (Maternal Host Plant, F1,347 = 1.8393, P = 0.1759), and the Backcross Type by Host Plant interaction term remained significant. In a complementary approach, we performed two separate analyses, one each for individuals whose mothers had been reared on maple versus willow, respectively. These analyses thus removed any maternal contributions to the results of our earlier ANOVAs. Nonetheless, both analyses again revealed highly significant Backcross by Host Plant interaction terms, indicating that our evidence for extrinsic isolation was not due to such maternal effects (Table 2). A lack of nongenetic maternal effects was supported further by the similar offspring fitnesses across maternal rearing environments within each test plant environment (Fig. 4).
Table 2.
ANOVA on relative growth rate for backcrosses analyzed separately by maternal host plant
| Effects | df | MS | F | P |
|---|---|---|---|---|
| Offspring with maple maternal host plant | ||||
| Cross type | 1 | 0.00017 | 0.05 | 0.8259 |
| Host environment | 1 | 0.00015 | 30.88 | <0.0001 |
| Cross type by host environment | 1 | 0.06829 | 13937.40 | <0.0001 |
| Family (cross type) | 16 | 0.00322 | 4.88 | <0.0001 |
| Family (cross type) by environment | 16 | 0.00000 | 0.01 | 0.99 |
| Residual | 173 | 0.00066 | ||
| Offspring with willow maternal host plant | ||||
| Backcross type | 1 | 0.00158 | 1.03 | 0.3217 |
| Host environment | 1 | 0.00139 | 1.57 | 0.2262 |
| Backcross type by host environment | 1 | 0.05235 | 59.26 | <0.0001 |
| Family (cross type) | 21 | 0.00154 | 1.57 | 0.0614 |
| Family (cross type) by environment | 18 | 0.00088 | 0.90 | 0.5781 |
| Residual | 173 | 0.00098 |
Fig. 4.
Mean ± SEM of the relative growth rate to day 14 for offspring deriving from dams that had been reared on either maple (Left) or willow (Right) test plants. This plot demonstrates a lack of effects on offspring performance as a function of maternal environment, because no differences were observed for any comparison (P > 0.05, see Results and Table 2 for details).
In this context, that both reciprocal F1 hybrid cross types nonetheless performed better on their maternal host than their paternal host is intriguing. In the apparent absence of an environmental explanation for this pattern, a genetic one might be sex linkage. This is consistent with the tendency for male leaf beetles to be the heterogametic sex and for genes responsible for host plant adaptation in herbivorous insects to be located on the X chromosome (38). However, the lack of sex-based differences in hybrids observed here suggests that any such X-linked genes likely exhibit dominance.
Generation 3: Quantifying and Comparing Reproductive Isolation for Backcross Hybrids.
We quantified the “individual contribution” of extrinsic isolation, that is, the magnitude of reproductive isolation that it would produce if acting alone, on a scale of 0.0 to 1.0, and compared it with values previously calculated for other reproductive barriers between these host forms (17, 19), yielding the following: habitat isolation = 0.39, premating immigrant inviability = 0.58, sexual isolation = 0.61, extrinsic postmating isolation = 0.36. We further found the fitness decline in both backcrosses to be appreciable when reared on the “wrong” test plant. It was also asymmetric. Specifically, the “maple-like” backcross type grew >45% faster on maple than that on willow, whereas the “willow-like” backcross type grew >70% faster on willow than that on maple.
Discussion
Rundle and Whitlock (30) emphasize that “any isolation detected by a comparison of F1 or F2 hybrids to the native parental form in each habitat can involve contributions of both intrinsic genetic and environment-dependent gene effects” (p 200) and thus conclude that “a reduction of F1 or F2 fitness relative to parental forms in a transplant experiment is not sufficient evidence for ecological speciation” (p 201). This argument is critical for two reasons. First, few studies of postmating isolation in natural populations have acquired data on the backcross generations necessary to make this distinction. Thus, little rigorous documentation of extrinsic postmating isolation exists. Second, although intrinsic isolation can evolve readily by various mechanisms (e.g., by genetic drift or even as a consequence of divergent selection), extrinsic postmating isolation is explained most easily as a by-product of ecologically adaptive divergence (7). Thus, rigorously documenting extrinsic isolation offers especially strong corroboration of the ecological speciation hypothesis. Extrinsic isolation further has been argued to evolve possibly commonly as populations initially adapt to different environments, thus playing a critical role in the early stages of speciation (1, 2). Indeed, various models of speciation with gene flow rely on assumptions of reduced fitness in phenotypically intermediate hybrids (39).
Our study of the maple and willow host forms of N. bebbianae leaf beetles was inspired by the papers that first developed (30) and empirically tested (36) the backcross approach for evaluating extrinsic isolation. Our rationale and results thus are compared most readily with this original investigation (36) plus a recent article (40) representing the only other application of this approach. These prior studies both treat ecologically divergent pairs of fish species—benthic and limnetic forms of three-spine stickleback and killifish adapted to divergent salinity regimes, respectively—and provide varying degrees and forms of evidence for extrinsic isolation. The present study extends the backcross approach by incorporating family-level effects and maternal rearing environment in our ANOVA models. These allowed us to evaluate genetic variation and potential nongenetic maternal influences on our results, respectively. Unlike the prior studies, all 14 possible cross types (Fig. 1) were evaluated.
Most importantly, our investigation provides strong statistical documentation of genetically based, ecologically dependent postmating isolation (i.e., extrinsic isolation) between the ecologically divergent maple- and willow-associated host forms of N. bebbianae leaf beetles. That is, we demonstrate that the relative fitnesses of the two reciprocal backcrosses switch across host environments (Fig. 3). Specifically, the “maple-like” backcross type (having a pure maple-associated parent) grew >45% faster on maple than that on willow, whereas the “willow-like” backcross type grew >70% faster on willow than that on maple. Such patterns cannot be explained sufficiently by intrinsic factors (30). Further, the complete match of rank fitnesses among cross types in each environment to predictions of ecological speciation had not been demonstrated previously (30, 36, 40). These results further corroborate accumulating evidence that these host forms are undergoing ecological speciation.
Multiple reproductive barriers, especially postmating barriers, to reproductive isolation have been evaluated for only a modest number of taxa, one being the present study system (17). Comparisons among four such barriers revealed extrinsic isolation to be 62% as strong as the strongest among them, indicating its appreciable contribution to ongoing speciation. The various forms of strong support for extrinsic isolation's importance provided by this study might reflect partly the nature of the alternative beetle environments evaluated here. For specialized insect herbivores, such as these beetles, the host plant is often the site of all life activities. Further, alternative host plants represent environments that are rather biologically discrete, as opposed to continuously varying. This is especially true when, as here, these alternative hosts belong to phylogenetically disparate plant families. In such situations, not only are hybrid-friendly biologically intermediate habitats nonexistent, but host-related selection pressures also are expected to be especially specific, strong, and divergent between host-associated populations. In turn, this may predispose such populations to divergent adaptation and the reproductive isolation predicted to accompany it under models of ecological speciation. This contrasts with hybridizing taxa that inhabit more continuous and less starkly differentiated environments. For example, sticklebacks inhabit benthic and open-water lake habitats that grade into each other (22), whereas big sagebrush experience habitat gradients along mountain slopes (41).
Nonetheless, despite the arguments for host-associated differentiation reviewed so far, other current findings combine with prior results to suggest that—and perhaps help explain why—these host forms have not progressed to biological species status. For example, some backcross individuals and families exhibited higher growth rates on the pure parental host than the pure parental types themselves (Fig. 3). This illustrates within-host-form genetic variation of a kind that might facilitate gene flow between host forms. Indeed, such gene flow is indicated not only by incomplete reproductive isolation but also by a recent study based on amplified fragment length polymorphisms of these and additional maple and willow host form populations (42). That study revealed genetic homogenization and close phylogenetic relationships between the present study populations at putatively neutral loci. Indeed, these sympatric maple and willow host form populations proved more similar to each other than to allopatric populations of the same host form at these loci, consistent with local gene flow. The study also found that a subset of loci apparently evolving under divergent host-related selection was differentiated highly between these sympatric host forms. Such results indicate that host-specific divergent selection is strong enough to maintain differentiation at genomic regions associated with host adaptation in the face of recurrent gene flow. Thus, the phenotypic results of the present study corroborate recent molecular ones in explaining how sympatric maple and willow host forms can remain differentiated ecologically despite incomplete reproductive isolation. They further satisfy various criteria hinting at their possible status as host races (43).
In summary, recent reviews have noted the lack of compelling tests of ecologically dependent postmating isolation (1, 7), even though it is a specific prediction of ecological speciation (30). Here, we rigorously document a clear example of such extrinsic isolation among sympatric, ecologically divergent leaf beetle populations representing two N. bebbianae host forms. We do so while controlling for potential contributions from intrinsic genetic incompatibilities, maternal effects, and family-level variation. Future work on this system, for example, will evaluate temporal changes in the proportion of hybrids across the life cycle of these populations using molecular markers. Such data will allow the quantification of hybrid cross type frequencies, the strength of selection acting on them, and patterns of gene flow between host forms (44, 45). These and additional investigations will provide further insights into the little-studied contributions of extrinsic isolation to ecological speciation.
Materials and Methods
Natural History of N. bebbianae Host Forms.
Neochlamisus bebbianae (Brown) is an eastern North American leaf beetle (Coleoptera: Chrysomelidae) that is univoltine and uses specific host plant species from six genera in five different families (46, 47). The suite of populations associated with each host plant is referred to as a particular host form (4), each of which exhibits host-specific adaptations (4, 33). The maple and willow host forms studied here are sympatric in moist and disturbed habitats across northeastern North America. All life activities, from oviposition through larval development and adult emergence, feeding, and mating, occur on the host plant, although adults fly between individual plants to find oviposition sites and mates. The normally obligate adult winter diapause of these beetles can be broken by manipulating greenhouse conditions, allowing the continual production of new generations for experiment.
Experimental Crosses and Larval Performance or Fitness Assays.
All Generation 1 test animals were collected on their host plants during the summer of 2007 from a site in Caledonia County, Vermont (44.402°N, 71.917°W). These were brought to Vanderbilt University and individually raised to maturity on cuttings of their native host plants (for details, see ref. 4). From these adults, F1 hybrid and pure parental offspring cross types were generated to form Generation 2. In turn, adults from this generation were used to create the backcross and pure parental cross types of Generation 3. See Fig. 1 for further details on the crossing design.
All test families were derived from individual male/female matings, and no beetle was mated more than once. Beetles were paired in 5-cm Petri dishes lined with moistened filter papers and continually observed for 2 h. If copulation was confirmed visually during this period, then the pair was left together overnight to facilitate further insemination. In the absence of copulation, females were paired later with a male of the same type as its original partner. Mated Generation 1 and Generation 2 females then were housed individually in 30 cm × 15 cm mesh bags that were tied over a meristem on a sapling tree of its native host plant, on which they oviposited, thus providing the next generation of test offspring. Bags were constructed of DelNet (DelStar Technologies). All saplings represented genotypes native to northeastern North America and were maintained in the Vanderbilt University greenhouse. Greenhouse conditions were maintained at 21–24 °C during the day, 18–21 °C at night, a 14:10 light/dark cycle, and >70% relative humidity, mimicking summer conditions at the collection locality. These conditions yielded the continual production of newly flushed leaves that prompted oviposition and provided test foliage during the September 2007–May 2008 period of this experiment.
Eggs were harvested from bags on a weekly basis and maintained in family-specific, filter-paper-lined Petri dishes that were stacked in sealed plastic boxes lined with moist paper towels. These boxes were kept in an incubator at 24 °C and a 14:10 light/dark cycle, and eggs were checked daily for larval emergence. Individual larval offspring were weighed (wt1) using a MX5 microbalance (Mettler Toledo) on the day of emergence (t1), and those from each family were assigned alternately to either maple or willow as the plant on which they would be reared, following their order of emergence. Each test larva then was maintained individually in a 5-cm Petri dish lined with moist filter paper and a cutting of its test plant. Dishes were cleaned, and foliage was replaced every 2 days. Larval weight (wt2) again was measured on day 14 (t2) of rearing to calculate RGR. RGR = [(ln wt2) − (ln wt1)]/(t2 − t1) and represents the proportional increase in mass per unit time, accounting for initial size and the nonlinear nature of growth over time (34).
Statistical Analysis.
Fitness comparisons of offspring from the reciprocal F1 hybrid and pure parental cross types of generation 2 used an ANOVA model that included Cross Type (MM, MW, WM, WW), Host Environment (maple or willow), and Cross Type by Host Environment as fixed effects and Family nested within Cross Type and Family by Environment nested within Cross Type as random effects. The focal Generation 3 analyses used two different approaches. First, separate ANOVAs using data for individuals from each rearing host were conducted to evaluate the effect of cross type per se on fitness. Here, Cross Type was a fixed effect, and Family nested within Cross Type was a random effect. Second, to more specifically test whether backcross fitness was ecologically dependent, an ANOVA model was used that included Backcross Type (BCmaple or BCwillow), Host Environment, and Backcross Type by Host Environment as fixed effects and Family nested within Backcross Type and Family by Environment nested within Backcross Type as random effects. Additionally, two analyses were performed to assess potential contributions of nongenetic host-associated maternal effects on our results. These used ANOVAs in a manner analogous to the main analyses just detailed and otherwise are described adequately in Results. All ANOVA models including random effects were fit using the restricted maximum likelihood method (48), and F ratios were constructed to test effects of a partially nested design (49). All posthoc treatments of mean comparisons were conducted using a Tukey HSD test, which accounts for multiple comparisons. All statistical analyses were performed using JMP, version 5.0.1a (50).
Quantifying Reproductive Isolation.
We calculated the individual contribution of extrinsic isolation (EI) to total reproductive isolation (following ref. 17) as EI = backcross fitness − (backcross fitness/pure parental fitness), where backcross and pure parental fitnesses were each estimated using mean RGR across all possible combinations of cross type and test plant environment. Analogous values from other reproductive barriers estimated for this system (17) using published formulas (19) also were compiled. Finally, we calculated the relative fitness reduction of the reciprocal backcross types on their nonnative test plants by comparing the relative growth rates of each backcross on each of the two test plants.
Acknowledgments.
We thank D. McCauley and an anonymous reviewer for statistical advice; C. Brown, N. Spiegel, and S. Gibson for assistance with beetle husbandry and data collection; and two anonymous reviewers for their useful comments. We also acknowledge New England Wetland Plants and especially Pterophylla Nursery for providing the sapling plants used in our experiments. This work was funded by grants to D.J.F. from the National Science Foundation (DEB No. 0221262) and Vanderbilt University's Discovery Grant program.
Footnotes
The authors declare no conflict of interest.
References
- 1.Coyne JA, Orr HA. Speciation. Sunderland, MA: Sinauer; 2004. [Google Scholar]
- 2.Schluter D. The Ecology of Adaptive Radiation. Oxford: Oxford Univ Press; 2000. [Google Scholar]
- 3.Schluter D. Ecology and the origin of species. Trends Ecol Evol. 2001;16:372–380. doi: 10.1016/s0169-5347(01)02198-x. [DOI] [PubMed] [Google Scholar]
- 4.Funk DJ. Isolating a role for natural selection in speciation: Host adaptation and sexual isolation in Neochlamisus bebbianae leaf beetles. Evolution. 1998;52:1744–1759. doi: 10.1111/j.1558-5646.1998.tb02254.x. [DOI] [PubMed] [Google Scholar]
- 5.Mayr E. Ecological factors in speciation. Evolution. 1947;1:263–288. [Google Scholar]
- 6.Funk DJ, Filchak KE, Feder JL. Herbivorous insects: Model systems for the comparative study of speciation ecology. Genetica. 2002;116:251–267. [PubMed] [Google Scholar]
- 7.Rundle HD, Nosil P. Ecological speciation. Ecol Lett. 2005;8:336–352. [Google Scholar]
- 8.Funk DJ, Nosil P, Etges WJ. Ecological divergence exhibits consistently positive associations with reproductive isolation across disparate taxa. Proc Natl Acad Sci USA. 2006;103:3209–3213. doi: 10.1073/pnas.0508653103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rundle HD, Nagel L, Wenrick Boughman J, Schluter D. Natural selection and parallel speciation in sympatric sticklebacks. Science. 2000;287:306–308. doi: 10.1126/science.287.5451.306. [DOI] [PubMed] [Google Scholar]
- 10.Nosil P. Divergent host plant adaptation and reproductive isolation between ecotypes of Timema cristinae walking sticks. Am Nat. 2007;169:151–162. doi: 10.1086/510634. [DOI] [PubMed] [Google Scholar]
- 11.Langerhans RB, Gifford ME, Joseph EO. Ecological speciation in Gambusia fishes. Evolution. 2007;61:2056–2074. doi: 10.1111/j.1558-5646.2007.00171.x. [DOI] [PubMed] [Google Scholar]
- 12.Bolnick DI, Near TJ, Wainwright PC. Body size divergence promotes post-zygotic reproductive isolation in centrarchids. Evol Ecol Res. 2006;8:903–913. [Google Scholar]
- 13.Vines TH, Schluter D. Strong assortative mating between allopatric sticklebacks as a by-product of adaptation to different environments. Proc R Soc London Ser B. 2006;273:911–916. doi: 10.1098/rspb.2005.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feder JL, et al. Host fidelity is an effective pre-mating barrier between sympatric races of the apple maggot fly. Proc Natl Acad Sci USA. 1994;91:7990–7994. doi: 10.1073/pnas.91.17.7990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lowry DB, Rockwood RC, Willis JH. Ecological reproductive isolation of coast and inland races of Mimulus guttatus. Evolution. 2008;62:2196–2214. doi: 10.1111/j.1558-5646.2008.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wood TK, Keese MC. Host-plant-induced assortative mating in Enchenopa treehoppers. Evolution. 1990;44:619–628. doi: 10.1111/j.1558-5646.1990.tb05942.x. [DOI] [PubMed] [Google Scholar]
- 17.Nosil P, Vines TH, Funk DJ. Perspective: Reproductive isolation caused by natural selection against immigrants from divergent habitats. Evolution. 2005;59:705–719. [PubMed] [Google Scholar]
- 18.Etges WJ. Premating isolation is determined by larval rearing substrates in cactophilic Drosophila mojavensis. IV. Correlated responses in behavioral isolation to artificial selection on a life-history trait. Am Nat. 1998;152:129–144. doi: 10.1086/286154. [DOI] [PubMed] [Google Scholar]
- 19.Ramsey J, Bradshaw HD, Jr, Schemske DW. Components of reproductive isolation between the monkeyflowers Mimulus lewisii and M. cardinalis (Phrymaceae) Evolution. 2003;57:1520–1534. doi: 10.1111/j.0014-3820.2003.tb00360.x. [DOI] [PubMed] [Google Scholar]
- 20.Lowry DB, Modliszewski JL, Wright KM, Wu CA, Willis JH. The strength and genetic basis of reproductive isolating barriers in flowering plants. Philos Trans R Soc London Ser B. 2008;363:3009–3021. doi: 10.1098/rstb.2008.0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rice WR, Hostert EE. Laboratory experiments on speciation: What have we learned in 40 years? Evolution. 1993;47:1637–1653. doi: 10.1111/j.1558-5646.1993.tb01257.x. [DOI] [PubMed] [Google Scholar]
- 22.Hatfield T, Schluter D. Ecological speciation in sticklebacks: Environment-dependent hybrid fitness. Evolution. 1999;53:866–873. doi: 10.1111/j.1558-5646.1999.tb05380.x. [DOI] [PubMed] [Google Scholar]
- 23.Gavrilets S. Fitness Landscapes and the Origin of Species. Princeton: Princeton Univ Press; 2004. [Google Scholar]
- 24.Thibert-Plante X, Hendry AP. Five questions on ecological speciation addressed with individual-based simulations. J Evol Biol. 2009;22:109–123. doi: 10.1111/j.1420-9101.2008.01627.x. [DOI] [PubMed] [Google Scholar]
- 25.Forister ML. Independent inheritance of preference and performance in hybrids between host races of Mitoura butterflies (Lepidoptera: Lycaenidae) Evolution. 2005;59:1149–1155. [PubMed] [Google Scholar]
- 26.Craig TP, Itami JK, Craig JV. Host plant genotype influences survival of hybrids between Eurosta solidaginis host races. Evolution. 2007;61:2607–2613. doi: 10.1111/j.1558-5646.2007.00209.x. [DOI] [PubMed] [Google Scholar]
- 27.Via S, Bouck AC, Skillman S. Reproductive isolation between divergent races of pea aphids on two hosts. II. Selection against migrants and hybrids in the parental environments. Evolution. 2000;54:1626–1637. doi: 10.1111/j.0014-3820.2000.tb00707.x. [DOI] [PubMed] [Google Scholar]
- 28.Bordenstein SR, Drapeau MD. Genotype-by-environment interactions and the Dobzhansky–Muller model of postzygotic isolation. J Evol Biol. 2001;14:490–501. [Google Scholar]
- 29.Hoffman AA, Merilä J. Heritable variation and evolution under favourable and unfavourable conditions. Trends Ecol Evol. 1999;14:96–101. doi: 10.1016/s0169-5347(99)01595-5. [DOI] [PubMed] [Google Scholar]
- 30.Rundle HD, Whitlock MC. A genetic interpretation of ecologically dependent isolation. Evolution. 2001;55:198–201. doi: 10.1111/j.0014-3820.2001.tb01284.x. [DOI] [PubMed] [Google Scholar]
- 31.Lynch M. The genetic interpretation of inbreeding depression and outbreeding depression. Evolution. 1991;45:622–629. doi: 10.1111/j.1558-5646.1991.tb04333.x. [DOI] [PubMed] [Google Scholar]
- 32.Funk DJ, Nosil P. In: The Evolutionary Biology of Herbivorous Insects: Specialization, Speciation, and Radiation. Tilmon K, editor. Berkeley: Univ of California Press; 2008. pp. 117–135. [Google Scholar]
- 33.Egan SP, Funk DJ. Individual advantages to ecological specialization: Insights on cognitive constraints from three conspecific taxa. Proc R Soc London Ser B. 2006;273:843–848. doi: 10.1098/rspb.2005.3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hunt R. Plant Growth Curves: The Functional Approach to Plant Growth Analysis. London: Edward Arnold; 1982. [Google Scholar]
- 35.Nylin S, Gotthard K. Plasticity in life-history traits. Annu Rev Entomol. 1998;43:63–83. doi: 10.1146/annurev.ento.43.1.63. [DOI] [PubMed] [Google Scholar]
- 36.Rundle HD. A test of ecologically dependent postmating isolation between sympatric sticklebacks. Evolution. 2002;56:322–329. doi: 10.1111/j.0014-3820.2002.tb01342.x. [DOI] [PubMed] [Google Scholar]
- 37.Rossiter MC. Incidence and consequence of inherited environmental effects. Annu Rev Ecol Syst. 1996;27:451–476. [Google Scholar]
- 38.Prowell DP. In: Endless Forms: Species and Speciation. Howard DJ, Berlocher SH, editors. New York: Oxford Univ Press; 1998. pp. 309–319. [Google Scholar]
- 39.Dieckmann U, Doebeli M, Metz JAJ, Tautz D. Adaptive Speciation. Cambridge, UK: Cambridge Univ Press; 2004. [Google Scholar]
- 40.Fuller RC. Genetic incompatibilities in killifish and the role of environment. Evolution. 2008;62:3056–3068. doi: 10.1111/j.1558-5646.2008.00518.x. [DOI] [PubMed] [Google Scholar]
- 41.Wang H, McArthur ED, Sanderson SC, Graham JH, Freeman DC. Narrow hybrid zone between two subspecies of big sagebrush (Artemisia tridentata: Asteraceae). IV. Reciprocal transplant experiments. Evolution. 1997;51:95–102. doi: 10.1111/j.1558-5646.1997.tb02391.x. [DOI] [PubMed] [Google Scholar]
- 42.Egan SP, Nosil P, Funk DJ. Selection and genomic differentiation during ecological speciation: Isolating the contributions of host association via a comparative genome scan of Neochlamisus bebbianae leaf beetles. Evolution. 2008;62:1162–1181. doi: 10.1111/j.1558-5646.2008.00352.x. [DOI] [PubMed] [Google Scholar]
- 43.Drès M, Mallet J. Host races in plant-feeding insects and their importance in sympatric speciation. Philos Trans R Soc London Ser B. 2002;357:471–492. doi: 10.1098/rstb.2002.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gow JL, Peichel CL, Taylor EB. Ecological selection against hybrids in natural populations of sympatric threespine sticklebacks. J Evol Biol. 2007;20:2173–2180. doi: 10.1111/j.1420-9101.2007.01427.x. [DOI] [PubMed] [Google Scholar]
- 45.Fitzpatrick BM, Shaffer HB. Hybrid vigor between native and introduced salamanders raises new challenges for conservation. Proc Natl Acad Sci USA. 2007;104:15793–15798. doi: 10.1073/pnas.0704791104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karren JB. A revision of the subfamily Chlamisinae of America north of Mexico (Coleoptera: Chrysomelidae) Univ Kans Sci Bull. 1972;49:875–988. [Google Scholar]
- 47.Funk DJ. Molecular systematics of cytochrome oxidase I and 16S from Neochlamisus leaf beetles and the importance of sampling. Mol Biol Evol. 1999;16:67–82. doi: 10.1093/oxfordjournals.molbev.a026039. [DOI] [PubMed] [Google Scholar]
- 48.Sall J, Creighton L, Lehman A. 2nd Ed. Pacific Grove, CA: SAS Inst/Duxbury; 2001. JMP Start Statistics: A Guide to Statistics and Data Analysis Using JMP and JMP IN Software. [Google Scholar]
- 49.Quinn GP, Keough MJ. Experimental Design and Data Analysis for Biologists. New York: Cambridge Univ Press; 2002. [Google Scholar]
- 50.SAS Institute. JMP. Cary, NC: SAS Inst; 2002. Version 5.0.1a. [Google Scholar]