Abstract
Climate variation affects surface ocean processes and the production of organic carbon, which ultimately comprises the primary food supply to the deep-sea ecosystems that occupy ≈60% of the Earth's surface. Warming trends in atmospheric and upper ocean temperatures, attributed to anthropogenic influence, have occurred over the past four decades. Changes in upper ocean temperature influence stratification and can affect the availability of nutrients for phytoplankton production. Global warming has been predicted to intensify stratification and reduce vertical mixing. Research also suggests that such reduced mixing will enhance variability in primary production and carbon export flux to the deep sea. The dependence of deep-sea communities on surface water production has raised important questions about how climate change will affect carbon cycling and deep-ocean ecosystem function. Recently, unprecedented time-series studies conducted over the past two decades in the North Pacific and the North Atlantic at >4,000-m depth have revealed unexpectedly large changes in deep-ocean ecosystems significantly correlated to climate-driven changes in the surface ocean that can impact the global carbon cycle. Climate-driven variation affects oceanic communities from surface waters to the much-overlooked deep sea and will have impacts on the global carbon cycle. Data from these two widely separated areas of the deep ocean provide compelling evidence that changes in climate can readily influence deep-sea processes. However, the limited geographic coverage of these existing time-series studies stresses the importance of developing a more global effort to monitor deep-sea ecosystems under modern conditions of rapidly changing climate.
The ocean occupies ≈71% of the Earth's surface with a mean depth of ≈3,700 m. Deep-sea regions >2,000-m depth cover ≈60% of the Earth's surface. A great proportion of this vast deep-sea expanse, the abyssal zone, has never been explored and much <1% has ever been observed directly. Sampling the deep sea has been constrained by the technological difficulties involved in placing instrumentation remotely in a corrosive medium under high hydrostatic pressure, especially for long time-series studies essential to understanding the effects of long-term processes such as climate change on deep-sea ecosystems. Recent biogeochemical research in the deep sea has been directed largely toward midocean ridges and the chemosynthetic ecosystems that can inhabit such areas. Although scientifically interesting, these areas occupy only a small percentage (<1%) of the deep-sea floor. The vast expanses of the sea floor covered with soft sediment have received less attention, even though their extent is orders of magnitude greater, they play a larger role in carbon cycling, and they are much more likely to be influenced by anthropogenic processes such as recent climate change, mineral and hydrocarbon extraction, and commercial fishing.
Communities of organisms occupying this soft-sediment-covered expanse of the deep sea, considered a food-limited environment, are ultimately fueled by organic matter produced through photosynthesis in surface waters. A portion of this food escapes the surface waters and ultimately sinks to the sea floor. This link between surface-ocean and deep-sea processes has been known since the late 19th century, but now the importance of temporal variation at both seasonal and interannual scales has been substantiated (1).
Deep-sea communities are sensitive to variation in food supply. The abundance of sediment community fauna is positively related to primary production in the overlying water (2, 3). Respiration of the sediment community, an estimate of organic carbon utilization, is positively linked to the amount of particulate organic matter reaching abyssal depths (4, 5). The megafauna, conspicuous inhabitants of the surface sediments, consume detrital material (6–8) that can be linked back to surface water plankton production (9, 10). Our current knowledge of the connections between food supply and benthic community processes in the abyssal ocean are examined here with an emphasis on results from two long-term study sites, one in the Northeast Pacific (Station M, ≈4,100-m depth) and one in the Northeast Atlantic [Porcupine Abyssal Plain (PAP), ≈4,850-m depth; Fig. 1]. The results have been arranged here from the surface to the deep ocean, beginning with variation in climate and upper ocean conditions and the resulting changes in photosynthetic production of sinking particulate organic carbon. These processes are tied to variations in the arrival of the food supply to the deep ocean and the subsequent variation in deep-sea communities and biogeochemical processes at the sea floor.
Fig. 1.
Locations of Station M (Sta. M) at ≈4,100 depth in the Northeast Pacific Ocean and PAP at ≈4,850-m depth in the Northeast Atlantic Ocean. Map was produced by using Google Maps.
Climate and Carbon Flux
The efficiency of particulate organic carbon export from the surface waters to depths of 500 m is estimated to range from 20% to 50% in the North Pacific (11). There is a general trend of decreasing organic carbon flux with increasing depth (12). Sinking particulate organic carbon (POC) flux collected in sedimentation traps at abyssal depths is generally <5% of production in surface waters. However, this minimal food supply sustains the biological communities that inhabit the greatest surface area of our planet, and any alteration in the sinking flux of POC to deep-sea ecosystems could produce pervasive changes.
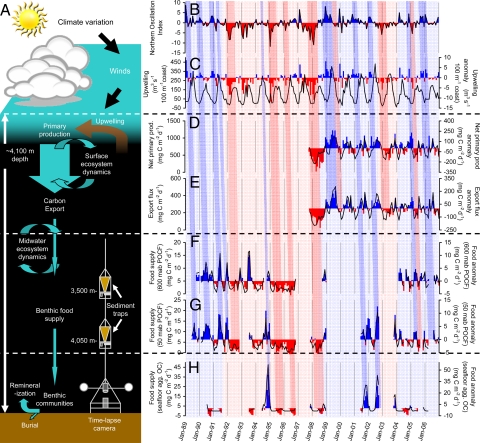
Estimates of food supply based on sinking particles collected in bottom-moored sedimentation traps at abyssal depths have been significantly (P < 0.05) correlated to satellite-estimated surface primary production and export flux when shifted (lagged) earlier by 1–2 months in long time-series studies conducted in both the Northeast Pacific (13) and Northeast Atlantic (14, 15). The magnitude of this food supply [POC flux (POCF)] measured at 3,500- and 4,050-m depth, 600 and 50 m above bottom, respectively, in the Northeast Pacific (Fig. 2A) over a 15-year period has been shown to be significantly correlated with climate indices on ocean basin and regional scales (16). The Pacific-basin scale Northern Oscillation Index (NOI) was significantly correlated with POCF when lagged earlier by 6 months (Fig. 2 B, F, and G). The regional scale Bakun Upwelling Index for an area over the Northeast Pacific site also was significantly correlated with POCF when lagged earlier by 2–3 months (Fig. 2 C, F, and G), indicating a strong relationship between the intensity of coastal upwelling that delivers nutrient-rich water for primary production and POCF to the abyss (16).
Fig. 2.
Schematic of processes and 18-year time series analyses (1989–2007) through the oceanic water column to the sea floor at the Northeast Pacific study site (Station M). (A) Schematic illustrating the simplified process by which climate can influence deep-sea ecology and biogeochemistry. (B) NOI (www.pfeg.noaa.gov/products/PFEL/modeled/indices/NOIX/noix.html), an indicator of El Niño-Southern Oscillation (ENSO) variation in the Northeast Pacific (70), is shown. Monthly data (black lines) and anomalies (positive in blue bars and negative in red bars) are from the Northeast Pacific. (C) Upwelling Index (71) for the California coastline in the vicinity of the Northeast Pacific study site (Station M). (D) Net primary production computed by using the carbon-based production model (72) applied to satellite data collected over monthly periods at a radius of 50 km around the study site. (E) Export flux calculated from net primary production and sea-surface temperature (73). (F and G) POCF to 600 m above bottom (mab) (3,500-m depth) (F) and 50 mab (4,050-m depth) (G). (H) Visibly detectable aggregate fluxes to the seafloor measured by using empirically calibrated time-lapse photography (21). Overlying the time series plots are light blue and light pink shading indicating time periods dominated by positive and negative NOI conditions, respectively. La Niña conditions are associated with the higher peaks in the NOI, as in early 1997 and late 1998, whereas El Niño conditions are associated with lower values of the NOI, as in early 1995 and early 1998. These conditions are then ultimately related to either higher than (light blue) or lower than average (light pink) food supplies. Darker blue and pink bars indicate similar associations on monthly time scales. The slant of the bars from the top to bottom panels is indicative of the time lags linking climate phenomena to seafloor processes.
A portion of the food supply to the deep ocean consists of large sinking aggregates of organic matter that are commonly observed as patches of phytoplankton detritus (phytodetritus) on the deep-sea floor (17, 18). Many of the larger detrital aggregates are believed to be under-sampled by sedimentation traps and represent an often-ignored food supply (15, 19, 20). The seasonal presence of aggregates on the sea floor in the Northeast Pacific has been correlated significantly to pulses of sinking POCF and surface water conditions such as upwelling intensity, primary production, and zooplankton abundance (ref. 21 and Fig. 2).
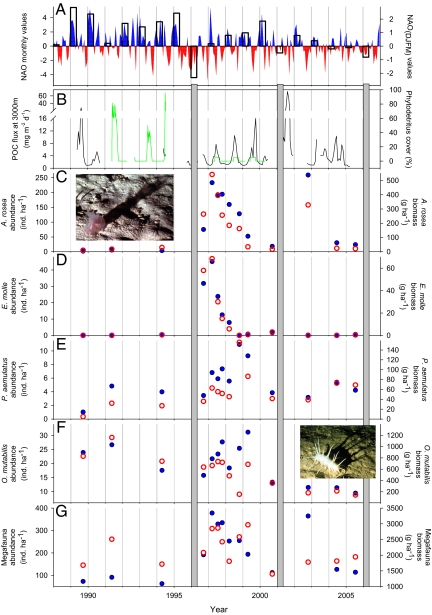
Similar conditions are found in the Northeast Atlantic where the North Atlantic Oscillation (NAO) influences winds, precipitation, storms, and surface ocean mixing especially in winter. Positive variations in the monthly NAO index have been linked to increases in the amount of POC exported from surface waters and ultimately to the amount of POC reaching sedimentation traps at 3,000-m depth (1,850 m above bottom). Increases in monthly surface production and export between 1989 and 1998 typically were followed by changes in abyssal POCF with lags of 0–3 months (refs. 15 and 16 and Fig. 3 A and B). More recent results suggest that year to year variation in POCF can approach an order of magnitude (22).
Fig. 3.
The NAO climate index compared with food supply and benthic community components in the Northeast Atlantic. (A) Monthly (positive in blue bars and negative in red bars) and winter (December, January, February, March; open bar) NAO index (www.cpc.noaa.gov/data/teledoc/nao.shtml). (B) POC flux (black line) to 3,000-m depth and visually detectable phytodetritus aggregate cover (green line) on the seafloor at 4,850-m depth. (C–G) Density (blue circles) and biomass (red open circles) of A. rosea (C), E. molle (D), P. aemulatus (E), O. mutabilis (F), and total megafauna (G). Three strongly negative NAO winters in 1996, 2001, and 2006 are indicated as gray-shaded vertical bars. No faunal data are available during or after the negative value in 2006.
As noted above, Northeast Atlantic climatology can be described by the NAO index (Fig. 3A) and has been linked with POC export from surface waters and supply to the abyssal seafloor (15, 16). In adjacent shallower seas (English Channel and North Sea) variations in the NAO index have been linked with changes in surface water communities from phytoplankton to fishes (23). The winter NAO index value for 1996 (December 1995–March 1996) was unusually low, representing one of the most extreme values over the past 100 years. The 1996 spring bloom was unusually dominated in biomass by small phytoplankton rather than by larger diatoms, as typically is the case. The highest POC fluxes to the deep sea were observed in 2001, another year with a mildly negative wintertime NAO. So, even though the NAO had a significant positive correlation with POCF to 3,000-m depth between 1989 and 2004, important unexplained variation also was observed, perhaps associated with variable efficiency of POCF transfer in the midwater or fluctuations in a process not connected to NAO variation. Data from the PAP also suggest that timing of mixed layer depth shoaling and the seasonal increase in POCF may have occurred progressively earlier over the course of the study period (22), although mechanistic understanding of this trend is currently incomplete. Northeast Atlantic climate variation nonetheless appears to influence POCF.
The apparent connectedness of variations in climate and deep-sea biogeochemical fluxes suggest that, if secular climate change results in altered export fluxes, those shifts would cascade to produce changes in deep-sea ecosystems. Numerical model simulations suggest that increased stratification under future climate change, caused by surface water warming, could result in long-term shifts in surface production and export fluxes (24–26). Empirical results also suggest that open-ocean and alongshore upwelling (27), aerosol and dust nutrient input (28, 29), depth of winter mixing (30), and water clarity or a combination of such mechanisms (31) could be important factors in future variations of export flux to the deep sea.
Atmospheric and upper-ocean warming already have been documented over the past four decades (32). Global warming has been predicted to increase stratification while reducing vertical mixing and nutrient exchange from deeper depths with resulting implications for phytoplankton production (33, 34). Such reduced mixing will enhance variability in primary production and carbon export to the deep sea (35). Nutrient depletion in surface waters caused by increasing stratification is suggested to favor small phytoplankton species over larger species such as diatoms (25) that sink more rapidly and export a greater fraction of primary production in the form of POC into the deep sea (11). Increasing CO2 concentrations resulting in decreasing pH could influence surface plankton production by affecting calcifying plankton such as coccolithophores and foraminiferans (36, 37), reducing their contribution to export flux to the deep sea (11). Research should nonetheless continue to consider the relative importance of other mechanisms, especially those not well-represented in current global climate models.
Deep-Sea Community Structure
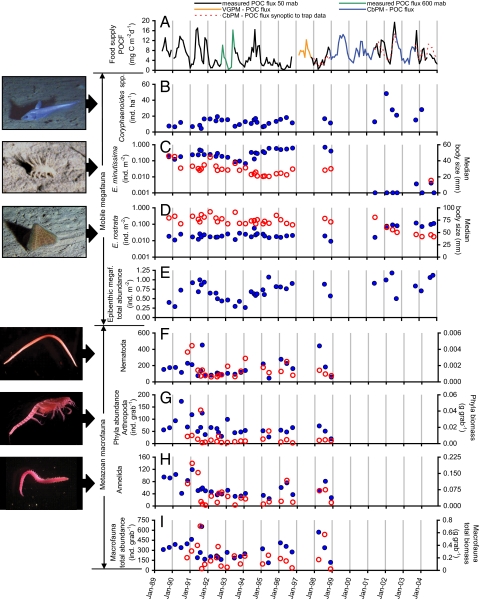
Common deep-sea demersal fishes associated with the sea floor are dominated by grenadiers (Macrouridae). At the Northeast Pacific site, two species of macrourid fishes, Coryphaenoides armatus and Coryphaenoides yaquinae, feed on a combination of prey, including carrion from epipelagic fishes and benthic animals (38). These macrourids more than doubled in abundance over a 15-year period from 1989 to 2004 (ref. 39 and Fig. 4B). It has been proposed that this change is linked to changing epipelagic carrion supply influenced by commercial fishing and climate variation (38). Similar links between commercial fishing and deep-sea fish abundances also have been observed in the Northeast Atlantic (40).
Fig. 4.
Food supply and benthic community components in the Northeast Pacific. (A) Black, green, orange, and blue time-series lines representing a composite of POCF estimates from 50 mab (black) and 600 mab (green) sediment traps and model-estimated flux using the vertically generalized production model (74) (orange) and the carbon-based production model (72) (blue) where possible. The red dashed line is unincorporated model data (16). The difference between the red dashed line and the black line indicates how well the model estimates correspond to measured POC flux values at 50 mab. (B) Density of Coryphaenoides spp. fishes (blue circles). (C and D) Density (blue circles) and median body size (red open circles) for E. minutissima (C) and E. rostrata (D). (E) Total mobile epibenthic megafauna density (blue circles). (F–H) Monthly density (blue circles) and biomass (open red circles) for Nematoda (F), Arthropoda (G), and Annelida (H). (I) Total metazoan macrofauna abundance (blue circles) and biomass (red open circles).
On the sea floor, the epibenthic megafauna are highly conspicuous and dominated by echinoderms, particularly mobile species of holothurians (sea cucumbers) and echinoids (sea urchins). In the Northeast Pacific, two species of holothurians, Elpidia minutissima and Peniagone vitrea, increased in abundance from 1989 through 1996 then decreased by three and two orders of magnitude, respectively, after 1998 (Fig. 4C). In contrast, three other species of holothurians and one species of echinoid, Echinocrepis rostrata, increased significantly in abundance after 2001 (refs. 41 and 42 and Fig. 4D). These shifts in epibenthic megafauna abundance are significantly correlated to El Niño/La Niña events expressed in the NOI climate index when lagged by 14–18 months (Figs. 2B and 4E). Species of epibenthic megafauna with elevated abundances exhibited decreases in body size, suggesting increased reproduction followed by recruitment of young individuals as a probable mechanism (42). Decreases in megafaunal abundance are believed to be related to less favorable competitive interactions and mortality (41, 42). Variations in food supply also have been significantly correlated to megafaunal behavior (43) and levels of sediment mixing by megafauna (44), and this mixing has been linked to changes in abundances of smaller fauna (45).
Smaller fauna (macrofauna) are largely undetected by photographic surveys and generally sampled by coring the sea floor. Initial results from a single year showed a significant seasonal increase in macrofaunal abundance from June to the following February at the Northeast Pacific site. However, there were no significant net changes across a 2-year time span (46). Over a longer 10-year period, total macrofaunal abundance and biomass were significantly correlated to climate-related changes in food supply, with lags of 4 and 8 months, respectively (ref. 47 and Figs. 2 F and G and 4 A and I). The three dominant taxa during this period were nematodes, crustaceans, and polychaetes (Fig. 4 F–H). The time lag from climate events to changes in abundance of these smaller fauna, which ranged from 8 to 9 months, was shorter than that found for the larger epibenthic megafauna (41), suggesting a more rapid response by macrofauna. As was the case for the epibenthic megafauna, the sediment macrofauna also showed changes in abundance and biomass that were significantly correlated to POCF (47). Clear seasonal responses of macrofauna to POCF also have been observed in the oligotrophic (low nutrient) central North Pacific at 4,730-m depth (48).
As in the Northeast Pacific, climate-driven changes in abyssal food supply at the Northeast Atlantic site are thought to influence the abundance and community structure of the benthos. Research in the Northeast Atlantic has examined the importance of food quantity (Fig. 3B) and quality, with potential competition between species in their responses to food inputs. Variations in megafaunal abundance have been dominated by a single species of small holothurian, Amperima rosea (Fig. 3C). This species is capable of boom-bust population dynamics, with densities varying by >2 orders of magnitude in 6-month periods (49). Other small holothurians, e.g., Ellipinion molle (Fig. 3D), also appear capable of rapid increases and decreases in abundance. Larger holothurian species, e.g., Psychropotes longicauda and Pseudostichopus aemulatus (Fig. 3E), do not exhibit population changes of such a large magnitude but nevertheless show clear responses to presumed food supply changes (50). As in the Northeast Pacific, species exhibiting significant population increases also showed shifts in body-size distributions to smaller individuals, suggesting reproduction and relatively rapid recruitment during periods of increased food availability (50). The abundance of another holothurian, Oneirophanta mutabilis, conversely declined as others increased (Fig. 3F), perhaps reflecting the importance of potential competitive interactions similar to those found in the Northeast Pacific. Rates of megafaunal movement across the sediment surface increased markedly as the total abundance and biomass of the megafauna shifted (ref. 49 and Fig. 3G) and are likely to influence sea floor biogeochemical processes such as burial and remineralization.
The changes observed in the abyssal megafauna at the Northeast Atlantic site may be related to changes in both the quantity and quality of food supply (51, 52). Biochemical indicators suggest that some holothurians are either selectively feeding on or selectively digesting different combinations of nutrient pigments (51, 53). This selectivity, combined with differences in feeding tentacle structure, provide a mechanism for resource partitioning, even among congeneric holothurians (54). Proportionally greater carotenoid selectivity in A. rosea, for example, may allow this species to gain a competitive advantage in acquiring reproductively important pigments (51). A. rosea and E. molle feeding on surface sediments also selectively remove sterols, which are not biosynthesized by deep-sea holothurians and are believed to be nutritionally important (55). Indeed, carotenoid pigments have been traced to the reproductive tissues of deep-sea holothurians (54, 56). As in the Northeast Pacific, some species in the Northeast Atlantic community showed opposing trends in abundance related to food availability. The reproductive potential of O. mutabilis (Fig. 3F) was notably reduced during increased A. rosea abundance, suggesting that O. mutabilis was disadvantaged during the time when A. rosea (Fig. 3C) was thriving (57).
Smaller members of the sediment community at the Northeast Atlantic site also have been examined. Foraminiferans (protists) significantly increased in abundance during periods of high A. rosea densities (58). One species (Quinqueloculina sp.) underwent a boom-bust cycle closely matched to those of A. rosea and E. molle. In contrast, another species (Epistominella exigua), a specialist feeding on phytodetritus (59), declined when A. rosea was more abundant. It is possible that E. exigua was out-competed for phytodetritus by mobile surface deposit-feeding megafauna (e.g., A. rosea and the ophiuroid Ophiocten hastatum; ref. 49). Among infaunal metazoans (meiofaunal and macrofaunal polychaetes), populations increased significantly during the period of enhanced A. rosea abundance.
There is a strong possibility that major changes in the fauna of the PAP are linked to climate-driven variations in the quantity and composition of sinking organic matter. Faunal change (see e.g., A. rosea, Fig. 3C) began in late 1996, after an extreme negative winter NAO, and peaked the following year (1997). A. rosea also underwent a second boom-bust cycle with a peak in 2002, again just after a negative NAO winter. The Northeast Atlantic time series also had significant correlations between NAO and the abundance of A. rosea and the species composition over the study period. The data records, though, are too short and of insufficiently high frequency to draw firm conclusions about this potential link between climate change and the abyssal fauna.
Deep-Sea Ecosystems and Carbon Cycle Processes
The net impact of climate-driven variation on the proportion of carbon either buried or remineralized is unknown, but understanding of the processes controlling carbon dynamics at the seabed is improving. Changes in the quantity and quality of food supply to the deep ocean have been related to variation in benthic community structure, with measurable implications for ecosystem functioning in the deep sea (60, 61). Because benthic communities seem to respond readily to food supply with fundamental changes in abundance, community structure, remineralization of carbon, bioturbation, and ecosystem function, the pervasive climate changes foreseen by the Intergovernmental Panel on Climate Change (IPCC) may have lasting impacts in the deep sea as well (62). Even seemingly subtle changes that persist beyond the year 2300, as projected by the IPCC, could have important implications for biogeochemical processes and other ecological interactions that affect the functioning of the oceans as a whole.
An indicator of benthic community remineralization of food supply (POCF) is the rate at which organic carbon is used by the sediment community. This estimate of food utilization is termed sediment community oxygen consumption (SCOC) and has been measured in situ with autonomous respiration chambers on the sea floor. Long time-series records have shown a strong seasonal signal in SCOC, which is highest in summer and fall and lowest in winter at the Northeast Pacific site and believed to be driven primarily by microbes and smaller fauna (46, 47, 63). Larger fauna have been observed to mix fresh phytodetritus centimeters into abyssal sediments, thus increasing the likelihood of carbon burial and facilitating aerobic life deeper into the sediment. Such facilitation is thought to explain the recently observed positive relationships between deep-sea biodiversity and ecosystem functioning (61).
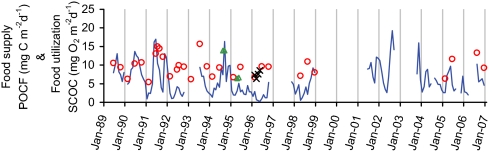
Over an extended time series in the Northeast Pacific, seasonal fluctuation in SCOC was in relative synchrony with the food supply but displayed less interannual variability (Fig. 5). However, a long-term discrepancy was recorded between food supply and utilization, with diminishing supply of POC to meet the utilization of the sediment community (64). The ratio of POC flux to SCOC (POCF/SCOC) declined from near unity in 1989 to 0.2 in 1996. The ratio rebounded to 0.4 in 1998 (13) but remained below unity from 2005 to 2007 after a 5-year hiatus in measurements (Fig. 4). Periods of deficient food supply in relation to food utilization at the Northeast Pacific site can be accommodated to some extent by depletion of standing stocks of organic carbon in the sediment but not indefinitely (13). It is possible that a portion of this deficit in food supply results from the undersampling by sedimentation traps of sinking POCF in the form of discrete detrital aggregates (15, 19, 20). However, even when direct measurements of POCF to the benthos are supplemented by an empirically derived estimate of carbon fluxes based on visible sea-floor detrital aggregates (Fig. 2H) there is still a deficit in available food supply during most of the existing time series (21). The quality of the available food during periods with long-term deficits is also thought to change, with less fresh POC and a greater reliance on material that is likely to be more refractory. Fluctuations in food supply driven by climate variation ultimately are linked to changes in benthic community structure and processes: higher POCF is significantly correlated with increased SCOC on both seasonal and interannual time scales (47). In particular, a long-term deficit in sinking particulate flux to the deep-sea floor would be expected to have a discernible impact on biogeochemical processes and ecological interactions that affect the functioning of the oceans as a whole. It is possible that the apparent discrepancy between sinking food supply and benthic community utilization can be reconciled by infrequent, episodic inputs of organic matter to the sea floor. Such events have been inadequately detected by individual sampling or monitoring equipment, especially given the small number of these kinds of instruments that have been deployed for long periods of time in the deep sea.
Fig. 5.
An 18-year time-series comparison of food supply and food utilization in the Northeast Pacific Station M. Monthly SCOC (food utilization) measured in situ with chambers deployed on free vehicles (red circles), chambers deployed by manned submersible (green triangles), and benthic rover measurements (black crosses) is shown. POCF (food supply) at 50 mab (blue line) is shown.
Conclusions
Long-term changes in climate will likely influence the ecology and biogeochemistry of the deep sea. Not only has flux efficiency been shown to vary spatially (65), but climate-driven temporal variation in POCF to the abyss also influences benthic communities over time scales as short as weeks to months. Processes over the greatest area of the Earth's surface are very poorly constrained in most carbon cycle models and rarely considered in discussions of global climate variation. This out-of-sight, out-of-mind mentality in ignoring the vast expanse of the deep ocean needs to be reversed in light of long-term datasets from two major ocean basins showing that the deep sea is strongly impacted by climate variation over a range of time scales. For example, efforts to evaluate the potential effects of artificially fertilizing the ocean with iron have focused on upper water column impacts (66), but the results summarized here suggest that those effects likely extend to the abyssal seafloor, as indeed appears to be the case in regions influenced by natural iron fertilization (67).
Major unanswered questions relate to the effects of global warming on surface primary production and the portion of this surface-derived food supply that reaches the deep ocean and is ultimately remineralized or sequestered in sediments. Another concern is increasing CO2 concentrations that could acidify the ocean and alter deep-sea communities (68). Existing long-term datasets from two major ocean basins show that deep-sea communities are strongly affected by climate variation and suggest that useful estimates of the magnitude of climate-change impacts on deep-sea ecosystems are both plausible and necessary. One limitation is the paucity of datasets that include long-term monitoring of deep-sea communities with high temporal resolution. Such time-series studies of the deep ocean are critical to understanding the impact of global warming on this vast, but poorly understood, ecosystem. Long-term monitoring of the abyssal realm is best accomplished with autonomous instrumentation that provides rapid feedback to shore-based scientific centers capable of analyzing data and adjusting sampling programs to best evaluate a changing ecosystem. Cabled observatories and seafloor-moored arrays in critical areas of the ocean are currently being designed and implemented, minimizing the need for costly ship-based measurements (69) that are difficult to sustain over long time periods and may not adequately capture episodic events critical to our interpretation of changes in the deep sea.
Acknowledgments.
This review is dedicated to our long-time colleague and friend, Roberta Baldwin, whose infectious enthusiasm and knowledge contributed greatly to our understanding of the deep ocean. This work was supported by the National Science Foundation and the David and Lucile Packard Foundation. The Northeast Atlantic time-series study at the PAP Sustained Observatory site is supported by the European Union Network of Excellence on Marine Biodiversity and Ecosystem Functioning Responsive Mode Project on Deep-Sea and Extreme Environments Patterns of Species and Ecosystem Time Series, the European Seas Observatory Network of Excellence, and the United Kingdom Natural Environment Research Council Strategic Research Project Oceans 2025.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Deuser WG, Ross EH. Seasonal change in the flux of organic carbon to the deep Sargasso Sea. Nature. 1980;283:364–365. [Google Scholar]
- 2.Rowe GT. In: Fertility of the Sea. Costlow JD, editor. New York: Gordon and Breach; 1971. pp. 441–445. [Google Scholar]
- 3.Johnson NA, et al. The relationship between the standing stock of deep-sea macrobenthos and surface production in the western North Atlantic. Deep-Sea Res I. 2007;54:1350–1360. [Google Scholar]
- 4.Smith KL., Jr Food energy supply and demand: A discrepancy between particulate organic carbon flux and sediment community oxygen consumption in the deep ocean. Limnol Oceanogr. 1987;32:201–220. [Google Scholar]
- 5.Pfannkuche O. Benthic response to the sedimentation of particulate organic matter at the BIOTRANS station, 47°N, 20°W. Deep-Sea Res II. 1993;40:135–149. [Google Scholar]
- 6.Billett DSM, Llewellyn C, Watson J. In: Echinoderm Biology. Burke RD, et al., editors. The Netherlands: A. A. Balkima, Rotterdam; 1988. pp. 421–429. [Google Scholar]
- 7.Lauerman LML, Smoak JM, Shaw TJ, Moore WS, Smith KL., Jr 234Th and 210Pb evidence for rapid ingestion of settling particles by mobile epibenthic megafauna in the abyssal NE Pacific. Limnol Oceanogr. 1997;42:589–595. [Google Scholar]
- 8.Demopoulos AWJ, Smith CR, DeMaster DJ, Fornes WL. Evaluation of excess 234Th activity in sediments as an indicator of food quality for deep-sea deposit feeders. J Mar Res. 2003;61:276–284. [Google Scholar]
- 9.Pfannkuche O, Lochte K. Open ocean pelago-benthic coupling: Cyanobacteria as tracers of sedimenting salp feces. Deep-Sea Res I. 1993;40:727–737. [Google Scholar]
- 10.Beaulieu SE, Smith KL., Jr Phytodetritus entering the benthic boundary layer and aggregated on the sea floor in the abyssal NE Pacific: Macro- and microscopic composition. Deep-Sea Res II. 1998;45:781–815. [Google Scholar]
- 11.Buesseler KO, et al. Revisiting carbon flux through the ocean's twilight zone. Science. 2007;316:567–570. doi: 10.1126/science.1137959. [DOI] [PubMed] [Google Scholar]
- 12.Lampitt RS, Antia AN. Particle flux in deep seas: Regional characteristics and temporal variability. Deep-Sea Res I. 1997;44:1377–1403. [Google Scholar]
- 13.Smith KL, Jr, Kaufmann RS, Baldwin RJ, Carlucci AF. Pelagic-benthic coupling in the abyssal eastern North Pacific: An 8-year time-series study of food supply and demand. Limnol Oceanogr. 2001;46:543–556. [Google Scholar]
- 14.Deuser WG, et al. Surface-ocean color and deep-ocean carbon flux: How close a connection? Deep-Sea Res. 1990;37:1331–1343. [Google Scholar]
- 15.Lampitt RS, et al. Material supply to the abyssal seafloor in the Northeast Atlantic. Prog Oceanogr. 2001;50:27–63. [Google Scholar]
- 16.Smith KL, Jr, et al. Climate effect on food supply to depths greater than 4,000 meters in the northeast Pacific. Limnol Oceanogr. 2006;51:166–176. [Google Scholar]
- 17.Billett DSM, Lampitt RS, Rice AL, Mantoura RFC. Seasonal sedimentation of phytoplankton to the deep-sea benthos. Nature. 1983;302:520–522. [Google Scholar]
- 18.Beaulieu SE. Accumulation and fate of phytodetritus on the sea floor. Oceanogr Mar Biol Ann Rev. 2002;40:171–232. [Google Scholar]
- 19.Baldwin RJ, Glatts RC, Smith KL., Jr Particulate matter fluxes into the benthic boundary layer at a long time-series station in the abyssal NE Pacific: Composition and fluxes. Deep-Sea Res II. 1998;45:643–666. [Google Scholar]
- 20.Robison BH, Reisenbichler KR, Sherlock RE. Giant larvacean houses: Rapid carbon transport to the deep sea floor. Science. 2005;308:1609–1611. doi: 10.1126/science.1109104. [DOI] [PubMed] [Google Scholar]
- 21.Smith KL, Jr, Ruhl HA, Kaufmann RS, Kahru M. Tracing abyssal food supply back to upper-ocean processes over a 17-year time-series in the northeast Pacific. Limnol Oceanogr. 2008;53:2655–2667. [Google Scholar]
- 22.Lampitt RS, et al. Long-term variability of downward particle flux in the deep Northeast Atlantic: Causes and trends. Deep-Sea Res. II. 2009 in press. [Google Scholar]
- 23.Irigoien X, Harris RP, Head RN, Harbour D. North Atlantic Oscillation and spring bloom phytoplankton composition in the English Channel. J Plank Res. 2000;22:2367–2371. [Google Scholar]
- 24.Pierce DW. Future changes in biological activity in the North Pacific due to anthropogenic forcing of the physical environment. Clim Change. 2004;62:389–418. [Google Scholar]
- 25.Bopp L, Aumont O, Cadule P, Alvain S, Gehlen M. Response of diatoms distribution to global warming and potential implications: A global model study. Geophys Res Lett. 2005 doi: 10.1029/2005GLO23653. [DOI] [Google Scholar]
- 26.Schneider B, et al. Climate-induced interannual variability of marine primary and export production in three global coupled climate carbon cycle models. Biogeosciences. 2008;5:597–614. [Google Scholar]
- 27.Rykaczewski RR, Checkley DM., Jr Influence of ocean winds on the pelagic ecosystem in upwelling regions. Proc Natl Acad Sci USA. 2008;105:1965–1970. doi: 10.1073/pnas.0711777105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Falkowski PG, Barber RT, Smetacek V. Biogeochemical controls and feedbacks on ocean primary production. Science. 1998;281:200–206. doi: 10.1126/science.281.5374.200. [DOI] [PubMed] [Google Scholar]
- 29.Jickells TD. Global iron connections between desert dust, ocean biogeochemistry, and climate. Science. 2005;308:67–71. doi: 10.1126/science.1105959. [DOI] [PubMed] [Google Scholar]
- 30.Henson SA, Dunne JP, Sarmiento JL. Decadal variability in North Atlantic phytoplankton blooms. J Geophys Res. 2009 doi: 10.1029/2008JC005139. [DOI] [Google Scholar]
- 31.Aksnes DL, Ohman MD. Multi-decadal shoaling of the euphotic zone in the southern sector of the California Current system. Limnol Oceanogr. 2009;54:1272–1281. [Google Scholar]
- 32.Barnett TP, et al. Penetration of human-induced warming into the world's oceans. Science. 2005;309:284–287. doi: 10.1126/science.1112418. [DOI] [PubMed] [Google Scholar]
- 33.Behrenfeld MJ, et al. Climate-driven trends in contemporary ocean productivity. Nature. 2006;444:752–755. doi: 10.1038/nature05317. [DOI] [PubMed] [Google Scholar]
- 34.Sarmiento JL, et al. Response of ocean ecosystems to climate warming. Glob Biogeochem Cycles. 2004 doi: 10.1029/2003GB002134. [DOI] [Google Scholar]
- 35.Huisman J, Thi NNP, Karl DM, Sommeijer B. Reduced mixing generates oscillations and chaos in the oceanic deep chlorophyll maximum. Nature. 2006;439:322–325. doi: 10.1038/nature04245. [DOI] [PubMed] [Google Scholar]
- 36.Feely RA, et al. Impact of anthropogenic CO2 on the CaCO3 system in the oceans. Science. 2004;305:362–366. doi: 10.1126/science.1097329. [DOI] [PubMed] [Google Scholar]
- 37.Iglesias-Rodriguez MD, et al. Phytoplankton calcification in a high-CO2 world. Science. 2008;320:336–340. doi: 10.1126/science.1154122. [DOI] [PubMed] [Google Scholar]
- 38.Drazen JC, et al. Bypassing the abyssal benthic food web: Macrourid diet in the eastern North Pacific inferred from stomach content and stable isotopes analyses. Limnol Oceanogr. 2008;53:2644–2654. [Google Scholar]
- 39.Bailey DM, Ruhl HA, Smith KL., Jr Long-term change in benthopelagic fish abundance in the abyssal northeast Pacific Ocean. Ecology. 2006;87:549–555. doi: 10.1890/04-1832. [DOI] [PubMed] [Google Scholar]
- 40.Bailey DM, Collins MA, Gordon JDM, Zuur AF, Priede IG. Long-term changes in deep-water fish populations in the northeast Atlantic: A deeper reaching effect of fisheries? Proc R Soc London Ser B. 2009 doi: 10.1098/rspb.2009.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruhl HA, Smith KL., Jr Shifts in deep-sea community structure linked to climate and food supply. Science. 2004;305:513–515. doi: 10.1126/science.1099759. [DOI] [PubMed] [Google Scholar]
- 42.Ruhl HA. Abundance and size distribution dynamics of abyssal epibenthic megafauna in the northeast Pacific. Ecology. 2007;88:1250–1262. doi: 10.1890/06-0890. [DOI] [PubMed] [Google Scholar]
- 43.Kaufmann RS, Smith KL., Jr Activity patterns of mobile epibenthic megafauna at an abyssal site in the eastern North Pacific: Results from a 17-month time-lapse photographic study. Deep-Sea Res I. 1997;44:559–579. [Google Scholar]
- 44.Vardaro MF, Ruhl HA, Smith KL., Jr Climate variation, carbon flux, and bioturbation in the abyssal North Pacific. Limnol Oceanogr. 2009;54:2081–2088. [Google Scholar]
- 45.Thistle D, Eckman JE, Paterson GLJ. Large, motile epifauna interact strongly with harpacticoid copepods and polychaetes at a bathyal site. Deep-Sea Res I. 2008;55:324–331. [Google Scholar]
- 46.Drazen JC, Baldwin RJ, Smith KL., Jr Sediment community response to a temporally varying food supply at an abyssal station in the NE Pacific. Deep-Sea Res II. 1998;45:893–913. [Google Scholar]
- 47.Ruhl HA, Ellena JA, Smith KL., Jr Connections between climate, food limitation, and carbon cycling in abyssal sediment communities. Proc Natl Acad Sci USA. 2008;105:17006–17011. doi: 10.1073/pnas.0803898105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith KL, Jr, Baldwin RJ, Karl DM, Boetius A. Benthic community responses to pulses in pelagic food supply: North Pacific Subtropical Gyre. Deep-Sea Res I. 2002;49:971–990. [Google Scholar]
- 49.Bett BJ, Malzone MG, Narayanaswamy BE, Wigham BD. Temporal variability in phytodetritus and megabenthic activity at the seabed in the deep Northeast Atlantic. Prog Oceanogr. 2001;50:349–368. [Google Scholar]
- 50.Billett DSM, et al. Long-term change in the megabenthos of the Porcupine Abyssal Plain (NE Atlantic) Prog Oceanogr. 2001;50:325–348. [Google Scholar]
- 51.Wigham BD, Hudson IR, Billett DSM, Wolff GH. Is long-term change in the abyssal Northeast Atlantic driven by qualitative changes in export flux? Evidence from selective feeding in deep-sea holothurians. Prog Oceanogr. 2003;59:409–441. [Google Scholar]
- 52.Billett DSM, Rice AL. The BENGAL programme: Introduction and overview. Prog Oceanogr. 2001;50:13–25. [Google Scholar]
- 53.Hudson IR, et al. Temporal variations in fatty acid composition of deep-sea holothurians: Evidence of bentho-pelagic coupling. Mar Ecol Prog Ser. 2004;281:109–120. [Google Scholar]
- 54.Smith T, et al. Phytopigments as biomarkers of selectivity in abyssal holothurians; interspecific differences in response to a changing food supply. Deep-Sea Res II. 2009 in press. [Google Scholar]
- 55.Ginger ML, et al. Organic matter assimilation and selective feeding by holothurians in the deep sea: Some observations and comments. Prog Oceanogr. 2001;50:407–421. [Google Scholar]
- 56.Hudson IR, Wigham BD, Billett DSM, Tyler PA. Seasonality and selectivity in the feeding ecology and reproductive biology of deep-sea bathyal holothurians. Prog Oceanogr. 2003;59:381–407. [Google Scholar]
- 57.Ramirez-Llodra E, Reid WDK, Billett DSM. Long-term changes in reproductive patterns of the holothurian Oneirophanta mutabilis from the Porcupine Abyssal Plain. Mar Biol. 2005;146:683–693. [Google Scholar]
- 58.Gooday AJ, Malzone MG, Bett BJ, Lamont PA. Decadal-scale changes in shallow-infaunal foraminiferal assemblages at the Porcupine Abyssal Plain, NE Atlantic. Deep-Sea Res II. 2009 in press. [Google Scholar]
- 59.Gooday AJ, Bett BJ, Pratt DN. Direct observation of episodic growth in an abyssal xenophyophore (Protista) Deep-Sea Res I. 1993;40:2131–2143. [Google Scholar]
- 60.Danovaro R. Exponential decline of deep-sea ecosystem functioning linked to benthic biodiversity loss. Curr Biol. 2008;18:1–8. doi: 10.1016/j.cub.2007.11.056. [DOI] [PubMed] [Google Scholar]
- 61.Loreau M. Biodiversity and ecosystem functioning: The mystery of the deep sea. Curr Biol. 2008;18:R126–R128. doi: 10.1016/j.cub.2007.11.060. [DOI] [PubMed] [Google Scholar]
- 62.Solomon S, et al. Intergovernmental Panel on Climate Change, Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK: Cambridge Univ Press; 2007. [Google Scholar]
- 63.Smith KL, Jr, Kaufmann RS, Baldwin RJ. Coupling of near-bottom pelagic and benthic processes at abyssal depths in the eastern North Pacific Ocean. Limnol Oceanogr. 1994;39:1101–1118. [Google Scholar]
- 64.Smith KL, Jr, Kaufmann RS. Long-term discrepancy between food supply and demand in the deep Eastern North Pacific. Science. 1999;284:174–177. doi: 10.1126/science.284.5417.1174. [DOI] [PubMed] [Google Scholar]
- 65.Lutz M, Dunbar R, Caldeira K. Regional variability in the vertical flux of particulate organic carbon in the ocean interior. Glob Biogeochem Cycl. 2002 doi: 10.1029/2000GB001383. [DOI] [Google Scholar]
- 66.Buesseler KO, et al. Ocean iron fertilization: Moving forward in a sea of uncertainty. Science. 2008;319:162–163. doi: 10.1126/science.1154305. [DOI] [PubMed] [Google Scholar]
- 67.Hughes JA, et al. Two abyssal sites in the Southern Ocean influenced by different organic matter inputs: Environmental characterization and preliminary observations on the benthic foraminifera. Deep-Sea Res II. 2007;54:2275–2290. [Google Scholar]
- 68.Barry JP, Buck KR, Lovera C, Kuhnz L, Whaling PJ. Utility of deep-sea CO2 release experiments in understanding the biology of a high-CO2 ocean: Effects of hypercapnia on deep sea meiofauna. J Geophys Res. 2005 doi: 10.1029/2004JC002629. [DOI] [Google Scholar]
- 69.Richardson AJ, Poloczanska ES. Under-resourced, under threat. Science. 2008;320:1294–1295. doi: 10.1126/science.1156129. [DOI] [PubMed] [Google Scholar]
- 70.Schwing FB, Murphree T, Green PM. The northern oscillation index (NOI): A new climate index for the northeast Pacific. Prog Oceanogr. 2002;53:115–139. [Google Scholar]
- 71.Bakun A. Coastal Upwelling Indices, West Coast of North America, 1946–1971. Washington, DC: U.S. Department of Commerce; 1973. National Oceanic and Atmospheric Administration Technical Report NMFS SSRF-671. [Google Scholar]
- 72.Behrenfeld MJ, Boss E, Siegel DA, Shea DM. Carbon-based ocean productivity and phytoplankton physiology from space. Glob Biogeochem Cycl. 2005 doi: 10.1029/2004GB002299. [DOI] [Google Scholar]
- 73.Laws EA. Export flux and stability as regulators of community composition in pelagic marine biological communities: Implications from regime shifts. Prog Oceanogr. 2004;60:343–354. [Google Scholar]
- 74.Behrenfeld MJ, Falkowski PG. Photosynthetic rates derived from satellite-based chlorophyll concentration. Limnol Oceanogr. 1997;42:1, 20. [Google Scholar]