Abstract
A T cell receptor transgenic mouse line reactive to a microbiota flagellin, CBir1, was used to define mechanisms of host microbiota homeostasis. Intestinal IgA, but not serum IgA, was found to block mucosal flagellin uptake and systemic T cell activation in mice. Depletion of CD4+CD25+ Tregs decreased IgA+ B cells, total IgA, and CBir1-specific IgA in gut within days. Repletion of T cell-deficient mice with either CD4+CD25+ or CD4+foxp3+ Tregs restored intestinal IgA to a much greater extent than their reciprocal CD4+ subsets, indicating that Tregs are the major helper cells for IgA responses to microbiota antigens such as flagellin. We propose that the major role of this coordinated Treg-IgA response is to maintain commensalism with the microbiota.
Keywords: T cells, TGF-beta, Tregs
The intestine harbors and is in constant contact with a microbiota consisting of some 100 trillion microbes spread among at least 15,000 strains in humans (1, 2). This microbiota has a major impact on many host systems, particularly on the development of the intestine and of the immune system. Despite this enormous bacterial challenge, the intestine lives in harmony with the microbiota, in part due to interactions of some members of the microbiota with the host to maintain intestinal homeostasis (3, 4). There are many host mechanisms that have evolved to regulate this relationship, and among these, one of the most important is Ig A (IgA). IgA regulates the microbiota, and bacteria in turn adapt to IgA by altering their gene expression patterns (5, 6). Although IgA also plays a role in host resistance to infection, specific pathogen free mice that have no pathogen exposure have abundant IgA whereas germ-free animals do not, arguing that the major role of IgA is in maintaining the balance between the host and its microbiota. IgA provides its functions through both high- and low-affinity binding systems. Low-affinity, polyspecific IgA blocks the adhesion of commensal bacteria to epithelial cells, whereas high affinity monospecific IgA neutralizes toxins and pathogens. The low affinity, B1 B cell pathway can be stimulated by DC-B cell or epithelial cell-B cell interactions (7–10), whereas the high affinity, B2 B cell pathway requires T cell help and a number of cytokines including TGFβ, IL-5, IL-6, and IL-10 (11–13). Although TGFβ has been shown to be required for Ig switching to IgA in vitro, and TGFβ-deficient mice are deficient in IgA in vivo (11, 12), the cellular source of the TGFβ needed for IgA switching in vivo is unknown.
T regulatory cells (Tregs) that respond to microbiota antigens are present in the gut and contribute to mucosal homeostasis. Both natural CD4+CD25+foxp3+ Treg cells that produce TGFß and bacterial antigen-specific Tr1 cells that produce IL-10 are present in normal mouse intestinal lamina propria (LP) (14–16). Although both intestinal Treg cells and IgA play a role in intestinal homeostasis, and there has been speculation that TGFβ production by Tregs might stimulate IgA responses (17, 18), there is sparse data that addresses how each of these systems responds to antigens of the microbiota, or whether these two systems interact in that effort.
We have recently identified flagellins as immunodominant antigens of the microbiota, using serologic expression cloning (19). Flagellin is both a potent antigen for an adaptive response and is also able to stimulate innate response through binding its receptor, TLR5, on innate cells (20). These microbiota flagellin antigens provide an opportunity to study the host immune response to its microbiota and the role of antigen-specific IgA on intestinal immune homeostasis. To this end, we have generated T cell receptor transgenic (Tg) mice specific for one of these flagellins, denoted CBir1 (19). We present data here that shows that intestinal IgA regulates the activation of peripheral, flagellin-specific CD4+ T cells. Moreover, Tregs control such intestinal IgA B cell responses in an antigen-specific manner, via production of TGFβ, and in an unexpectedly dynamic fashion; that is, depletion of CD4+CD25+ Tregs substantially reduced intestinal IgA levels within days, in part due to interruption of survival signals to LP IgA B cells provided by Tregs. These data are consistent with Tregs being the major helper T cells for induction and maintenance of intestinal IgA B cell responses.
Results
Generation of TCR Tg Mice Specific for CBir1 Flagellin.
To study the host immune response to defined commensal bacterial antigen, a TCR transgenic mouse line specific for CBir1 flagellin, one of the immunodominant commensal bacterial antigens (19), was generated (see Materials and Methods). TCR transgenic mice were detected by PCR amplification of tail DNA using the appropriate TCR-specific primers (Fig. S1A). Most peripheral CD4+ T cells from CBir1 TCR Tg mice expressed Vß 8.3 (Fig. S1B). Blood CD4+ T cells from CBir1 Tg mice responded in vitro by proliferation to relevant CBir1 peptide (456–475), to purified CBir1 flagellin, as well as to a cluster of CBir1-like flagellins that share the same epitope, but not to an irrelevant CBir1 peptide (p56–75), or flagellins of Salmonella, E. coli, or H. pylori (Fig. S1C), indicating this transgenic TCR can serve as a “reporter” for T cell response to this cluster of commensal flagellins. As in normal B6 mice (9), there was no serum IgG response against CBir1 but there was a high level of CBir1 flagellin-specific secretory IgA in the intestinal lumen in B6.CBir1/TCR Tg mice (Fig. S2 A and B). CBir1 flagellin was detected in the luminal content of both normal B6 mice and B6.CBir1 TCR Tg mice (Fig. S2C). Because adoptive transfer of a CBir1 flagellin-specific CD4+ T cell line into immunodeficient mice induced colitis in the recipients (19), we expected that CBir1 Tg mice would spontaneously develop colitis over time. Surprisingly, there was no inflammation observed in intestinal tissues even in mice older than 8 months (Fig. S2D).
Blockade of CBir1 Tg T Cell Response to Mucosally Delivered Antigen by Intestinal IgA.
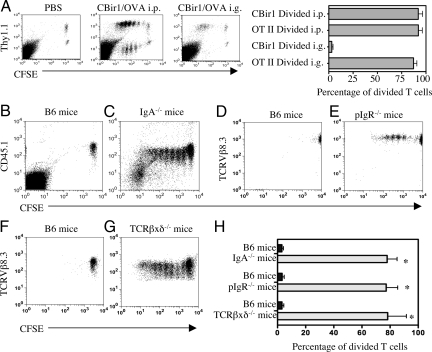
To investigate the CBir1 Tg CD4+ T cell response to CBir1 flagellin challenge in vivo, CFSE-labeled Thy1.2+ CBir1 Tg CD4+ T cells (TCRVβ8.3+) were adoptively transferred i.v. into B6 mice. CFSE-labeled Thy1.1 OT II CD4+ T cells (TCRVβ5.1+) specific for ovalbumin (OVA) were adoptively transferred into the same B6 recipient mice as a control. Adoptive recipients were then challenged 24 h later with CBir1 flagellin and OVA i.p. or orally, and the proliferation of CBir1 Tg T cells and OT II T cells in spleen and MLN were determined by flow cytometry 48 h (for i.p. immunization) and 72 h (for oral immunization) later. Both CBir1 Tg T cells and OT II T cells proliferated well in response to i.p. challenge of CBir1 flagellin and OVA, respectively (Fig. 1A). OT II T cells responded very well to gavaged OVA, however, CBir1 Tg T cells did not proliferate in response to gavaged CBir1 flagellin in either spleen or in MLN (Fig. 1A).
Fig. 1.
CBir1 Tg T cells proliferated in response to systemic stimulation, but not to mucosal stimulation with CBir1 flagellin in B6.WT mice (A). CFSE-labeled CD4+ T cells (1 × 106) from Thy1.2. CBir1 Tg mice and 1 × 106 CFSE-labeled CD4+ T cells from Thy1.1. OT-II mice were co-transferred into wild-type Thy1.2. B6 mice. The recipient mice were injected i.p. with 50 μg CBir1 and 100 μg OVA or gavaged with 100 μg CBir1 and 100 μg OVA the next day respectively. Spleen CD4+ T cell proliferation was analyzed 48 h later from the i.p. injected recipients and 72 h later from the gavaged recipient mice. The percentage of divided Thy1.1+ OT II T cells and Thy1.1− CBir1 Tg T cells was calculated by flow cytometry by gating on Thy1.1+ and Thy1.1− cells. *, P ≤ 0.01 compared to divided CBir1 Tg T cells in response to CBir1 flagellin given i.p. (B and C) CFSE-labeled CD45.1+ CBir1 Tg T cells (1 × 106) were transferred into wild-type B6 or B6.IgA KO mice. (D and E) CFSE-labeled TCRVß8.3+ CBir1 Tg T cells (1 × 106) were transferred into wild-type B6 or B6.pIgR KO mice. (F and G) CFSE-labeled TCRVß8.3+ CBir1 Tg T cells (1 × 106) were transferred into wild-type B6 or and B6.TCRβ×δ KO mice. The recipient mice were gavaged with CBir1 the next day. Splenic CD4+ T cell proliferation was analyzed 72 h later. CFSE-CBir1 Tg T cells proliferated in response to gavaged CBir1 Flagellin in B6.IgA KO mice (C), B6.pIgR KO mice (E), and B6.TCRβxδ KO mice (G), but not in WT B6 mice (B, D, and F). Representative data of three experiments is shown. (H) The percentage of divided CBir1 Tg T cells from six recipient mice of each group was shown as mean ± SE. *, P ≤ 0.01 compared to divided cells in B6 recipients.
To exclude that CBir1 flagellin, which is normally present in intestinal lumen, was inducing CBir1 Tg T cell anergy to mucosal CBir1 stimulation, CFSE-labeled CBir1 Tg CD4+ T cells were adoptively transferred into B6 mice and the recipients were gavaged 24 h later with CBir1 flagellin. Splenic CD4+ T cells were re-isolated 72 h later. When re-stimulated in vitro with CBir1 flagellin, these recovered CBir1 Tg CD4+ T cells proliferated well (Fig. S3), and produced IL-2, IL-10, and IFNγ, but not IL-4, a cytokine profile typical of naive CD4+ T cells, indicating that these CBir1 Tg CD4+ T cells were not anergic. Instead, the naive CD4+ T cell cytokine profile indicated that splenic CBir1 Tg CD4+ TCR was not activated by CBir1 flagellin in vivo despite the gavage of a large amount of CBir1 flagellin. Consistent with these results MLN DCs recovered from B6 mice after gavage with OVA and CBir1 flagellin stimulated OTII T cells, but not CBir1 Tg T cells (Fig. S4).
Because IgA specific for CBir1 flagellin was present in the intestinal lumen in B6 mice, CFSE-labeled CBir1 Tg CD4+ T cells were adoptively transferred into B6.IgA−/− mice, and into control wild-type B6 mice, which were gavaged with CBir1 flagellin 24 h later. As shown in Fig. 1 B, C, and H, CBir1 Tg CD4+ T cells proliferated in B6.IgA−/− mice but not in control B6 mice. CFSE-labeled CBir1 Tg CD4+ T cells also proliferated in response to gavaged CBir1 flagellin when adoptively transferred into B6.pIgR−/− mice (which have normal systemic IgA and IgM but no secretory IgA or IgM (Fig. 1 D, E, and H), or into TCRβ×δ−/− mice, which only have low levels of CBir1 specific secretory IgA production (Fig. 1 F–H). Collectively, these data show that antigen-specific intestinal IgA plays a critical role in regulation of CD4+ T cell response to commensal bacterial antigens.
Because both natural (polyspecific) and antigen-specific secretory IgA are present in the intestinal lumen, the question arose as to whether IgA exclusion of flagellin required antigen-specific secretory IgA. We used B6 mice gnotobiotic for altered Schaedler's flora (ASF), which is comprised of eight defined commensal bacterial strains, to address this question. B6 ASF mice had normal total secretory IgA, but lacked intestinal IgA anti-CBir1 flagellin (Fig. S5). CBir1 Tg T cells adoptively transferred to ASF mice responded strongly to mucosal CBir1 flagellin challenge indicating that IgA exclusion of commensal bacterial flagellin was antigen-specific, and not due to polyspecific, or natural IgA (Fig. S5).
Depletion of CD4+CD25+ Treg Cells Decreased Intestinal IgA Responses.
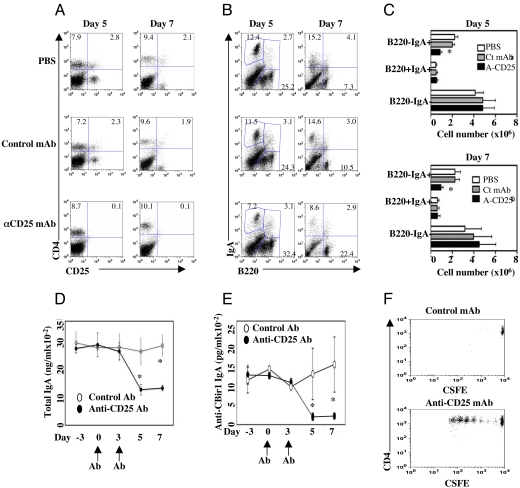
Tregs are abundant in the intestine and produce cytokines important in IgA production. To determine the role of CD25+ Treg cells in intestinal IgA responses, B6 mice were injected with anti-CD25 mAb or control antibody i.p. at days 0 and 3 to deplete CD25+ Treg cells. At days 5 and 7, CD25+ T cells were depleted almost completely in mice receiving anti-CD25 mAb but not control antibody (Fig. 2A). Depletion of CD25+ Treg cells resulted in decrease of LP B220−IgA+ B cells (IgA+ plasma cells), but not B220+IgA+ B cells (postclass-switch recombination IgA+ B cells) (Fig. 2 B and C). B-1 cells have been implicated as major resources of T cell-independent intestinal IgA production. Interestingly, depletion of CD25+ Treg cells decreased CD5−IgA+ B cells (B-2 cells) but had no effect on CD5+IgA+ B-1 cells (Fig. S6), indicating that depletion of CD25+ Treg cells affected mucosal IgA+ B-2 cells but not IgA+ B-1 B cells. To determine the effect of depletion of CD25+ Treg cells on commensal bacterial antigen specific secretory IgA production, as described above, stool pellet IgA anti-CBir1 flagellin and total IgA were assessed by ELISA before and after depletion. Depletion of CD25+ Treg cells significantly decreased total intestinal IgA and CBir1-specific IgA production by 5 days after anti-CD25 mAb injection (Fig. 2 D and E) with no change after control antibody injection. To determine the effect of such decreased CBir1-specific secretory IgA in vivo, CFSE-labeled CD4+ T cells from CBir1 Tg mice were transferred into CD25+ depleted or control B6 mice at day 3, the same day when recipient mice were administered the second antibody injection. Recipient mice were then gavaged with CBir1 flagellin the next day (day 4) and CBir1 Tg CD4+ T cell proliferation was determined 3 days later (day 7). As shown in Fig. 2F, CBir1 Tg CD4+ T cells proliferated in response to gavaged CBir1 flagellin in the mice administered anti-CD25 mAb, which had much lower levels of CBir1-specific intestinal IgA, but not in mice administered control antibody, which had normal levels of CBir1-specific intestinal IgA.
Fig. 2.
Depletion of CD4+CD25+ Treg cells decreased intestinal IgA responses. B6 mice were injected with 100 μg anti-CD25 mAb twice at days 0 and 3. Stool pellets were collected at days −3, 0, 3, 5, and 7. LP IgA B cells were determined by flow cytometry at days 5 and 7. (A) CD4+CD25+ T cells were depleted in LP of the mice treated with anti-CD25 but not control mAb. (B) IgA+ B220− B cells were decreased in LP of the mice treated with anti-CD25 but not control mAb. (C) B cell numbers from four mice of each group. *, P ≤ 0.05 compared to control mAb treated mice. (D) Total pellet IgA production, assessed by ELISA. (E) Pellet anti-CBir1 IgA production, assessed by ELISA. *, P ≤ 0.05 compared to control Ab-treated group. (F) CFSE-labeled CD4+ T cells (1 × 106) from CBir1 Tg mice were transferred into CD25-depleted or control B6 mice at day 3, the same day that recipient mice were administered the second mAb injection. Recipient mice were gavaged with CBir1 flagellin the next day (day 4) and CBir1 Tg CD4+ T cell proliferation was determined 3 days later (day 7). One representative of three experiments is shown.
Repletion of CD25+ foxp3+ Tregs Promotes Intestinal IgA B Cells and IgA Anti-CBir1 Flagellin in TCRβxδ−/− Mice.
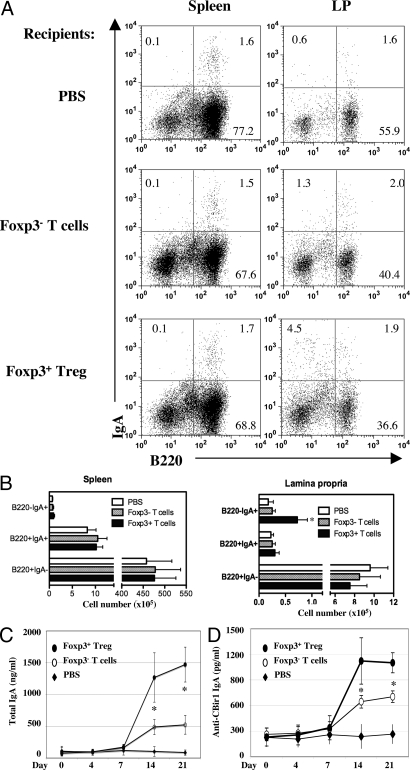
We next asked whether adoptive transfer of CD25+ Treg cells could restore CBir1 specific intestinal IgA production in TCRβ×δ−/− mice, which have only low levels of intestinal IgA anti-CBir1 flagellin (Fig. S5C). CD4+ CD25+ Treg cells were sorted from B6 mice by flow cytometry and transferred into B6.TCRβ×δ−/− mice at 1 × 106/mouse. CD4+CD25− T cells were transferred also for comparison. Stool pellets were collected at days 0, 4, 7, 14, and 21, and CBir1-specific IgA and total IgA were determined by ELISA. Adoptive transfer of CD4+CD25+ Treg cells greatly increased CBir1 specific secretory IgA as well as total intestinal IgA production in pellets, whereas transfer of CD4+CD25− T cells only modestly increased such IgA production (Fig. S7A). Because CD25 is not the sole marker of Treg cells and memory CD4+ T cells can also express CD25, CD4+foxp3+ Treg cells were sorted from B6.GFP-foxp3 reporter mice by flow cytometry and transferred into B6. TCRβ×δ−/− mice in a similar fashion. CD4+ foxp3− T cells were transferred as a control. As shown in Fig. 3 A and B, transfer of CD4+foxp3+ Treg cells or CD4+foxp3− T cells did not affect IgA+ B cells in spleen. Adoptive transfer of CD4+ foxp3− T cells had no significant effects on LP B220+IgA+ B cells or B220−IgA+ B cells. In contrast, adoptive transfer of CD4+foxp3+ Treg cells significantly increased LP B220+IgA+ B cells and B220−IgA+ B cells. Adoptive transfer of CD4+foxp3+ Treg cells increased total and CBir1-specific IgA in stool pellets to a much greater extent than did transfer of CD4+foxp3− T cells (Fig. 3 C and D). Consistent with these results, transfer of WT B6 spleen B cells into B6.IgA−/− mice restored intestinal IgA responses, and depletion of CD25+ T cells in the recipients reduced such repletion (Fig. S7B).
Fig. 3.
Adoptive transfer of CD4+foxp3+ Treg cells restored intestinal IgA in B6.TCRβ×δ−/− mice. CD4+ foxp3+ Treg cells and CD4+ foxp3− T cells from B6.GFP-foxp3 mice were transferred into B6.TCRβ×δ−/− mice at 1 × 106/mouse. (A) LP IgA+ B cells were analyzed by flow cytometry at day 21. (B) B cell numbers from four mice of each group. *, P ≤ 0.05 compared to the recipient mice of CD4+ foxp3− T cells. (C and D) Stool pellets were collected at days 0, 4, 7, 14, and 21. Total IgA (C) and anti-CBir1 IgA (D) were assessed by ELISA. *, P ≤ 0.05 compared to foxp3− T cell group.
CD4+foxp3+ Treg Cells Provided Help to IgA B Cells via TGFβ and Promoted Survival of Lamina Propria IgA+ B Cells.
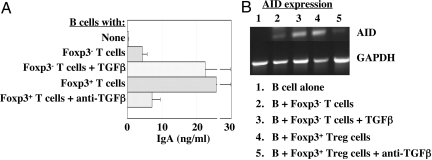
TGFβ is known to induce IgA class switch recombination and maturation. To determine whether TGFβ was involved in Treg stimulation of B cell production of IgA, splenic IgD+ B cells were cultured with flow sorted CD4+foxp3+ Treg cells or CD4+foxp3− T cells in the presence of αCD3 and αCD28 mAbs. CD4+foxp3+ Treg cells stimulated IgA production, and this increase in B cell IgA production was blocked by anti-TGFß (Fig. 4A). B cells that were cultured with CD4+foxp3+ Treg cells in the absence of αCD3, and anti-CD28 mAbs did not produce IgA. CD4+foxp3− T cells increased B cell IgA production slightly, but addition of TGFβ greatly enhanced B cell IgA production in such co-cultures. Expression of mRNA encoding activation-induced cytidine deaminase (AID), an enzyme essential for class-switch recombination, was up-regulated in B cells cultured with CD4+foxp3+ Treg cells plus anti-CD3/CD28, and such enhanced B cell AID expression was inhibited by blockade of TGFβ (Fig. 4B). In contrast, culture of B cells with CD4+foxp3− T cells only slightly up-regulated B cell AID expression unless TGFβ was added to cultures. Collectively, these data indicate that Treg cells promoted B cell AID expression and IgA production through production of TGFβ.
Fig. 4.
CD25+ foxp3+ Treg cells provided help to IgA B cells via TGFβ. Splenic IgD+ B cells (2.5 × 106) from B6 mice were cultured with 1 × 106 flow sorted CD4+foxp3+ T cells or 1 × 106 CD4+foxp3− T cells in the presence of 20 ng/mL TGFβ or 10 μg/mL anti-TGFβ mAb in the plate coated with 5 μg/mL of anti-CD3 and 10 μg/mL soluble anti-CD28 mAb. B cells were stimulated only by the activated T cells. (A) Supernatant was collected at day 5 and IgA was measured by ELISA. (B) B cells were re-isolated at day 3 and RNA was isolated from B cells for determination of AID using RT-PCR. One representative of three experiments is shown.
The rapidity of the depletion of LP B220−IgA+ B cells after CD25+ T cell depletion suggested that CD25+ Tregs might provide survival signals for them. This possibility was confirmed by Annexin V staining of LP IgA+ B cells after anti-CD25 treatment that found significantly increased apoptosis of these LP B cells compared to control antibody-treated mice (Fig. S8).
Treg Cells Promoted B Cell IgA Production in an Antigen-Specific Manner.
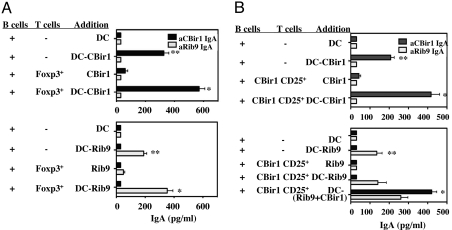
Peyer's patches B cells were cultured with MLN CD4+foxp3+ Treg cells in the presence of bone-marrow-derived dendritic cells (BMDCs) that had been pulsed with a recombinant intestinal bacterial antigen (9), either with rIB9 or CBir1 flagellin. Antigen-specific IgA in supernatants was measured by ELISA. As shown in Fig. 5A, when cultured with CBir1-pulsed BMDCs alone, PP B cells produced IgA specific for CBir1 flagellin but not IgA anti-rIB9. CD4+foxp3+ Treg cells enhanced PP B cell production of IgA anti-CBir1 flagellin but not IgA anti-rIB9 in the culture with CBir1-pulsed BMDCs. In a similar fashion, PP B cells cultured with rIB9-pulsed BMDCs produced IgA specific for rIB9 but not for CBir1 flagellin, and CD4+ foxp3+ Treg cells enhanced PP B cell production of IgA anti-rIB9 but not IgA anti-CBir1 flagellin in these cultures. Notably, there was no IgA induced in co-cultures of Tregs plus B cells plus antigen in the absence of BMDCs. Collectively, these data demonstrated that promotion of B cell IgA production required B cell and Treg cell activation in an antigen-specific manner.
Fig. 5.
Treg cells promoted B cell IgA production in an antigen-specific manner. (A) PP B220+ B cells (2.5 × 106) from B6 mice were cultured with 1 × 106 CD4+foxp3+ T cells in the presence of 1 × 106 CBir1-pulsed BMDCs or rIB9-pulsed BMDCs. Culture supernatant was collected at day 5 and IgA was measured by ELISA. (B) PP B220+ B cells (2.5 × 106) from B6 mice were cultured with 1 × 106 CD4+CD25+ Treg cells from CBir1 Tg mice in the presence of 1 × 106 CBir1-pulsed BMDCs or rIB9-pulsed BMDCs. Culture supernatant was collected at day 5 and IgA was measured by ELISA. *, P ≤ 0.05 compared to B cells cultured with antigen-pulsed DC alone; **, P ≤ 0.01 compared to B cells cultured with DCs without antigen.
To further examine whether Treg cell help for IgA production was antigen-specific, we tested flow sorted CBir1 Tg CD4+CD25+ Treg cells in a similar fashion. As shown above, when cultured with antigen-pulsed BMDCs, PP B cells produced a low level of IgA only to the BMDC-pulsed antigen. CBir1 Tg CD4+CD25+ Treg cells enhanced PP B cell production of IgA specific for CBir1 flagellin but not for rIB9 in cultures with CBir1-pulsed BMDCs. When cultured with BMDCs that were pulsed with both antigens, CBir1 Tg CD25+ Treg cells enhanced mainly IgA anti-CBir1 flagellin, and to a lesser extent, IgA anti-rIB9 (Fig. 5B). Collectively, these data demonstrated that Treg cell helper activity required antigen-specific stimulation via TCR. Once activated, Treg cells promoted B cell IgA production specific for the relevant antigen but could also provide bystander help for an unrelated commensal bacterial antigen. Confirming these in vitro data, CBir1 Tg CD25+ cells induced predominately IgA anti-CBir1 in vivo, after transfer to B6.TCRß×δ−/− recipients (Fig. S9).
Discussion
An understanding of the mechanisms by which the host immune system lives in relative peace with its microbiota is just emerging. Of particular interest is how T cell responses to the microbiota are controlled, in that unrestrained effector CD4+ T cell responses to the microbiota result in intestinal inflammation and tissue damage. To this end, we generated a CD4+ T cell receptor transgenic mice line specific for a microbiota flagellin, CBir1, which is a dominant commensal bacterial antigen in experimental colitis and in patients with Crohn's disease (19). We demonstrated in this report that flagellin-specific secretory IgA played a key role in control of systemic CD4+ T cell responses by immune exclusion. Importantly, such IgA production was regulated by T regulatory cells in an antigen-specific manner. Thus, the Treg-IgA axis plays an important role in maintenance of intestinal immune homeostasis to commensal bacteria.
Although intestinal IgA can be produced by both T cell-dependent and T cell-independent pathways, the former pathway is dominant in that T cell-deficient TCRβ×δ−/− mice have much lower levels of intestinal total IgA and antigen-specific IgA. Dendritic cells interact with both T cells and B cells to induce IgA responses and this plays an important role in intestinal homeostasis. There are many mucosal DC subsets and these vary among PP, LP, and MLN sites (21–23). Mucosal DCs have distinctive properties, particularly the production of retinoic acid, which has many biologic effects that include stimulation of Tregs in concert with TGFß (22, 23), and induction of gut homing receptors α4ß7 and CCR9 on B cells and T cells (24, 25). Antigen-pulsed DCs can interact with B cells directly to stimulate IgA responses (26, 27). This has been demonstrated mainly with B1 B cells, but the present studies demonstrate that DCs can stimulate B2 B cell production of IgA as well. Two DC subsets have been linked to mucosal IgA responses. An iNOS+, TNFα+ DC subset regulates expression of the TGFßRII on B cells that is required for IgA class switch recombination (CSR). This subset induces expression of BAFF and APRIL, which are needed for T cell-independent IgA CSR (28). A second mucosal DC subset affecting IgA production is a CD11chiCD11bhiTLR5+ DC that increases B1 B cell IgA differentiation via RA production (29). The BMDCs used in the present experiments do not express TLR5 or iNOS and we can only speculate that mucosal DCs may stimulate IgA anti-flagellin responses even more than did BMDCs.
TGFß has been well defined as crucial for IgA CSR (27). Thus, B cells deficient in TGFßRII−/− do not make IgA (11, 30) and mice deficient in SMAD2 and SMAD3 signaling factors downstream of the TGFß-R also have deficient IgA responses (13, 31). Because multiple cell types can produce TGFß, including epithelial cells, dendritic cells, and non-regulatory helper T cells, which are all well represented in the intestine, the cellular source of TGFß that is needed for IgA CSR has been unclear. The intestine not only contains abundant IgA, but also is home to large numbers of T regulatory cells, including both foxp3+CD25+ Treg and IL-10-producing Tr1 cells (32, 33). CD25+ natural Treg cells have been shown to express large amounts of TGFβ, and their inhibitory function requires TGFβ production (34, 35). These considerations make Tregs prime candidates for the source of TGFß needed for IgA CSR. This conclusion is supported by the repletion studies in which either CD25+ Tregs or foxp3+ Tregs greatly increased IgA anti-flagellin and total IgA responses in the gut of TCRßδ−/− recipients, and the repletion provided by Tregs was substantially better than that achieved by CD25− or foxp3−CD4+ T cells containing Th1, Th2, and other T cell subsets. The role of TGFβ in these CD4+CD25−- or foxp3−CD4+-T cell effects on IgA was confirmed in in vitro studies in which blockade of TGFβ inhibited both CD4+CD25+ and CD4+foxp3+ Treg stimulation of IgA production.
Activation of Treg cells via their TCR was required for their stimulation of B cell IgA, in that CD4+CD25+ and CD4+foxp3+ Treg cells promoted B cell IgA production only if activated via the TCR. T cell-dependent IgA CSR is known to involve CD40-CD40L interactions in addition to TGFß signals, and that is likely the case with Tregs as well. In a similar fashion, CBir1 Tg CD25+ T cells increased B cell IgA production only after activation with CBir1 flagellin, but not in its absence. However, once activated, CBir1-flagellin-specific Treg cells could provide bystander help for B cell production of IgA for an unrelated commensal bacterial antigen. Collectively, these data demonstrated that Treg cell helper activity required antigen-specific stimulation.
These studies don't address the “geography” of the Treg-IgA B cell interaction; that is, whether it might be occurring in the Peyer's patches (PP), isolated lymphoid follicles (ILF), or in the LP, because IgA B cells can be induced in all three sites (26). Induction of T cell-dependent IgA responses is known to occur in PP and ILF and require T cell CD40L–B cell CD40 interactions plus TGFß (18). IgA CSR can occur by other mechanisms involving epithelial-B cell or dendritic cell–B cell interactions plus TNF family members BAFF and/or APRIL, mainly for T-independent antigens (7, 10, 18, 36). The low level of IgA anti-flagellin in TCRßδ−/− mice deficient in T cells indicates that this pathway is minor for antigens such as flagellin. Foxp3+ Tregs are present in both PP and LP (33).
The rapidity of the reduction of IgA+ B cells in LP upon CD4+CD25+ Treg depletion suggested that Tregs may be providing a survival factor for IgA+ B cells in the intestinal LP. Indeed, we found that the proportion of LP IgA+ B cells undergoing apoptosis as measured by Annexin V staining was significantly increased in mice receiving anti-CD25 compared to those receiving control mAb. Taken together, these data indicate that Tregs have a dual effect on intestinal IgA B cell responses, one being the on induction of B cell CSR and the second the provision of a survival signal for IgA+ B cells in the LP. Whether the latter is a direct or indirect effect of Tregs, and whether TGFß is involved, is presently unknown.
These data have important implications for mucosal vaccine development. Most vaccine candidates are of microbial origin and are T cell-dependent antigens. The present results might explain some of the difficulty experienced in the development of effective mucosal vaccines in that the default pathway to antigen in the intestine appears to be induction of Tregs that, in turn, stimulate IgA responses. It is known that mucosally administered antigen can induce both systemic tolerance (Tregs) and mucosal IgA responses, but these outcomes have been thought to be parallel but independent consequences, due to shared cytokine pathways (17). The present data alter that concept by showing that Tregs and IgA are not acting in parallel but in series, with Tregs functioning as helper T cells for IgA B cells. Effective mucosal vaccines will require subversion or bypass of the Treg response to get a systemic response. This does happen during intestinal infections and with the mucosal adjuvant, cholera toxin. The stimulation of this Treg-IgA pathway might explain the transient nature of the IgA response even with living vectors such as Salmonella, which provide an initial response that wanes over ensuing weeks. Certainly presentation of vaccine antigens by co-evolved commensal bacteria seems likely to stimulate Tregs preferentially, rather than T effector cells, because at least some of these bacteria are able to induce anti-inflammatory pathways in the host (3, 37) that would likely favor the Treg-IgA pathway.
The major function of IgA is usually presented in the context of protection against pathogens. In this context, the induction of Tregs that in turn stimulate IgA as a protective strategy against pathogens seems counterintuitive. Instead, the present data support the idea that the major purpose of IgA and Tregs in the intestine is the maintenance of commensalisms with the microbiota, and the restriction of gut inflammation that can perturb such commensalism (2, 38). The microbiota provides very effective protection against pathogens via colonization resistance. Thus, IgA, Tregs, and the microbiota acting together as a unit would be protective against pathogens, and may be more effective than IgA would be alone. We speculate that Tregs evolved to be the major helper cells for IgA rather than one of the other helper T cell subsets because intestinal infections or inflammation alters the normal microbiota with a decrease in beneficial anaerobes (and probably with a concomitant decrease in colonization resistance). Thus, suppression of intestinal inflammation by Tregs would serve to restore the resident microbiota and in turn restore normal pathogen resistance. This would be a survival advantage that could provide selection during the co-evolution with our microbiota.
Materials and Methods
See SI for methods used to generate TCR transgenic mice, isolate lamina propria cells, generate dendritic cells, perform reverse transcription PCR, assess intracellular cytokines by flow cytometry, and analyze histopathology.
Statistics.
For immunologic assays such as T cell proliferation and cytokine production, data were expressed as mean ± SD of replicate measurements. Statistical analysis used the non-parametric Mann Whitney U test.
Supplementary Material
Acknowledgments.
We thank Dr. Stephan J. McSorley (Univ. Minnesota, Minneapolis, MN) for advice and reagents needed for generation of the T cell receptor transgenic line, Wayne Duck and Lifang Li for expert technical assistance, and Dr. Casey Weaver for advice and critical review of the manuscript. This work was supported by National Institutes of Health grants PO1 DK071176, DK06400, DK079918, and RR-20136 and Digestive Diseases Research Development Center (DK064400), and National Institutes of Health RR-20136.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0812681106/DCSupplemental.
References
- 1.Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124:837–848. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 2.Frank DN, et al. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA. 2007;104:13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelly D, et al. Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shuttling of PPAR-gamma and RelA. Nat Immunol. 2004;5:104–112. doi: 10.1038/ni1018. [DOI] [PubMed] [Google Scholar]
- 4.Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–625. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki K, et al. Aberrant expansion of segmented filamentous bacteria in IgA-deficient gut. Proc Natl Acad Sci USA. 2004;101:1981–1986. doi: 10.1073/pnas.0307317101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peterson DA, McNulty NP, Guruge JL, Gordon JI. IgA response to symbiotic bacteria as a mediator of gut homeostasis. Cell Host Microbe. 2007;2:328–339. doi: 10.1016/j.chom.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 7.Litinsky MB, et al. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat Immunol. 2002;3:822–829. doi: 10.1038/ni829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Macpherson AJ, Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science. 2004;303:1662–1665. doi: 10.1126/science.1091334. [DOI] [PubMed] [Google Scholar]
- 9.Konrad A, Cong Y, Duck W, Borlaza R, Elson CO. Tight mucosal compartmentation of the murine immune response to antigens of the enteric microbiota. Gastroenterology. 2006;130:2050–2059. doi: 10.1053/j.gastro.2006.02.055. [DOI] [PubMed] [Google Scholar]
- 10.He B, et al. Intestinal bacteria trigger T cell-independent immunoglobulin A(2) class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity. 2007;26:812–826. doi: 10.1016/j.immuni.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 11.Cazac BB, Roes J. TGF-beta receptor controls B cell responsiveness and induction of IgA in vivo. Immunity. 2000;13:443–451. doi: 10.1016/s1074-7613(00)00044-3. [DOI] [PubMed] [Google Scholar]
- 12.Brandtzaeg P, Johansen FE. Mucosal B cells: Phenotypic characteristics, transcriptional regulation, and homing properties. Immunol Rev. 2005;206:32–63. doi: 10.1111/j.0105-2896.2005.00283.x. [DOI] [PubMed] [Google Scholar]
- 13.Klein J, et al. B cell-specific deficiency for Smad2 in vivo leads to defects in TGF-beta-directed IgA switching and changes in B cell fate. J Immunol. 2006;176:2389–2396. doi: 10.4049/jimmunol.176.4.2389. [DOI] [PubMed] [Google Scholar]
- 14.Cong Y, Weaver CT, Lazenby A, Elson CO. Bacterial-reactive T regulatory cells inhibit pathogenic immune responses to the enteric flora. J Immunol. 2002;169:6112–6119. doi: 10.4049/jimmunol.169.11.6112. [DOI] [PubMed] [Google Scholar]
- 15.Makita S, et al. CD4+CD25bright T cells in human intestinal lamina propria as regulatory cells. J Immunol. 2004;173:3119–3130. doi: 10.4049/jimmunol.173.5.3119. [DOI] [PubMed] [Google Scholar]
- 16.Guo Z, et al. CD4+CD25+ regulatory T cells in the small intestinal lamina propria show an effector/memory phenotype. Int Immunol. 2008;20:307–315. doi: 10.1093/intimm/dxm143. [DOI] [PubMed] [Google Scholar]
- 17.Mestecky J, Russell MW, Elson CO. Perspectives on mucosal vaccines: Is mucosal tolerance a barrier? J Immunol. 2007;179:5633–5638. doi: 10.4049/jimmunol.179.9.5633. [DOI] [PubMed] [Google Scholar]
- 18.Cerutti A, Rescigno M. The biology of intestinal immunoglobulin A responses. Immunity. 2008;28:740–750. doi: 10.1016/j.immuni.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lodes MJ, et al. Bacterial flagellin is a dominant antigen in Crohn disease. J Clin Invest. 2004;113:1296–1306. doi: 10.1172/JCI20295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith KD, et al. Toll-like receptor 5 recognizes a conserved site on flagellin required for protofilament formation and bacterial motility. Nat Immunol. 2003;4:1247–1253. doi: 10.1038/ni1011. [DOI] [PubMed] [Google Scholar]
- 21.Kelsall BL, Strober W. Distinct populations of dendritic cells are present in the subepithelial dome and T cell regions of the murine Peyer's patch. J Exp Med. 1996;183:237–247. doi: 10.1084/jem.183.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coombes JL, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun CM, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mora JR, et al. Selective imprinting of gut-homing T cells by Peyer's patch dendritic cells. Nature. 2003;424:88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- 25.Mora JR, et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157–1160. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- 26.Macpherson AJ. The immune geography of IgA induction and function. Mucosal Immunol. 2007;1:12–22. doi: 10.1038/mi.2007.6. [DOI] [PubMed] [Google Scholar]
- 27.Cerutti A. The regulation of IgA class switching. Nat Rev Immunol. 2008;8:421–434. doi: 10.1038/nri2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tezuka H, et al. Regulation of IgA production by naturally occurring TNF/iNOS-producing dendritic cells. Nature. 2007;448:929–933. doi: 10.1038/nature06033. [DOI] [PubMed] [Google Scholar]
- 29.Uematsu S, et al. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nat Immunol. 2008;9:769–776. doi: 10.1038/ni.1622. [DOI] [PubMed] [Google Scholar]
- 30.Borsutzky S, Cazac BB, Roes J, Guzman CA. TGF-beta receptor signaling is critical for mucosal IgA responses. J Immunol. 2004;173:3305–3309. doi: 10.4049/jimmunol.173.5.3305. [DOI] [PubMed] [Google Scholar]
- 31.Ashcroft GS, et al. Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response. Nat Cell Biol. 1999;1:260–266. doi: 10.1038/12971. [DOI] [PubMed] [Google Scholar]
- 32.Izcue A, Coombes JL, Powrie F. Regulatory T cells suppress systemic and mucosal immune activation to control intestinal inflammation. Immunol Rev. 2006;212:256–271. doi: 10.1111/j.0105-2896.2006.00423.x. [DOI] [PubMed] [Google Scholar]
- 33.Maynard CL, et al. Regulatory T cells expressing interleukin 10 develop from Foxp3+ and Foxp3- precursor cells in the absence of interleukin 10. Nat Immunol. 2007;8:931–941. doi: 10.1038/ni1504. [DOI] [PubMed] [Google Scholar]
- 34.Marie JC, Letterio JJ, Gavin M, Rudensky AY. TGF-beta1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J Exp Med. 2005;201:1061–1067. doi: 10.1084/jem.20042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 36.Castigli E, et al. TACI and BAFF-R mediate isotype switching in B cells. J Exp Med. 2005;201:35–39. doi: 10.1084/jem.20032000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neish AS, et al. Prokaryotic regulation of epithelial responses by inhibition of IkappaB-alpha ubiquitination. Science. 2000;289:1560–1563. doi: 10.1126/science.289.5484.1560. [DOI] [PubMed] [Google Scholar]
- 38.Lupp C, et al. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe. 2007;2:119–129. doi: 10.1016/j.chom.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 39.Elson CO, et al. Monoclonal anti-interleukin 23 reverses active colitis in a T cell-mediated model in mice. Gastroenterology. 2007;132:2359–23570. doi: 10.1053/j.gastro.2007.03.104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.