Abstract
Evarcha culicivora (Araneae, Salticidae) feeds indirectly on vertebrate blood by choosing, as preferred prey, bloodcarrying female mosquitoes. Mutual mate-choice behavior is also pronounced in this species. Here we show that, when E. culicivora feeds indirectly on blood, it acquires a diet-related odor that makes it more attractive to the opposite sex. The mate-choice decisions of the adults of both sexes were investigated in a series of experiments based on comparing how long the test spider remained close to the odor of one source spider on one day and to the odor of a different source spider on the following day. Four different maintenance diets for source spiders were used in these experiments: bloodfed female mosquitoes (Culicidae, Anopheles gambiae ss), sugar-fed female mosquitoes, male mosquitoes, and lake flies (Chironomidae, Nilodorum brevibucca). Both sexes of E. culicivora spent more time close to the odor of opposite-sex conspecifics that had been on a diet of bloodfed mosquitoes (blood diet) instead of any of the three nonblood diets. Opposite-sex conspecifics that had been on a nonblood diet became more attractive once they were switched to a blood diet. That the attractive odor from blood dissipates was shown when spiders became less attractive once they were switched to a nonblood diet or subjected to a fast. However, there was no evident preference for the odor of a same-sex conspecific on a blood diet instead of a lake fly diet. These findings are discussed in the context of sexual selection and sensory exploitation.
Keywords: mosquitoes, olfaction, predation, sensory exploitation, sexual selection
Chemoreception, including olfaction, widely acknowledged as the most ancient sensory modality (1, 2), is used by animals in many different contexts (3). For example, chemoreception might play a particularly important role in the context of reproduction when an animal needs to distinguish mates from rivals and conspecific individuals from heterospecific individuals (3), as well as determine the relatedness (4) and the health of a potential mate (5). Here we consider how diet-derived odor might increase the attractiveness of the opposite sex. This is an important step in our ongoing research on the mate-choice decisions of Evarcha culicivora, a jumping spider (Araneae: Salticidae) that is known for having unusual prey-choice behavior (6) and for being a species in which mutual mate choice is pronounced (7). Although all spiders probably rely to some substantial extent on chemoreception (8), salticids are better known for having unique, complex eyes (9) and vision based on a level of spatial acuity that is unrivalled by animals of comparable size (10). Yet salticids use tactile, auditory, and percussion signals, either in conjunction with or as alternatives to vision-based signals (11, 12). Salticids are also one of the spider families for which we have the most experimental evidence of refined ability to use chemical signals (13, 14).
E. culicivora is an unusual salticid species because it feeds indirectly on vertebrate blood by actively choosing bloodcarrying mosquitoes as prey (6, 15). However, the dominant mosquito-size dipterans in E. culicivora's habitat are nonbiting midges (known locally as “lake flies”) from the families Chaoboridae and Chironomidae (16), and E. culicivora can readily discriminate between the lake fly and the bloodcarrying mosquito by sight and by olfaction (6).
Evarcha culicivora also identifies opposite-sex conspecific individuals (i.e., potential mates) not only by sight but also by odor (7, 17). Here we consider whether there is a link between this predator's unusual diet and its mate-choice decisions, our hypothesis being that, by feeding on bloodcarrying mosquitoes, E. culicivora acquires an odor (or ‘perfume’) that is preferred by potential mates. Our findings come from using an olfactometer designed for retention testing (17), each individual test spider being presented with odor from one source spider on one day and odor from a different source spider on the following day (odor presented on first day determined at random). The rationale for this test design was an expectation that the spider would stay near a more preferred odor for longer than near a less preferred odor.
Results
How the Source Spider's Maintenance Diet Influences Retention Time.
As predicted by our hypothesis, retention times for test spiders were significantly longer when the opposite-sex source spider had been maintained on a blood diet instead of a nonblood diet (Table 1). This trend held regardless of whether source spiders had or had not already mated (mated compared with virgin source spiders; male test spiders: Z = 0.993, P = 0.321; female test spiders: Z = 1.399, P = 0.162).
Table 1.
Scores for Evarcha culicivora when tested for discrimination of diet-related odor
| Test spider | Source spider 1 | Source spider 2 | Median score | First quartile | Third quartile | Wilcoxon test |
|---|---|---|---|---|---|---|
| Male | Female on blood diet | Female on male-mosquito diet | 14.5 | 6.0 | 22.0 | Z = 4.870, P < 0.001 |
| Female | Male on blood diet | Male on male-mosquito diet | 25.5 | 13.0 | 32.0 | Z = 5.691, P < 0.001 |
| Male | Female on blood diet | Female on sugar-fed female-mosquito diet | 9.0 | −2.0 | 20.0 | Z = 3.398, P < 0.001 |
| Female | Male on blood diet | Male on sugar-fed female-mosquito diet | 20.0 | 9.0 | 37.0 | Z = 5.426, P < 0.001 |
| Male | Female on blood diet | Female on lake-fly diet | 18.5 | −1.0 | 27.0 | Z = 4.810, P < 0.001 |
| Female | Male on blood diet | Male on lake-fly diet | 19.0 | 9.0 | 31.0 | Z = 5.476, P < 0.001 |
| Male | Mated female on blood diet | Mated female on lake-fly diet | 11.5 | −1.0 | 24.0 | Z = 3.929, P < 0.001 |
| Female | Mated male on blood diet | Mated male on lake-fly diet | 13.0 | −1.0 | 31.0 | Z = 3.577, P < 0.001 |
| Male | Male on blood diet | Male on lake-fly diet | −0.50 | −6.0 | 3.0 | Z = 1.394, P = 0.163 |
| Female | Female on blood diet | Female on lake-fly diet | 0.0 | −4.0 | 3.0 | Z = 0.643, P = 0.511 |
Source spiders maintained on a variety of diets. Unless stated otherwise, all test and source spiders virgins. For each row, n = 50. Score: latency (min) to leave holding chamber when tested with source spider 2 minus latency (min) to leave holding chamber when tested with source spider 1.
Diet did not appear to influence retention time when same-sex source spiders were used (Table 1), and ‘scores’ (defined in Tables 1–4 and Materials and Methods) using opposite-sex source spiders were significantly higher than scores using same-sex source spiders on the same diets (male test spiders: Z = 3.816, P < 0.001; female test spiders: Z = 6.136, P < 0.001).
Table 2.
Scores for Evarcha culicivora when tested for effect of switching source-spider diet from lake flies to blood
| Test spider | Source spider 1 | Source spider 2 | Median score | First quartile | Third quartile | Wilcoxon test |
|---|---|---|---|---|---|---|
| Male | Female on blood diet for 1 day | Female on lake-fly diet | 7.0 | −1.0 | 21.0 | Z = 3.228, P = 0.001 |
| Female | Male on blood diet for 1 day | Male on lake-fly diet | 1.0 | −5.0 | 13.0 | Z = 1.556, P = 0.120 |
| Male | Female on blood diet for 14 days | Female on blood diet for 1 day | 6.0 | −2.0 | 18.0 | Z = 2.554, P = 0.011 |
| Female | Male on blood diet for 14 days | Male on blood diet for 1 day | 10.0 | −1.0 | 18.0 | Z = 3.765, P < 0.001 |
| Male | Female on blood diet for 28 days | Female on blood diet for 14 days | 5.0 | −8.0 | 15.0 | Z = 2.273, P = 0.023 |
| Female | Male on blood diet for 28 days | Male on blood diet for 14 days | 0.0 | −13.0 | 16.0 | Z = 0.554, P = 0.580 |
All test and source spiders virgins. For each row, n = 50. Score: latency (min) to leave holding chamber when tested with source spider 2 minus latency (min) to leave holding chamber when tested with source spider 1.
Table 3.
Scores for Evarcha culicivora when tested for effect of switching source-spider diet from blood to lake flies
| Test spider | Source Spider 1 | Source Spider 2 | Median score | First quartile | Third quartile | Wilcoxon test |
|---|---|---|---|---|---|---|
| Male | Female on blood diet | Female on lake-fly diet for 1 day | 12.0 | 0.0 | 24.0 | Z = 3.820, P < 0.001 |
| Female | Male on blood diet | Male on lake-fly diet for 1 day | 5.5 | −2.0 | 22.0 | Z = 3.051, P = 0.002 |
| Male | Female on lake-fly diet for 1 day | Female on lake-fly diet for 14 days | 4.0 | −7.0 | 18.0 | Z = 2.597, P = 0.009 |
| Female | Male on lake-fly diet for 1 day | Male on lake-fly diet for 14 days | 5.5 | −9.0 | 26.0 | Z = 2.635, P = 0.008 |
All test and source spiders virgins. For each row, n = 50. Score: latency (min) to leave holding chamber when tested with source spider 2 minus latency (min) to leave holding chamber when tested with source spider 1.
Table 4.
Scores for Evarcha culicivora when tested for effect of fasting
| Test spider | Source Spider 1 | Source Spider 2 | Median score | First quartile | Third quartile | Wilcoxon result |
|---|---|---|---|---|---|---|
| Male | Female on blood diet | Female on blood diet then fasted 7 days | 7.0 | 2.0 | 17.0 | Z = 3.377, P = 0.001 |
| Female | Male on blood diet | Male on blood diet then fasted 7 days | 12.0 | 0.0 | 20.0 | Z = 3.939, P < 0.001 |
| Male | Female on blood diet then fasted 7 days | Female on blood diet then fasted 14 days | 10.0 | 1.0 | 19.0 | Z = 4.113, P < 0.001 |
| Female | Male on blood diet then fasted 7 days | Male on blood diet then fasted 14 days | 10.5 | −1.0 | 27.0 | Z = 3.605, P < 0.001 |
All test and source spiders virgins. For each row, n = 50. Score: latency (min) to leave holding chamber when tested with source spider 2 minus latency (min) to leave holding chamber when tested with source spider 1.
How Switching to a Blood Diet Influences Retention Time.
For males, retention times were significantly longer when the source spiders were females that had been switched from a lake fly diet to a blood diet 1 day earlier, instead of remaining on a lake fly diet. Retention times were also significantly longer when the female source spiders had been switched to a blood diet 14 days earlier instead of 1 day earlier, and significantly longer when the female source spiders had been switched to a blood diet 28 days earlier instead of 14 days earlier (Table 2).
For females, retention times were significantly longer when the male source spiders had been switched from a lake fly diet to a blood diet 14 days earlier instead of 1 day earlier (Table 2). However, retention times were not significantly longer when the male source spiders had been switched to a blood diet 1 day earlier instead of remaining on a lake fly diet, and not significantly longer when the male source spiders had been switched to a blood diet 28 days previously, instead of 14 days previously.
On the whole, these findings appear to show that, even in the short term, a blood diet makes the odor of opposite-sex conspecifics more attractive, and attractiveness seems to increase when the duration of blood feeding increases. Although two of the comparisons that were significant for males were not significant for females, scores for females were not significantly different from scores for males, regardless of whether the opposite-sex source spiders had been on a blood diet for 1 day instead of remaining on a lake fly diet (Z = 1.512, P = 0.131), had been on a blood diet for 14 days instead of for only 1 day (Z = 1.165, P = 0.244), or had been on a blood diet for 28 days instead of for 14 days (Z = 0.762, P = 0.446).
How Switching to a Lake-Fly Diet and Fasting Influence Retention Time.
Retention times for male and female test spiders were significantly shorter when source spiders were opposite-sex individuals that had been switched from a blood diet to a lake fly diet 1 day earlier, instead of remaining on a blood diet. Retention times were also significantly shorter when opposite-sex source spiders had been switched from a blood diet to a lake fly diet 14 days earlier instead of only 1 day earlier (Table 3).
Moreover, retention times were significantly shorter when the source spiders were opposite-sex individuals that, after being maintained on a blood diet, were subjected to a 7-day fast instead of not fasting and were also significantly shorter when opposite-sex source spiders had fasted for 14 days instead of for 7 days (Table 4).
Discussion
Our experimental findings show that feeding on bloodcarrying mosquitoes makes the odor of E. culicivora males and females more attractive to the opposite sex, with even a single blood meal sufficing to make either sex more attractive to the other and with attractiveness tending to increase when the time on a blood diet increased. Continued access to blood meals appears to be important, as the attractiveness opposite-sex source spiders gained from a blood diet was lost when access to this diet was brought to an end either by a switch to a nonblood diet or by a fast.
Although retention testing showed that opposite-sex individuals were more attractive (i.e., test spiders were more inclined to stay near them) when they had been maintained on a blood diet for longer, our experimental design precluded spiders actually mating. An important next step will be to determine whether a blood diet makes spiders more successful at mating. However, an earlier study (7) showed that both sexes of E. culicivora prefer larger mates in choice tests where mating is precluded and also in choice tests where spiders could mate. These earlier findings suggest that the experimental design we used in the present study, despite precluding actual mating, is nonetheless an accurate indicator of mate-choice decisions that would be made in real life situations.
This is evidence that E. culicivora's unusual prey-choice behavior might be linked to this species' mating strategy, but it may be common for animal courtship routines to be based in part on the use of odors that have salience in another context. Examples include pheromones of noctuid moths mimicking the plant volatiles used by females for locating oviposition sites (18), male fruit flies attracting potential mates by using ginger root oil as a perfume (19), and bird species that incorporate aromatic plant material in their nests (20, 21). There is also evidence from studies on salamanders and voles that odor derived from high-quality diets makes individuals more attractive to the opposite sex (5, 22).
The highly specific prey-choice behavior by which E. culicivora appears to specialize on a difficult-to-satisfy diet suggests that good-genes hypotheses (23) might be useful for explaining why this species bases mate-choice decisions on diet-related odor, and an important next step will be to determine whether there is heritable variation in the spider's ability to acquire the diet-related attractive odor. It will also be useful to investigate more direct effects, including the possibility that females on a blood diet produce more viable eggs or the possibility that males on a blood diet produce more viable sperm. However, another perspective on sexual selection, sensory exploitation (24), may be especially relevant for understanding E. culicivora's odor-based mate-choice decisions.
Sensory-exploitation hypotheses have been proposed in other research on salticid courtship behavior (11, 25). For example, males may sometimes attract the female's attention during courtship by using specific movement patterns that simulate the movement-related cues by which females normally detect prey. However, a closer parallel to how E. culicivora uses diet-derived odor may be found in how male euglossine bees store odor-generating compounds from orchid flowers in special sacs on their hind leg tibia and use this odor to attract females (26), but with some important differences. The bees do not rely on orchid odor for finding food, but E. culicivora uses blood odor in the context of feeding (6), as well as in the context of courtship. For the euglossines, it is only the male that collects odor-generating compounds (27), whereas both sexes of E. culicivora acquire odor from mosquitoes. For euglossine males, there is a problem of acquired odor attracting the unwanted attention of other males (28), but there is no evidence that, for E. culicivora, acquired odor is attractive to same-sex conspecific individuals.
The role of sensory exploitation in E. culicivora's biology appears to be less than straightforward, as the odor derived from preying on bloodcarrying mosquitoes does not seem to attract the opposite sex simply by exploiting a predisposition to respond to this odor as a prey-identification cue. What we found was instead evidence of acquired odor making individuals more attractive only when the source spider was an opposite-sex conspecific individual. One possibility is that the attractant is an odor derived by some additional processing after ingestion. Another hypothesis is that a prerequisite for the acquired odor to render an individual more attractive to the opposite sex is for this odor to be paired with another odor that identifies the individual's sex. Yet another hypothesis is that the odor of a same-sex conspecific may override any attractive odor from feeding on a blood diet. As a step toward investigating these hypotheses, we are currently working on identifying the volatile compounds in odor plumes from prey and mates so that we can base future experiments on using the compound blends that are especially salient when E. culicivora finds and chooses prey and mates.
Materials and Methods
General.
Standard procedures were used for rearing and maintenance (29). All individuals used as test and source spiders (see below) were from the F2 or F3 laboratory generations (cultures derived from individuals collected at our field site, Mbita Point, western Kenya). Once juveniles dispersed from their egg sacs, they were kept isolated from encounters with other conspecific individuals until used in an experiment. Source spiders were fed three times a week, and were assigned at random to one of four maintenance diets (6). There was a ‘blood diet’ (prey always female mosquitoes that had fed on human blood 4–5 h before used as prey for the spider) and there were three ‘nonblood diets’, namely spiders fed only on lake flies, only on male mosquitoes or only on female mosquitoes that were sustained on sugar alone (no blood meals). Test spiders were fed three times a week and were maintained on a diet of both blood and of lake flies in about equal numbers. Findings from preliminary work suggested that test spiders on other diets did not respond any differently in the olfactometer. All mosquitoes were Anopheles gambiae ss from culture. All lake flies were Nilodorum brevibucca (Chironomidae), collected locally as needed. Test spiders and, if not fasted, source spiders were always used in experiments on the next day after feeding. Findings from preliminary work suggested that the hunger level of test spiders did not influence experimental outcome.
For spiders, the terms ‘male’ and ‘female’ always refer to adult males and adult females (both sexes 5-mm in body length). Virgin: no contact with conspecific individuals after emerging from egg sac (reached maturity 14 days before tested). Mated: like virgin, except mated on the seventh day after reaching maturity and then tested 7 days later. Mated females had not oviposited.
Experimental Methods.
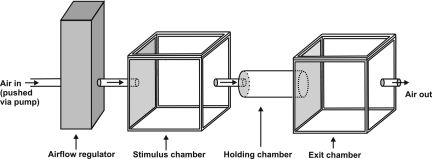
During testing, air was pushed successively through a stimulus chamber, a holding chamber and an exit chamber (Fig. 1). Airflow was always adjusted to 1,500 mL/min (Matheson FM-1000 airflow regulator) and there was no evidence that this airflow setting impaired locomotion or had any adverse effects on E. culicivora's behavior. The stimulus chamber was a glass cube (inner dimensions, 70 × 70 × 70 mm) made from 5-mm-thick glass and each cube had two holes (diameter 20 mm, centered on opposite sides of the cube, each hole plugged with a rubber stopper). Source spiders were put in the stimulus chamber 30 min before testing began.
Fig. 1.
Olfactometer for retention testing (not drawn to scale). Arrows indicate direction of airflow. Source spider held in stimulus chamber. Testing started with test spider in holding chamber at end distal to exit chamber. Test spider has access to exit chamber during test. Test spider's view of odor source obstructed by black paper taped to outside of stimulus-chamber wall that faced holding chamber.
The holding chamber was a glass tube (length 90 mm, inner diameter 15 mm, rubber stopper in one end, other end open). The open end of the holding chamber fit securely in the hole in the exit chamber, flush with the inner wall of the exit chamber. At the other end of the holding chamber there was a hole in the stopper with a glass tube going through to the stimulus chamber. A nylon-netting screen over the stopper (new netting for each test) ensured that the test spider could not enter the stimulus chamber, with the only way out of the holding chamber being via the opening into the exit chamber. The exit chamber was another glass cube identical to the stimulus chamber.
The test spider was first kept in the holding chamber for 2 min, with the holding chamber not yet connected to stimulus and exit chambers. The end of the holding chamber that would go into the exit chamber was plugged with a rubber stopper. For starting a test, this stopper was removed and the holding chamber was positioned between the stimulus and exit chamber, but with a prerequisite that the test spider had to be in the half of the holding chamber distal to the exit chamber. If this prerequisite was not met at the end of the 2-min pretest period, starting was delayed until the spider moved on its own accord into the distal half of the chamber and remained there for 2 min. Testing was aborted if this criterion was still not met after waiting 15 min, but aborted tests were rare (<5% for any given experiment).
All tests began between 8:00 AM and 2:00 PM (laboratory photoperiod 12L:12D, lights on at 7:00 AM) and lasted for a maximum of 60 min. Once testing began, we recorded the test spider's latency to leave the holding chamber (i.e., time elapsing between test beginning and spider entering exit chamber; maximum time allowed, 60 min). By default, the spider's latency to leave was recorded as 60 min whenever the 60-min test period ended with the test spider still in the holding chamber. No individual was used in more than one pair of retention tests and no individual was used more than once as a source spider. Between tests, the olfactometer was dismantled and cleaned with 80% ethanol, followed by distilled water, and then dried. For access to the interior when cleaning, there was a removable top on each stimulus and exit chamber.
Data Analysis.
As our data often failed to meet the assumptions required for parametric analyses, we used Wilcoxon tests for paired comparisons (null hypothesis: latency to leave holding chamber when tested with source spider 1 matched latency to leave holding chamber when tested with source spider 2). A score was calculated for each test spider by subtracting latency to leave holding chamber when tested with source spider 2 from latency to leave holding chamber when tested with source spider 1 (positive score: spider spent more time in the holding chamber when tested with source spider 1; negative score: spider spent more time in the holding chamber when tested with source spider 2). Using Mann-Whitney U tests, we also compared the scores for different groups of test spiders (spiders tested with virgin source spiders vs. spiders tested with mated source spiders; spiders tested with opposite-sex source spiders vs. spiders tested with same-sex source spiders; males tested with source spiders that had switched to blood diets vs. females tested with source spiders that had switched to blood diets; null hypothesis that scores for one group matched scores for another group). Wilcoxon test results are in Tables 1–4, whereas Mann-Whitney test results are in text (for details about statistical procedures, see 30).
Acknowledgments.
We thank Godfrey Otieno Sune, Stephen Abok Aluoch, and Jane Atieno Obonyo for their assistance at the International Centre of Insect Physiology and Ecology. This work was supported by the Royal Society of New Zealand Marsden Fund (to R.R.J. and S.D.P.), a James Cook Fellowship (to R.R.J.), the National Geographic Society (R.R.J.), and a University of Canterbury Doctoral Scholarship (to F.R.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Davis SF, Ludvigson HW. Odor memory in nonhumans. In: Schab FR, Crowder RG, editors. Memory for Odors. Mahwah, New Jersey: Lawrence Erlbaum Associates, Inc; 1995. pp. 133–158. [Google Scholar]
- 2.Freeman WJ. How Brains Make up their Minds. London: Phoenix; 1999. [Google Scholar]
- 3.Wyatt TD. Pheromones and Animal Behaviour. Cambridge: Cambridge Univ Press; 2003. [Google Scholar]
- 4.Brennan PA, Kendrick KM. Mammalian social odours: Attraction and individual recognition. Phil Trans R Soc London Ser B. 2006;361:2061–2078. doi: 10.1098/rstb.2006.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferkin MH, Sorokin ES, Johnston RE, Lee CJ. Attractiveness of scents varies with protein content of the diet in meadow voles. Anim Behav. 1997;53:133–141. [Google Scholar]
- 6.Jackson RR, Nelson XJ, Sune GO. A spider that feeds indirectly on vertebrate blood by choosing female mosquitoes as prey. Proc Natl Acad Sci USA. 2005;102:15155–15160. doi: 10.1073/pnas.0507398102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cross FR, Jackson RR, Pollard SD. Male and female mate-choice decisions by Evarcha culicivora, an East African jumping spider. Ethology. 2007;113:901–908. [Google Scholar]
- 8.Huber BA. Sexual selection research on spiders: Progress and biases. Biol Rev. 2005;80:363–385. doi: 10.1017/s1464793104006700. [DOI] [PubMed] [Google Scholar]
- 9.Land MF, Nilsson DE. Animal eyes. Oxford: Oxford Univ Press; 2002. [Google Scholar]
- 10.Harland DP, Jackson RR. Portia perceptions: The Umwelt of an araneophagic jumping spider. In: Prete FR, editor. Complex Worlds from Simpler Nervous Systems. Cambridge, Massachusetts: MIT Press; 2004. pp. 5–40. [Google Scholar]
- 11.Jackson RR, Pollard SD. Jumping spider mating strategies: Sex among cannibals in and out of webs. In: Choe JC, Crespi BJ, editors. The Evolution of Mating Systems in Insects and Arachnids. Cambridge: Cambridge Univ Press; 1997. pp. 340–351. [Google Scholar]
- 12.Elias DO, Hebets EA, Hoy RR, Mason AC. Seismic signals are crucial for male mating success in a visual specialist jumping spider (Araneae: Salticidae) Anim Behav. 2005;69:931–938. [Google Scholar]
- 13.Pollard SD, Macnab AM, Jackson RR. Communication with chemicals: Pheromones and spiders. In: Nentwig W, editor. Ecophysiology of Spiders. Berlin: Springer-Verlag; 1987. pp. 133–141. [Google Scholar]
- 14.Jackson RR, Clark RJ, Harland DP. Behavioural and cognitive influences of kairomones on an araneophagic jumping spider. Behaviour. 2002;139:749–775. [Google Scholar]
- 15.Wesolowska W, Jackson RR. Evarcha culicivora sp. nov., a mosquito-eating jumping spider from East Africa (Araneae: Salticidae) Ann Zool. 2003;53:335–338. [Google Scholar]
- 16.Okedi J. Lake flies in Lake Victoria: Their biomass and potential for use in animal feeds. Insect Sci Appl. 1992;13:137–144. [Google Scholar]
- 17.Cross FR, Jackson RR. Mate-odour identification by both sexes of Evarcha culicivora, an East African jumping spider. Behav Processes. 2009;81:74–79. doi: 10.1016/j.beproc.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Phelan PL. Evolution of mate-signalling in moths: Phylogenetic considerations and predictions from the asymmetric tracking hypothesis. In: Choe JC, Crespi BJ, editors. The Evolution of Mating Systems in Insects and Arachnids. Cambridge: Cambridge Univ Press; 1997. pp. 240–256. [Google Scholar]
- 19.Shelly TE, Edu J, Pahio E, Nishimoto J. Scented males and choosy females: Does male odor influence female mate choice in the Mediterranean fruit fly? J Chem Ecol. 2007;33:2308–2324. doi: 10.1007/s10886-007-9394-y. [DOI] [PubMed] [Google Scholar]
- 20.Gwinner H. The function of green plants in nests of European starlings (Sturnus vulgaris) Behaviour. 1997;134:337–351. [Google Scholar]
- 21.Brouwer L, Komdeur J. Green nesting material has a function in mate attraction in the European starling. Anim Behav. 2004;67:539–548. [Google Scholar]
- 22.Walls SC, Mathis A, Jaeger RG, Gergits WF. Male salamanders with high-quality diets have faeces attractive to females. Anim Behav. 1989;38:546–548. [Google Scholar]
- 23.Byers JA, Waits L. Good genes sexual selection in nature. Proc Natl Acad Sci USA. 2006;103:16343–16345. doi: 10.1073/pnas.0608184103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arnqvist G. Sensory exploitation and sexual conflict. Phil Trans R Soc B. 2006;361:375–386. doi: 10.1098/rstb.2005.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clark DL, Uetz GW. Signal efficacy and the evolution of male dimorphism in the jumping spider, Maevia inclemens. Proc Natl Acad Sci USA. 1993;90:11954–11957. doi: 10.1073/pnas.90.24.11954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lunau K. Evolutionary aspects of perfume collection in male euglossine bees (Hymenoptera) and of nest deception in bee-pollinated flowers. Chemoecology. 1992;3:65–73. [Google Scholar]
- 27.Eltz T, Roubik DW, Lunau K. Experience-dependent choices ensure species-specific fragrance accumulation in male orchid bees. Behav Ecol Sociobiol. 2005;59:149–156. [Google Scholar]
- 28.Zimmermann Y, Roubik DW, Eltz T. Species-specific attraction to pheromonal analogues in orchid bees. Behav Ecol Sociobiol. 2006;60:833–843. [Google Scholar]
- 29.Cross FR, Jackson RR, Pollard SD. Complex display behaviour of Evarcha culicivora, an East African mosquito-eating jumping spider. NZ J Zool. 2008;35:151–187. [Google Scholar]
- 30.Howell DC. Statistical Methods for Psychology. Belmont, California: Wadsworth; 2002. [Google Scholar]