Abstract
The influential role of Wnt5a in tumor progression underscores the requirement for developing molecules that can target Wnt5a-mediated cellular responses. In the aggressive skin cancer, melanoma, elevated Wnt5a expression promotes cell motility and drives metastasis. Two approaches can be used to counteract these effects: inhibition of Wnt5a expression or direct blockade of Wnt5a signaling. We have investigated both options in the melanoma cell lines, A2058 and HTB63. Both express Frizzled-5, which has been implicated as the receptor for Wnt5a in melanoma cells. However, only the HTB63 cell line expresses and secretes Wnt5a. In these cells, the cytokine, TGFβ1, controlled the expression of Wnt5a, but due to the unpredictable effects of TGFβ1 signaling on melanoma cell motility, targeting Wnt5a signaling via TGFβ1 was an unsuitable strategy to pursue. We therefore attempted to target Wnt5a signaling directly. Exogenous Wnt5a stimulation of A2058 cells increased adhesion, migration and invasion, all crucial components of tumor metastasis, and the Wnt5a-derived N-butyloxycarbonyl hexapeptide (Met-Asp-Gly-Cys-Glu-Leu; 0.766 kDa) termed Box5, abolished these responses. Box5 also inhibited the basal migration and invasion of Wnt5a-expressing HTB63 melanoma cells. Box5 antagonized the effects of Wnt5a on melanoma cell migration and invasion by directly inhibiting Wnt5a-induced protein kinase C and Ca2+ signaling, the latter of which we directly demonstrate to be essential for cell invasion. The Box5 peptide directly inhibits Wnt5a signaling, representing an approach to anti-metastatic therapy for otherwise rapidly progressive melanoma, and for other Wnt5a-stimulated invasive cancers.
Keywords: inhibitory peptide, malignant melanoma, tumor cell invasion
Wnt ligands comprise a family of 19 human secreted signaling proteins, which coordinate essential processes required for development and maintenance of tissue homeostasis. Misregulation of Wnt signaling can lead to cancer progression (1). The Wnt ligands are secreted glycoproteins that can be divided based on their ability to activate different intracellular signals, in a tissue-dependent manner. One group primarily activates canonical signaling that controls β-catenin stability, while the other is loosely described as β-catenin-transcriptionally independent (non-canonical Wnt signaling). However, cross-talk between the two signaling networks does exist (2).
Wnt5a is in most situations characterized as a non-canonical Wnt ligand that elicits intracellular signals through association with distinct receptors and co-receptors in a cell specific manner. Wnt5a has been shown to stimulate increases in intracellular Ca2+ levels in developmental models (3) and mammalian cell lines, including breast and thyroid cancer cells (4–6), giving rise to the model of a non-canonical Wnt/Ca2+ signaling pathway. Wnt5a-mediated intracellular increases in Ca2+ levels enables the activation of Ca2+-regulated proteins, such as protein kinase C (PKC) in a context dependent manner, as reviewed recently (7).
Wnt5a expression has been linked to cancer progression in a variety of tumor types, but its role in tumorigenesis is complex. In breast, colon, thyroid, and liver cancers, Wnt5a functions as a tumor suppressor (6, 8–10). However, in other cancers such as malignant melanoma, Wnt5a actually promotes cancer progression (11–13). Therefore depending on the type of cancer, Wnt5a signaling has the potential to be manipulated to block tumor progression, either by mimicking its effects as for example in breast cancer (4, 14) or, as in the case of melanoma, by inhibiting its function. Currently the molecular components of the Wnt5a signaling pathway in melanoma are not fully elucidated. It has been shown to signal through the G protein-coupled receptor, Frizzled-5 (FZD5), which results largely in a β-catenin-independent signaling response in melanoma cells (12). Moreover, Wnt5a stimulates PKC activation to induce an epithelial to mesenchymal transition, resulting in increased adhesion, migration, and invasion (12, 15). Furthermore, Wnt5a has been shown to control cell polarity, orientation, and directional movement in melanoma cells (16). These findings highlight how Wnt5a can increase melanoma metastasis, but it is still unknown how the ligand directly elicits these activities and how the expression of Wnt5a itself is controlled in these cells.
Although cutaneous melanoma represents only 4% of all diagnosed skin cancers, due to its highly metastatic nature it accounts for 80% of all skin cancer-related deaths (17). If the melanoma has metastasized to lymph nodes or distant organs there are very limited therapeutic options to prolong survival or cure the disease (18). Consequently, cutaneous malignant melanoma poses a serious healthcare issue, and it is imperative that new drug therapies are identified and developed that can antagonize the metastatic process. Currently, there is a lack of effective therapeutics for hindering the progression of metastatic melanoma.
Given the distinct lack of therapeutics available for melanoma progression and the potency of Wnt5a to increase melanoma cell invasiveness and metastasis, we believe that inhibition of Wnt5a expression and signaling would be an excellent therapeutic approach for this disease, a concept already suggested by others (19). The promise of such a therapeutic approach is highlighted by the striking ability of Wnt5a to increase melanoma metastases in vivo (19). To identify a compound capable of antagonizing the effects of Wnt5a in melanoma cells, we investigated the Wnt5a signaling pathway in more detail. Although we report here that signaling via the potent cytokine, tumor growth factor β1 (TGFβ1), controls Wnt5a expression, loss of TGFβ1 signaling led to unpredictable effects on melanoma cell motility, highlighting the importance of targeting Wnt5a signaling directly. Therefore, we have developed and characterized a Wnt5a-specific antagonist peptide with the capacity to inhibit Wnt/Ca2+ signaling in melanoma cells. The inhibition of these Wnt5a-induced signals significantly reduced Wnt5a-mediated migration and invasion of melanoma cells, essential events in tumor metastasis. We believe this peptide represents a candidate for the development of an anti-metastatic therapy for Wnt5a-stimulated invasive cancers such as malignant melanoma.
Results
Due to the strong correlation between elevated Wnt5a levels and enhanced melanoma progression (11, 13), we investigated how Wnt5a expression is controlled in melanoma cell lines to test if blocking its expression could be a means to control melanoma cell invasiveness. To begin, we identified two human melanoma cell lines with differential Wnt5a expression patterns: A2058 cells with low endogenous expression and HTB63 cells that express and secrete high levels of Wnt5a (Fig. S1). Both cell lines expressed the putative melanoma Wnt5a receptor, Frizzled5 (FZD5) (Fig. S1). MCF-7 breast cancer cells were used as a positive control (20) (Fig. S1). Aside from the Wnt5a expression, these cell lines are actually similar in other aspects. Both these lines were originally isolated from metastases of patients with cutaneous melanoma (21, 22), and both carry the V599E BRAF mutation (23). Interestingly, the anti-proliferative cyclin-dependent kinase inhibitor, p16ink4a, is not expressed in the HTB63 cells, but is expressed and functional in the A2058 cells (24). This is in agreement with previous findings, showing that in patient material there is an inverse correlation between p16ink4a and Wnt5a expression in melanoma progression (13).
The complementary expression of Wnt5a in melanoma cells at the mRNA and protein levels (Fig. S1 A and B), suggests expression is controlled at the transcriptional level. Interestingly, TGFβ1 has been shown to transcriptionally regulate Wnt5a in the mouse mammary gland (25), and maintain Wnt5a expression during mammary tumor formation (26). We found that the selective TGFβ1 type I receptor inhibitor SB431542 and recombinant TGFβ1 decreased and induced Wnt5a expression in the HTB63 and A2058 cell lines, respectively (Fig. S2). These data demonstrate that TGFβ1 is a positive regulator of Wnt5a expression in human melanoma cells. In accordance, we demonstrated that TGFβ1 signaling could increase melanoma cell adhesion and migration (Fig. S3). Paradoxically, however, TGFβ1 signaling had a negative effect on melanoma cell invasion in a 3-D cell culture assay (Fig. S4), which has also been observed in murine melanoma cells (27). These findings suggest that TGFβ1 is an unpredictable target for blocking Wnt5a-mediated melanoma cell motility presumably due to its well characterized multiple downstream effects.
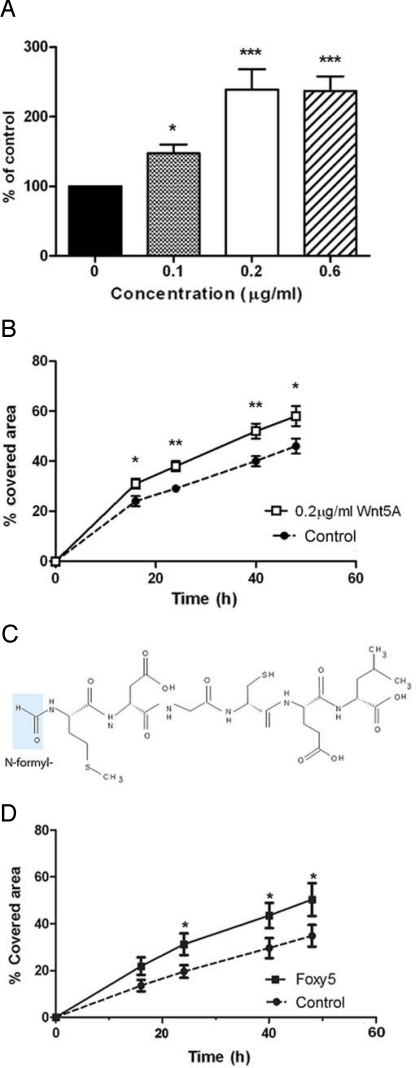
The above results made us conclude that direct targeting of Wnt5a signaling could provide a more direct and therefore potentially safer alternative for therapeutics that antagonize Wnt5a activity in melanoma cells. We set about developing a molecule with the capacity to antagonize Wnt5a-induced melanoma cell migration. We first ascertained that Wnt5a could increase adhesion and migration in A2058 melanoma cells (Fig. 1 A and B) as previously demonstrated in other melanoma cell lines (12). Previously we identified and characterized a Wnt5a-derived, N-formylated hexapeptide (Foxy5) (Fig. 1C) that functions as an agonist of Wnt5a signaling. This peptide mimics the anti-migratory and anti-invasive effects of Wnt5a in breast cancer cell lines (4, 14), and has anti-tumorigenic effects on breast cancer in vivo (14, 28). We found that Foxy5 could also mimic the pro-migratory effects of Wnt5a in A2058 melanoma cells (compare Fig. 1 B and D), suggesting this peptide functions as a Wnt5a agonist in diverse cell types. Interestingly, it has previously been shown that modification of a formylated bacterially derived chemotactic peptide (formyl-Met-Leu-Phe), converted the molecule from an agonist to an antagonist analogue (29). Specifically, the modification involved substitution of the N-terminal formyl group for a t-butoxycarbonyl (t-boc) group. We hypothesized that such a modification of Foxy5 could also change its Wnt5a agonist functions to that of an antagonist. We synthesized and purified such a t-boc-Met-Asp-Gly-Cys-Glu-Leu peptide, hereafter referred to as Box5 (Fig. 2A).
Fig. 1.
Wnt5a and Foxy5 promote melanoma cell migration. (A) A2058 cells (low Wnt5a expressing melanoma cell line, Fig. S1) were stimulated with the indicated concentrations of Wnt5a for 16 h, detached with versene and allowed to adhere for 1 h. Non-adherent cells were washed away, while the adherent cells were stained and their relative number determined. This number is represented as a percentage of non-Wnt5a-stimulated cells. (B) Wnt5a (0.2 μg/mL, open symbols and solid line) increased the migration of A2058 cells in a wound-healing assay compared to untreated cells (control, closed symbols and broken line) over a 2-day time course. The wound-healing data are expressed as a percentage of the wound area closed after 0, 16, 24, 40, and 48 h. (C) Foxy5 structure (formyl group in blue). (D) Foxy5 (50 μM; solid squares and solid line) increased the migration of A2058 cells in a wound-healing assay compared to untreated cells (control, closed circles and broken line) over a 2-day time course. The wound-healing data are expressed as a percentage of the wound area closed after 0, 16, 24, 40, and 48 h. Error bars, SEM. Paired t-tests; *, P < 0.05.
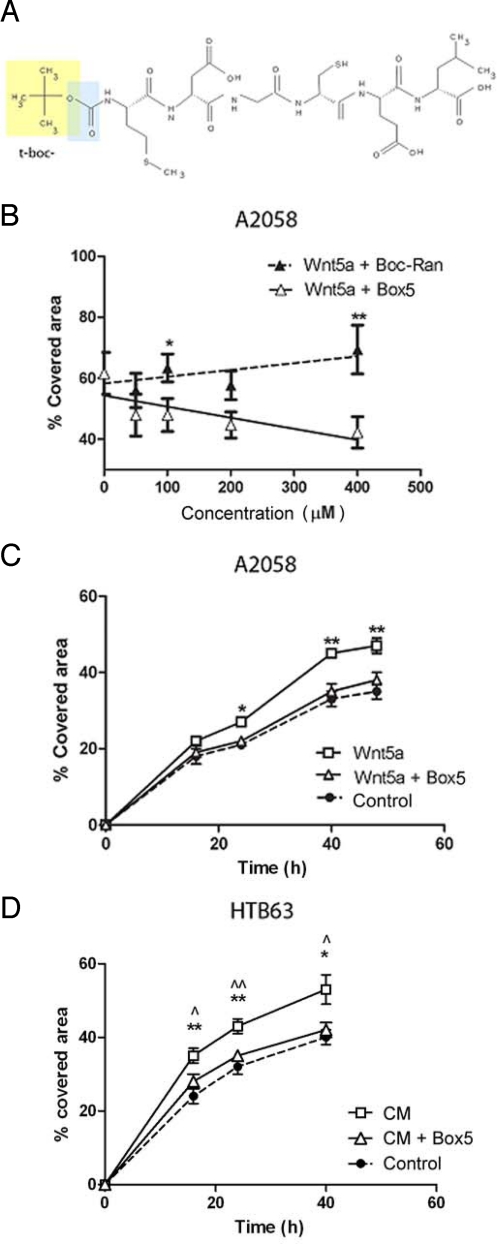
Fig. 2.
Box5 is a modified analogue of Foxy5 and functions as an antagonist of Wnt5a in melanoma cells. (A) Box5 structure (t-boc group in yellow and blue, yellow represents the additional modification as compared to the formyl group). (B) Box5 (open symbols and solid line) inhibits Wnt5a-mediated A2058 cell migration (wound-healing analysis) in a dose-dependent manner. A t-boc-conjugated random peptide, Boc-Ran (closed symbols and broken line), could not antagonize Wnt5a-mediated migration. Cells were incubated with either of the peptides at the indicated concentrations, and 0.2 μg/mL recombinant Wnt5a (rWnt5a) for 40 h. (C) Wound-healing analysis of A2058 cells during a 48-h time course. The cells were incubated with rWnt5a (0.2 μg/mL) alone (open squares and solid line) or rWnt5a (0.2 μg/mL) and 100 μM Box5 (open triangles and solid line), or without any additive (control, closed symbols and broken line). (D) Wound-healing analysis of HTB63 cells (high Wnt5a expressing melanoma cell line) (Fig. S1) during a 40-h time course. The HTB63 cells were incubated in conditioned medium (open squares and solid line), or conditioned medium containing and 100 μM Box5 (open triangles and solid line), or fresh serum-free medium without any additive (control, closed symbol and broken line). * t-tests indicate conditioned medium values compared to control, while analysis of conditioned medium compared to Box5 are indicated by ⋀, P < 0.05, ⋀⋀, P < 0.01. In A–D, error bars, SEM. Paired t-tests: *, P < 0.05; **, P < 0.01.
Dose-response analysis of Box5 indicated there was a strong inhibitory effect on Wnt5a-induced A2058 cell migration, with a statistically significant effective concentration at 100 μM (Fig. 2B). A t-boc-conjugated random hexamer (Met-Ser-Ala-Asp-Val-Gly; Boc-Ran) was unable to inhibit cell migration (Fig. 2B), confirming selectivity of Box5. Over a 48 h time course, Box5 had the ability to inhibit Wnt5a-mediated migration of A2058 cells (Fig. 2C) and could also antagonize the migration of HTB63 cells (endogenously secreting Wnt5a), to the same extent as changing the conditioned media (containing secreted Wnt-5a) to fresh serum-free media (lacking Wnt-5a) (Fig. 2D). However, Box5 did not affect the intrinsic migration of A2058 cells that lack endogenous Wnt5a expression (Fig. S5A). We also found that TGFβ1-mediated migration of A2058 cells could be blocked by preincubation with Box5 (Fig. S5B), further highlighting that direct blockade of Wnt5a-signaling downstream of TGFβ1 is an effective approach to inhibit Wnt5a-mediated melanoma migration.
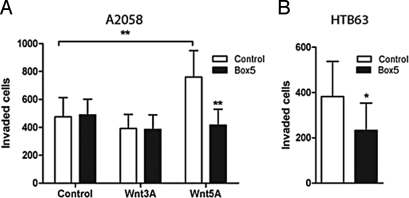
During the metastatic process, tumor cells need to invade through the extracellular matrix, so we tested the efficacy of Box5 to block cell invasion in a 3-D matrigel cell culture model. Addition of Box5 abolished Wnt5a-induced invasion of A2058 cells, an effect not seen when the cells were stimulated with the canonical Wnt ligand, Wnt3a (Fig. 3A). Furthermore, Box5 also had the ability to inhibit invasion of HTB63 cells, by antagonizing the effects of endogenous and secreted Wnt5a from these cells (Fig. 3B). Collectively these data show that Box5 is a potent, selective antagonist of Wnt5a-mediated migration and invasion of melanoma cells, both of which are essential components of the metastatic process.
Fig. 3.
(A) Box5 inhibits Wnt5a-mediated melanoma cell invasion through matrigel. A2058 cells were preincubated with/without Box5 (100 μM) for 40 min, followed by the addition of rWnt3a (0.05 μg/mL), rWnt5a (0.2 μg/mL), or no stimuli at all. (B) Invasion of HTB63 cells is inhibited by addition of 100 μM Box5. All error bars, SEM. Paired t-tests for all experiments; *, P < 0.05, **, P < 0.01.
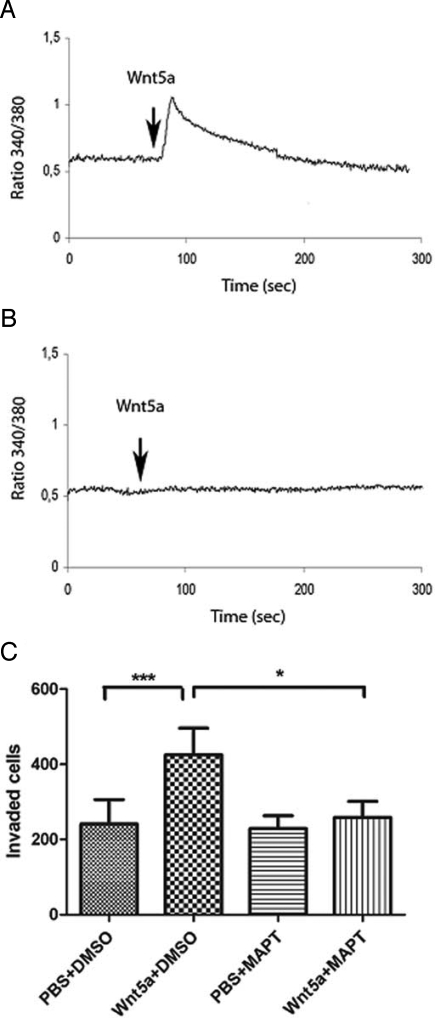
To identify the molecular basis for the antagonistic functions of Box5, we investigated components of Wnt5a signaling in melanoma cells that are essential for cell invasion. A recent study showed that Wnt5a signaling in melanoma cells resulted in cleavage of filamin A and remodeling of the cytoskeleton, leading to increased cell motility, where filamin A cleavage was mediated by Ca2+-activated proteases (30). This suggested that intracellular Ca2+ fluxes are required for Wnt5a-mediated cell invasion. We found that Wnt5a stimulated a rapid cytosolic Ca2+ signal in A2058 cells (Fig. 4A), which could be inhibited by preloading these cells with the intracellular Ca2+ chelator, MAPT (Fig. 4B). We used MAPT-Ca2+ chelation to assess the invasive capacity of melanoma cells in the absence of Wnt5a-induced Ca2+ signaling. The pro-invasive effect of Wnt5a on the A2058 cells was completely abolished by incubation with MAPT (Fig. 4C). This demonstrates that the Ca2+ signaling component of Wnt5a stimulation is required for mediating melanoma cell invasion.
Fig. 4.
The Wnt/Ca2+ signaling pathway is essential for Wnt5a-mediated melanoma cell invasion. (A) rWnt5a (0.1 μg/mL; addition indicated by arrow) triggers a rapid cytosolic Ca2+ signal in A2058 cells. (B) Preincubation of A2058 cells with 10 μM MAPT/AM for 30 min abolishes rWnt5a (0.1 μg/mL) stimulation (indicated by arrow) of cytosolic Ca2+. (C) MAPT/AM abolishes Wnt5a-induced A2058 cell invasion. Cells were preincubated with 10 μM MAPT/AM or vehicle alone for 30 min, then stimulated with/without rWnt5a (0.2 μg/mL), and then with 1 μM MAPT/AM or vehicle alone throughout the duration of the invasion experiment (24 h), where the latter treatment condition had the same chelating effect on Ca2+ as 10 μM of MAPT/AM for 30 min, shown in A. Error bars, SEM. Paired t-tests; *, P < 0.05, ***, P < 0.001.
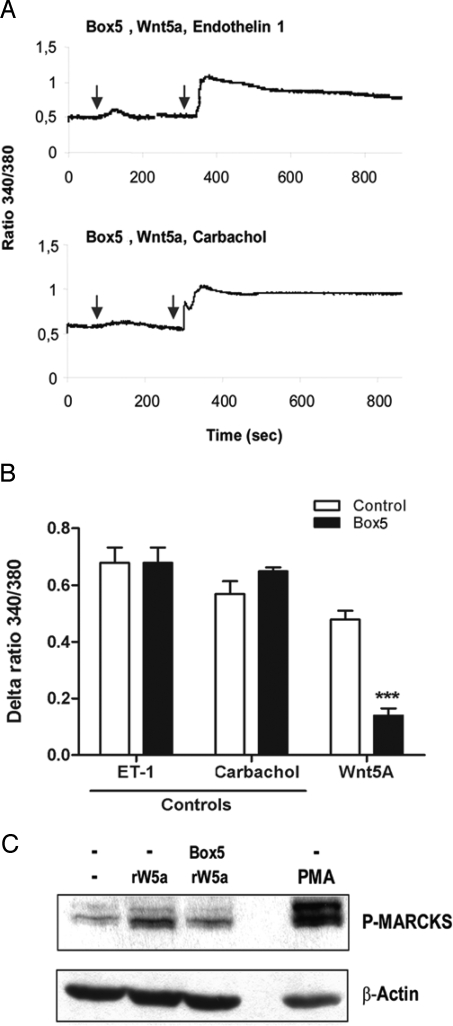
Next we analyzed the ability of Box5 to modulate Wnt5a-induced Ca2+ signaling in melanoma cells. Box5 selectively inhibited Wnt5a-induced intracellular Ca2+ signaling, but not that induced by either endothelin-1 (ET-1) or carbachol (Fig. 5A), both of which trigger G protein coupled receptor-induced increases in cytosolic Ca2+ (Fig. S6). Accumulated results revealed that there was >70% inhibition of Wnt5a-induced Ca2+ signaling by Box5, but the peptide had no such effect on the Ca2+ signal induced by either ET-1 or carbachol (Fig. 5B).
Fig. 5.
Box5 functions as a Wnt5a antagonist by inhibiting Wnt5a-mediated Ca2+ signaling. (A) Preincubation with Box5 (100 μM) overnight blocks rWnt5a-induced (0.1 μg/mL) Ca2+ release. Representative Ca2+ traces of A2058 cells stimulated with rWnt5a (first arrow), and then with (second arrow) either endothelin-1 (ET-1; 10 nM) or carbacol (5 μM). (B) Accumulated ΔCa2+ changes in ratio values recorded from A2058 cells stimulated with the various ligands described for A, either in the presence or absence of 100 μM Box5. Error bars, SEM. Paired t-test; ***, P < 0.001. (C) Preincubation with Box5 (100 μM) overnight inhibits myristoylated alanine-rich C kinase substrate (MARCKS) phosphorylation after 45 min of rWnt5a stimulation (0.2 μg/mL). 1 nM of the PKC activator, phorbol 12-myristate 13-acetate (PMA) was used as a positive indicator for MARCKS phosphorylation.
Previous studies have suggested that there is a downstream effect of Wnt-5a-induced PKC activation on the regulation of melanoma cell migration (12, 15). In the present study, we assessed Ser-152/156 phosphorylations of the endogenous PKC substrate, Myristoylated Alanine-Rich C Kinase Substrate (MARCKS) (31), as a direct means of estimating the level of PKC activity. Wnt5a stimulation of A2058 cells resulted in increased phosphorylation of MARCKS, which was inhibited in the presence of the Box5 peptide (Fig. 5C). These data show that Box5 functions to inhibit melanoma cell invasion by directly antagonizing Wnt5a-stimulated Ca2+ and PKC signaling, both of which are essential for Wnt5a-mediated melanoma cell invasion. We conclude that Box5 functions as a selective antagonist of Wnt5a-induced melanoma cell migration and invasion by inhibiting Wnt5a signaling.
Discussion
Malignant melanoma is a particularly aggressive cancer, and once metastasis has occurred, there is an extremely poor overall prognosis with limited treatment options, highlighting the crucial need for new therapies to counteract the metastatic process in these patients. The fact that Wnt5a expression and signaling has been strongly associated with driving malignant melanoma metastasis (11–13, 15, 19), not only highlights the relevance of Wnt5a as a prognostic marker for melanoma, but also makes Wnt5a an attractive drug target for patients suffering from this form of aggressive skin cancer. Two strategies can be exploited to block the positive effects of Wnt5a on melanoma metastases development; blocking Wnt5a expression in these cells, or inhibiting its signaling directly. We have investigated both these options. We found that TGFβ1 signaling controls Wnt5a expression in melanoma cells, and also regulates melanoma cell motility. However, while we found it promoted cell migration, it unexpectedly inhibited cell invasion. Thus, selective TGFβ inhibitors appear to be unsuitable treatment options for malignant melanoma due to the multiple and unpredictable effects of this potent cytokine. We propose instead that direct blocking of Wnt5a-induced signaling provides a more specific and therefore more effective approach for the development of anti-metastatic drugs in tumors that express high constitutive levels of Wnt5a.
Our study demonstrates that the Box5 hexapeptide effectively inhibits Wnt5a signaling in a selective and dose-dependent manner in melanoma cells, and is a direct antagonist of Wnt5a signaling. The cell lines we chose to test Box5 on were originally derived from cutaneous melanoma metastases with BRAF mutations, thus they represent the most common form of the malignancy and therefore our data suggests that Box5 could have broad potential as an anti-metastatic drug for melanoma. To test this, in vivo studies are required to help predict the potential clinical efficacy of the Box5 peptide. As such, we have currently initiated experiments using melanoma xenograft models to test the peptide in an in vivo setting. A previous study on substituting the N-terminal formyl group for different carbamate groups on the chemotactic tripeptide Fmet-Leu-Phe (29), indicated that the overall size and shape of the N-terminal modifying group were key factors in agonist versus antagonist activity conversion. It is reasonable to also suggest that the present substitution of the formyl group with the carbamate butyloxycarbonyl on the Wnt5a-derived agonist hexapeptide (4) modifies this peptide for antagonist activity based on the size and shape of the butyloxycarbonyl group.
The Box5 peptide significantly inhibited Wnt5a-induced Ca2+ and PKC signaling, both of which are necessary for Wnt5a-mediated melanoma cell invasion. Intracellular Ca2+ and PKC signaling are often intimately linked events (7). Consequently, our present finding that the Wnt5a-induced Ca2+ signal is essential for Wnt5a mediated melanoma cell migration and invasion has several possible interpretations. Firstly, Wnt5a-induced Ca2+ signaling is required for subsequent activation of a Ca2+-sensitive PKC isofom(s). In accordance, Wnt5a has been shown to activate Ca2+ dependent PKC isoforms in melanoma cells (12). Alternatively, the essential role of the Wnt5a-induced Ca2+ signal is independent of PKC activation and instead required for Wnt-5a-induced remodeling of the cytoskeleton in a PKC-independent manner, which is essential for melanoma cell motility (30). Finally, of course both of these alternatives could be involved.
Although the FZD5 receptor has been implicated in Wnt5a signaling in melanoma cells (12), the precise receptors/co-receptors that are involved remain undetermined. Therefore our approach of developing a Wnt5a inhibitor was based on our previous identification of possible secondary/solvent accessible surface exposed regions in the Wnt5a molecule, as previously described (4). We believe that this ligand-focused approach, rather than one based on targeting a particular receptor, is a more relevant strategy based on current understanding. Box5 is derived from the Wnt5a agonist peptide, Foxy5, also developed in our lab (4). The two peptides share the same amino acid sequence but differ in that Foxy5 has a formyl group and Box5 a butyloxycarbonyl group to the N-terminal methionine of this hexapeptide. Previously, we found that the effects of Foxy5 were lost if the cells were pretreated with a FZD5-specific blocking antibody, suggesting that Foxy5 mediates its effect on breast cancer cells via the G protein-coupled receptor FZD5 (4). The previous and present findings that the amino acid sequence is essential for the described effects of Foxy5 (4) and Box5, makes it logical to speculate that Box5 functions, like Foxy5, via binding to the FZD5 receptor. The relevance of this assumption is something we are actively investigating in our laboratory at present.
It should be noted that not all melanoma metastasis express high levels of Wnt5a (13, 19); this finding is of clinical relevance. With Box5 targeting, the need to screen patient's tumors for Wnt5a expression and active signaling will be required to identify those patients most likely to benefit from Box5 chemotherapy. Whilst Box5 may not be a suitable target for all patients suffering from melanoma, the most vulnerable group are most likely to benefit from the treatment given that Wnt5a expression is strongly associated with poor outcome (13). In a clinical setting, Box5 would probably be used in conjunction with existing therapies, defining it as an adjuvant agent for melanoma therapy.
In summary, we describe a hexapeptide (Box5) that antagonizes signaling by the pro-invasive signaling ligand, Wnt5a, in melanoma cells. The effects of Box5 suggest it could be a lead compound for the development of anti-metastatic therapeutics for malignant melanoma patients. Most cancer-related deaths are due to metastatic spread, highlighting the need for effective anti-metastatic therapeutics. Wnt5a has also been implicated in promoting cell migration in other tumors such as prostate (32), pancreatic (33), gastric (34), and non-small-cell lung cancers (35), suggesting that Box5 could have far wider potential use as an anti-metastatic drug. In a broader context, Wnt5a is also thought to promote chronic inflammatory diseases such as psoriasis and rheumatoid arthritis (36–38), highlighting further possible applications for Box5.
Materials and Methods
Cells, Chemicals, and Peptides.
Details of cells, culture conditions, and chemicals are provided in SI Materials and Methods. The Foxy5, Box5, and Boc-Ran peptides were synthesized by Inbiolabs (several different production batches of the peptides were used). Peptides were purified by reverse-phase high performance liquid chromatography and the purity of each synthesis was >95% (mass spectrometry, Inbiolabs).
Cell Adhesion Assay.
Pretreated cells, stimulated as described for each experiment were detached with versene, counted, resuspended in growth media at 30,000 cells per well, and plated in a 96-well plate. The cells were allowed to adhere for 1 h using normal growth conditions and then the non-adherent cells washed away with PBS. The remaining, adherent cells were then fixed in 1% glutaraldehyde for 10 min and stained with 0.5% crystal violet in 20% methanol. After washing with PBS, the stain from each well was dissolved in 50% acetic acid. The absorbance of the dissolved stain was measured in a plate reader (Fluostar, BMG Lab Technologies) at 544 nm. Individual samples were analyzed in quadruplicate against a background of blank wells.
Wound Healing/Cell Migration.
Confluent cell monolayers had a scratch inflicted and were incubated in serum-free media containing the relevant stimuli for each experiment. Images of the scratches were taken as indicated and migration was measured as percentage of the scratch area closed over time. The wound-healing data are expressed as a percentage of the wound area closed after 0, 16, 24, 40, and 48 h.
Cell Invasion.
Three-dimensional invasion assays were carried out using Matrigel invasion chambers (BD) with 8-μm pore size membranes, in a 24-well plate format. Cell invasion was performed as described previously (5). Briefly, cells were resuspended in serum-free media (25,000 cells/well). For treatments with Box5 and MAPT/AM, the cells were preincubated for 40 min with gentle agitation, and stimulated as required upon seeding. Cells were allowed to invade for 24 h, fixed (4% paraformaldehyde), stained with crystal violet, and counted. As an additional control for the migration and invasion assays, 0.2 μg/mL of recombinant Wnt5a (rWnt5a) was not found to affect cell proliferation or apoptosis.
Determination of Cytosolic Free Calcium Levels.
This was carried out as previously described (5). Fura-2 fluorescence was measured during stimulations with Wnt5a, endothelin-1, or carbachol. Cells were stimulated with half the concentration of rWnt5a as used for most other assays, so that similar Ca2+ responses as with ET-1 (10 nM) and carbachol (5 μM) could be observed. For experiments with MAPT/AM, cells were incubated with 10 μM MAPT/AM in Ca2+-containing medium for 30 min, after initial incubation with 4 μM Fura-2/AM. All Ca2+ traces shown are representative of a minimum of three repeats for each experimental condition tested.
Western Blotting.
Cells were lysed in 50 mM Tris-HCl (pH 7.5), 1% Triton X-100, 100 mM NaCl, 10 mM MgCl2, 20% glycerol, 1 mM Na3VO4, and protease inhibitors (20 μg/mL aprotinin, 1 μg/mL leupeptin, 2.5 mM benzamidine, and 2 mM pefbloc), and spun at 15,000 rpm for 5 min at 4 °C. For blotting of secreted proteins, cell medium was first concentrated in Amicon Ultra-15 concentrator columns (Millipore), according to the manufacturer's instructions. Immunoblotting was conducted as previously described (4).
Statistical Analysis.
Means and standard error of the means (SEM; indicated as error bars on all graphs), were calculated and plotted using GraphPad Prism 4. Several repeats were carried out of each experiment (all experiments were conducted at least in triplicate). Two-tailed, unpaired t-tests were used to determine the significance of experimental data. The following symbols were used to denote statistical significance on the graphs: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Supplementary Material
Acknowledgments.
We thank Kristi Kurg at Inbiolabs for excellent technical assistance. The work was supported by the Swedish Cancer Foundation (to T.A.), Swedish Research Council (to T.A.), Malmö University Hospital Research Foundations (to T.A.), Gunnar Nilsson's Cancer Foundation (to T.A.), and the Royal Physiografic Society (to V.S., V.J., and J.H.).
Footnotes
Conflict of interest statement: V.J., V.S., J.H, A.S., L.A., and T.A. have filed a patent on Box5 and its use as an anti-metastatic compound.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0909409106/DCSupplemental.
References
- 1.Klaus A, Birchmeier W. Wnt signalling and its impact on development and cancer. Nat Rev Cancer. 2008;8:387–398. doi: 10.1038/nrc2389. [DOI] [PubMed] [Google Scholar]
- 2.Quaiser T, Anton R, Kühl M. Kinases and G proteins join the Wnt receptor complex. Bioessays. 2006;28:339–343. doi: 10.1002/bies.20386. [DOI] [PubMed] [Google Scholar]
- 3.Slusarski DC, Yang-Snyder J, Busa WB, Moon RT. Modulation of embryonic intracellular Ca2+ signaling by Wnt-5A. Dev Biol. 1997;182:114–120. doi: 10.1006/dbio.1996.8463. [DOI] [PubMed] [Google Scholar]
- 4.Säfholm A, Leandersson K, Dejmek J, Nielsen CK, Villoutreix BO, Andersson T. A formylated hexapeptide ligand mimics the ability of Wnt-5a to impair migration of human breast epithelial cells. J Biol Chem. 2006;281:2740–2749. doi: 10.1074/jbc.M508386200. [DOI] [PubMed] [Google Scholar]
- 5.Dejmek J, Säfholm A, Kamp Nielsen C, Andersson T, Leandersson K. Wnt-5a/Ca2+-induced NFAT activity is counteracted by Wnt-5a/Yes-Cdc42-casein kinase 1alpha signaling in human mammary epithelial cells. Mol Cell Biol. 2006;26:6024–6036. doi: 10.1128/MCB.02354-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kremenevskaja N, von Wasielewski R, Rao AS, Schofl C, Andersson T, Brabant G. Wnt-5a has tumor suppressor activity in thyroid carcinoma. Oncogene. 2005;24:2144–2154. doi: 10.1038/sj.onc.1208370. [DOI] [PubMed] [Google Scholar]
- 7.Angers S, Moon RT. Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol. 2009;10:468–477. doi: 10.1038/nrm2717. [DOI] [PubMed] [Google Scholar]
- 8.Dejmek J, Dejmek A, Säfholm A, Sjölander A, Andersson T. Wnt-5a protein expression in primary dukes B colon cancers identifies a subgroup of patients with good prognosis. Cancer Res. 2005;65:9142–9146. doi: 10.1158/0008-5472.CAN-05-1710. [DOI] [PubMed] [Google Scholar]
- 9.Jönsson M, Dejmek J, Bendahl PO, Andersson T. Loss of Wnt-5a protein is associated with early relapse in invasive ductal breast carcinomas. Cancer Res. 2002;62:409–416. [PubMed] [Google Scholar]
- 10.Liu XH, et al. Expression of Wnt-5a and its clinicopathological significance in hepatocellular carcinoma. Dig Liver Dis. 2008;40:560–567. doi: 10.1016/j.dld.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 11.Bittner M, et al. Molecular classification of cutaneous malignant melanoma by gene expression profiling. Nature. 2000;406:536–540. doi: 10.1038/35020115. [DOI] [PubMed] [Google Scholar]
- 12.Weeraratna AT, et al. Wnt5a signaling directly affects cell motility and invasion of metastatic melanoma. Cancer Cell. 2002;1:279–288. doi: 10.1016/s1535-6108(02)00045-4. [DOI] [PubMed] [Google Scholar]
- 13.Da Forno PD, et al. WNT5A expression increases during melanoma progression and correlates with outcome. Clin Cancer Res. 2008;14:5825–5832. doi: 10.1158/1078-0432.CCR-07-5104. [DOI] [PubMed] [Google Scholar]
- 14.Säfholm A, Tuomela J, Rosenkvist J, Dejmek J, Härkönen P, Andersson T. The Wnt-5a-derived hexapeptide Foxy-5 inhibits breast cancer metastasis in vivo by targeting cell motility. Clin Cancer Res. 2008;14:6556–6563. doi: 10.1158/1078-0432.CCR-08-0711. [DOI] [PubMed] [Google Scholar]
- 15.Dissanayake SK, et al. The Wnt5A/protein kinase C pathway mediates motility in melanoma cells via the inhibition of metastasis suppressors and initiation of an epithelial to mesenchymal transition. J Biol Chem. 2007;282:17259–17271. doi: 10.1074/jbc.M700075200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Witze ES, Litman ES, Argast GM, Moon RT, Ahn NG. Wnt5a control of cell polarity and directional movement by polarized redistribution of adhesion receptors. Science. 2008;320:365–369. doi: 10.1126/science.1151250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Houghton AN, Polsky D. Focus on melanoma. Cancer Cell. 2002;2:275–278. doi: 10.1016/s1535-6108(02)00161-7. [DOI] [PubMed] [Google Scholar]
- 18.Tarhini AA, Agarwala SS. Cutaneous melanoma: Available therapy for metastatic disease. Dermatol Ther. 2006;19:19–25. doi: 10.1111/j.1529-8019.2005.00052.x. [DOI] [PubMed] [Google Scholar]
- 19.Dissanayake SK, et al. Wnt5A regulates expression of tumor-associated antigens in melanoma via changes in signal transducers and activators of transcription 3 phosphorylation. Cancer Res. 2008;68:10205–10214. doi: 10.1158/0008-5472.CAN-08-2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hansen C, et al. Wnt-5a-induced phosphorylation of DARPP-32 inhibits breast cancer cell migration in a CREB-dependent manner. J Biol Chem. 2009;284:27533–27543. doi: 10.1074/jbc.M109.048884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fabricant RN, De Larco JE, Todaro GJ. Nerve growth factor receptors on human melanoma cells in culture. Proc Natl Acad Sci USA. 1977;74:565–569. doi: 10.1073/pnas.74.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fogh J, Trempe G. Human Tumor Cells in Vitro. In: Fogh J, editor. New York: Plenum Publishing Corp.; 1975. pp. 115–159. [Google Scholar]
- 23.Davies H, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 24.Castellano M, et al. CDKN2A/p16 is inactivated in most melanoma cell lines. Cancer Res. 1997;57:4868–4875. [PubMed] [Google Scholar]
- 25.Roarty K, Serra R. Wnt5a is required for proper mammary gland development and TGF-beta-mediated inhibition of ductal growth. Development. 2007;134:3929–3939. doi: 10.1242/dev.008250. [DOI] [PubMed] [Google Scholar]
- 26.Roarty K, Baxley SE, Crowley MR, Frost AR, Serra R. Loss of TGF-beta or Wnt5a results in an increase in Wnt/beta-catenin activity and redirects mammary tumor phenotype. Breast Cancer Res. 2009;11:R19. doi: 10.1186/bcr2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramont L, Pasco S, Hornebeck W, Maquart FX, Monboisse JC. Transforming growth factor-beta1 inhibits tumor growth in a mouse melanoma model by down-regulating the plasminogen activation system. Exp Cell Res. 2003;291:1–10. doi: 10.1016/s0014-4827(03)00336-7. [DOI] [PubMed] [Google Scholar]
- 28.Ford CE, Ekström EJ, Andersson T. Wnt-5a signaling restores tamoxifen sensitivity in estrogen receptor-negative breast cancer cells. Proc Natl Acad Sci USA. 2009;106:3919–3924. doi: 10.1073/pnas.0809516106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Derian CK, et al. Selective inhibition of N-formylpeptide-induced neutrophil activation by carbamate-modified peptide analogues. Biochemistry. 1996;35:1265–1269. doi: 10.1021/bi952087k. [DOI] [PubMed] [Google Scholar]
- 30.O'Connell MP, et al. Wnt5A activates the calpain-mediated cleavage of filamin A. J Invest Dermatol. 2009;129:1782–1789. doi: 10.1038/jid.2008.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blackshear PJ. The MARCKS family of cellular protein kinase C substrates. J Biol Chem. 1993;268:1501–1504. [PubMed] [Google Scholar]
- 32.Cao J, Chiarelli C, Richman O, Zarrabi K, Kozarekar P, Zucker S. Membrane type 1 matrix metalloproteinase induces epithelial-to-mesenchymal transition in prostate cancer. J Biol Chem. 2008;283:6232–6240. doi: 10.1074/jbc.M705759200. [DOI] [PubMed] [Google Scholar]
- 33.Ripka S, et al. WNT5A-target of CUTL1 and potent modulator of tumor cell migration and invasion in pancreatic cancer. Carcinogenesis. 2007;28:1178–1187. doi: 10.1093/carcin/bgl255. [DOI] [PubMed] [Google Scholar]
- 34.Kurayoshi M, et al. Expression of Wnt-5a is correlated with aggressiveness of gastric cancer by stimulating cell migration and invasion. Cancer Res. 2006;66:10439–10448. doi: 10.1158/0008-5472.CAN-06-2359. [DOI] [PubMed] [Google Scholar]
- 35.Huang CL, et al. Wnt5a expression is associated with the tumor proliferation and the stromal vascular endothelial growth factor–an expression in non-small-cell lung cancer. J Clin Oncol. 2005;23:8765–8773. doi: 10.1200/JCO.2005.02.2871. [DOI] [PubMed] [Google Scholar]
- 36.Sen M, Lauterbach K, El-Gabalawy H, Firestein GS, Corr M, Carson DA. Expression and function of wingless and frizzled homologs in rheumatoid arthritis. Proc Natl Acad Sci USA. 2000;97:2791–2796. doi: 10.1073/pnas.050574297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Romanowska M, et al. Wnt5a exhibits layer-specific expression in adult skin, is upregulated in psoriasis, and synergizes with type 1 interferon. PLoS One. 2009;4:e5354. doi: 10.1371/journal.pone.0005354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reischl J, Schwenke S, Beekman JM, Mrowietz U, Sturzebecher S, Heubach JF. Increased expression of Wnt5a in psoriatic plaques. J Invest Dermatol. 2007;127:163–169. doi: 10.1038/sj.jid.5700488. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.