Abstract
Interleukin-6 (IL-6) is a well-established, independent indicator of multiple distinct types of cardiovascular disease and all-cause mortality. In this review, we present current understanding of the multiple roles that IL-6 and its signaling pathways through glycoprotein 130 (gp130) play in cardiovascular homeostasis. IL-6 is highly inducible in vascular tissues through the actions of the angiotensin II (Ang II) peptide, where it acts in a paracrine manner to signal through two distinct mechanisms, the first being a classic membrane receptor initiated pathway and the second, a trans-signaling pathway, being able to induce responses even in tissues lacking the IL-6 receptor. Recent advances and new concepts in how its intracellular signaling pathways operate via the Janus kinase (JAK)-Signal Transducer and Activator of Transcription (STAT) are described. IL-6 has diverse actions in multiple cell types of cardiovascular importance, including endothelial cells, monocytes, platelets, hepatocytes and adipocytes. We discuss central roles of IL-6 in endothelial dysfunction, cellular inflammation by affecting monocyte activation/differentiation, cellular cytoprotective functions from reactive oxygen species (ROS) stress, modulation of pro-coagulant state, myocardial growth control, and its implications in metabolic control and insulin resistance. These multiple actions indicate that IL-6 is not merely a passive biomarker, but actively modulates adaptive and pathological responses to cardiovascular stress.
Summary:
IL-6 is a multifunctional cytokine whose presence in the circulation is linked with diverse types of cardiovascular disease and is an independent risk factor for atherosclerosis. In this review, we examine the mechanisms by which IL-6 signals and its myriad effects in cardiovascular tissues that modulate the manifestations of vascular inflammation.
Key Words: IL-6/ gp-130/ angiotensin II/ STAT3/ vascular inflammation.
INTRODUCTION
IL-6 is a multifunctional cytokine that has been widely implicated in cardiovascular disease. Produced by a wide spectrum of cell types in the cardiovascular system, IL-6 secretion is upregulated in response to inflammation, angiotensin II (Ang II), oxidative stress and vascular injury [1-3]. Because of its ability to sense cardiovascular stress, IL-6 has become a marker of vascular inflammation, where its increase in the circulation is epidemiologically associated with a variety of clinically significant outcomes. Although IL-6 is clinically considered to be a biomarker of cardiovascular disease, emerging evidence indicates that IL-6 signaling plays a central, significant biological role in cardiovascular regulation. In this review, we will discuss new studies which elucidate its signaling pathways, and implicate its actions in mediating systemic inflammation (hepatic acute phase induction and modification of thrombotic pathways), homeostatic functions (cellular cytoprotection from ROS stress), endothelial dysfunction, cellular inflammation (monocyte activation), growth control (intimal proliferation and cardiac hypertrophy), and metabolic control (insulin resistance). These multiple actions indicate that IL-6 is not merely a passive biomarker, but actively modulates responses to cardiovascular disease.
IL-6 AND CARDIOVASCULAR DISEASE
Although it is outside the scope of this review to detail all potential roles of IL-6 (or its downstream product, C-reactive protein (CRP)) as a cardiovascular biomarker in different cardiovascular diseases, it is important to emphasize that elevation of IL-6 is associated with diverse pathologies. Increased circulating IL-6 is associated with a number of cardiac risk factors, including atherosclerotic disease, cardiomyopathies, and metabolic syndromes. For example, in seminal observations emanating from the Physicians Health Study, baseline plasma concentration of IL-6 is associated with increasing risk of myocardial infarction (MI). Here, individuals with the highest quartile of IL-6 values have a 2.3-fold increased relative risk of having an MI relative to those with the lowest IL-6 values [4]. Importantly, in this study the IL-6 association remained significant even after adjusting for conventional cardiovascular risk factors. In patients admitted for acute coronary syndromes, increases in circulating IL-6 in the first two days of hospitalization are positively correlated with risk of reinfarction and in-hospital complications [5]. In apparently healthy middle-aged men, multiple measures of blood pressure strongly correlate with circulating IL-6 levels [6]. In congestive heart failure, circulating IL-6 inversely correlates with AHA functional classification, ejection fraction, and survival [7,8]. In studies designed to identify plasma predictors of peripheral arterial disease (PAD), the downstream induced protein of IL-6, CRP, is strongly and independently associated with symptomatic PAD [9]. Additionally, in obesity, serum IL-6 levels are positively correlated with extent of obesity [10,11] and risk for subsequent development of overt diabetes [12]. Together, these observations indicate that circulating IL-6 is a marker for common pathophysiologic processes underlying clinically significant cardiovascular disease.
CARDIOVASCULAR INDUCERS OF IL-6
IL-6 is highly inducible in response to cytokines (IL-1, TNFα), viral infection, and Ang II [13,14]. Because of its central role in mediating cardiovascular inflammation, the mechanisms by which Ang II activates IL-6 have been intensively investigated. In smooth muscle cells and hepatocytes, Ang II activates IL-6 expression via the type 1 Ang II receptor (AT1R) [3]. Studies by our group have shown the essential role of the NF-κB transcription factor in mediating inducible IL-6 expression [13]. NF-κB is a cytoplasmic transcription factor that has been implicated in cardiovascular inflammation and is known to be regulated by several distinct pathways that control its cytoplasmic-to-nuclear partitioning [Reviewed in [15]]. Our recent work has shown that Ang II induces NF-κB via an entirely distinct mechanism-one that activates the latent transcriptional activity of the RelA transcriptional subunit, mediated by the Rho family of GTPases. This process culminates in enhancing phosphorylation in the RelA COOH transactivation domain at serine residue 536 and formation of a nuclear complex with the NF-κB inducing kinase (NIK) [16]. IL-6 gene expression results when the activated phosphorylated form exchanges with inactive unphosphorylated RelA bound to the IL-6 promoter [13]. Recent studies from the Lucas laboratory have defined further key signaling intermediates of the Ang II signaling pathway converging on NF-κB [17]. This group has identified a requirement of three additional signaling molecules that form an activated membrane bound complex. These proteins include: (i) CARMA3 [caspase recruitment domain (CARD)], a tissue specific member of the membrane associated guanylate-kinase superfamily of scaffolding proteins, which serves to integrate the upstream signal of activated protein kinase C with downstream factors, (ii) Bcl10, an intermediate bridging factor; and (iii) MALT1, an effector protein that oligomerizes through interaction with Bcl10 [17]. The interaction of the CARMA3/MALT1/Bcl10 complex with the NF-κB signaling pathway is actively under investigation and should provide novel therapeutic targets to selectively disrupt Ang II-induced vascular inflammation without affecting pathways controlling adaptive immunity and cellular apoptosis.
Recent studies indicate that several tissues are affected by enhanced IL-6 associated with vascular inflammation. First, IL-6 has actions locally in the vessel wall. For example, IL-6 production has been identified locally in coronary atherosclerotic plaques [18], where it co-localizes with Ang II [18], as well as aortic atherosclerotic plaque in experimental rodents [2]. In Ang II-stimulated vessels, IL-6 is the most abundantly secreted cytokine detected. Here IL-6 is predominantly expressed by fibroblasts and activated macrophages in the adventitial layer of the proximal ascending aorta, with lesser amounts in the media and intimal layers [2]. Moreover, these studies demonstrated that the IL-6 signaling pathway was locally activated in both adventitial and endothelial layers [2]. These data indicate that Ang II activates a local IL-6 signaling pathway in the aortic adventitia during very early phases of Ang II-induced atherosclerosis.
MECHANISMS OF IL-6 SIGNALING
Classical Membrane IL-6 Signaling
IL-6 is the prototype for one of the most pleiotropic cytokine family in mammals, a family that includes IL-11, oncostatin M (OSM), cardiotrophin-1 (CT-1), ciliary neurotrophic factor (CNTF), cardiotrophin-like cytokine (CLC), leukemia inhibitory factor (LIF), and the recently identified IL-27p28 [19-21]. This family contains structures with four long α-helices arranged in an up and down topology. IL-6 is a highly inducible cytokine secreted by several different cell types of cardiovascular relevance, including macrophages, lymphocytes, fibroblasts, endothelial cells and smooth muscle cells [22-26]. Since IL-6 is the major hormonal mediator of the hepatic acute-phase reaction, mechanisms for IL-6 signaling have been intensely studied. Currently we know that IL-6 activates target cells through a classical signaling pathway by binding cell surface IL-6 receptor α-subunits (IL-6Rα).
Molecular events in IL-6 signaling are initiated by binding to its receptor subunit IL-6Rα (which has no intrinsic kinase activity) with low affinity at the cell surface. The IL-6∙IL-6Rα complex then triggers ligand-mediated oligomerization with the ubiquitously expressed transmembrane gp130 β-subunit, inducing gp130 homodimerization, and subsequent formation of a hexameric IL-6∙IL-Rα∙gp130 high-affinity complex [27] (Fig. 1). Receptor ligation induces conformational changes in the cytoplasmic domains of gp130 that bring Janus tyrosine kinases (JAKs) into close proximity. This molecular interaction results in trans-autophosphorylation of JAK1, a specific Janus kinase mediating IL-6 signaling [21,28,29]. JAK1, in turn, phosphorylates gp130 on the docking sites for the signal transducer and activator of transcription (STAT); STAT isoforms -1 and -3 are then recruited, where they, too, become phosphorylated [21]. In addition to STAT activation, phosphorylation of gp130 on membrane proximal Tyr 759 residue is necessary and sufficient for binding of SRC homology domain 2-containing tyrosine phosphatase 2 (SHP-2). SHP-2 is then phosphorylated, and by itself or together with another docking protein, Grb2 (growth factor receptor binding protein 2)-associated binder-1 (Gab1), and initiation of the Ras-ERK-MAPK cascade occurs [19,21,28,30].
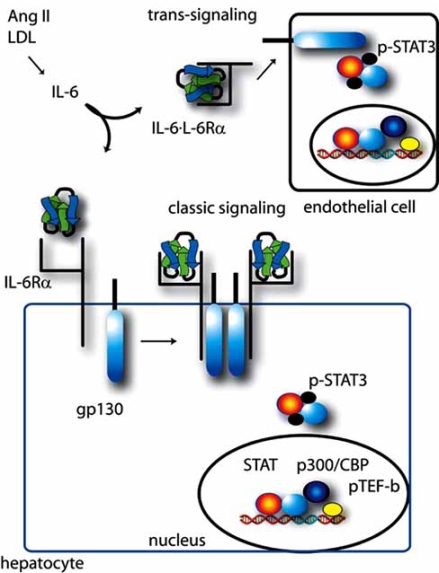
Fig. (1).
IL-6 induced classical and trans-signaling pathways. Shown is a schematic view of classical IL-6 signaling via the IL-6Rα receptor and gp130 for a representative hepatocyte. IL-6Rα bound to the IL-6 ligand results in complex formation with gp130, activating tyrosine kinase activity, including and culminating in tyrosine phosphorylation of STAT3. The IL-6 trans-signaling pathway is diagrammed at top, using a representative endothelial cell. Circulating IL-6∙IL-6Rα engages with gp130 expressed on cells, enabling activation of the IL-6 signaling pathway in cells lacking IL-6Rα. See text for further details.
Of the signaling pathways downstream of the IL-Rα∙gp130 complex, STAT appears to play a major role. Tyr phosphorylated STATs-1 and -3 then form intermolecular associations, homo- and hetero-dimerize and translocate into the nucleus, where they bind specific DNA sequences (for example, acute phase or IL-6 response elements) and enhance transcription of target genes [21,29,31]. Analyses of complex formation with STAT3-dependent transcriptional enhancers have shown that STAT3 undergoes additional post-translational modifications that permits interactions with co-factors and co-activators [32] .
Recent studies have shown that STAT activities are modulated by their interactions with co-factors which positively or negatively regulate their activity. For example, upon entry into the nucleus, STAT3 associates with the p300/ CREB-binding protein (CBP) coactivator, an enhancer protein with intrinsic histone acetyltranferase (HAT) activity which is able to open chromatin structure, allowing other chromatin-modifying proteins to bind to DNA and activate transcription [33-35] (Fig. 2). The p300/CBP association requires both the NH2-terminal modulatory domain and the COOH-terminal transactivation domain of STAT3 [35,36]. Interestingly, STAT3 itself can also be acetylated by p300/ CBP at these two domains in response to IL-6. Acetylation on Lys 685 on its COOH-terminal region is critical for stable dimer formation and DNA-binding activity [36]. Studies from our laboratory first described two novel acetylation sites on the STAT3 NH2 terminus at Lys 49 and -87 that are required to stabilize the STAT3-p300/CBP complex through an additional interaction mediated by the modified STAT3 NH2 terminus [37].
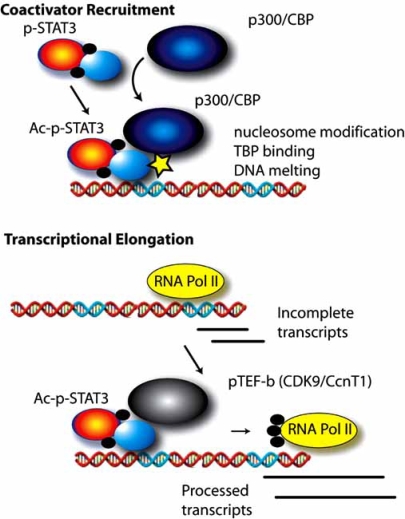
Fig. (2).
Discrete mechanisms for IL-6 induction of target genes. Top, coactivator recruitment mechanism. Tyrosine phosphorylated STAT3 binds to p300/CBP, resulting in STAT3 acetylation (Ac) on its NH2 terminus, and stabilization of the STAT3-p300/CBP complex. The acetylated-phosphorylated STAT3-p300/CBP complex then binds to high affinity IL-6 response elements in the promoters of target genes. This complex induces nucleosomal reorganization via the p300 histone acetylase activity, pre-initiation complex formation, recruiting TATA box binding protein, and enhanced RNA polymerase II activity. Bottom, transcriptional elongation. In a subset of IL-6 responsive promoters, RNA polymerase (Pol) II is engaged with the promoter producing incomplete transcripts. During the process of activation, tyrosine phosphorylated STAT3 complexes with the positive transcriptional elongation factor (PTEF-b), a complex containing CDK9. CDK9 phosphorylates the COOH terminal domain of RNA polymerase II, enabling it to enter productive elongation mode, producing full length RNA transcripts.
Further, we have recently discovered that STAT3 also regulates downstream gene expression by promoting transcription elongation [38] (Fig. 2). This function is realized by the interaction between STAT3 and Positive Transcription Elongation Factor (PTEF-b) [38,39], a complex that phosphorylates Ser 2 on the heptad repeat of the COOH terminal domain of RNA polymerase II. COOH terminal phosphorylation permits Pol II to escape from transcription arrest and produce full length mRNA transcripts. Specifically, we found that activated nuclear STAT3 forms complexes with Cyclin-Dependent Kinase 9 (CDK9), the major component of PTEFb, through both its NH2- and COOH-terminal domains. STAT3 binding then results in CDK9 being recruited to the promoter and downstream coding region of target genes. Importantly, induction of STAT3 target genes, such as γ-FBG and p21waf1, are significantly reduced when CDK9 kinase activity is inhibited [38], suggesting that it is possible to modulate cellular response induced by the IL-6-STAT3 pathway by targeting CDK9 (Fig. 2).
IL-6 Trans-Signaling
Recently it has been observed that the IL-6 tissue response is significantly enhanced by a phenomenon termed “trans-signaling” [29,31] (Fig. 1). This mechanism is suggested by the findings that the IL-6Rα also exists in a soluble plasma form lacking the transmembrane domain, and that membrane association of the IL-6Rα subunit with gp130 is not required for signal initiation. Although soluble IL-6Rα can be formed by alternative splicing of the receptor transcript, the chief means of its production is through ectodomain shedding from activated leukocytes by a disintegrin and metalloproteinases (ADAMs)-17 and -10 [26,29,40,41]. Although the membrane-associated IL-6Rα is specific to only certain cell-types, gp130 is ubiquitously expressed. Thus, the soluble IL-6-IL-6Rα complex can initiate IL-6 signaling on any cell type. This of course, expands the repertoire of IL-6 responsive cells to virtually any cell in the body [29].
Also recently, it has been shown that IL-6 signaling in inflammatory disease utilizes classic or trans-signaling mechanisms to different degrees depending on specific cell-types and pathologies. There also exists a little understood and naturally occurring soluble form of gp130 [29,31]; this protein can be used as a tool to differentiate classic cell surface IL-6 signaling versus trans-signaling processes because soluble gp130 competitively inhibits trans-signaling without affecting membrane-bound signaling. In one study, administration of soluble recombinant gp130 in a rodent model demonstrated that Ang II-dependent hypertension required IL-6 trans-signaling, but concomitant vascular hypertrophy, down-regulation of the AT1R, and STAT3 activation were responses mediated through classic cell surface IL-6R signaling [42]. In another elegant study, using a transgenic mouse overexpressing soluble gp130, effectively inhibiting IL-6 trans-signaling [43], mononuclear cell-dominated inflammatory processes were selectively inhibited, indicating that mononuclear inflammation relied on trans-signaling rather than classic signaling. Further, some inflammatory processes in these mice were blocked to the same degree as in an IL-6 knockout mouse [43]. These approaches hold promise to elucidate these two mechanisms of IL-6 signaling in various pathological processes.
Negative Regulation of IL-6 Signaling
Several negative feedback mechanisms provide temporal control of IL-6 signaling (Fig. 3). Ligand-induced internalization and degradation of IL-6Rα and gp130 has been identified as a proximal mechanism for negating signaling [31]. IL-6 signaling is particularly sensitive to the downstream STAT3-dependent recruitment of suppressor of cytokine signaling 3 (SOCS3) to the gp130 Tyr 759 residue, a site near where JAK1 binds. SOCS3, itself inducible by STAT3, inhibits JAK1 activity through an unknown mechanism involving its kinase inhibitor domain [21,44,45]. IL-6-gp130 signaling is also attenuated by a phosphorylation-dependent induction of SHP-2 tyrosine phosphatase activity which dephosphorylate gp130 and JAKs [19,46]. Finally, SOCS proteins have been observed to recruit the elongin BC ubiquitin-ligase complex to JAKs, and perhaps other components of the receptor complex, promoting ubiquitination and subsequent proteosomal degradation [21,28,44]. Together these inhibitory mechanisms ensure transient IL-6 action.
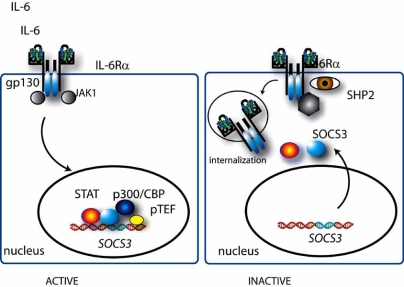
Fig. (3).
Negative regulation of the JAK-STAT pathway. Shown are the major negative autoregulatory pathways of IL-6 induced STAT3 signaling. IL-6 activated STAT3 both engages the suppressor of cytokine signaling (SOCS3) gene, inducing its expression and recruits SOC3 to gp130 where it subsequently terminates STAT3 activation via JAK1 inactivation. SHP2 phosphatase activity also inactivates gp130 and JAKs.
CELLULAR TARGETS AND ACTIONS OF IL-6
Endothelial Cells
Enhanced ROS stress has been implicated in the pathogenesis of atherosclerosis, hypertension, diabetes, aging and mechanical injury [47-52]. ROS are either oxygen-centered free radicals that include superoxide (O2-), hydroxyl radicals (. OH) and lipid (L) hydroperoxides (LOO), or reactive non-radical compounds that include (H2O2), singlet oxygen (1O2), hypochlorous acid (HOCl) and chloramines (RNHCl) [53,54]. Under normal conditions the rate and magnitude of oxidant formation is balanced by the rate of oxidant elimination (an enzymatic activity influenced by IL-6). However, oxidant overproduction produces an imbalance that over-whelms cellular antioxidant capacity, damaging cellular lipids, membranes, proteins and DNA [55,56]. In addition, ROS can act as second messengers in an autocrine or paracrine fashion to modulate endothelium-dependent vasorelaxation, smooth muscle cell and endothelial cell growth and survival, and vascular remodeling. Each of these responses, when uncontrolled, contributes to vascular disease [57].
Because of these important activities of ROS, the mechanisms by which they are generated have been extensively investigated [49,58]. Although macrophages are the major source of most ROS in the vessel wall, other cells, such as endothelial, smooth muscle and adventitial cells, produce ROS. Ang II and lipid (oxidized) LDL appear to be potent inducers of ROS [59]. In fact, Ang II infusion doubles O2- production in aortic segments [49]. As a result, during the early stage of Ang II-induced atherosclerosis, the nonadhesive function of endothelium which controls vasomotor tone, is disturbed. This process is mediated by Ang II–induced production of ROS, which results in the chemical inactivation of nitric oxide (NO), blunting its ability to vasodilate [60]. Among the variety of ROS generators in VSMCs are the mitochondrion and cellular enzymes, such as xanthine oxidase, cyclooxygenase, lipoxygenase, NO synthase, heme oxygenases, peroxidases, and the membrane-associated NAD(P)H oxidases, the latter having been shown to be of foremost physiological importance [58]. Inhibition of vascular ROS production through administration of superoxide dismutase increases acetylcholine-induced relaxation, suggesting that ROS species themselves are responsible for the endothelial dysfunction [49]. In humans, it has been shown that treatment with selective inhibitors of AT1R reverses endothelial dysfunction in large arteries [28].
Because IL-6 upregulates AT1R gene expression, it may lead to increased Ang II-mediated vasoconstriction and ROS production, and thereby play an important role in mediating endothelial dysfunction [61]. Consistent with this idea, it was observed that IL-6 deficiency protects against Ang II – induced endothelial dysfunction [62]. This IL-6 effect occurs locally within the vessel wall, independent of increases in blood pressure. Schrader et al. have reported that an O2- scavenger restored endothelial responses in Ang II-treated arteries [62]. These findings further suggest that the effect of Ang II on endothelial function is attributable to O2--mediated inactivation of NO [49,63]. Importantly, Ang II increases in ROS tone are absent in mice deficient in IL-6 or Nox2 genes. Together, these data suggest that NAD(P)H oxidase is a major source of O2- and a primary mediator of endothelial dysfunction. Thus, IL-6 may be a critical link in NAD(P)H-derived, O2.--mediated impairment of NO-induced vascular relaxation. Whether activation of NAD(P)H oxidase by Ang II occurs upstream or downstream of IL-6 expression remains unclear. IL-6 expression may be an important link between Ang II–induced increases in NAD(P)H oxidase activity, limiting the bioavailability of NO for normal vascular responses.
Interestingly, endothelium does not express transmembrane IL-6Rα and is unresponsive to IL-6; however, endothelium can be activated by the IL-6 trans-signaling pathway discussed earlier [64]. IL-6 trans-signaling may play important roles in other endothelial-dependent functions. For example, in addition to vasodilation, the endothelium also plays a central role in regulating hemostasis, expressing anti-coagulant and anti-adhesion molecules [65]. Both acute vascular inflammation and chronic injury can cause inappropriate activation of endothelium, converting it to a prothrombotic surface [66]. The actions of endothelial cells on haemostasis are tightly regulated by a network of cytokines in autocrine or paracrine mechanisms [67]. Inflammatory stimuli, such as lipopolysaccharide (LPS) or cytokines, can activate endothelial cells, resulting in the synthesis of IL-1, IL-5, IL-6, IL-8, IL-11, IL-15; as well as colony-stimulating factors and chemokines. These secreted cytokines not only affect the local microenvironment by inducing local inflammation and thrombosis, but also influence the systemic inflammatory responses and global hemostatic balance. Specific inducers of IL-6 production by vascular cells include IL-1 [64], LPS [68], TNFα [69] and IL-4 [70]. Co-stimulation of endothelial cells with IL-4 and interferon-γ or IL-4 with IL-1 further amplify the synthesis of IL-6 mRNA [70,71]. Interactions between IL-6 and endothelial cells regulate recruitment of leukocytes and expression of chemokines. IL-6-/- mice show defective leukocyte accumulation to inflammatory sites, which is associated with decreased synthesis of chemokines by endothelial cells and reduced surface expression of adhesion molecules [72]. Cultured endothelial cells (HUVEC) have been shown to produce MCP-1, -3, IL-8, as well as IL-6. Upregulation of intercellular adhesion molecule-1 (ICAM-1) also is observed in the presence of a trans-signaling complex, IL-6·IL-R, at physiological concentrations [72]. The multiple inducers mentioned above promote interactions between endothelial cells and leukocytes, platelets or red blood cells, leading to further activation and damage of endothelium in an autocrine amplification pathway.
Monocytes
Macrophages play an important role in vascular inflammation where they locally secrete cytokines, chemokines, and matrix metalloproteinases that promote further cellular infiltration and vascular remodeling. Vascular macrophages are derived from peripheral blood monocytes which locally differentiate into macrophages. This is a complex, multi-step process; importantly, IL-6 is a prominent cytokine that promotes monocyte-to-macrophage differentiation.
IL-6 promotes macrophage differentiation, growth arrest and eventual apoptosis in vitro [73-79]. Experiments using IL-6 stimulation of myeloid leukemia cell lines have shown that IL-6 induces development of mature macrophages [73,74,80,81]. IL-6 stimulation causes these cells to increase in size, develop a large vacuolar cytoplasm, develop irregularly shaped nuclei [74-76,82] and become surface adherent [77]. Functionally, these cells have increased esterase and phagocytic activities [74]. Surface expression of C3 complement receptors, Fc receptors, and macrophage-colony stimulating factor (M-CSF) receptors along with F4/80, a marker for mature macrophages, also are up-regulated [76, 77,83]. CD36, an oxidized lipid uptake receptor, has been shown to be induced by IL-6 in mouse peritoneal macrophages [84]. Furthermore, IL-6 induces expression of genes typical of macrophages, including the early response genes c-Jun, jun B, jun D, interferon-regulatory factor 1 (IRF1), JAK3, and Egr-1 [74,76,77,79]. However, expression levels of c-myc mRNA go down within hours of IL-6 stimulation, facilitating growth arrest and differentiation [74,77-79]. Bcl-2 and cyclin D1 are down-regulated subsequently, increasing susceptibility to apoptosis [76,85]. IL-6 also regulates late response genes, including lysozyme and ferritin light-chain, genes that are normally induced during terminal macrophage differentiation [77,86]. Moreover, upon stimulation with IL-6, monocytic cells up-regulate MCP-1 mRNA and protein, a chemokine more strongly expressed in macrophages than monocytes [87].
Additional work has shown that IL-6 favors monocyte-to-macrophage differentiation rather than monocyte to dendritic cell differentiation [83,88,89]. Monocytes cultured in the presence of IL-4 and GM-CSF become CD1a+CD14- dendritic cells (DCs), but the addition of IL-6 alone causes CD1a-CD14+ macrophage differentiation, [88], where morphological and functional characteristics of macrophages are seen [83,88]. Interestingly, this effect is not seen with the addition of other IL-6 family member cytokines, including IL-11, LIF, and OSM; monocytes become dendritic cells in their presence [88]. The favoring of macrophage differentiation by IL-6 is also seen in monocyte and fibroblast co-culture where the cell-cell interaction is thought to induce large amounts of IL-6 [83]. Even in the presence of IL-4 and GM-CSF in this system, the dendritic cell differentiation program is overridden by IL-6 signaling, and monocytes develop into macrophages [83]. This may be due to the observation that IL-6 up-regulates the number of functional M-CSF receptors on moncytes, thereby increasing their sensitivity to M-CSF [83].
gp130 and downstream signaling is required for IL-6-induced macrophage differentiation [79]. Expression of gp130 mutations that are unable to activate STAT3 prevents the subsequent growth arrest and differentiation of M1 myeloid cells [79]. Furthermore, downstream STAT3 activation is required because dominant-negative forms of STAT3 block differentiation [78,90]. Specifically, Minami et al. showed that inhibiting STAT3 prevented induction of Fc receptors, ferritin light chain, and lysozyme; moreover, c-myc was not down-regulated and the cells continued to proliferate [78]. Likewise, over-expression of JAK3, which also is induced rapidly by IL-6, accelerates macrophage differentiation [91]. Therefore, IL-6 activates the gp130-JAK/STAT signaling pathway leading to differentiation of monocytes.
Other transcription factors have been shown to modulate the IL-6-induced macrophage differentiation phenomenon. GATA-1, an erythroid nuclear protein that regulates globin gene expression, inhibits IL-6-induced macrophage differentiation and apoptosis [76]. Overexpression of GATA-1 in M1 cells leads to megakaryocytic or erythroid differentiation even in the presence of IL-6 and normal STAT3 signaling [76,92]. Tanaka et al. reported that expression of bcl-2 and cyclin D1 in these cells remain sustained, thereby disrupting the effect of IL-6 [76]. Thus, although the STAT3 pathway is necessary for IL-6-induced macrophage differentiation, altered gene transcription induced by other pathways can modulate this process. In contrast to GATA-1, the zinc finger transcription factor Egr-1 is thought to be essential for macrophage differentiation [77,93]. Knocking down Egr-1 with anti-sense oligonucleotides blocks M1, HL-60, and U-937 myeloid cell lines from differentiating into morphologically mature macrophages while overexpression of Egr-1 leads to activation of macrophage differentiation even without the presence of IL-6 [77,93]. Increased Egr-1 activity, by itself, decreases growth rate and expression of c-myc, increases Fc and C3 receptors, and elevates expression of jun B, ferritin light-chain, and lysozyme [77]. Simulation with IL-6 accelerates this process, resulting in increased cell adherence to culture dishes and a doubling of the percentage of morphologically-mature macrophages as compared to Egr-1 over-expressing cells alone [77]. However, the lack of Egr-1 in mice does not impair macrophage differentiation and activation in vivo [94]. This might be due to the possibility that other members of the Egr-1 family (Egr-2,-3, and-4) have redundant activities [77].
Despite the wealth of in vitro data, there is little evidence that IL-6 plays a critical role in macrophage differentiation in vivo. Macrophages from IL-6 deficient and wild-type mice are similar, and there is no report on decreased numbers of mature macrophages in the IL-6 deficient mouse. Peritoneal macrophages from IL-6 knockout mice express levels of major histocompatibility complex (MHC) class II and F4/80 comparable to wild-types, and they can be stimulated in vitro by LPS and IFN-γ to produce almost equal amounts of nitric oxide [95,96]. However, it is well known that IL-6 deficiency results in impaired protection against bacterial infections in vivo, particularly to Listeria monocytogenes [96-99]. L. monocytogenes is a bacterium that primarily infects and proliferates intracellularly in macrophages; activation of macrophages is required to destroy the bacterium. Interestingly, IL-6 deficient mice fail to control infections by L. monocytogenes [95-97]. Bluethmann et al. have proposed that this reduced anti-bacterial defense might be due to a defect in differentiation of macrophages in bone marrow [98]. In fact, the number of myeloid progenitors (CFU-GM) that give rise to granulocytic-monocyte lineage are reduced by half in the bone marrow of IL-6 deficient mice and are increased 4-fold in the spleen [100]. Although the total number of CFU-GM does not increase overall, the redistribution from bone marrow to the spleen may affect the differentiation and/or function of the macrophages. Except for the decreased ability to fight infections, there are no other reported differences in macrophages obtained from IL-6 deficient mice. Thus, other factors that promote macrophage differentiation may compensate for the lack of IL-6. IL-6 might be sufficient, but it does not seem to be necessary for macrophage differentiation in vivo.
Nevertheless, IL-6 probably contributes to macrophage differentiation in vascular inflammation where IL-6 levels are highly elevated in serum and cardiovascular tissues. In Ang II-infused mice, IL-6 secretion in aortic tissue is increased 4-fold over basal levels [2]. The location of IL-6 production and the site of activated STAT3 is predominantly in the adventitia, a location, coincidently, where the majority of macrophages reside [2,101]. This strong co-localization suggests that the monocytes recruited into the adventitia are most likely stimulated by the IL-6 present, along with other pro-macrophage factors, to become macrophages. Although not directly studying macrophages recruited to vascular tissue, Keidar et al. reported that CD36 was up-regulated on peritoneal macrophages in association with increased IL-6 serum levels in Ang II-infused mice [84]. CD36 was further shown to be directly inducible by IL-6 [84]. This study suggests that IL-6 may promote macrophage differentiation in vascular tissue by up-regulating CD36 on recruited monocytes. Clearly, more research is needed to elucidate the roles of IL-6 in differentiating monocytes to macrophages in vascular tissue itself.
Platelets
Platelets are not only essential for blood coagulation, but also for modulating inflammatory processes and contributing to wound healing by producing cytokines, chemokines, growth factor and other inflammatory mediators [102]. IL-6 is known as an important regulator of megakaryocyte differentiation and maturation in vitro [103,104], particularly when stimulated in conjunction with IL-1α and IL-3 [105]. Several in vivo studies provide evidence that IL-6 induces thrombocytosis. It has been shown that recombinant IL-6 and other IL-6 family members, including IL-11, OSM, and LIF, significantly enhance peripheral blood platelet count in experimental animals [106-109]. The relationship between IL-6 and thrombocytosis also was observed in a correlative study in which 83 % of patients with secondary thrombocytosis had elevated serum IL-6 levels [110]. IL-6 not only augments platelet count, but also affects platelet function. IL-6-treatment enhances platelet responsiveness to thrombin stimulation and increases P-selectin expression, a sensitive marker of platelet activation [111,112]. Also, incubation of platelets with IL-6 in vitro caused a dose-dependent enhancement of agonist induced maximum aggregation (AIMA) and secretion of thromboxane B2 (TXB2), indicating the activation of platelets [113,114]. Involvement of arachidonic acid metabolism is suggested since both AIMA and TXB2 production were inhibited by indomethacin and dazoxiben [113].
Myocardiocytes
Although the individual roles of the IL-6 family of cytokines are not fully elucidated, the gp130-signaling pathway is known to play a key role in hypertrophic response of the myocardium to acute pressure overload. For example, genetic modifications that result in tonic gp130 activation produce cardiovascular hypertrophy in mice [115]. Because organism-wide knock-out of gp130, STAT3, LIF or CT-1 is embryonically lethal with exhibition of multiple organ defects, the cardiovascular role of these molecules has been difficult to discern. The development of cre-lox technology for tissue-specific gp130 knockout in ventricular muscle resulted in the surprising observation that gp130 signaling was not required for cardiac development or baseline indices of cardiac function [116]. However, when these animals were challenged in a model of acute pressure overload by aortic banding, instead of compensatory hypertrophy, gp130 knockout mice rapidly showed signs of cardiac failure, including reduced fractional systolic shortening and increased LV end diastolic pressure, followed later by development of dilated cardiomyopathy with increased cardiomyocyte apoptosis [116]. Other studies have also found that the JAK-STAT3 pathway is activated in acute MI, where the greatest induction appears at the border between the infarct and viable tissue, and inhibition of signaling produces increased myocardial apoptosis [117]. Together, these studies indicate that the gp130 signaling pathway mediates the interface between compensatory hypertrophy and cardiomyocyte apoptosis in response to pressure overload and ischemic insults. Because of the multiple redundant actions of the IL-6/LIF/OSM/CT-1 cytokine family, the individual cytokines that mediate the early gp130 activation have not been definitively determined.
Hepatocytes
IL-6 is a key effector cytokine in hepatic physiology, including inducing hepatoprotection, mitogenesis, and the acute phase response [118,119]. The acute phase response has been extensively reviewed elsewhere [1,120] and will not be further discussed here. IL-6 has recently been shown to modulate hepatocyte survival. For example, the cell death-inducing ligand, Fas, and toxin-mediated liver injury produce direct mitochondrial damage, ROS generation and subsequent hepatocyte necrosis (with a lesser degree of apoptosis). IL-6 signaling via STAT3 induces both antioxidant Ref-1 and caspase inhibitors, including Bcl-2, FLIP, and Bcl-XL, to induce hepatoprotective state [121]. Separately, IL-6 also promotes liver regeneration, to restore liver mass after necrotic or apoptotic injury has occurred.
Haga et al. has identified ROS as a component of Fas-mediated liver injury and identified an endogenous antioxidant, Ref-1, as a target of STAT3 [121]. Expression of Ref-1 provided hepatoprotection, strongly suggesting that Ref-1 is a critical component of STAT3-mediated hepatoprotection. Ref-1, a dual-function protein upregulated by increases in ROS, is an endonuclease in the base excision repair pathway and a reducing agent that facilitates the DNA-binding properties of redox-sensitive transcription factors [122,123]. Ref-1 is able to suppress ROS generation and hepatic apoptosis.
A recent study demonstrated that IL-6 also has a protective effect on Fas-mediated liver injury similar to the effect seen with STAT3 by upregulating c-FLIP, Bcl-2 and Bcl-xL [124]. The data of Haga et al. implicate that this effect of IL-6 is mediated by STAT3 [121]. More work will be required to dissect the inter-relationships between Ref-1 and STAT3 in IL-6 signaling and cellular survival.
Of the myriad hepatic proteins that IL-6 induces with cardiovascular activities, it is increasingly clear that IL-6 is a major modulator of the coagulation pathway [125-127], where its actions shift hemostatic balance to prothrombosis, thereby increasing the risk of cardiovascular diseases. IL-6 promotes coagulation by a number of mechanisms. First, it increases the expression of procoagulant factors, such as fibrinogen (FBG), tissue factor (TF) and factor VIII [128-130], and reduces the production of antithrombotic factors, such as antithrombin and protein S [112,131]. Second, as discussed earlier, IL-6 is involved in the activation of endothelial cells and plays a central role in hemostasis [67,132]. Third, IL-6 contributes to thrombosis by increasing platelet numbers and regulating their functions [133].
Fibrinogen (FBG) is a large glycoprotein consisting of three pairs of non-identical polypeptides (Aα, Bβ, and γ) which are encoded by separate genes [134]. It is not only a rapid and sensitive marker of the acute phase response, but also an important mediator of hemostasis by participating in clot formation, platelet aggregation and clot retraction [135]. IL-6 can stimulate mammalian hepatocytes to produce FBG in a dose-dependent manner [128]. IL-6 response elements have been identified in the promoter regions of all human FBG Aα, Bβ, and γ genes [136-138]. Analysis of the 5’-flanking region of human FBG Aα identified six potential IL-6 responsive sequences, among which a single sequence of CTGGGA localized from -122 to -127 bp is a functional element [136]. Also, a CCAAT/enhancer binding protein site (C/EBP, -134 to -749 bp) was found adjacent to the functional IL-6 response element (IL-6RE), which might modulate and further increase the magnitude of IL-6 response [136]. In addition, a hepatocyte nuclear factor 1 (HNF-1) binding site, present from -47 to -59 bp, also was essential for the expression of the human fibrinogen Aα gene [136]. A similar finding was observed in the promoter of the human FBG Bβ gene. The identified DNA sequences essential for full IL-6-induced expression of fibrinogen Bβ included three distinct cis-acting DNA elements: an HNF-1 site at ~85 bp upstream of the transcription start site; a C/EBP binding site between nucleotides -124 and -133; and an IL-6 responsive element (IL-6RE) present just 4 bp upstream of the C/EBP consensus binding site [138-140]. The γ chain of fibrinogen (γ-FBG) plays a crucial role in fibrinogen function by inducing platelet aggregation and leukocyte recruitment in inflammation [141,142], concentrating growth factors and cytokines for wound healing [143-145], and mediating fibrin clot formation. Consequently, transcriptional control mechanisms regulating inducible γ-FBG expression have been extensively investigated. These studies have shown that three IL-6 REs are found in the promoter region of the γ-FBG gene [137,146]. Although all of them contributed to the full promoter activity induced by IL-6, one site (site II) was the major functional IL-6 responsive site [137,146]. Further studies using gel mobility shift assays have shown that the binding affinity of STAT3 to these three elements inversely correlated with their functional activities [147]. In contrast to Aα and Bβ-FBG genes, the promoter activity of γ-FBG was not affected by overexpression of C/EBPβ and C/EBPδ isoforms [137]. Recent findings from our lab indicate that γ-FBG expression in hepatocytes also is regulated by the interaction between STAT3 and coactivators p300/CBP [36,37] and CDK9 [38]. Considering the key role of FBG in blood coagulation, IL-6 exerts its effects on hemostasis by regulating the levels of FBG in circulation.
Tissue factor (TF) is well known for its primary role in the initiation of the extrinsic pathway of coagulation. After vessel injury, TF forms a complex with factor VIIIa, which promotes the activation of factor V, leading to thrombin generation, fibrin deposition and finally clot formation [148]. A two-year follow-up study of 120 patients with congestive heart failure (CHF) revealed a strong correlation between TF and IL-6 levels, suggesting a close link between inflammation and thrombogenesis in CHF [149]. Also, patients with CHF and high IL-6 and TF levels have a poorer prognosis, raising the possibility that IL-6 contributes to the prothrombotic state in CHF through its affects on TF expression [149]. In human mononuclear leukocytes, recombinant IL-6 rapidly induces TF mRNA and protein expression [150]. Also, IL-6 induces an increase in TF surface expression on monocytes, and the upregulation of TF is accompanied by an enhanced monocyte procoagulant activity (PCA) [150]. The actions of IL-6 on TF expression also may be indirectly mediated by CRP, an acute phase reactant that is markedly induced by IL-6 [22]. Highly purified human CRP causes a significant increase of TF mRNA after 4 hours of stimulation and a 75-fold increase in the PCA of human peripheral blood monocular cells that is dependent on TF protein synthesis [129]. In a study of 106 outpatients with atrial fibrillation (AF), it was reported that AF patients have higher levels of IL-6, CRP, TF, and plasma viscosity compared with controls, and significant correlations were reported between these inflammatory markers (IL-6, CRP) and prothrombotic plasma markers [151].
Plasminogen activator inhibitor-1 (PAI-1), the major physiologic inhibitor of fibrinolysis, is also induced by pro-inflammatory cytokines, including IL-1 and IL-6 [152,153], in the human hepatoma cell line, HepG2. Incubation of HepG2 cells with IL-1 caused a rapid and dramatic increase in PAI-1 mRNA expression in a dose-dependent manner. IL-6 alone only had a modest effect on PAI-1 mRNA synthesis, however, when combined with IL-1, a significant accumulation of PAI mRNA was observed [153]. A specific region (-239 to -210 bp) of the PAI-1 promoter was shown to be necessary for IL-1β-inducible expression and mediated the combined induction by IL-1β and IL-6 [154,155]. An increased in the binding activity of C/EBPδ to PAI-1 promoter was induced by either IL-1β or IL-6 stimulation. Downregulation of PAI-1 induction by siRNA against C/EBPδ confirmed the critical role of C/EBPδ in the inducible PAI-1 expression [155]. Both IL-1β and IL-6 increase C/EBPδ mRNA expression [154,155]. Although C/EBPδ functions as a common mediator of PAI-1 expression in IL-1β and IL-6 signaling, different upstream kinases were involved. Activation of C/EBPδ by IL-1β required all three of the mitogen-activated protein kinase pathways (MAPK), while JAK signaling contributed to IL-6-inducible expression of PAI-1 [155]. Interestingly, the HMG-CoA reductase inhibitor (mevastatin) abrogated PAI-1 production induced by IL-1 and IL-6, which was mediated by decreasing the levels of C/EBPδ mRNA and protein, as well as by inhibiting C/EBPδ binding to PAI-1 promoter [154,155]. These findings suggest that statins prevent vascular inflammation, at least in part, by inhibiting C/EBPδ-induced PAI-1 expression.
IL-6 also contributes to a pro-coagulant state by reducing synthesis of antithrombotic proteins. For example, IL-6 negatively regulated the production of antithrombin, a potent inhibitor of coagulation, both in vivo and in vitro [131]. Protein S, a cofactor to Protein C in the inactivation of factors Va and VIIIa, is another IL-6-regulated anti-coagulant. The function of protein S is regulated by forming an inactive complex with complement protein C4b [156]. In a canine model, exogenous IL-6 significantly decreased levels of free protein S, which recovered to normal levels after the cytokine exposure was discontinued [112].
Adipocytes
The incidence of obesity and its co-morbidities has increased dramatically worldwide, and is characterized by systemic inflammation with an important, central role for IL-6. Visceral obesity is an independent risk factor for numerous chronic cardiovascular diseases, including atherosclerosis, arterial hypertension, renal glomerulopathies with proteinuria, and diabetes. Diabetes is a major risk factor for premature cardiovascular, cerebrovascular, and peripheral vascular disease. Over the last decade, our understanding of fat tissue has changed dramatically from a simple energy storage organ to an important endocrine organ modulating appetite, energy expenditure, insulin sensitivity, metabolism, endocrine and reproductive systems, inflammation, and immunity [86,157-161]. These effects are mediated by adipocytokines, including leptin, resistin, adiponectin, visfatin, as well as more classical cytokines, including TNF-α, MCP-1, and IL-6. Since adipose tissue is a major source of IL-6, it is generally considered an adipokine. These adipokines act on immune cells, leading to local and systemic inflammation, as well as on vascular cells, leading to obesity-related disorders associated with the metabolic syndrome (including hypertension, atherosclerosis, insulin resistance), diabetes, and cancer [86].
Serum IL-6 levels are positively correlated with extent of obesity based on body mass index [10,11]. Visceral adipocytes harvested from severely obese, nondiabetic patients produce substantially more IL-6 than subcutaneous adipocytes harvested from the same individual. This finding partly explains the relationship between visceral adipose fat deposits and the increased risk of cardiovascular disease in humans. Interestingly, visceral adipocytes account for only 10 % of total adipose tissue production of IL-6 [162]. The remainder is produced by non-adipose stromal cells, vascular endothelial cells, and monocyte/macrophages [162,163]. Nevertheless, adipose tissue contributes significantly to the serum pool of IL-6, and the observation that the venous drainage of omental adipose tissue flows directly into the liver suggests an important metabolic impact, particularly on VLDL secretion and hypertriglyceridemia, which could impact on atherosclerosis. In addition, it is well appreciated that IL-6 induces hepatic CRP production, which is an independent risk marker of cardiovascular disease [164,165]. It has been estimated that white adipose tissue, representing the major portion of fat tissue and the major site for energy storage, contributes ~25 % of the circulating IL-6 in the absence of acute inflammation [159,165,166].
IL-6 plasma levels also correlate with the development of the metabolic syndrome [167] and predict future risk for type 2 diabetes [168,169]. It is important to note that these studies show an association with type 2 diabetes, not causality. It has been suggested that association between IL-6 and progression to type 2 diabetes may reflect an attempt to counter-regulate the low-grade inflammation induced by other inflammatory mediators such as TNFα [170]. An increase in adipose tissue mass is associated with insulin resistance, hyperglycemia, dyslipidemia, and hypertension – all components of the metabolic syndrome. IL-6 gene expression is related to adipose cell size [171], and IL-6 plasma concentration increases postprandially [172]. The role of IL-6 in insulin resistance remains controversial [170,173]. Nevertheless, human and experimental animal studies do suggest that IL-6 is involved in the development of insulin resistance in adipose tissue, skeletal muscle, and particularly in hepatocytes [160,170,174]. While the mechanism remains unclear, cytokines such as IL-6 and TNFα are able to decrease insulin action [175-177].
Perivascular adipose tissue increasingly is recognized as an important source of adipokines and proinflammatory cytokines, including IL-6 [178-180], but its role in cardiovascular disease remains unclear [181]. Perivascular adipose tissue plays a role in the regulation of arterial tone since it has been reported that adventitial adipose tissue attenuates responsiveness of rat aortic rings to phenylephrine and norepinephrine [182]. The identity of this perivascular fat-derived, vascular relaxing factor remains unknown, although leptin and/or adiponectin have been proposed. Nevertheless, these observations are intriguing and suggest that perivascular fat may have beneficial, protective effects under normal physiological conditions, but “perivascular adipose tissue dysfunction” may be deleterious under disease conditions such as obesity and diabetes [181].
SUMMARY
In summary, IL-6 is a major indicator of significant cardiovascular disease of diverse etiologies and has emerged as a multi-faceted regulator of vascular tone and cellular inflammation. In this review, we have illustrated its complex mechanisms of signaling, mediated by classic membrane receptor or trans-signaling modalities, which act in concert to promote the targets and spectrum of IL-6 effects. Recent advances in understanding the molecular signaling pathway initiated by IL-6 through the STAT3 transcription factor has led to the discovery of novel coactivators required for STAT3 genomic effects that may be targets for vascular therapies. From this work it is clear that IL-6 has diverse actions including modulating endothelial-dependent vasorelaxation, monocyte differentiation, platelet function, procoagulant state, myocardial hypertrophy, and effects on obesity and intermediary metabolism. These studies underscore the central relevance of the IL-6-gp130 signaling pathway in vascular pathologies.
ACKNOWLEDGEMENTS
Work in our lab was supported by NHLBI 1 P50 HL083794 (to ARB and AR), NHLBI RO1 HL70925 (to ARB), AHA Grant # 06651294 (to SR), and an ADA Grant # 7-07-RA-169 (to RT). Core Laboratory support was from NIEHS grant P30 ES06676 (to J. Halpert, UTMB) and BAA-HL-02-04 (A. Kurosky, UTMB).
REFERENCES
- 1.Brasier AR, Recinos A III, Eledrisi MS. Vascular inflammation and the renin-angiotensin system. Arterioscler Thromb Vasc Biol. 2002;22(8):1257–66. doi: 10.1161/01.atv.0000021412.56621.a2. [DOI] [PubMed] [Google Scholar]
- 2.Recinos III A, LeJeune WS, Sun H, et al. Angiotensin II induces IL-6 expression and the Jak-STAT3 pathway in aortic adventitia of LDL receptor-deficient mice. Atherosclerosis. 2007;194(1):125–33. doi: 10.1016/j.atherosclerosis.2006.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han Y, Runge MS, Brasier AR. Angiotensin II induces interleukin-6 transcription in vascular smooth muscle cells through pleiotropic activation of nuclear factor-kappa B transcription factors. Circ Res. 1999;84(6):695–703. doi: 10.1161/01.res.84.6.695. [DOI] [PubMed] [Google Scholar]
- 4.Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma Concentration of Interleukin-6 and the Risk of Future Myocardial Infarction Among Apparently Healthy Men. Circulation. 2000;101(15):1767–72. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- 5.Biasucci LM, Liuzzo G, Fantuzzi G, et al. Increasing Levels of Interleukin (IL)-1Ra and IL-6 During the First 2 Days of Hospitalization in Unstable Angina Are Associated With Increased Risk of In-Hospital Coronary Events. Circulation. 1999;99(16):2079–84. doi: 10.1161/01.cir.99.16.2079. [DOI] [PubMed] [Google Scholar]
- 6.Chae CU, Lee RT, Rifai N, Ridker PM. Blood Pressure and Inflammation in Apparently Healthy Men. Hypertension. 2001;38(3):399–403. doi: 10.1161/01.hyp.38.3.399. [DOI] [PubMed] [Google Scholar]
- 7.Tsutamoto T, Hisanaga T, Wada A, et al. Interleukin-6 spillover in the peripheral circulation increases with the severity of heart failure, and the high plasma level of interleukin-6 is an important prognostic predictor in patients with congestive heart failure. J Am Coll Cardiol. 1998;31(2):391–8. doi: 10.1016/s0735-1097(97)00494-4. [DOI] [PubMed] [Google Scholar]
- 8.Roig E, Orus J, Pare C, et al. Serum Interleukin-6 in congestive heart failure secondary to idiopathic dilated cardiomyopathy. Am J Cardiol. 1998;82(5):688–+. doi: 10.1016/s0002-9149(98)00388-9. [DOI] [PubMed] [Google Scholar]
- 9.Blake GJ, Ridker PM. Inflammatory bio-markers and cardiovascular risk prediction. J Intern Med. 2002;252(4):283–94. doi: 10.1046/j.1365-2796.2002.01019.x. [DOI] [PubMed] [Google Scholar]
- 10.Vgontzas AN, Papanicolaou DA, Bixler EO, et al. Elevation of plasma cytokines in disorders of excessive daytime sleepiness: role of sleep disturbance and obesity. J Clin Endocrinol Metab. 1997;82(5):1313–6. doi: 10.1210/jcem.82.5.3950. [DOI] [PubMed] [Google Scholar]
- 11.Fernandez-Real JM, Vayreda M, Richart C, et al. Circulating interleukin 6 levels, blood pressure, and insulin sensitivity in apparently healthy men and women. J Clin Endocrinol Metab. 2001;86(3):1154–9. doi: 10.1210/jcem.86.3.7305. [DOI] [PubMed] [Google Scholar]
- 12.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-Reactive Protein, Interleukin 6, and Risk of Developing Type 2 Diabetes Mellitus. JAMA. 2001;286(3):327–34. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 13.Cui R, Tieu B, Recinos A, Tilton RG, Brasier AR. RhoA mediates angiotensin II-induced phospho-Ser536 nuclear factor kappaB/RelA subunit exchange on the interleukin-6 promoter in VSMCs. Circ Res. 2006;99(7):723–30. doi: 10.1161/01.RES.0000244015.10655.3f. [DOI] [PubMed] [Google Scholar]
- 14.Akira S, Kishimoto T. IL-6 and NF-IL6 in acute-phase response and viral infection. Immunol Rev. 1992;127:25–50. doi: 10.1111/j.1600-065x.1992.tb01407.x. [DOI] [PubMed] [Google Scholar]
- 15.Brasier AR. The NF-kB regulatory network. Cardiovasc Toxicol. 2006;6:111–30. doi: 10.1385/ct:6:2:111. [DOI] [PubMed] [Google Scholar]
- 16.Choudhary S, Lu M, Cui R, Brasier AR. Involvement of a novel Rac/RhoA guanosine triphosphatase-nuclear factor-{kappa}B inducing kinase signaling pathway mediating angiotensin II-Induced RelA transactivation. Mol Endocrinol. 2007;21(9):2203–17. doi: 10.1210/me.2006-0465. [DOI] [PubMed] [Google Scholar]
- 17.McAllister-Lucas LM, Ruland J, Siu K, et al. CARMA3/Bcl10/ MALT1-dependent NF-{kappa}B activation mediates angiotensin II-responsive inflammatory signaling in nonimmune cells. Proc Natl Acad Sci USA. 2007;104(1):139–44. doi: 10.1073/pnas.0601947103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schieffer B, Schieffer E, Hilfiker-Kleiner D, et al. Expression of angiotensin II and interleukin 6 in human coronary atherosclerotic plaques: potential implications for inflammation and plaque instability. Circulation. 2000;101(12):1372–8. doi: 10.1161/01.cir.101.12.1372. [DOI] [PubMed] [Google Scholar]
- 19.Ernst M, Jenkins BJ. Acquiring signalling specificity from the cytokine receptor gp130. Trends Genet. 2004;20(1):23–32. doi: 10.1016/j.tig.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Murakami M, Kamimura D, Hirano T. New IL-6 (gp130) family cytokine members, CLC/NNT1/BSF3 and IL-27. Growth Factors. 2004;22(2):75–7. doi: 10.1080/08977190410001715181. [DOI] [PubMed] [Google Scholar]
- 21.Kurdi M, Booz GW. Can the protective actions of JAK-STAT in the heart be exploited therapeutically? Parsing the regulation of lnterleukin-6-type cytokine signaling. J Cardiovasc Pharmacol. 2007;50(2):126–41. doi: 10.1097/FJC.0b013e318068dd49. [DOI] [PubMed] [Google Scholar]
- 22.Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990;265(3):621–36. doi: 10.1042/bj2650621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link?. [Review] [44 refs] Atherosclerosis. 2000;148(2):209–14. doi: 10.1016/s0021-9150(99)00463-3. [DOI] [PubMed] [Google Scholar]
- 24.Hojo Y, Ikeda U, Takahashi M, Shimada K. Increased levels of monocyte-related cytokines in patients with unstable angina. Atherosclerosis. 2002;161(2):403–8. doi: 10.1016/s0021-9150(01)00636-0. [DOI] [PubMed] [Google Scholar]
- 25.Marrone D, Pertosa G, Simone S, et al. Local activation of interleukin 6 signaling is associated with arteriovenous fistula stenosis in hemodialysis patients. Am J Kidney Dis. 2007;49(5):664–73. doi: 10.1053/j.ajkd.2007.02.266. [DOI] [PubMed] [Google Scholar]
- 26.Jones SA, Horiuchi S, Topley N, Yamamoto N, Fuller GM. The soluble interleukin 6 receptor: mechanisms of production and implications in disease. FASEB J. 2001;15(1):43–58. doi: 10.1096/fj.99-1003rev. [DOI] [PubMed] [Google Scholar]
- 27.Boulanger MJ. Hexameric Structure and Assembly of the Interleukin-6/IL-6 [alpha]-Receptor/gp130 Complex.[Report] Science. 2003;300(5628):2101–4. doi: 10.1126/science.1083901. [DOI] [PubMed] [Google Scholar]
- 28.Heinrich PC, Behrmann I, Haan S, et al. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vardam TD, Zhou L, Appenheimer MM, et al. Regulation of a lymphocyte-endothelial-IL-6 trans-signaling axis by fever-range thermal stress: hot spot of immune surveillance. [Review] [151 refs] Cytokine. 2007;39(1):84–96. doi: 10.1016/j.cyto.2007.07.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakaoka Y, Nishida K, Fujio Y, et al. Activation of gp130 transduces hypertrophic signal through interaction of scaffolding/docking protein Gab1 with tyrosine phosphatase SHP2 in cardiomyocytes. Circ Res. 2003;93(3):221–9. doi: 10.1161/01.RES.0000085562.48906.4A. [DOI] [PubMed] [Google Scholar]
- 31.Scheller J, Rose-John S. Updating interleukin-6 classic- and transsignaling. Signal Transduct. 2006;6:240–59. [Google Scholar]
- 32.Sun W, Snyder M, Levy DE, Zhang JJ. Regulation of Stat3 transcriptional activity by the conserved LPMSP motif for OSM and IL-6 signaling. FEBS Lett. 2006;580(25):5880–4. doi: 10.1016/j.febslet.2006.09.054. [DOI] [PubMed] [Google Scholar]
- 33.Bhattacharya S, Eckner R, Grossman S, et al. Cooperation of Stat2 and p300/CBP in signalling induced by interferon-α. Nature. 1996;383:344–7. doi: 10.1038/383344a0. [DOI] [PubMed] [Google Scholar]
- 34.Pfitzner E, Jahne R, Wissler M, Stoecklin E, Groner B. p300/CREB-binding protein enhances the prolactin-mediated transriptional induction through direct interaction with the transactivation domain of STAT5, but does not participate in the Stat5-mediated suppression of the glucorticoid response. Mol Endocrinol. 1998;12(10):1582–93. doi: 10.1210/mend.12.10.0180. [DOI] [PubMed] [Google Scholar]
- 35.Ray S, Sherman CT, Lu M, Brasier AR. Angiotensinogen gene expression is dependent on signal transducer and activator of transcription 3-mediated p300/cAMP response element binding protein-binding protein coactivator recruitment and histone acetyl-transferase activity. Mol Endocrinol. 2002;16:824–36. doi: 10.1210/mend.16.4.0811. [DOI] [PubMed] [Google Scholar]
- 36.Yuan ZL, Guan YJ, Chatterjee D, Chin YE. Stat3 dimerization regulated by reversible acetylation of a single lysine residue. Science. 2005;307(5707):269–73. doi: 10.1126/science.1105166. [DOI] [PubMed] [Google Scholar]
- 37.Ray S, Boldogh I, Brasier AR. STAT3 NH2-terminal acetylation is activated by the hepatic acute-phase response and required for IL-6 induction of angiotensinogen. Gastroenterology. 2005;129:1616–32. doi: 10.1053/j.gastro.2005.07.055. [DOI] [PubMed] [Google Scholar]
- 38.Hou T, Ray S, Brasier AR. The functional role of an interleukin 6-inducible CDK9.STAT3 complex in human gamma-fibrinogen gene expression. J Biol Chem. 2007;282(51):37091–102. doi: 10.1074/jbc.M706458200. [DOI] [PubMed] [Google Scholar]
- 39.Giraud S, Hurlstone A, Avril S, Coqueret O. Implication of BRG1 and cdk9 in the STAT3-mediated activation of the p21waf1 gene. Oncogene. 2004;23(44):7391–8. doi: 10.1038/sj.onc.1207972. [DOI] [PubMed] [Google Scholar]
- 40.Lust JA, Donovan KA, Kline MP, et al. Isolation of an mRNA encoding a soluble form of the human interleukin-6 receptor. Cytokine. 1992;4(2):96–100. doi: 10.1016/1043-4666(92)90043-q. [DOI] [PubMed] [Google Scholar]
- 41.Chalaris A, Rabe B, Paliga K, et al. Apoptosis is a natural stimulus of IL6R shedding and contributes to the proinflammatory transsignaling function of neutrophils. Blood. 2007;110(6):1748–55. doi: 10.1182/blood-2007-01-067918. [DOI] [PubMed] [Google Scholar]
- 42.Coles B, Fielding CA, Rose-John S, et al. Classic interleukin-6 receptor signaling and interleukin-6 trans-signaling differentially control angiotensin II-dependent hypertension, cardiac signal transducer and activator of transcription-3 activation, and vascular hypertrophy In vivo. Am J Pathol. 2007;171(1):315–25. doi: 10.2353/ajpath.2007.061078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rabe B, Chalaris A, May U, et al. Transgenic blockade of interleukin 6 transsignaling abrogates inflammation. Blood. 2008;111(3):1021–8. doi: 10.1182/blood-2007-07-102137. [DOI] [PubMed] [Google Scholar]
- 44.Kile BT, Alexander WS. The suppressors of cytokine signalling (SOCS) Cell Mol Life Sci. 2001;58(11):1627–35. doi: 10.1007/PL00000801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krebs DL, Hilton DJ. SOCS proteins: Negative regulators of cytokine signaling. Stem Cells. 2001;19(5):378–87. doi: 10.1634/stemcells.19-5-378. [DOI] [PubMed] [Google Scholar]
- 46.Lehmann U, Schmitz J, Weissenbach M, et al. SHP2 and SOCS3 contribute to Tyr-759-dependent attenuation of interleukin-6 signaling through gp130. J Biol Chem. 2003;278(1):661–71. doi: 10.1074/jbc.M210552200. [DOI] [PubMed] [Google Scholar]
- 47.Mueller CF, Laude K, McNally JS, Harrison DG. ATVB in focus: redox mechanisms in blood vessels. Arterioscler Thromb Vasc Biol. 2005;25:274–8. doi: 10.1161/01.ATV.0000149143.04821.eb. [DOI] [PubMed] [Google Scholar]
- 48.Ohara Y, Peterson TE, Harrison DG. Hypercholesterolemia Increases Endothelial Superoxide Anion Production. J Clin Invest. 1993;91(6):2546–51. doi: 10.1172/JCI116491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rajagopalan S, Kurz S, Munzel T, et al. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation - Contribution to alterations of vasomotor tone. J Clin Invest. 1996;97(8):1916–23. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kawasaki H, Abe K, Kanno M, Hattori Y. Superoxide dismutase recovers altered endothelium-dependent relaxation in diabetic rat aorta. Am J Physiol. 1991;261:1086–94. doi: 10.1152/ajpheart.1991.261.4.H1086. [DOI] [PubMed] [Google Scholar]
- 51.van der Loo B, Labugger R, Skepper JN, et al. Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med. 2000;192:1731–44. doi: 10.1084/jem.192.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laurindo FRM, de Souza HP, Pedro MD, Janiszewski M. Redox aspects of vascular response to injury. Redox Cell Biol Genet, Part A. 2002;352:432–54. doi: 10.1016/s0076-6879(02)52039-5. [DOI] [PubMed] [Google Scholar]
- 53.Touyz RM. Reactive oxygen species and angiotensin II signaling in vascular cells -- implications in cardiovascular disease. Related Articles, Links Touyz RM. Braz J Med Biol Res. 2004;37:1263–73. doi: 10.1590/s0100-879x2004000800018. [DOI] [PubMed] [Google Scholar]
- 54.Swindle EJ, Metcalfe DD. The role of reactive oxygen species and nitric oxide in mast cell-dependent inflammatory processes. Immunol Rev. 2007;217:186–205. doi: 10.1111/j.1600-065X.2007.00513.x. [DOI] [PubMed] [Google Scholar]
- 55.Faraci FM. Oxidative stress: the curse that underlies cerebral vascular dysfunction? Stroke. 2005;36:186–8. doi: 10.1161/01.STR.0000153067.27288.8b. [DOI] [PubMed] [Google Scholar]
- 56.Madamanchi NR, Vendrov A, Runge MS. Oxidative stress and vascular disease. Arterioscler Thromb Vasc Biol. 2005;25:29–38. doi: 10.1161/01.ATV.0000150649.39934.13. [DOI] [PubMed] [Google Scholar]
- 57.Griendling KK, Harrison DG. Dual role of reactive oxygen species in vascular growth. Circ Res. 1999;85(6):562–3. doi: 10.1161/01.res.85.6.562. [DOI] [PubMed] [Google Scholar]
- 58.Griendling KK, Sorescu D, Lassegue B, Ushio-Fukai M. Modulation of protein kinase activity and gene expression by reactive oxygen species and their role in vascular physiology and pathophysiology. [Review] [132 refs] Arterioscler Thromb Vasc Biol. 2000;20(10):2175–83. doi: 10.1161/01.atv.20.10.2175. [DOI] [PubMed] [Google Scholar]
- 59.Harrison D, Griendling KK, Landmesser U, Hornig B, Drexler H. Role of oxidative stress in atherosclerosis. Am J Cardiol. 2003;91(3):7A–11A. doi: 10.1016/s0002-9149(02)03144-2. [DOI] [PubMed] [Google Scholar]
- 60.Harrison DG. Cellular and molecular mechanisms of endothelial cell dysfunction. J Clin Invest. 1997;100(9):2153–7. doi: 10.1172/JCI119751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wassmann S, Stumpf M, Strehlow K, et al. Interleukin-6 induces oxidative stress and endothelial dysfunction by overexpression of the angiotensin II type 1 receptor. Circ Res. 2004;94(4):534–41. doi: 10.1161/01.RES.0000115557.25127.8D. [DOI] [PubMed] [Google Scholar]
- 62.Schrader LI, Kinzenbaw DA, Johnson AW, Faraci FM, Didion SP. IL-6 deficiency protects against angiotensin II - Induced endothelial dysfunction and hypertrophy. Arterioscler Thromb Vasc Biol. 2007;27(12):2576–81. doi: 10.1161/ATVBAHA.107.153080. [DOI] [PubMed] [Google Scholar]
- 63.Didion SP, Kinzenbaw DA, Faraci FM. Critical role for CuZn-superoxide dismutase in preventing angiotensin II-induced endothelial dysfunction. Hypertension. 2005;46:1147–53. doi: 10.1161/01.HYP.0000187532.80697.15. [DOI] [PubMed] [Google Scholar]
- 64.Sironi M, Breviario F, Proserpio P, et al. IL-1 stimulates IL-6 production in endothelial cells. J Immunol. 1989;142(2):549–53. [PubMed] [Google Scholar]
- 65.Verhamme P, Hoylaerts MF. The pivotal role of the endothelium in haemostasis and thrombosis. Acta Clin Belg. 2006;61(5):213–9. doi: 10.1179/acb.2006.036. [DOI] [PubMed] [Google Scholar]
- 66.Gross PL, Aird WC. The endothelium and thrombosis. Semin Thromb Hemost. 2000;26(5):463–78. doi: 10.1055/s-2000-13202. [DOI] [PubMed] [Google Scholar]
- 67.Bierhaus A, Chen J, Liliensiek B, Nawroth PP. LPS and cytokine-activated endothelium. Semin Thromb Hemost. 2000;26(5):571–87. doi: 10.1055/s-2000-13214. [DOI] [PubMed] [Google Scholar]
- 68.Modat G, Dornand J, Bernad N, et al. LPS-stimulated bovine aortic endothelial cells produce IL-1 and IL-6 like activities. Agents Actions. 1990;30(3-4):403–11. doi: 10.1007/BF01966305. [DOI] [PubMed] [Google Scholar]
- 69.Pober JS. Effects of tumour necrosis factor and related cytokines on vascular endothelial cells. Ciba Found Symp. 1987;131:170–84. doi: 10.1002/9780470513521.ch12. [DOI] [PubMed] [Google Scholar]
- 70.Howells G, Pham P, Taylor D, Foxwell B, Feldmann M. Interleukin 4 induces interleukin 6 production by endothelial cells: synergy with interferon-gamma. Eur J Immunol. 1991;21(1):97–101. doi: 10.1002/eji.1830210115. [DOI] [PubMed] [Google Scholar]
- 71.Colotta F, Sironi M, Borre A, et al. Interleukin 4 amplifies monocyte chemotactic protein and interleukin 6 production by endothelial cells. Cytokine. 1992;4(1):24–8. doi: 10.1016/1043-4666(92)90032-m. [DOI] [PubMed] [Google Scholar]
- 72.Romano M, Sironi M, Toniatti C, et al. Role of IL-6 and its soluble receptor in induction of chemokines and leukocyte recruitment. Immunity. 1997;6(3):315–25. doi: 10.1016/s1074-7613(00)80334-9. [DOI] [PubMed] [Google Scholar]
- 73.Shabo Y, Lotem J, Rubinstein M, et al. The myeloid blood cell differentiation-inducing protein MGI-2A is interleukin-6. Blood. 1988;72(6):2070–3. [PubMed] [Google Scholar]
- 74.Oritani K, Kaisho T, Nakajima K, Hirano T. Retinoic acid inhibits interleukin-6-induced macrophage differentiation and apoptosis in a murine hematopoietic cell line, Y6. Blood. 1992;80(9):2298–305. [PubMed] [Google Scholar]
- 75.Matas D, Milyavsky M Shats I, et al. p53 is a regulator of macrophage differentiation. Cell Death Differ. 2004;11(4):458–67. doi: 10.1038/sj.cdd.4401379. [DOI] [PubMed] [Google Scholar]
- 76.Tanaka H, Matsumura I, Nakajima K, et al. GATA-1 blocks IL-6-induced macrophage differentiation and apoptosis through the sustained expression of cyclin D1 and bcl-2 in a murine myeloid cell line M1. Blood. 2000;95(4):1264–73. [PubMed] [Google Scholar]
- 77.Krishnaraju K, Hoffman B, Liebermann DA. The zinc finger transcription factor Egr-1 activates macrophage differentiation in M1 myeloblastic leukemia cells. Blood. 1998;92(6):1957–66. [PubMed] [Google Scholar]
- 78.Minami M, Inoue M, Wei S, et al. STAT3 activation is a critical step in gp130-mediated terminal differentiation and growth arrest of a myeloid cell line. Proc Natl Acad Sci USA. 1996;93(9):3963–6. doi: 10.1073/pnas.93.9.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yamanaka Y, Nakajima K, Fukada T, Hibi M, Hirano T. Differentiation and growth arrest signals are generated through the cytoplasmic region of gp130 that is essential for Stat3 activation. EMBO J. 1996;15(7):1557–65. [PMC free article] [PubMed] [Google Scholar]
- 80.Miyaura C, Onozaki K, Akiyama Y, et al. Recombinant human interleukin 6 (B-cell stimulatory factor 2) is a potent inducer of differentiation of mouse myeloid leukemia cells (M1) FEBS Lett. 1988;234(1):17–21. doi: 10.1016/0014-5793(88)81293-6. [DOI] [PubMed] [Google Scholar]
- 81.Chiu CP, Lee F. IL-6 is a differentiation factor for M1 and WEHI-3B myeloid leukemic cells. J Immunol. 1989;142(6):1909–15. [PubMed] [Google Scholar]
- 82.Haviernik P, Lahoda C, Bradley HL, et al. Tissue inhibitor of matrix metalloproteinase-1 overexpression in M1 myeloblasts impairs IL-6-induced differentiation. Oncogene. 2004;23(57):9212–9. doi: 10.1038/sj.onc.1208096. [DOI] [PubMed] [Google Scholar]
- 83.Chomarat P, Banchereau J, Davoust J, Palucka AK. IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nat Immunol. 2000;1(6):510–4. doi: 10.1038/82763. [DOI] [PubMed] [Google Scholar]
- 84.Keidar S, Heinrich R, Kaplan M, Hayek T, Aviram M. Angiotensin II administration to atherosclerotic mice increases macrophage uptake of oxidized ldl: a possible role for interleukin-6. Arterioscler Thromb Vasc Biol. 2001;21(9):1464–9. doi: 10.1161/hq0901.095547. [DOI] [PubMed] [Google Scholar]
- 85.Oritani K, Tomiyama Y, Kincade PW, et al. Both Stat3-activation and Stat3-independent BCL2 downregulation are important for interleukin-6-induced apoptosis of 1A9-M cells. Blood. 1999;93(4):1346–54. [PubMed] [Google Scholar]
- 86.Goralski KB, Sinal CJ. Type 2 diabetes and cardiovascular disease: getting to the fat of the matter. Can J Physiol Pharmacol. 2007;85(1):113–32. doi: 10.1139/y06-092. [DOI] [PubMed] [Google Scholar]
- 87.Biswas P, Delfanti F, Bernasconi S, et al. Interleukin-6 induces monocyte chemotactic protein-1 in peripheral blood mononuclear cells and in the U937 cell line. Blood. 1998;91(1):258–65. [PubMed] [Google Scholar]
- 88.Mitani H, Katayama N, Araki H, et al. Activity of interleukin 6 in the differentiation of monocytes to macrophages and dendritic cells. Br J Haematol. 2000;109(2):288–95. doi: 10.1046/j.1365-2141.2000.02020.x. [DOI] [PubMed] [Google Scholar]
- 89.Jansen JH, Kluin-Nelemans JC, Van Damme J, et al. Interleukin 6 is a permissive factor for monocytic colony formation by human hematopoietic progenitor cells. J Exp Med. 1992;175(4):1151–4. doi: 10.1084/jem.175.4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nakajima K, Yamanaka Y, Nakae K, et al. A central role for Stat3 in IL-6-induced regulation of growth and differentiation in M1 leukemia cells. EMBO J. 1996;15(14):3651–8. [PMC free article] [PubMed] [Google Scholar]
- 91.Mangan JK, Rane SG, Kang AD, et al. Mechanisms associated with IL-6-induced up-regulation of Jak3 and its role in monocytic differentiation. Blood. 2004;103(11):4093–101. doi: 10.1182/blood-2003-06-2165. [DOI] [PubMed] [Google Scholar]
- 92.Yamaguchi Y, Zon LI, Ackerman SJ, Yamamoto M, Suda T. Forced GATA-1 expression in the murine myeloid cell line M1: induction of c-Mpl expression and megakaryocytic/erythroid differentiation. Blood. 1998;91(2):450–7. [PubMed] [Google Scholar]
- 93.Nguyen HQ, Hoffman-Liebermann B, Liebermann DA. The zinc finger transcription factor Egr-1 is essential for and restricts differentiation along the macrophage lineage. Cell. 1993;72(2):197–209. doi: 10.1016/0092-8674(93)90660-i. [DOI] [PubMed] [Google Scholar]
- 94.Lee SL, Wang Y, Milbrandt J. Unimpaired macrophage differentiation and activation in mice lacking the zinc finger transplantation factor NGFI-A (EGR1) Mol Cell Biol. 1996;16(8):4566–72. doi: 10.1128/mcb.16.8.4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dalrymple SA, Lucian LA, Slattery R, et al. Interleukin-6-deficient mice are highly susceptible to Listeria monocytogenes infection: correlation with inefficient neutrophilia. Infect Immun. 1995;63(6):2262–8. doi: 10.1128/iai.63.6.2262-2268.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kopf M, Baumann H, Freer G, et al. Impaired immune and acutephase responses in interleukin-6-deficient mice. Nature. 1994;368(6469):339–42. doi: 10.1038/368339a0. [DOI] [PubMed] [Google Scholar]
- 97.Akira S, Kishimoto T. Role of interleukin-6 in macrophage function. Curr Opin Hematol. 1996;3(1):87–93. doi: 10.1097/00062752-199603010-00013. [DOI] [PubMed] [Google Scholar]
- 98.Bluethmann H, Rothe J, Schultze N, Tkachuk M, Koebel P. Establishment of the role of IL-6 and TNF receptor 1 using gene knockout mice. J Leukoc Biol. 1994 Nov;56(5):565–70. doi: 10.1002/jlb.56.5.565. [DOI] [PubMed] [Google Scholar]
- 99.Tanaka T, Akira S, Yoshida K, et al. Targeted disruption of the NF-IL6 gene discloses its essential role in bacteria killing and tumor cytotoxicity by macrophages. Cell. 1995;80(2):353–61. doi: 10.1016/0092-8674(95)90418-2. [DOI] [PubMed] [Google Scholar]
- 100.Bernad A, Kopf M, Kulbacki R, et al. Interleukin-6 is required in vivo for the regulation of stem cells and committed progenitors of the hemaopoietic system. Immunity. 1994;1:725–31. doi: 10.1016/s1074-7613(94)80014-6. [DOI] [PubMed] [Google Scholar]
- 101.Daugherty A, Manning MW, Cassis LA. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J Clin Invest. 2000;105(11):1605–12. doi: 10.1172/JCI7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zarbock A, Polanowska-Grabowska RK, Ley K. Platelet-neutrophil-interactions: linking hemostasis and inflammation. Blood Rev. 2007;21(2):99–111. doi: 10.1016/j.blre.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 103.Ishibashi T, Kimura H, Uchida T, et al. Human interleukin 6 is a direct promoter of maturation of megakaryocytes in vitro. Proc Natl Acad Sci USA. 1989;86(15):5953–7. doi: 10.1073/pnas.86.15.5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kimura H, Ishibashi T, Uchida T, et al. Interleukin 6 is a differentiation factor for human megakaryocytes in vitro. Eur J Immunol. 1990;20(9):1927–31. doi: 10.1002/eji.1830200909. [DOI] [PubMed] [Google Scholar]
- 105.Bruno E, Cooper RJ, Briddell RA, Hoffman R. Further examination of the effects of recombinant cytokines on the proliferation of human megakaryocyte progenitor cells. Blood. 1991;77(11):2339–46. [PubMed] [Google Scholar]
- 106.Asano S, Okano A, Ozawa K, et al. In vivo effects of recombinant human interleukin-6 in primates: stimulated production of platelets. Blood. 1990;75(8):1602–5. [PubMed] [Google Scholar]
- 107.Metcalf D, Nicola NA, Gearing DP. Effects of injected leukemia inhibitory factor on hematopoietic and other tissues in mice. Blood. 1990;76(1):50–6. [PubMed] [Google Scholar]
- 108.Neben TY, Loebelenz J, Hayes L, et al. Recombinant human interleukin-11 stimulates megakaryocytopoiesis and increases peripheral platelets in normal and splenectomized mice. Blood. 1993;81(4):901–8. [PubMed] [Google Scholar]
- 109.Wallace PM, MacMaster JF, Rillema JR, et al. Thrombocytopoietic properties of oncostatin M. Blood. 1995;86(4):1310–5. [PubMed] [Google Scholar]
- 110.Hollen CW, Henthorn J, Koziol JA, Burstein SA. Elevated serum interleukin-6 levels in patients with reactive thrombocytosis. Br J Haematol. 1991;79(2):286–90. doi: 10.1111/j.1365-2141.1991.tb04534.x. [DOI] [PubMed] [Google Scholar]
- 111.Peng J, Friese P, George JN, Dale GL, Burstein SA. Alteration of platelet function in dogs mediated by interleukin-6. Blood. 1994;83(2):398–403. [PubMed] [Google Scholar]
- 112.Burstein SA, Peng J, Friese P, et al. Cytokine-induced alteration of platelet and hemostatic function. Stem Cells. 1996;14(Suppl 1):154–62. doi: 10.1002/stem.5530140720. [DOI] [PubMed] [Google Scholar]
- 113.Oleksowicz L, Mrowiec Z, Zuckerman D, et al. Platelet activation induced by interleukin-6: evidence for a mechanism involving arachidonic acid metabolism. Thromb Haemost. 1994;72(2):302–8. [PubMed] [Google Scholar]
- 114.Oleksowicz L, Puszkin E, Mrowiec Z, Isaacs R, Dutcher JP. Alterations in platelet function in patients receiving interleukin-6 as cytokine therapy. Cancer Invest. 1996;14(4):307–16. doi: 10.3109/07357909609012156. [DOI] [PubMed] [Google Scholar]
- 115.Hirota H, Yoshida K, Kishimoto T, Taga T. Continuous Activation of gp130, a Signal-Transducing Receptor Component for Interleukin 6-Related Cytokines, Causes Myocardial Hypertrophy in Mice. Proceedings of the National Academy of Sciences. 1995;92(11):4862–6. doi: 10.1073/pnas.92.11.4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hirota H, Chen J, Betz UAK, et al. Loss of a gp130 cardiac muscle cell survival pathway is a critical event in the onset of heart failure during biomechanical stress. Cell. 1998;97:189–98. doi: 10.1016/s0092-8674(00)80729-1. [DOI] [PubMed] [Google Scholar]
- 117.Negoro S, Kunisada K, Tone E, et al. Activation of JAK/STAT pathway transduces cytoprotective signal in rat acute myocardial infarction. Cardiovasc Res. 2000;47(4):797–805. doi: 10.1016/s0008-6363(00)00138-3. [DOI] [PubMed] [Google Scholar]
- 118.Cressman DE, Diamond RH, Taub R. Rapid Activation of the Stat3 Transcription Complex in Liver-Regeneration. Hepatology. 1995;21(5):1443–9. [PubMed] [Google Scholar]
- 119.Cressman DE, Greenbaum LE, DeAngelis RA, et al. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science. 1996;274(5291):1379–83. doi: 10.1126/science.274.5291.1379. [DOI] [PubMed] [Google Scholar]
- 120.Kushner I. The acute phase response: an overview. Methods Enzymol. 1988;163:373–83. doi: 10.1016/0076-6879(88)63037-0. [DOI] [PubMed] [Google Scholar]
- 121.Haga S, Terui K, Zhang HQ, et al. Stat3 protects against Fas-induced liver injury by redox-dependent and -independent mechanisms. J Clin Invest. 2003;112(7):989–98. doi: 10.1172/JCI17970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Grosch S, Fritz G, Kaina B. Apurinic endonuclease (Ref-l) is induced in mammalian cells by oxidative stress and involved in clastogenic adaptation. Cancer Research. 1998;58(19):4410–6. [PubMed] [Google Scholar]
- 123.Nakamura H, Nakamura K, Yodoi J. Redox regulation of cellular activation. Annu Rev Immunol. 1997;15:351–69. doi: 10.1146/annurev.immunol.15.1.351. [DOI] [PubMed] [Google Scholar]
- 124.Kovalovich K, Li W, DeAngelis R, et al. Interleukin-6 protects against Fas-mediated death by establishing a critical level of anti-apoptotic hepatic proteins FLIP, Bcl-2, and Bcl-xL. J Biol Chem. 2001;276:26605–13. doi: 10.1074/jbc.M100740200. [DOI] [PubMed] [Google Scholar]
- 125.Williams JL. Cross talk between the inflammation and coagulation systems. Clin Lab Sci. 2007;20(4):224–9. [PubMed] [Google Scholar]
- 126.Esmon CT. The interactions between inflammation and coagulation. Br J Haematol. 2005;131(4):417–30. doi: 10.1111/j.1365-2141.2005.05753.x. [DOI] [PubMed] [Google Scholar]
- 127.Levi M, van der PT. Two-way interactions between inflammation and coagulation. Trends Cardiovasc Med. 2005;15(7):254–9. doi: 10.1016/j.tcm.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 128.Amrani DL. Regulation of fibrinogen biosynthesis: glucocorticoid and interleukin-6 control. Blood Coagul Fibrinolysis. 1990;1(4-5):443–6. [PubMed] [Google Scholar]
- 129.Cermak J, Key NS, Bach RR, et al. C-reactive protein induces human peripheral blood monocytes to synthesize tissue factor. Blood. 1993;82(2):513–20. [PubMed] [Google Scholar]
- 130.Stirling D, Hannant WA, Ludlam CA. Transcriptional activation of the factor VIII gene in liver cell lines by interleukin-6. Thromb Haemost. 1998;79(1):74–8. [PubMed] [Google Scholar]
- 131.Niessen RW, Lamping RJ, Jansen PM, et al. Antithrombin acts as a negative acute phase protein as established with studies on HepG2 cells and in baboons. Thromb Haemost. 1997;78(3):1088–92. [PubMed] [Google Scholar]
- 132.Hooper WC, Phillips DJ, Evatt BL. Endothelial cell protein S synthesis is upregulated by the complex of IL-6 and soluble IL-6 receptor. Thromb Haemost. 1997;77(5):1014–9. [PubMed] [Google Scholar]
- 133.Burstein SA. Cytokines, platelet production and hemostasis. Platelets. 1997;8(2-3):93–104. doi: 10.1080/09537109709169324. [DOI] [PubMed] [Google Scholar]
- 134.Ernst E, Resch KL. Fibrinogen as a cardiovascular risk factor: a meta-analysis and review of the literature. Ann Intern Med. 1993;118(12):956–63. doi: 10.7326/0003-4819-118-12-199306150-00008. [DOI] [PubMed] [Google Scholar]
- 135.Rooney MM, Parise LV, Lord ST. Dissecting clot retraction and platelet aggregation. Clot retraction does not require an intact fibrinogen gamma chain C terminus. J Biol Chem. 1996;271(15):8553–5. doi: 10.1074/jbc.271.15.8553. [DOI] [PubMed] [Google Scholar]
- 136.Hu CH, Harris JE, Davie EW, Chung DW. Characterization of the 5'-flanking region of the gene for the alpha chain of human fibrinogen. J Biol Chem. 1995;270(47):28342–9. doi: 10.1074/jbc.270.47.28342. [DOI] [PubMed] [Google Scholar]
- 137.Mizuguchi J, Hu CH, Cao Z, et al. Characterization of the 5'-flanking region of the gene for the gamma chain of human fibrinogen. J Biol Chem. 1995;270(47):28350–6. doi: 10.1074/jbc.270.47.28350. [DOI] [PubMed] [Google Scholar]
- 138.Anderson GM, Shaw AR, Shafer JA. Functional characterization of promoter elements involved in regulation of human B beta-fibrinogen expression. Evidence for binding of novel activator and repressor proteins. J Biol Chem. 1993;268(30):22650–5. [PubMed] [Google Scholar]
- 139.Gervois P, Vu-Dac. N, Kleemann R, et al. Negative regulation of human fibrinogen gene expression by peroxisome proliferator-activated receptor alpha agonists via inhibition of CCAAT box/enhancer-binding protein beta. J Biol Chem. 2001;276(36):33471–7. doi: 10.1074/jbc.M102839200. [DOI] [PubMed] [Google Scholar]
- 140.Dalmon J, Laurent M, Courtois G. The human beta fibrinogen promoter contains a hepatocyte nuclear factor 1-dependent interleukin-6-responsive element. Mol Cell Biol. 1993;13(2):1183–93. doi: 10.1128/mcb.13.2.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hawiger J. Adhesive ends of fibrinogen and its antiadhesive peptides: the end of a sage? Semin Hematol. 1995;32(2):99–109. [PubMed] [Google Scholar]
- 142.Ugarova TP, Lishko VK, Podolnikova NP, et al. Sequence gamma 377-395(P2), but not gamma 190-202(P1), is the binding site for the alpha MI-domain of integrin alpha M beta 2 in the gamma C-domain of fibrinogen. Biochemistry. 2003;42(31):9365–73. doi: 10.1021/bi034057k. [DOI] [PubMed] [Google Scholar]
- 143.Sahni A, Altland OD, Francis CW. FGF-2 but not FGF-1 binds fibrin and supports prolonged endothelial cell growth. J Thromb Haemost. 2003;1(6):1304–10. doi: 10.1046/j.1538-7836.2003.00250.x. [DOI] [PubMed] [Google Scholar]
- 144.Sahni A, Guo M, Sahni SK, Francis CW. Interleukin-1beta but not IL-1alpha binds to fibrinogen and fibrin and has enhanced activity in the bound form. Blood. 2004;104(2):409–14. doi: 10.1182/blood-2004-01-0126. [DOI] [PubMed] [Google Scholar]
- 145.Sahni A, Francis CW. Vascular endothelial growth factor binds to fibrinogen and fibrin and stimulates endothelial cell proliferation. Blood. 2000;96(12):3772–8. [PubMed] [Google Scholar]
- 146.Zhang Z, Fuentes NL, Fuller GM. Characterization of the IL-6 responsive elements in the gamma fibrinogen gene promoter. J Biol Chem. 1995;270(41):24287–91. doi: 10.1074/jbc.270.41.24287. [DOI] [PubMed] [Google Scholar]
- 147.Duan HO, Simpson-Haidaris PJ. Functional analysis of interleukin 6 response elements (IL-6REs) on the human gamma-fibrinogen promoter: binding of hepatic Stat3 correlates negatively with transactivation potential of type II IL-6REs. J Biol Chem. 2003;278(42):41270–81. doi: 10.1074/jbc.M304210200. [DOI] [PubMed] [Google Scholar]
- 148.Osterud B, Rapaport SI. Activation of factor IX by the reaction product of tissue factor and factor VII: additional pathway for initiating blood coagulation. Proc Natl Acad Sci USA. 1977;74(12):5260–4. doi: 10.1073/pnas.74.12.5260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chin BS, Blann AD, Gibbs CR, et al. Prognostic value of interleukin-6, plasma viscosity, fibrinogen, von Willebrand factor, tissue factor and vascular endothelial growth factor levels in congestive heart failure. Eur J Clin Invest. 2003;33(11):941–8. doi: 10.1046/j.1365-2362.2003.01252.x. [DOI] [PubMed] [Google Scholar]
- 150.Neumann FJ, Ott I, Marx N, et al. Effect of human recombinant interleukin-6 and interleukin-8 on monocyte procoagulant activity. Arterioscler Thromb Vasc Biol. 1997;17(12):3399–405. doi: 10.1161/01.atv.17.12.3399. [DOI] [PubMed] [Google Scholar]
- 151.Conway DS, Buggins P, Hughes E, Lip GY. Relationship of interleukin-6 and C-reactive protein to the prothrombotic state in chronic atrial fibrillation. J Am Coll Cardiol. 2004;43(11):2075–82. doi: 10.1016/j.jacc.2003.11.062. [DOI] [PubMed] [Google Scholar]
- 152.Dong J, Fuji S, Goto D, et al. Increased expression of plasminogen activator inhibitor-1 by mediators of the acute phase response: a potential progenitor of vasculopathy in hypertensives. Hypertension Research. 2003;26(9):723–9. doi: 10.1291/hypres.26.723. [DOI] [PubMed] [Google Scholar]
- 153.Healy AM, Gelehrter TD. Induction of Plasminogen-Activator Inhibitor-1 in Hepg2 Human Hepatoma-Cells by Mediators of the Acute-Phase Response. J Biol Chem. 1994;269(29):19095–100. [PubMed] [Google Scholar]
- 154.Dong J, Fujii S, Li HM, et al. Interleukin-6 and mevastatin regulate plasminogen activator inhibitor-1 through CCAAT/enhancer-binding protein-delta. Arterioscler Thromb Vasc Biol. 2005;25(5):1078–84. doi: 10.1161/01.ATV.0000159701.24372.49. [DOI] [PubMed] [Google Scholar]
- 155.Dong J, Fujii S, Kaneko T, et al. IL-1 and IL-6 induce hepatocyte plasminogen activator inhibitor-1 expression through independent signaling pathways converging on C/EBP delta. Circulation. 2006;114(18):116. doi: 10.1152/ajpcell.00157.2006. [DOI] [PubMed] [Google Scholar]
- 156.Dahlback B. Protein S and C4b-binding protein: components involved in the regulation of the protein C anticoagulant system. Thromb Haemost. 1991;66(1):49–61. [PubMed] [Google Scholar]
- 157.Ritchie SA, Connell JM. The link between abdominal obesity, metabolic syndrome and cardiovascular disease. Nutr Metab Cardiovasc Dis. 2007;17(4):319–26. doi: 10.1016/j.numecd.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 158.Trayhurn P, Wood IS. Signalling role of adipose tissue: adipokines and inflammation in obesity. Biochem Soc Trans. 2005;33(Pt 5):1078–81. doi: 10.1042/BST0331078. [DOI] [PubMed] [Google Scholar]
- 159.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115(5):911–9. doi: 10.1016/j.jaci.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 160.Bastard JP, Maachi M, Van Nhieu JT, et al. Adipose tissue IL-6 content correlates with resistance to insulin activation of glucose uptake both in vivo and in vitro. J Clin Endocrinol Metab. 2002;87(5):2084–9. doi: 10.1210/jcem.87.5.8450. [DOI] [PubMed] [Google Scholar]
- 161.Havel PJ. Update on adipocyte hormones: regulation of energy balance and carbohydrate/lipid metabolism. Diabetes. 2004;53(Suppl 1):S143–S151. doi: 10.2337/diabetes.53.2007.s143. [DOI] [PubMed] [Google Scholar]
- 162.Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab. 1998;83(3):847–50. doi: 10.1210/jcem.83.3.4660. [DOI] [PubMed] [Google Scholar]
- 163.Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology. 2004;145(5):2273–82. doi: 10.1210/en.2003-1336. [DOI] [PubMed] [Google Scholar]
- 164.Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107(3):363–9. doi: 10.1161/01.cir.0000053730.47739.3c. [DOI] [PubMed] [Google Scholar]
- 165.Kralisch S, Bluher M, Paschke R, Stumvoll M, Fasshauer M. Adipokines and adipocyte targets in the future management of obesity and the metabolic syndrome. Mini Rev Med Chem. 2007;7(1):39–45. doi: 10.2174/138955707779317821. [DOI] [PubMed] [Google Scholar]
- 166.Mohamed-Ali V, Goodrick S, Rawesh A, et al. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab. 1997;82(12):4196–200. doi: 10.1210/jcem.82.12.4450. [DOI] [PubMed] [Google Scholar]
- 167.Sjoholm A, Nystrom T. Inflammation and the etiology of type 2 diabetes. Diabetes Metab Res Rev. 2006;22(1):4–10. doi: 10.1002/dmrr.568. [DOI] [PubMed] [Google Scholar]
- 168.Hu FB, Meigs JB, Li TY, Rifai N, Manson JE. Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes. 2004;53(3):693–700. doi: 10.2337/diabetes.53.3.693. [DOI] [PubMed] [Google Scholar]
- 169.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286(3):327–34. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 170.Kristiansen OP, Mandrup-Poulsen T. Interleukin-6 and diabetes: the good, the bad, or the indifferent? Diabetes. 2005;54(Suppl 2):S114–S124. doi: 10.2337/diabetes.54.suppl_2.s114. [DOI] [PubMed] [Google Scholar]
- 171.Burstein SA, Peng J, Friese P, et al. Cytokine-induced alteration of platelet and hemostatic function. Stem Cells. 1996;14(Suppl 1):154–62. doi: 10.1002/stem.5530140720. [DOI] [PubMed] [Google Scholar]
- 172.Orban Z, Remaley AT, Sampson M, Trajanoski Z, Chrousos GP. The differential effect of food intake and beta-adrenergic stimulation on adipose-derived hormones and cytokines in man. J Clin Endocrinol Metab. 1999;84(6):2126–33. doi: 10.1210/jcem.84.6.5747. [DOI] [PubMed] [Google Scholar]
- 173.Carey AL, Febbraio MA. Interleukin-6 and insulin sensitivity: friend or foe? Diabetologia. 2004;47(7):1135–42. doi: 10.1007/s00125-004-1447-y. [DOI] [PubMed] [Google Scholar]
- 174.Bastard JP, Jardel C, Bruckert E, et al. Elevated levels of interleukin 6 are reduced in serum and subcutaneous adipose tissue of obese women after weight loss. J Clin Endocrinol Metab. 2000;85(9):3338–42. doi: 10.1210/jcem.85.9.6839. [DOI] [PubMed] [Google Scholar]
- 175.Mooney RA, Senn J, Cameron S, et al. Suppressors of cytokine signaling-1 and -6 associate with and inhibit the insulin receptor. A potential mechanism for cytokine-mediated insulin resistance. J Biol Chem. 2001;276(28):25889–93. doi: 10.1074/jbc.M010579200. [DOI] [PubMed] [Google Scholar]
- 176.Lagathu C, Bastard JP, Auclair M, et al. Chronic interleukin-6 (IL-6) treatment increased IL-6 secretion and induced insulin resistance in adipocyte: prevention by rosiglitazone. Biochem Biophys Res Commun. 2003;311(2):372–9. doi: 10.1016/j.bbrc.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 177.Grimble RF. Inflammatory status and insulin resistance. Curr Opin Clin Nutr Metab Care. 2002;5(5):551–9. doi: 10.1097/00075197-200209000-00015. [DOI] [PubMed] [Google Scholar]
- 178.Gollasch M, Dubrovska G. Paracrine role for periadventitial adipose tissue in the regulation of arterial tone. Trends Pharmacol Sci. 2004;25(12):647–53. doi: 10.1016/j.tips.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 179.Gao YJ. Dual modulation of vascular function by perivascular adipose tissue and its potential correlation with adiposity/lipoatrophy-related vascular dysfunction. Curr Pharm Des. 2007;13(21):2185–92. doi: 10.2174/138161207781039634. [DOI] [PubMed] [Google Scholar]
- 180.Galvez B, de CJ, Herold D, et al. Perivascular adipose tissue and mesenteric vascular function in spontaneously hypertensive rats. Arterioscler Thromb Vasc Biol. 2006;26(6):1297–302. doi: 10.1161/01.ATV.0000220381.40739.dd. [DOI] [PubMed] [Google Scholar]
- 181.Guzik TJ, Marvar PJ, Czesnikiewicz-Guzik M, Korbut R. Perivascular adipose tissue as a messenger of the brain-vessel axis: role in vascular inflammation and dysfunction. J Physiol Pharmacol. 2007 Dec;58(4):591–610. [PubMed] [Google Scholar]
- 182.Soltis EE, Cassis LA. Influence of perivascular adipose tissue on rat aortic smooth muscle responsiveness. Clin Exp Hypertens A. 1991;13(2):277–96. doi: 10.3109/10641969109042063. [DOI] [PubMed] [Google Scholar]