Abstract
The incidence of diabetes is increasing in the general population because of increasing obesity, and is likely to result in a higher incidence of coronary artery disease. It was recently reported that diabetics (types I and II) dying suddenly from coronary artery disease have greater macrophage and T lymphocyte infiltration in atherosclerotic plaques, as well as larger necrotic cores compared with nondiabetics. The inflammatory cell infiltrates showed human leukocyte antigen-DR expression, which was greater in diabetics. The receptors for advanced glycosylation end-products expression, demonstrated by immunohistochemistry, was greater in diabetics than in nondiabetics in macrophages, smooth muscle cells and endothelial cells, and was associated with apoptosis of macrophages and smooth muscle cells, but not of endothelial cells. There is also a higher incidence of healed plaque ruptures and healed myocardial infarct in type II diabetics. Plaque burden is higher in diabetics than in nondiabetics; however, distal plaque burden was only significantly different in type II diabetics compared with nondiabetics. There was greater positive remodelling in diabetic coronary arteries than in nondiabetic ones, which correlated with the per cent necrotic core. Further studies are needed to better understand the mechanisms that govern greater inflammation and plaque burden in diabetics.
Keywords: Coronary artery disease, Diabetes, Inflammation, Sudden death
Abstract
L’incidence du diabète est en hausse dans la population générale à cause de l’augmentation des cas d’obésité, et elle risque fort d’entraîner à sa suite une forte incidence de la maladie coronarienne. On a récemment signalé que comparativement aux non-diabétiques, les diabétiques (de type I et de type II) décédés subitement de coronaropathie présentaient une infiltration plus importante des macrophages et des lymphocytes T dans leurs plaques d’athérome et des noyaux nécrotiques plus volumineux. Les infiltrats cellulaires inflammatoires ont révélé une expression plus marquée de l’antigène HLA-DR chez les diabétiques que chez les non-diabétiques. Les récepteurs de l’expression des produits terminaux de la glycosylation avancée observés en immunohistochimie étaient plus présents dans les macrophages, les cellules des muscles lisses et les cellules endothéliales des diabétiques que des non-diabétiques et étaient associés à l’apoptose des macrophages et des cellules des muscles lisses, mais non des cellules endothéliales. On note en outre une incidence plus forte de plaques rompues cicatrisées et d’infarctus du myocarde cicatrisés chez les diabétiques de type II. Le rôle de la plaque est plus important chez les diabétiques que chez les non-diabétiques. Par contre, la différence ne s’est révélée significative qu’entre les diabétiques de type II et les non-diabétiques. On a noté un remodelage positif plus marqué au niveau des coronaires des diabétiques, phénomène qui était en corrélation avec le pourcentage de noyaux nécrotiques. Il faudra poursuivre la recherche pour mieux comprendre les mécanismes qui sont à l’origine de l’inflammation plus marquée et du rôle plus prépondérant de la plaque chez les diabétiques.
Diabetes affects over 100 million people worldwide (1). Of these, 5% to 10% have type I diabetes (juvenile diabetes) and 90% to 95% have type II diabetes, also known as adult-onset diabetes mellitus. Because of increasing obesity due to lifestyle changes in children in the United States, it is expected that the incidence of type II diabetes will increase in the next few decades, and it is predicted to affect as many as 35% to 40% of the population (2).
Diabetes is associated with the development of accelerated coronary atherosclerotic artery disease, which results in increased morbidity and cardiovascular complications. Angioscopic studies suggest an increased incidence of coronary thrombosis in diabetics (3). Studies using ultrafast computed tomography scans have shown a greater extent of calcification (4). Intravascular ultrasound studies have suggested a decrease in adaptive remodelling of atheroma in diabetics compared with nondiabetics (5). Pathological specimens obtained during coronary atherectomy or carotid endarterectomy have shown that there is an increase in macrophage infiltration in diabetics compared with nondiabetics (6).
Diabetes results in a variety of metabolic imbalances with different vascular effects. The combination of hyperglycemia, elevated free fatty acids and insulin resistance increases oxidative stress, upregulates protein kinase C and activates receptors for advanced glycosylation end-products (RAGE) (7). Within the endothelium, there is increased vasoconstriction, adhesion molecule activation, inflammation, and thrombosis via hyper-coagulation and platelet activation (7). The mechanisms by which diabetes cause differences in coronary plaque morphology are complex and poorly understood, but likely related to the proinflammatory and prothrombotic diabetic state.
MORPHOLOGICAL FINDINGS FROM THE REGISTRY OF SUDDEN CORONARY DEATH
We have recently reported morphological findings from our sudden coronary death registry in both type I and type II diabetics and compared these with age- and sex-matched nondiabetics dying suddenly from coronary artery atherosclerotic disease (8). The basis for inclusion into the registry of sudden coronary death included either presence of an acute coronary thrombus or severe coronary atherosclerosis (one or more epicardial arteries with 75% or more cross-sectional luminal narrowing) and the absence of noncoronary causes of death at autopsy or prior surgery. Sixty-six diabetics were selected on the basis of history of type I diabetes mellitus treated with insulin, or the presence of type II diabetes. Type II diabetes was ascertained by history of oral hypoglycemics, or postmortem glycohemoglobin 10% or higher in the absence of type I diabetes. A total of 16 patients had type I diabetes, 50 had type II diabetes, and the findings in these patients were compared with 66 age- and sex-matched nondiabetics that were dying from severe coronary artery disease (Table 1). The body mass index was higher in type II diabetics than in nondiabetics. The rate of smoking and hypertension were similar in nondiabetics and diabetics (Table 2). There was a trend toward higher total cholesterol and lower high density lipoprotein (HDL) cholesterol in type II diabetics. The ratio of total cholesterol to HDL cholesterol was significantly higher in type II diabetics than in nondiabetics.
TABLE 1.
Patient characteristics
| Characteristics | Type I DM | Type II DM | No DM |
|---|---|---|---|
| Patients (n) | 16 | 50 | 66 |
| Age ± SD (years) | 50.3±13.2 | 50.2±11.0 | 50.6±12.3 |
| Female sex (%) | 25 | 30 | 29 |
| Black (%) | 20 | 30 | 29 |
| Body mass index ± SD (kg/m2) | 25.6±6.4 | 30.5±7.41* | 26.6±5.4 |
P=0.001 versus those with no diabetes mellitus (DM)
TABLE 2.
Association between diabetes and other risk factors
| Risk factors | Type I DM | P* | Type II DM | P* | No DM |
|---|---|---|---|---|---|
| Glycohemoglobin ± SD (%) | 12.2±2.5 | 0.0001 | 10.7±2.6 | 0.0001 | 6.2±0.6 |
| Smokers (%) | 42 | 0.4 | 58 | 0.8 | 55 |
| Hypertension (%) | 29 | 0.9 | 35 | 0.6 | 30 |
| Total cholesterol ± SD (mg/dL) | 183±52 | 0.3 | 227±83 | 0.3 | 211±79 |
| High density lipoprotein cholesterol ± SD (mg/dL) | 37±14 | 0.8 | 33±16 | 0.1 | 38±18 |
| Total cholesterol/high density lipoprotein cholesterol ± SD (mg/dL) | 5.8±2.9 | 0.7 | 7.9±3.9 | 0.02 | 6.3±3.4 |
Versus those with no diabetes mellitus (DM)
Acute thrombi due to rupture or erosions were relatively uncommon in type I diabetics (Table 3); in type II diabetics, the proportion of ruptures was similar to that of nondiabetics, however, plaque erosions were significantly less frequent. The mean heart weight was significantly increased in type II diabetes; and healed infarcts were significantly more frequent in type II diabetics.
TABLE 3.
Incidence of acute thrombi, heart weight and healed infarcts
| Type I DM | P* | Type II DM | P* | No DM | |
|---|---|---|---|---|---|
| Acute plaque ruptures (%) | 6 | 0.09 | 32 | 0.6 | 27 |
| Erosions (%) | 6 | 0.02 | 12 | 0.04 | 29 |
| Heart weight ± SD (g) | 425±119 | 0.7 | 524±140 | 0.004 | 434±121 |
| Corrected heart weight† ± SD (g) | 428±94 | 0.3 | 508±134 | 0.03 | 460±106 |
| Healed infarcts (%) | 33 | 0.7 | 73 | 0.0001 | 37 |
Versus those with no diabetes mellitus (DM).
Corrected for body weight
The mean per cent plaque area composed of necrotic core was greater in type I (P=0.05) and type II (P=0.004) diabetics compared with nondiabetics (Table 4). The mean per cent calcified area was greatest in type II diabetics and smallest in type I diabetics, but the differences were not significant (P≥0.1). The mean number of fibrous cap atheromas was greater in type II diabetics than in nondiabetics (P=0.02) (Table 4). The number of healed plaque ruptures was greater in type II diabetics (Table 4).
TABLE 4.
Plaque characteristics
| Type I DM | P* | Type II DM | P* | No DM | |
|---|---|---|---|---|---|
| Necrotic core plaque area, mean ± SD (%) | 12.0±5.7 | 0.05† | 11.6±8.4 | 0.004† | 9.4±9.3 |
| Calcified matrix plaque area, mean ± SD (%) | 7.8±9.1 | 0.9† | 12.1±11.2 | 0.05† | 11.4±13.5 |
| Macrophage plaque area, mean ± SD (mm) | 0.15±0.02 | 0.03† | 0.13±0.03 | 0.03† | 0.10±0.02‡ |
| Fibrous cap atheroma, n ± SD | 7.1±5.0 | 0.9 | 8.8±4.3 | 0.02 | 6.9±4.7 |
| Thin cap atheroma, n ± SD | 1.0±1.3 | 0.5 | 0.8±0.8 | 0.8 | 0.7±0.8 |
| Healed plaque ruptures, n ± SD | 2.6±2.1 | 0.2 | 2.6±1.8 | 0.04 | 1.9±1.8 |
| Total plaque burden ± SD (%) | 275±129 | 0.04 | 358±114 | 0.0001 | 232±128 |
| Distal plaque burden ± SD (%) | 310±114 | 0.8 | 630±263 | 0.0001 | 331±199 |
Versus those with no diabetes mellitus (DM).
P calculated using log normalized data;
P=0.006 versus type I and II diabetes combined
By multivariate analysis, there was a positive correlation between mean per cent necrotic core size and glycohemoglobin, independent of total cholesterol/HDL cholesterol, HDL cholesterol, age, smoking and sex (P=0.005, T=2.9). There was a similar correlation with body mass index (Table 5). There was a strong correlation between macrophage area and glycohemoglobin (P=0.004, T=2.9; Table 5). There was a stronger relationship between numbers of fibrous cap atheromas and total cholesterol/HDL cholesterol (Table 5) than with other risk factors, including glycohemoglobin.
TABLE 5.
Relationship of risk factors, including diabetes, to plaque characteristics: A multivariate analysis
| Necrotic core area, % of plaque area | Macrophage area, % of plaque area | Fibrous cap atheromas, n | ||||
|---|---|---|---|---|---|---|
| T | P | T | P | T | P | |
| Glycohemoglobin (%) | 2.8 | 0.005 | 2.9 | 0.004 | 1.7 | 0.09 |
| Total cholesterol/HDL cholesterol | 2.5 | 0.01 | 1.3 | 0.19 | 3.0 | 0.0003 |
| Body mass index | 3.5 | 0.006 | 1.5 | 0.14 | 0.57 | 0.57 |
| Smoking | −0.4 | 0.7 | −0.6 | 0.5 | −1.1 | 0.24 |
| Age | −1.2 | 0.2 | −5.4 | 0.0001 | −1.2 | 0.2 |
HDL High density lipoprotein
The present study demonstrates that type II diabetics dying suddenly with severe coronary disease have extensive disease, including distal involvement of their coronary arteries, compared with nondiabetics. Part of the reason for increased plaque burden may be because of the observed high rate of healed plaque ruptures, indicating subclinical ruptures that may participate in plaque progression (9). The effect of diabetes on plaque burden has been demonstrated by calcium imaging studies (10). The implications of these findings are unclear, but suggest a direct atherogenic effect of type II diabetes, probably related to the development of lipid-rich cores. The known risk of diabetics following coronary artery bypass graft surgery (11,12) may in part be due to distal disease, as shown in our study, which may impair blood flow distal to graft anastomoses.
REMODELLING
In our sudden death registry, the mean ± SD of the internal elastic lamina (IEL) area adjusted for the distance from the coronary ostium was greater in type I and II diabetics than in nondiabetics (18.2±6.6 mm2, 16.5±4.4 mm2 and 16.0±4.5 mm2, respectively). The mean IEL was also significantly greater in type I (P=0.001) and type II diabetics (P=0.01). By multivariate analysis, there was a correlation between type I diabetes and IEL area independent of heart weight, plaque area, per cent necrotic core and per cent plaque calcification (P=0.0004). This analysis (per cent necrotic core, P=0.05; plaque area, P<0.0001; and heart weight, P=0.05) showed a positive correlation with IEL area. Clinical studies have shown that diabetes is associated with positive or a negative (absence) of remodelling (13,14). Our findings support the notion that diabetics are more likely to show positive remodelling. These data are consistent with previous findings from our laboratory that the necrotic core and macrophage infiltrates are associated with expansion of the IEL independent of plaque size (15).
INFLAMMATORY INFILTRATE IN DIABETICS
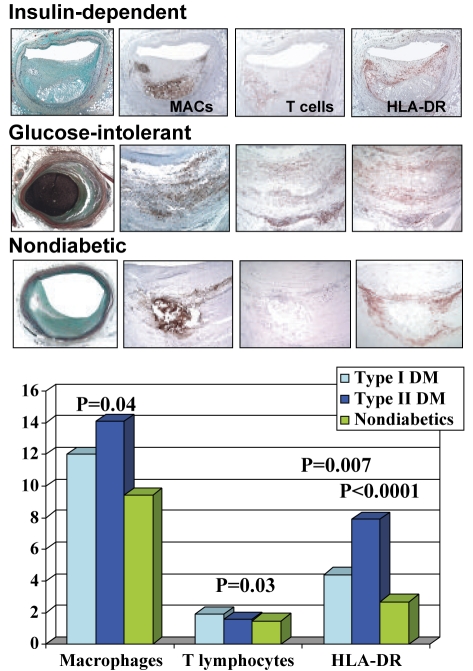
In our studies from the sudden death registry, the mean per cent plaque area composed of necrotic core was greater in type I (P=0.05) and type II (P=0.004) diabetics than in nondiabetics. Macrophage plaque area and T-cell infiltration were also significantly greater in diabetics than in nondiabetics (P=0.03), along with human leukocyte antigen-DR expression (Figure 1). The fact that T-cell infiltration was greater in type I diabetics is consistent with the fact that type I diabetes is an autoimmune disease with a common genetic susceptibility to other disorders, like autoimmune thyroiditis, which may also be of pathophysiological significance in coronary plaque pathology (Figure 1).
Figure 1).
Top Coronary lesions (fibroatheromas) illustrating the extent of inflammatory infiltrate (macrophages [MACs] and T cells [CD45RO]) and human leukocyte antigen-DR (HLA-DR) expression in patients with type I and II diabetes mellitus (DM) and nondiabetics. Bottom Bar graph showing a semiquantitative comparison of the extent of macrophage and T cell infiltration, and HLA-DR expression in coronary arteries from diabetics and nondiabetics. Plaque T cell infiltration was maximal in type I diabetics, while HLA-DR expression is significantly higher in type I and II diabetics
Cipollone et al (16) have shown that carotid plaques from diabetics have more macrophages, T lymphocytes and human leukocyte antigen-DR-positive cells (P<0.0001), more immunoreactivity for RAGE (P<0.0001), activated nuclear factor-kappa B, cyclooxygenase-2(COX-2)/membrane-associated protein eicosanoid and glutathione metabolism synthase-1 (mPGES-1), and matrix metalloproteinases (MMPs), increased (P<0.0001) gelatinolytic activity, reduced (P<0.0001) collagen content, and increased (P<0.0001) lipid and oxidized low density lipoprotein content (16). Interestingly, RAGE, COX-2/mPGES-1 and MMP expression were linearly correlated with plasma concentration of hemoglobin A1c (16). Therefore, in humans, carotid diabetic plaque RAGE overexpression, along with enhanced inflammatory reaction and COX-2/mPGES-1 expression in macrophages, may contribute to plaque destabilization by inducing culprit metalloproteinase expression. Also, experimental studies of murine models of diabetic aortic atherosclerosis have demonstrated that RAGE blockade by soluble RAGE decreases atheroma formation (17). The same group has also shown again in carotid atherosclerotic plaques that prostaglandin E2 pathway was significantly prevalent in symptomatic carotid plaques, whereas the platelet-derived growth factor 2 pathway was overexpressed in asymptomatic ones, and was associated with nuclear factor-kappa B inactivation and MMP-9 expression (18). In vitro COX-2 inhibition in monocytes was associated with reduced MMP-9 release only when the platelet-derived growth factor 2 pathway overcame prostaglandin E2. These results suggested to the researchers that COX-2 may have proinflammatory and anti-inflammatory properties as a function of expression of downstream prostaglandin H2 isomerases (18).
Recently, it has been demonstrated that extracellular newly identified RAGE-binding protein (EN-RAGE, or S100 A12) is a natural ligand for RAGE and is a proinflammatory cytokine expressed especially in macrophages (19). An association between soluble S100 proteins and human inflammatory bowel disease has been demonstrated (20). The interactions between S100 Ca2+-modulated proteins and RAGE receptors in the fine regulation of leukocyte trafficking and proliferation has been reviewed (21), but the role of EN-RAGE in atherosclerosis-related inflammation has not been studied. We applied immunohistochemical antibodies against RAGE and EN-RAGE (S100 A12) to coronary plaques of diabetics and nondiabetics (8). Although RAGE was found localized to macrophages, smooth muscle cells and endothelial cells, in both diabetics and nondiabetics, the overall expression of RAGE, as graded semiquantitatively, was significantly greater in diabetics (16.8±6.2 for diabetics and 10.0±6.1 for nondiabetics; P=0.004). Extensive RAGE staining was noted in the macrophages and necrotic core of diabetics and nondiabetics but the expression was dependent on the extent of cellular infiltration. Fewer smooth muscle cells expressing RAGE were observed but were greater in type II diabetics. RAGE expression was often associated with apoptotic smooth muscle cells and macrophages, whereas endothelial cells positive for RAGE were generally negative for apoptosis. EN-RAGE expression was most prominent in macrophages and, to a lesser degree, in smooth muscle cells in the core regions of plaques from diabetics.
The role of RAGE/EN-RAGE upregulation in atherosclerotic plaque is likely complex, and associated with both apoptosis and necrotic cell death; the precise triggers of the inflammatory response, which culminate in the formation in some plaques of large necrotic cores and in others, in fibrocalcific plaques, remain unknown.
SUMMARY
Diabetes is associated with greater inflammatory infiltrate (macrophages and T lymphocytes), larger necrotic core size and more diffuse atherosclerosis in the coronary arteries. Severe coronary atherosclerosis in diabetics is accompanied by the presence of healed myocardial infarction and cardiomegaly. Further studies are needed to better understand the relationship of hyperglycemia and insulin resistance to the greater induction of inflammation seen in atherosclerotic arteries and controlling of either or both would result in a decrease in inflammation in atherosclerotic plaques overtime.
REFERENCES
- 1.Amos AF, McCarty DJ, Zimmet P. The rising global burden of diabetes and its complications: Estimates and projections to the year 2010. Diabet Med. 1997;14(Suppl 5):S1–85. [PubMed] [Google Scholar]
- 2.Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP. The continuing epidemics of obesity and diabetes in the United States. JAMA. 2001;286:1195–200. doi: 10.1001/jama.286.10.1195. [DOI] [PubMed] [Google Scholar]
- 3.Silva JA, Escobar A, Collins TJ, Ramee SR, White CJ. Unstable angina. A comparison of angioscopic findings between diabetic and nondiabetic patients. Circulation. 1995;92:1731–6. doi: 10.1161/01.cir.92.7.1731. [DOI] [PubMed] [Google Scholar]
- 4.Schurgin S, Rich S, Mazzone T. Increased prevalence of significant coronary artery calcification in patients with diabetes. Diabetes Care. 2001;24:335–8. doi: 10.2337/diacare.24.2.335. [DOI] [PubMed] [Google Scholar]
- 5.Kornowski R, Mintz GS, Lansky AJ, et al. Paradoxic decreases in atherosclerotic plaque mass in insulin-treated diabetic patients. Am J Cardiol. 1998;81:1298–304. doi: 10.1016/s0002-9149(98)00157-x. [DOI] [PubMed] [Google Scholar]
- 6.Moreno PR, Murcia AM, Palacios IF, et al. Coronary composition and macrophage infiltration in atherectomy specimens from patients with diabetes mellitus. Circulation. 2000;102:2180–4. doi: 10.1161/01.cir.102.18.2180. [DOI] [PubMed] [Google Scholar]
- 7.Creager MA, Luscher TF, Cosentino F, Beckman JA. Diabetes and vascular disease: Pathophysiology, clinical consequences, and medical therapy: Part I. Circulation. 2003;108:1527–32. doi: 10.1161/01.CIR.0000091257.27563.32. [DOI] [PubMed] [Google Scholar]
- 8.Burke AP, Kolodgie FD, Zieske A, et al. Morphologic findings of coronary atherosclerotic plaques in diabetics: A postmortem study. Arterioscler Thromb Vasc Biol. 2004;24:1266–71. doi: 10.1161/01.ATV.0000131783.74034.97. [DOI] [PubMed] [Google Scholar]
- 9.Burke AP, Kolodgie FD, Farb A, et al. Healed plaque ruptures and sudden coronary death: Evidence that subclinical rupture has a role in plaque progression. Circulation. 2001;103:934–40. doi: 10.1161/01.cir.103.7.934. [DOI] [PubMed] [Google Scholar]
- 10.Mielke CH, Shields JP, Broemeling LD. Coronary artery calcium, coronary artery disease, and diabetes. Diabetes Res Clin Pract. 2001;53:55–61. doi: 10.1016/s0168-8227(01)00239-x. [DOI] [PubMed] [Google Scholar]
- 11.van Brussel BL, Plokker HW, Voors AA, et al. Multivariate risk factor analysis of clinical outcome 15 years after venous coronary artery bypass graft surgery. Eur Heart J. 1995;16:1200–6. doi: 10.1093/oxfordjournals.eurheartj.a061076. [DOI] [PubMed] [Google Scholar]
- 12.Takazawa K, Hosoda Y, Yamamoto T, Kawasaki S, Sasaguri S. Coronary artery bypass grafting. Late result of actual 10-years follow-up in 376 patients. Jpn J Thorac Cardiovasc Surg. 1999;47:110–5. doi: 10.1007/BF03217953. [DOI] [PubMed] [Google Scholar]
- 13.Gyongyosi M, Yang P, Hassan A, et al. Coronary risk factors influence plaque morphology in patients with unstable angina. Coron Artery Dis. 1999;10:211–9. doi: 10.1097/00019501-199906000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Weissman NJ, Sheris SJ, Chari R, et al. Intravascular ultrasonic analysis of plaque characteristics associated with coronary artery remodeling. Am J Cardiol. 1999;84:37–40. doi: 10.1016/s0002-9149(99)00188-5. [DOI] [PubMed] [Google Scholar]
- 15.Burke AP, Kolodgie FD, Farb A, Weber D, Virmani R. Morphological predictors of arterial remodeling in coronary atherosclerosis. Circulation. 2002;105:297–303. doi: 10.1161/hc0302.102610. [DOI] [PubMed] [Google Scholar]
- 16.Cipollone F, Iezzi A, Fazia M, et al. The receptor RAGE as a progression factor amplifying arachidonate-dependent inflammatory and proteolytic response in human atherosclerotic plaques: Role of glycemic control. Circulation. 2003;108:1070–7. doi: 10.1161/01.CIR.0000086014.80477.0D. [DOI] [PubMed] [Google Scholar]
- 17.Bucciarelli LG, Wendt T, Qu W, et al. RAGE blockade stabilizes established atherosclerosis in diabetic apolipoprotein E-null mice. Circulation. 2002;106:2827–35. doi: 10.1161/01.cir.0000039325.03698.36. [DOI] [PubMed] [Google Scholar]
- 18.Cipollone F, Fazia M, Iezzi A, et al. Balance between PGD synthase and PGE synthase is a major determinant of atherosclerotic plaque instability in humans. Arterioscler Thromb Vasc Biol. 2004;24:1259–65. doi: 10.1161/01.ATV.0000133192.39901.be. [DOI] [PubMed] [Google Scholar]
- 19.Hofmann MA, Drury S, Fu C, et al. RAGE mediates a novel proinflammatory axis: A central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889–901. doi: 10.1016/s0092-8674(00)80801-6. [DOI] [PubMed] [Google Scholar]
- 20.Lugering N, Stoll R, Schmid KW, et al. The myeloic related protein MRP8/14 (27E10 antigen) – usefulness as a potential marker for disease activity in ulcerative colitis and putative biological function. Eur J Clin Invest. 1995;25:659–64. doi: 10.1111/j.1365-2362.1995.tb01982.x. [DOI] [PubMed] [Google Scholar]
- 21.Donato R. S100: A multigenic family of calcium-modulated proteins of the EF-hand type with intracellular and extracellular functional roles. Int J Biochem Cell Biol. 2001;33:637–68. doi: 10.1016/s1357-2725(01)00046-2. [DOI] [PubMed] [Google Scholar]