Abstract
Atherosclerosis is a disease of blood vessel walls that is thought to be initiated as a reaction of insults to the endothelium. The complex sequence of cellular events that begins with focal inflammation leads to the accumulation of leukocytes in the subendothelial layer and unrestricted uptake of oxidized lipoproteins by macrophages and smooth muscle cells, leading to foam cell formation. Vascular endothelial cells do not undergo the foam cell transformation and do not accumulate cholesterol in atherosclerotic plaques to the same extent as macrophages or smooth muscle cells. However, vascular endothelial cells express receptors for oxidized lipoproteins, and have the biochemical pathways for sterol synthesis and receptor-mediated endocytosis of lipoproteins. Data from the authors’ laboratory show that high density lipoproteins but not lipid-free apolipoprotein A-I promote cellular cholesterol efflux in human umbilical vascular endothelial cells and human aortic endothelial cells. Gene expression microarrays were used to examine the differential expression of genes after cholesterol loading. While sterol regulatory element-binding protein-sensitive genes were downregulated, the authors identified a novel transporter, the ATP-binding cassette G1 (ABCG1) to be highly expressed in response to both cellular cholesterol loading and stimulation with the liver X receptor agonist 22-hydroxycholesterol. The ABCA1 gene and protein, the major modulator of cellular cholesterol efflux in macrophages and in peripheral and hepatic tissues, are only weakly expressed in human umbilical vascular endothelial cells and human aortic endothelial cells. These data suggest that endothelial cells maintain cholesterol homeostasis by downregulating cholesterol synthesis and low density lipoprotein receptors and by a cellular cholesterol efflux mechanism onto low-affinity but high-capacity high density lipoproteins. The role of ABC-type transporters, including ABCG1, requires further examination.
Keywords: ABC transporters, Cellular cholesterol efflux, Genes expression, Vascular endothelial cells
Abstract
L’athérosclérose est une maladie de la paroi des vaisseaux sanguins présumée s’amorcer en réaction à des agressions de l’endothélium. La séquence complexe d’événements cellulaires, qui débute par une inflammation localisée, entraîne l’accumulation de leucocytes dans la couche subendothéliale et la captation non-restrictive de lipoprotéines oxydées par des macrophages et cellules musculaires lisses conduisant à la formation de cellules spumeuses. Dans la plaque athéromateuse, les cellules endothéliales vasculaires ne subissent pas de transformation spumeuse et n’accumulent pas de cholestérol de manière comparable aux macrophages et cellules musculaires lisses. Cependant, ces cellules expriment des récepteurs pour les lipoprotéines oxydées et sont pourvues des voies métaboliques pour la synthèse de stérols et l’endocytose de lipoprotéines dépendant de récepteurs. Les données de ce laboratoire démontrent que les lipoprotéines de haute densité, mais non l’apolipoprotéine A-I sans lipide, provoque l’efflux de cholestérol cellulaire dans des cellules endothéliales vasculaires humaines d’origine ombilicale et aortique. L’expression de gènes sur micro-puces à ADN fut examinée pour évaluer l’expression différentielle des gènes dans des conditions de surcharge en cholestérol cellulaire. Alors que des gènes sensibles aux sterol regulatory element-binding proteins apparaissaient sous-régulés, les auteurs identifièrent un nouveau transporteur, l’ATP binding cassette G1 (ABCG1), hautement exprimé en réponse à une surcharge de cholestérol cellulaire, de même qu’en présence d’un agoniste du liver X receptor, le 22-hydroxycholestérol. Le gène et la protéine ABCA1, modulateur important de l’efflux du cholestérol cellulaire dans les macrophages, les tissus périphériques et hépatiques, paraissaient faiblement exprimés dans les cellules endothéliales vasculaires ombilicales et les cellules endothéliales aortiques humaines. Ces données suggèrent que les cellules endothéliales maintiennent une homéostasie du cholestérol par régulation négative de la synthèse de cholestérol et des récepteurs à lipoprotéines de basse densité, ainsi que par un mécanisme d’efflux de cholestérol cellulaire dépendant de lipoprotéines de haute densité de faible affinité mais grande capacité. Le rôle des transporteurs de type ABC, notamment l’ABCG1, exigent des investigations poussées.
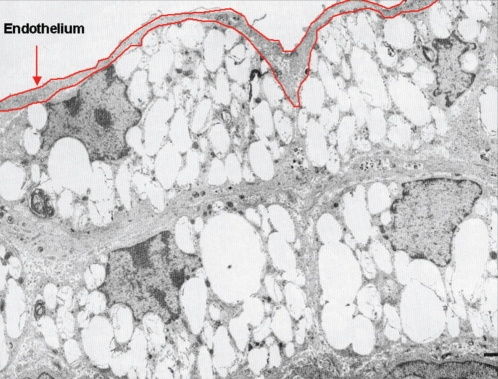
Vascular endothelial cells constitute a structurally simple but functionally sophisticated organ that regulates complex processes as diverse as hemostasis, fibrinolysis, inflammation, vessel tone and blood pressure, lipoprotein metabolism and angiogenesis (1). Although the endothelium consists of a monolayer of cells, it has an important aggregate surface area and mass, roughly equivalent to that of the liver. One of the primary events in atherosclerosis is the accumulation of cholesterol within specific and specialized cell types, especially the macrophage. However, vascular endothelial cells do not undergo the foam cell phenotypic change seen in macrophages and smooth muscle cells in atherosclerotic plaques, a change characterized by the unrestricted accumulation of cellular cholesterol (2) (Figure 1). Understanding the mechanisms by which vascular endothelial cells maintain tight cholesterol homeostasis may shed light on mechanisms of cellular sterol transport. High density lipoprotein (HDL)-dependent reverse cholesterol transport (RCT) is believed to be the most important mechanism for removal of cholesterol from extrahepatic cells. Although it has become generally accepted that RCT is one of the major mechanisms by which HDL may protect against coronary artery disease (CAD) (3), the mechanisms that protect the body from the pathological accumulation of lipid and cholesterol that cause CAD are not well understood. Studies have indicated that ATP-binding cassette transporter A1 (ABCA1) is a critical cell surface protein required for the transfer of cellular lipids and maintenance of HDL levels in plasma, and is likely important for the first step of RCT from peripheral tissues, including macrophages in the vessel wall (4).
Figure 1).
Electron microphotograph of an atherosclerotic plaque in a nonhuman primate fed an atherogenic diet (2). From reference 2 with permission
Surprisingly, we observed that the expression of ABCA1 in endothelial cells is very low and does not seem to be sensitive to treatment with cholesterol or 22-hydroxycholesterol, suggesting that ABCA1 does not mediate endothelial cholesterol efflux. Indeed, several mechanisms have been proposed for endothelial cholesterol excretion, such as cholesterol oxidation as an alternative and/or a complement to HDL-mediated RCT (5). At the same time, recent work in both cell culture and mice experiments indicates that HDL causes robust activation of endothelial nitric oxide synthase (eNOS), and this effect is mediated in endothelial cell caveolae by scavenger receptor (SR)-B1 through a process that requires apoliprotein (Apo) A-I binding followed by eNOS phosphorylation by protein kinase C and cyclic (c)AMP-dependent protein kinase (6). Thus, HDL, ApoA-I and the principal high-affinity HDL receptor, the SR-B1, are believed to be antiatherogenic and beneficial to the response to vascular injury. However, the fundamental mechanisms underlying these properties remain complex and not well understood.
We and others have identified ABCA1 as the key protein involved in active cellular lipid efflux (7) and consequently contributing to plasma HDL-cholesterol (8). Recent work in our laboratory has focused on the expression of potential candidate proteins that may be involved in the regulation of endothelial cholesterol. Although it is clear that endothelial cells have a significant expression of ABCG1 transporter and SR-B1, which are believed to play an important role in the RCT process, the molecular events involved in endothelial cholesterol homeostasis are still largely unknown.
BACKGROUND
ApoA-I, HDL and cardiovascular disease
Plasma levels of HDL are inversely proportional to the risk of CAD in humans. HDL and its major protein component ApoA-I are thought to accomplish this by sequestering cellular cholesterol in the periphery and transporting it to the liver, the major site of cholesterol catabolism and excretion into the bile. This RCT pathway likely works against the pathological accumulation of lipid in blood vessels that eventually leads to atherosclerosis, the major underlying cause of CAD. Lipid-poor forms of ApoA-I may be particularly efficient at promoting lipid efflux from cells in the vessel wall during the first step of this process. Once associated with phospholipids and cholesterol, ApoA-I forms disc-shaped HDL particles that are quickly remodelled in the circulation by lecithin:cholesterol acyltransferase, which esterifies a fatty acid onto cholesterol (9,10). The resulting cholesteryl ester is packaged into a hydrophobic core to form the spherical HDL2 and HDL3 particles that comprise the bulk of plasma HDL. HDL then interacts with SR-B1 to deliver cholesterol to the liver and adrenals for bile acid and steroid hormone synthesis, respectively. In addition, HDL can prevent oxidation of low density lipoprotein (LDL) (11), act as an anticoagulant (12) and have anti-inflammatory properties (13), all of which may contribute to the antiatherogenic effects of HDL.
ApoA-I-mediated lipid efflux
The transfer of cholesterol from peripheral cells to HDL occurs via two general mechanisms. The first is a bidirectional exchange of lipid between the cell membrane and small, phospholipid-rich HDL particles. This aqueous diffusion mechanism is subject to the laws of mass action. The second pathway is apo-mediated cholesterol efflux. In this mechanism, ApoA-I associates with the cell membrane, removes cellular lipids and leaves the cell as a nascent HDL particle. The pathway is rapid, unidirectional and may account for a significant portion of the cholesterol efflux that occurs in vivo (14).
ABC transporters subfamily and RCT
ABCA1:
The importance of ABCA1 in HDL biogenesis has been demonstrated by the identification of mutations in the ABCA1 gene locus such as the molecular defect of Tangier disease and familial HDL deficiency (7,15–17). These patients are characterized by extremely low HDL-C levels, caused by inadequate transport of cellular cholesterol and phospholipids to the extracellular space, leading to hypercatabolism of lipid-poor nascent HDL particles. The interaction between ApoA-I and ABCA1 is of critical importance for active ApoA-I lipidation (18,19). Transcriptional control of ABCA1 occurs via the nuclear hormone receptors liver X receptor (LxR) and retinoid X receptor (RxR) (20).
ABC G subfamily (ABCG1):
The ABCG1 gene and its putative gene product were independently recognized by two groups as the drosophila ‘white’ gene homologue. This half transporter contains one ATP-binding cassette and six trans-membrane domains. Human ABCG1 messenger (m)RNA is expressed primarily in the heart, spleen, brain, liver, lung, skeletal muscle, kidney and placenta (21). In human macrophages, elevated expression of ABCG1 mRNA was identified subsequent to cholesterol loading, oxidized LDL treatment, or upon the addition of LxR and RxR agonists. Thus, a growing body of evidence indicates ABCG1 involvement in lipid/sterol regulation (22,23). According to initial studies in human cells, endogenous ABCG1 was found to localize to both plasma membrane and internal membranes. On the other hand, human ABCG4 was identified independently in two laboratories based on its homology and close similarity to ABCG1 (21,24). ABCG4 gene expression was also found to be regulated by oxysterols and retinoids in a similar manner to ABCG1. ABCG1 and ABCG4 share 72% identity at the amino acid level. Based on high sequence similarity and the observation that both transporter mRNAs are upregulated upon stimulation of sterol pathways, ABCG1 and ABCG4 make good candidates for heterodimer partners. Expression of both ABCG1 and ABCG4 in insect cells suggest that the ABCG1/4 heterodimer may function as a functional unit (25). Interestingly, overexpression of ABCG1 in the liver of mice using recombinant ABCG1 vectors results in decreased plasma HDL levels and indicates that ABCG1 can modulate plasma lipoprotein levels in vivo. The potential importance of ABCG1 in RCT has not been definitively established.
SR-B1
The SR-B1 (also known as CLA-1 in humans) is an HDL receptor that can mediate selective uptake of HDL cholesteryl esters by cells, but can also promote cellular free cholesterol efflux to HDL and the reorganization of a cholesterol oxidase-sensitive pool of cellular cholesterol. In endothelial cells, SR-B1 is required for eNOS activation by HDL. SR-B1 has been localized within caveolae, cholesterol-rich plasma membrane microdomains that are abundant in endothelial cells (6,26).
Cellular cholesterol transport
Numerous processes, including de novo synthesis of cholesterol, lipoprotein uptake, cholesterol esterification and reverse cholesterol transport, control cellular cholesterol metabolism (9,27). Genes that govern RCT may be particularly important in determining the fate of cells within the atherosclerotic plaque.
The movement of cholesterol between various cell compartments and subcellular organelles is complex and poorly understood (27). Cholesterol uptake from plasma lipoproteins by receptor-mediated endocytosis (especially the LDL receptor) has been relatively well characterized. The formation of endocytic vesicles within the early endosomal compartment, the recycling of the LDL receptor in the endocytic recycling compartment and the formation of the late endosomal compartment have been previously described (27). Some free cholesterol generated within the endosome is recycled to the plasma membrane within the endocytic recycling compartment but most is thought to remain in the late endosome compartment. From there, free cholesterol must proceed to the endoplasmic reticulum (ER) for esterification or directly to the plasma membrane or the Golgi apparatus. Both vesicular and non-vesicular transport are thought to mediate this cholesterol transport from ER to the plasma membrane, with nonvesicular transport being predominant (27). The transport from ER to plasma membrane is dynamic and experimental data suggest that the plasma membrane pool cycles to the ER with a half-time of approximately 40 min (28), despite a large concentration gradient of cholesterol from the ER (with 0.1% to 2.0% of cellular cholesterol) to the plasma membrane (with 65% to 90% of cellular cholesterol).
The role of ABC transporters in mediating some of the intracellular cholesterol transport has been emphasized by elegant experiments showing the colocalization of ABCA1 to the late endosomal pathway and cycling to the plasma membrane (29). It is not established what role, if any, ABC transporters have in intracellular cholesterol transport, rather than in cholesterol and phospholipid efflux. Specific transport systems for cholesterol include the steroidogenic acute regulatory protein-related lipid transfer gene family, which includes at least 16 different members; their roles are currently under investigations (30). Specific steroidogenic acute regulatory protein-related lipid transfer gene family proteins (steroidogenic acute response proteins) are involved in mediating the transfer of cholesterol to mitochondria for oxidation. Other proteins, including sterol carrier protein-2, mediate the transport of various lipid molecules within the cytosol and are considered to be nonspecific lipid transfer proteins. The mechanisms by which cytosolic cholesterol shuttles from one compartment to the other is critical in determining cellular functions. Cholesterol exerts regulatory control of cholesterol synthesis and uptake via the sterol regulatory element-binding protein 2 (SREBP-2) pathway, while the SREBP-1a and SREBP-1c regulate fatty acid metabolism. Oxysterols regulate many processes, including cellular cholesterol efflux via the LxR/RxR pathway. Finally, cholesterol content modulates acyl-CoA:cholesterol acyltransferase activity within the ER. Understanding the mechanisms of cellular cholesterol homeostasis is therefore important in providing an insight into cellular function.
Vascular endothelial cells
Vascular endothelial cells provide an impermeable barrier between the blood and tissues. Their major role is to provide oxygen to tissues by vasodilation and to maintain the integrity of the blood vessel in case of injury. The mechanisms by which these functions are accomplished are complex (1,6). The interaction between plasma lipoproteins (especially HDL) and endothelial cells has recently been reviewed (31). Nitric oxide (NO) is produced by constitutive eNOS in response to fluid shear stress and exposure to the neurohumoral factors acetylcholine, bradykinin, serotonin and substance P. Although short-lived, NO is a potent vasodilator that induces smooth muscle relaxation through the activation of guanylate cyclase. NO also inhibits platelet aggregation and leukocyte adhesion to the endothelium. Inhibition of endothelium-dependent relaxation due to the decrease in the bioavailability of NO is perhaps the most prominent feature of endothelial dysfunction. An association between atherogenic lipids (LDL) and a decrease in acetylcholine-induced endothelium-dependent relaxation or even a paradoxical vasoconstrictive response is well documented. High HDL-C in humans, however, is associated with normal endothelium-dependent relaxation in response to acetylcholine. Recently, direct infusion of ApoA-I-containing proteoliposomes has been shown to improve flow-mediated vasodilatation in subjects with low HDL-C (32).
Matsuda et al (33) suggested that HDL acts by preventing the transfer of lysophosphatidylcholine (LPC) from oxidized LDL to endothelial cells. LPC is a major lipid component of oxidized LDL that can mimic many of the atherogenic effects of oxidized LDL, including the decrease in endothelium-dependent vasodilation. Incubation of endothelial cells with oxidized LDL depletes plasma membrane caveolae of cholesterol and translocates eNOS from caveolae to an internal membrane compartment, making eNOS insensitive to stimulation by acetylcholine. HDL opposes this phenomenon by donating cholesteryl esters to endothelial cell caveolae and preventing the defective localization of eNOS (34). eNOS-receptor uncoupling is believed to be important in the early progression of endothelial dysfunction, but in the long term it is thought that decreases in NO bioavailability are due to an increase in the generation of superoxide anion. Superoxide anions react with NO to form peroxynitrate, a less effective vasodilator than NO. HDL is an important antioxidant and may improve the bioavailability of NO by bolstering the antioxidant status of endothelial cells and decreasing the formation of superoxide anions (35).
The purpose of the present review is to examine potential mechanisms by which vascular endothelial cells resist cholesterol accumulation. We have previously reported (36) the potential role of a novel transporter, ABCG1, in vascular endothelial cells.
METHODS
Cell culture
Human umbilical vein endothelial cells (HUVEC) and human aortic endothelial cells (HAEC) were obtained from Cambrex, USA. Skin fibroblasts were obtained from normal volunteers and from patients with Tangier disease, as previously described (8). Endothelial cells were cultured in media obtained from Cambrex (EGM-2) as previously described (36), while fibroblasts were cultured in Dulbecco’s Modified Eagle Medium (Invitrogen, Canada) supplemented with 100 U/mL penicillin and 100 U/mL streptomycin (Invitrogen), nonessential amino acids (Invitrogen), and 10% fetal bovine serum (Wisent, Canada). Bovine serum albumin (BSA), cholesterol, 22(R)-hydroxycholesterol and 8-bromo-cAMP were from Sigma, Canada.
Northern blot
For the Northern blot, 250,000 HUVEC, HAEC and human skin fibroblasts were seeded on 100 mm cell culture plates. Upon reaching confluence, growth media was changed to serum-free media with 2 mg/mL BSA and either 20 μg/mL cholesterol in an ethanol carrier, 0.5 mM 8-bromo-cAMP, or ethanol carrier alone and incubated for 24 h. Total RNA was isolated with the RNeasy Mini Kit (Qiagen, Canada) and 15 μg of RNA was loaded on a 1% formaldehyde agarose gel. RNA was then transferred to a Hybond N+ membrane (GE Healthcare, USA) and hybridized with a 460 bp ABCG1 probe prepared by polymerase chain reaction amplification (forward = 5′-GTA TGG GTT CGA AGG GGT CAT-3, reverse = 5′-CAC CAA TCT GCC TAC ATC TTC-3′). ′ A phosphorimager (Molecular Dynamics, USA) was used to capture the 32P-image. The membrane was stripped and rehybridized with a ABCA1 probe prepared as described previously (36). The signal from a glyceraldehyde-3-phosphate dehydrogenase DNA probe was used to control for consistent loading. Radiolabelled probes were prepared using a nick translation kit and Redivue 32P-dCTP supplied by Amersham. ImageQuant 5.1 software (Molecular Dynamics) was used to quantify RNA expression.
Cholesterol efflux
Cells (100,000) were seeded in six-well plates. Cellular cholesterol pools were radiolabelled for 24 h with media containing 1 μCi/mL [3H]-cholesterol (Amersham). The cells were then washed and incubated for 24 h in serum-free media containing 1 mg/mL BSA and 20 μg/mL cholesterol, as previously described (8). Cholesterol efflux was determined after 24 h incubation with serum-free media containing 0.2 mg/mL BSA, serum-free media containing 50 μg/mL ApoA-I (Biodesign, USA), or serum-free media containing 50 μg/mL HDL3 (extracted from normal plasma by ultra-centrifugation). Cellular lipid efflux was expressed as the radioactivity counted in the media divided by the sum of the radioactivity in the media and the cells. Each experiment was performed in triplicate.
Immunoblotting
Cell lysates were harvested in lysis buffer (20 mM Tris, 0.5 mM EDTA, 0.5 mM EGTA, 0.5% Triton X-100 and complete protease inhibitors [Roche, Canada]), homogenized, and centrifuged for 10 min at 3500 rpm in a tabletop centrifuge at 4°C to pellet nuclei. The protein concentration of postnuclear supernatants was measured with the bicinchoninic acid protein assay (Pierce, USA). We loaded 45 μg of cell lysate onto an 8% polyacrylamide gel. After electrophoresis, proteins were transferred to an Immobilon P membrane (Millipore, Canada). Primary anti-SR-BI antibody (Novus Biologicals, USA) was used at a concentration of 1:750, anti-V5 antibody (Invitrogen) was used at 1:2500, anti-ABCA1 antibody was used at 1:500 (Novus Biologicals), anti-ABCG1 antibody was used at 1:100 (Santa Cruz Biotechnologies, USA) and anti-heat shock protein 70 antibody was used at 1:1000 (Transduction Laboratories, USA). Antimouse and antirabbit secondary antibodies coupled to horseradish peroxidase (Amersham) were used at a concentration of 1:5000, while the donkey antigoat secondary antibody (Santa Cruz Biotechnologies) was used at a concentration of 1:10,000 (36). Signal was revealed using an enhanced chemiluminescence kit (Pierce) and captured with X-Omat Blue XB-1 film (Kodak, USA). ImageQuant software was used to quantify protein signals. For the dose-response experiments, semiquantitative exposures were selected.
RESULTS
The pattern of gene expression in HUVEC in response to cellular cholesterol loading shows marked downregulation of genes involved in cholesterol synthesis and the LDL receptor. These genes are under the regulatory control of SREBP-2. In conditions of cholesterol excess, SREBP-2 production is decreased and the cholesterol biosynthetic pathway and the LDL receptor are downregulated. Conversely, a novel ABC transporter, ABCG1, is markedly upregulated in HUVEC and HAEC in response to cholesterol loading (36).
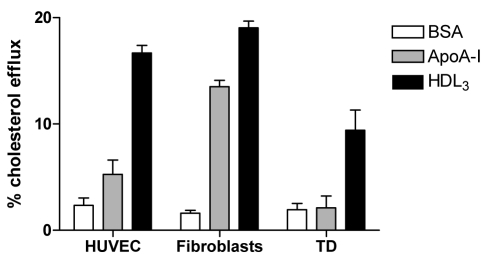
The mechanisms of cellular cholesterol efflux in HUVEC mediated by ApoA-I or HDL were investigated. Unlike human fibroblasts, lipid-free ApoA-I does not promote cholesterol efflux from HUVEC (Figure 2) and HAEC (data not shown). HDL3, however, is capable of mediating cellular cholesterol efflux in a time-dependent fashion. To investigate potential genes involved in lipid transport and homeostasis in HUVEC, the authors examined sterol-sensitive genes in cells grown under basal conditions and after cholesterol loading. They have previously reported the identification of the ABCAG1 gene in RNA expression microarrays in HUVEC, and also determined that ABCG1 is regulated by sterols and confirmed these results by northern blot and immunoblotting (Figure 3). Subsequently, other groups have reported the importance of hydroxysterols in the regulation of ABC transporters in general, and of ABCG1 specifically (37).
Figure 2).
Cellular cholesterol efflux in human umbilical vein endothelial cells (HUVEC) and fibroblasts. Cells were loaded with 3H cholesterol (20 μg/mL) and incubated in the presence of apoliprotein (apo) AI (50 μg/mL) or high density lipoprotein (HDL3) (50 μg/mL), as previously described (11,20). BSA Bovine serum albumin; TD Tangier disease
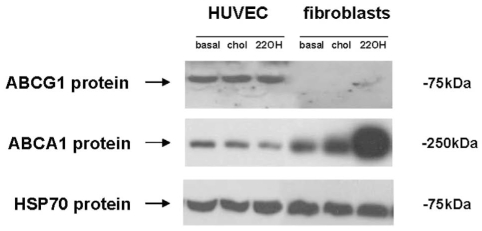
Figure 3).
ATP-binding cassette transporter A1 (ABCA1) and ABCG1 protein expression in human umbilical vein endothelial cells (HUVEC) and fibroblasts. Cells were stimulated with cholesterol (chol) (20 μg/mL) or 22(R)-hydroxycholesterol (22OH) (2.5 μg/mL), as previously described (20) (ligand for the liver-specific receptor liver X receptor that upregulates the ABCA1 and ABCG1 transporters). HSP70 Heat shock protein 70
The authors studied the regulation of ABCG1 and ABCA1 in HAEC and HUVEC cells. They treated endothelial cells and human fibroblasts with free cholesterol and 22(R)-hydroxycholesterol, a ligand for the LxR transcriptional regulatory protein. LxR binds to oxysterols and heterodimerizes with the retinoic acid receptor RxR, which uses 9-cis-retinoic acid as its ligand. Total RNA from cells was analyzed by Northern blot using specific radiolabelled ABCA1 and ABCG1 DNA probes (36). In HUVEC, cholesterol and 22(R)-hydroxycholesterol induced ABCG1 mRNA and protein expression, while ABCA1 expression was low or absent throughout. This pattern was reversed in fibroblasts, where ABCA1 is highly induced by cholesterol and 22(R)-hydroxycholesterol, but ABCG1 levels remained low. A similar pattern was observed in HAEC (not shown).
SR-B1 has been shown to play a major role in vascular endothelial cells and modulates the expression of eNOS. SR-B1 has been shown to mediate selective uptake of HDL-derived cholesterol esters. Previous studies (38) have shown that SR-B1, under certain conditions, can also mediate cellular efflux of free cholesterol onto HDL particles. The authors have previously shown that cholesterol loading of HUVEC leads to a downregulation of SR-B1 protein expression. Furthermore, blocking SR-B1 did not affect HDL-mediated cellular cholesterol efflux in HUVEC (36).
DISCUSSION
A key feature of atherosclerosis is the accumulation of cholesterol in macrophages that have become foam cells and in smooth muscle cells that have adapted their phenotype to the local environment by accumulating oxidized lipoproteins by receptor-mediated endocytosis. Endothelial cells that are exposed to plasma and subendothelial natural and oxidized lipoproteins do not undergo the unrestricted accumulation of cholesteryl esters. This does not occur despite the expression of receptors for native and oxidized lipoproteins. One possible explanation for this observation is that endothelial cells are able to shut down cellular cholesterol synthesis. We have shown that this is the case in response to cholesterol loading by gene expression microarrays. This mechanism, however, is not sufficient in macrophages and smooth muscle cells to prevent sterol accumulation. We postulated, therefore, that endothelial cells must have developed a capacity to efflux lipids to a high-capacity (HDL) pool in plasma.
The major cellular cholesterol and phospholipid transporter, ABCA1, is not expressed to a significant extent in HUVEC and HAEC; furthermore, ApoA-I does not appear to mediate cellular cholesterol efflux. In contrast, we have identified a novel ABC transporter, ABCG1, as a potential mechanism for cellular cholesterol efflux. In the present study, we show that HDL3, but not ApoA-I, mediates cellular cholesterol efflux in HUVEC. We have also shown that ABCG1 is expressed in HUVEC in response to cholesterol loading and to 22-hydroxycholesterol (a member of the hydroxysterol family).
Sterol accumulation in vascular endothelial cells is deleterious and leads to a loss of vasodilatory function, with a decrease in eNOS expression, possibly related to abnormal caveolae structure (6). We speculate, therefore, that vascular endothelial cells have developed two important mechanisms to prevent sterol accumulation. First, cholesterol synthesis is downregulated, as shown by the mRNA expression data previously reported (36). This mechanism is mediated via the SREBP-2 pathway, which, in the presence of cellular cholesterol, is markedly decreased and leads to a downregulation in the genes involved in sterol biosynthesis. The same mechanism is also responsible for the decrease in LDL-R expression, the classic pathway for native LDL particle endocytosis.
We now postulate that a second major mechanism, mediated via the LxR/RxR pathway of transcriptional regulators, leads to cellular cholesterol efflux onto HDL (but not ApoA-I). We provide preliminary data that this can be, in part, mediated via the ABC transporter ABCG1. ABCG1, however, is a half-transporter and must either dimerize or heterodimerize with another half-transporter. It is likely that ABCG1 forms functional homodimers. Another potential candidate for heterodimerization is ABCG4, as shown by Cserepes et al (25). However, ABCG4 is expressed predominantly in the brain and its pattern of expression in non-neuronal vascular tissues is not known. Another possibility to explain the HDL-mediated cellular cholesterol efflux is either another transporter or a novel, as yet unidentified, cellular cholesterol efflux mechanism.
Unravelling the mechanisms by which specific cell types avoid the accumulation of cholesterol may have therapeutic implications to prevent the formation of foam cells.
REFERENCES
- 1.Assmann G, Nofer JR. Atheroprotective effects of high-density lipoproteins. Annu Rev Med. 2003;54:321–41. doi: 10.1146/annurev.med.54.101601.152409. [DOI] [PubMed] [Google Scholar]
- 2.Faggiotto A, Ross R, Harker L. Studies of hypercholesterolemia in the nonhuman primate. 1. Changes that lead to fatty streak formation. Arteriosclerosis. 1984:4323–40. doi: 10.1161/01.atv.4.4.323. [DOI] [PubMed] [Google Scholar]
- 3.Tall AR. Plasma cholesteryl ester transfer protein. J Lipid Res. 1993;34:1255–74. [PubMed] [Google Scholar]
- 4.Brewer HB, Jr, Santamarina-Fojo S. 2003. New insights into the role of the adenosine triphosphate-binding cassette transporters in high-density lipoprotein metabolism and reverse cholesterol transport Am J Cardiol 2003913E–11E. [DOI] [PubMed] [Google Scholar]
- 5.Babiker A, Andersson O, Lund E, Xiu RJ, Deeb S, Reshef A, Leitersdorf E, Diczfalusy U, Bjorkhem I. Elimination of cholesterol in macrophages and endothelial cells by the sterol 27-hydroxylase mechanism. Comparison with high-density lipoprotein-mediated reverse cholesterol transport. J Biol Chem. 1997;272:26253–61. doi: 10.1074/jbc.272.42.26253. [DOI] [PubMed] [Google Scholar]
- 6.Mineo C, Shaul PW. HDL stimulation of endothelial nitric oxide synthase: A novel mechanism of HDL action. Trends Cardiovasc Med. 2003;13:226–31. doi: 10.1016/s1050-1738(03)00098-7. [DOI] [PubMed] [Google Scholar]
- 7.Brooks-Wilson A, Marcil M, Clee SM, et al. Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat Genet. 1999;22:336–45. doi: 10.1038/11905. [DOI] [PubMed] [Google Scholar]
- 8.Marcil M, Bissonnette R, Vincent J, Krimbou L, Genest J. Cellular phospholipid and cholesterol eflux in high-density lipoprotein deficiency. Circulation. 2003;107:1366–71. doi: 10.1161/01.cir.0000056764.53152.f9. [DOI] [PubMed] [Google Scholar]
- 9.Genest J, Gotto A, Libby P. Lipoprotein disorders and CAD. In: Braunwald E, Lippb P, editors. Braunwald’s Heart Disease. New York: Saunders; 2004. pp. 1013–33. [Google Scholar]
- 10.Jonas A. Lecithin-cholesterol acyltransferase in the metabolism of high-density lipoproteins. Biochim Biophys Acta. 1991;1084:205–20. doi: 10.1016/0005-2760(91)90062-m. [DOI] [PubMed] [Google Scholar]
- 11.Klimov AN, Gurevich VS, Nikiforova AA, et al. Antioxidative activity of high density lipoproteins in vivo. Atherosclerosis. 1993;100:13–8. doi: 10.1016/0021-9150(93)90063-z. [DOI] [PubMed] [Google Scholar]
- 12.Epand RM, Stafford A, Leon B, et al. HDL and apolipoprotein A-I protect erythrocytes against the generation of procoagulant activity. Arterioscler Thromb. 1994;14:1775–83. doi: 10.1161/01.atv.14.11.1775. [DOI] [PubMed] [Google Scholar]
- 13.Cockerill GW, Huehns TY, Weerasinghe A, et al. Elevation of plasma high-density lipoprotein concentration reduces interleukin-1-induced expression of E-selectin in an in vivo model of acute inflammation. Circulation. 2001;103:108–12. doi: 10.1161/01.cir.103.1.108. [DOI] [PubMed] [Google Scholar]
- 14.Hara H, Yokoyama S. Interaction of free apolipoproteins with macrophages. Formation of high density lipoprotein-like lipoproteins and reduction of cellular cholesterol. J Biol Chem. 1991;266:3080–6. [PubMed] [Google Scholar]
- 15.Marcil M, Brooks-Wilson A, Clee SM, et al. Mutations in the ABC1 gene in familial HDL deficiency with defective cholesterol efflux. Lancet. 1999;354:1341–6. doi: 10.1016/s0140-6736(99)07026-9. [DOI] [PubMed] [Google Scholar]
- 16.Rust S, Rosier M, Funke H, et al. Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nat Genet. 1999;22:352–5. doi: 10.1038/11921. [DOI] [PubMed] [Google Scholar]
- 17.Bodzioch M, Orso E, Klucken J, et al. The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nat Genet. 1999;22:347–51. doi: 10.1038/11914. [DOI] [PubMed] [Google Scholar]
- 18.Denis M, Haidar B, Marcil M, Bouvier M, Krimbou L, Genest J., Jr Molecular and cellular physiology of apolipoprotein A-I lipidation by the ATP-binding cassette transporter A1 (ABCA1) J Biol Chem. 2004;279:7384–94. doi: 10.1074/jbc.M306963200. [DOI] [PubMed] [Google Scholar]
- 19.Denis M, Haidar B, Marcil M, Bouvier M, Krimbou L, Genest J. Characterization of oligomeric human ATP binding cassette transporter A1. Potential implications for determining the structure of nascent high density lipoprotein particles. J Biol Chem. 2004;279:41529–36. doi: 10.1074/jbc.M406881200. [DOI] [PubMed] [Google Scholar]
- 20.Repa JJ, Turley SD, Lobaccaro JA, et al. Regulation of absorption and ABC1-mediated efflux of cholesterol by RxR heterodimers. Science. 2000;289:1524–9. doi: 10.1126/science.289.5484.1524. [DOI] [PubMed] [Google Scholar]
- 21.Oldfield S, Lowry C, Ruddick J, Lightman S. ABCG4: A novel human white family ABC-transporter expressed in the brain and eye. Biochim Biophys Acta. 2002;1591:175–9. doi: 10.1016/s0167-4889(02)00269-0. [DOI] [PubMed] [Google Scholar]
- 22.Schmitz G, Langmann T, Heimerl S. Role of ABCG1 and other ABCG family members in lipid metabolism. J Lipid Res. 2001;42:1513–20. [PubMed] [Google Scholar]
- 23.O’Connell BJ, Genest J., Jr High-density lipoproteins and endothelial function. Circulation. 2001;104:1978–83. doi: 10.1161/hc3901.096667. [DOI] [PubMed] [Google Scholar]
- 24.Annilo T, Tammur J, Hutchinson A, Rzhetsky A, Dean M, Allikmets R. Human and mouse orthologs of a new ATP-binding cassette gene, ABCG4. Cytogenet Cell Genet. 2001;94:196–201. doi: 10.1159/000048816. [DOI] [PubMed] [Google Scholar]
- 25.Cserepes J, Szentpetery Z, Seres L, et al. Functional expression and characterization of the human ABCG1 and ABCG4 proteins: Indications for heterodimerization. Biochem Biophys Res Commun. 2004;320:860–7. doi: 10.1016/j.bbrc.2004.06.037. [DOI] [PubMed] [Google Scholar]
- 26.Rigotti A, Miettinen HE, Krieger M. The role of the high-density lipoprotein receptor SR-BI in the lipid metabolism of endocrine and other tissues. Endocr Rev. 2003;24:357–87. doi: 10.1210/er.2001-0037. [DOI] [PubMed] [Google Scholar]
- 27.Soccio RE, Breslow JL. Intracellular cholesterol transport. Arterioscler Thromb Vasc Biol. 2004;24:1150–60. doi: 10.1161/01.ATV.0000131264.66417.d5. [DOI] [PubMed] [Google Scholar]
- 28.Lange Y. Cholesterol movement from plasma membrane to rough endoplasmic reticulum. Inhibition by progesterone. J Biol Chem. 1994;269:3411–4. [PubMed] [Google Scholar]
- 29.Neufeld EB, Remaley AT, Demosky SJ, et al. Cellular localization and trafficking of the human ABCA1 transporter. J Biol Chem. 2001;276:27584–90. doi: 10.1074/jbc.M103264200. [DOI] [PubMed] [Google Scholar]
- 30.Soccio RE, Breslow JL. StAR-related lipid transfer (START) proteins: Mediators of intracellular lipid metabolism. J Biol Chem. 2003;278:22183–6. doi: 10.1074/jbc.R300003200. [DOI] [PubMed] [Google Scholar]
- 31.Assmann G, Gotto AM., Jr HDL cholesterol and protective factors in atherosclerosis. Circulation. 2004;109:III8–14. doi: 10.1161/01.CIR.0000131512.50667.46. [DOI] [PubMed] [Google Scholar]
- 32.Bisoendial RJ, Hovingh GK, Levels JH, et al. Restoration of endothelial function by increasing high-density lipoprotein in subjects with isolated low high-density lipoprotein. Circulation. 2003;107:2944–8. doi: 10.1161/01.CIR.0000070934.69310.1A. [DOI] [PubMed] [Google Scholar]
- 33.Matsuda Y, Hirata K, Inoue N, et al. High density lipoprotein reverses inhibitory effect of oxidized low density lipoprotein on endothelium-dependent arterial relaxation. Circ Res. 1993;72:1103–9. doi: 10.1161/01.res.72.5.1103. [DOI] [PubMed] [Google Scholar]
- 34.Uittenbogaard A, Shaul PW, Yuhanna IS, Blair A, Smart EJ. High density lipoprotein prevents oxidized low density lipoprotein-induced inhibition of endothelial nitric-oxide synthase localization and activation in caveolae. J Biol Chem. 2000;275:11278–83. doi: 10.1074/jbc.275.15.11278. [DOI] [PubMed] [Google Scholar]
- 35.Tomasian D, Keane JF, Vita JA. Antioxidants and the bioactivity of endothelium-derived nitric oxide. Cardiovasc Res. 2000;47:426–35. doi: 10.1016/s0008-6363(00)00103-6. [DOI] [PubMed] [Google Scholar]
- 36.O’Connell BJ, Denis M, Genest J. Cellular physiology of cholesterol efflux in vascular endothelial cells. Circulation. 2004;110:2881–8. doi: 10.1161/01.CIR.0000146333.20727.2B. [DOI] [PubMed] [Google Scholar]
- 37.Schmitz G, Langmann T. Transcriptional regulatory networks in lipid metabolism control ABCA1 expression. Biochim Biophys Acta. 2005;1735:1–19. doi: 10.1016/j.bbalip.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 38.Yancey PG, Bortnick AE, Kellner-Weibel G, de la Llera-Moya M, Phillips MC, Rothblat GH. Importance of different pathways of cellular cholesterol efflux. Arterioscler Thromb Vasc Biol. 2003;23:712–9. doi: 10.1161/01.ATV.0000057572.97137.DD. [DOI] [PubMed] [Google Scholar]