Abstract
The understanding of the pathophysiology of atherosclerosis has advanced greatly in the past decade. Cardiovascular risk factors increase the likelihood of an adverse event by having a detrimental effect on the blood vessel wall. Abnormal interactions among cholesterol, inflammatory mediators, platelets and the vascular wall lead to atherogenesis and cardiac events. In an effort to better understand this process, develop surrogate end points for clinical trials and, ultimately, better risk stratify individuals, a variety of measures of arterial function have been studied. These include measures of endothelial health and arterial compliance. The current paper reviews the various techniques available for the study of vascular health. While not yet routinely used for clinical care, these measurements provide important insights into the pathophysiology and treatment of atherosclerosis.
Keywords: Arterial stiffness, Atherosclerosis, Compliance, Endothelium
Abstract
La compréhension de la physiopathologie de l’athérosclérose a beaucoup progressé depuis dix ans. Les facteurs de risque cardiovasculaire augmentent la possibilité d’événement indésirable en raison de leur effet néfaste sur la paroi des vaisseaux sanguins. Des interactions anormales entre le cholestérol, les médiateurs inflammatoires, les plaquettes et la paroi vasculaire provoquent une athérogénèse et des troubles cardiaques. Afin de mieux comprendre ce processus, d’élaborer des paramètres ultimes de substitution en vue d’essais cliniques et, en bout de ligne, de mieux stratifier les individus selon leur risque, diverses mesures de la fonction artérielle ont été étudiées. Ces mesures incluent celles de la santé endothéliale et de la compliance artérielle. Le présent article permet d’analyser les diverses techniques disponibles pour étudier la santé vasculaire. Bien qu’elles ne soient pas encore utilisées systématiquement en soins cliniques, ces mesures donnent d’importants aperçus de la physiopathologie et du traitement de l’athérosclérose.
The understanding of the pathophysiology of atherosclerosis and its vascular complications has increased dramatically over the past decade. Numerous measurable biomarkers play a role in the atherosclerosis process, and are associated with clinically important vascular events. Not only has their evaluation advanced our knowledge of the atherosclerotic process but it has also accelerated a search for new diagnostic tools to aid in risk stratification. Putative factors include soluble biomarkers and imaging assessments of vascular structure and function. The endothelium plays a key role in vascular homeostasis through the release of a variety of paracrine factors that interact with platelets, inflammatory cells and the vessel wall. The endothelium’s central location allows it to sense and respond to a variety of perturbations. Endothelial dysfunction is an early marker of disease. Risk factors can also affect mechanical properties such as arterial stiffness, which is now recognized as a major contributor to systolic hypertension in elderly people. Methods of assessing endothelial function and arterial stiffness are available for clinical research and recent evidence suggests that these measures may provide important prognostic information.
ATTRIBUTES OF A SURROGATE BIOMARKER
Epidemiological studies, such as Framingham, have allowed longitudinal assessment of cardiovascular events and the development of simple scoring tables for risk calculation (1,2). These factors involved include blood pressure, lipid profile, smoking status and age. More recently, many factors thought to be associated with the atherosclerotic process have been studied. Some of these have been shown to correlate with clinical outcomes and have been deemed biomarkers (3,4). However, association does not necessarily imply causation and, due to a variety of limitations, most of these markers will not be useful for the clinical evaluation of risk or drug therapy (5). To become clinically important, a biomarker must fulfill the criteria for a surrogate marker. A surrogate substitutes for clinically important end points with respect to how an individual feels, functions or survives. Classic examples include blood pressure and low density lipoprotein cholesterol. These are targets of treatment. A surrogate should meet the following criteria:
Adds independent information above currently used criteria;
Is reliably and reproducibly measured;
Accounts for a significant proportion of disease risk;
Provides good predictive value;
Is reasonably associated with the underlying pathophysiology;
If it is to be used in risk prevention, is present before the clinical appearance of the outcome; and
Is available and practical for widespread application (5–8).
Surrogates must also stand the rigours of time and be well validated in multiple studies in a wide range of patient populations. The new biomarker that most closely meets these criteria is C-reactive protein (4,9–11).
ARTERIAL STIFFNESS
Blood pressure has long been known to be an important risk factor for coronary and cerebrovascular disease. While initial work concentrated on diastolic pressure, more recently, it has been recognized that systolic and pulse (difference between systolic and diastolic) pressures play a more important role, particularly with advancing age (12). Systolic pressure is influenced by arterial stiffness and increases continuously with age. It has long bothered physiologists that the shape of the waveforms in the proximal aorta differ markedly for pressure and flow. In the prevailing frequency-domain theory of blood pressure, pressure and flow waveforms are separated into mean and oscillatory components. This leads to the concept of reflected (backward-going) waves that can reinforce a forward-moving pressure wave (13–15). Recent elegant work by Wang et al (16) has suggested an alternative hypothesis. The arterial system can be thought of as a Windkessel type reservoir in which the level is controlled by peripheral resistance. A second component is very closely related to forward-travelling flow waves as a result of ventricular ejection (16). Aortic pressure is then the instantaneous summation of the reservoir pressure and the effects of the flow wave. In a situation in which velocity is increased within the system (increased arterial stiffness), a backward-travelling reflected wave could arrive back in the aorta in systole, further augmenting systolic pressure. This has a detrimental effect by increasing cardiac afterload and decreasing diastolic coronary filling. Alterations in the speed at which waves travel and the change in the arterial contour serve as the basis of evaluating structural properties of the arterial tree (17).
Arterial stiffness is determined in large part by the elastin to collagen ratio in their walls. The proximal large arteries (aorta and major branches) are quite elastic due to high elastin content. Aging leads to progressive arterial stiffness as a result of elastic fibre degeneration and atherosclerosis development. In addition, the elasticity of a given arterial segment is not constant. With increasing distending pressure, there is recruitment of collagen fibres leading to greater stiffness. Increases in heart rate can also change arterial characteristics and must be considered in these measurements as well (18,19).
Noninvasive methods of assessing arterial stiffness
Relating change in volume to distending pressure:
There are many methods that have been used to measure arterial stiffness in vivo in humans (19). This discussion is restricted to those of a noninvasive nature. Arterial compliance is the relationship between change in vessel diameter (or area) for a given change in pressure. Bank et al (18) have described an ultrasound technique used to measure changes in brachial artery area while changing pressure over a wide range, with progressive occlusion with a blood pressure cuff. Radial artery waveforms are measured with applanation tonometry. This allowed the calculation of the following parameters:
Compliance is the first derivative of area versus pressure curve
Circumferential wall stress = pressure × midwall radius/intimal-medial thickness
Circumferential strain = midwall radius/unstressed midwall radius (radius at 0 distending pressure)
Young’s incremental elastic modulus (Einc) = 0.75 × stress/strain.
Pulse wave velocity (PWV) = [(Einc × intimal-medial thickness)/(2 blood density × radius)]1/2 by the Moens-Korteweg equation.
In the study by Bank et al (18), nitroglycerin increased the compliance of the artery, decreased wall stress and decreased the elastic modulus (decreased stiffness). While this technique is reproducible and elegant, it is somewhat limited in that considerable expertise is required for the various measurements undertaken. In addition, it has not been extensively used in patients with risk factors to determine if differences can be detected in arterial compliance among different risk groups. Kinlay et al (20) used a modification of this technique and determined that nitric oxide (NO) is a regulator of arterial stiffness. This suggests an interplay between endothelial-derived factors that alter tone and structural properties of the arterial wall. Some investigators have assessed stiffness parameters by looking at diameter changes between systole and diastole (during a cardiac cycle). Ultrasound and tonometry would allow these measurements. While less physiological, these studies are easier to perform and the carotid artery can be studied directly (21). When applied at a population level, it has been clearly demonstrated that arterial stiffness increases with age (22,23). Magnetic resonance imaging has the potential to be useful in the measurement of aortic distensibility as well (24). A limitation of all of these approaches is that peripheral rather than central blood pressure was used in the calculation of stiffness. This can only be overcome by invasive approaches that use catheters within the aorta.
PWV:
While PWV can be measured by the above approach, it can also be directly measured by simultaneous assessment of arterial waveforms in two locations. Velocity is then calculated as distance/time. Both applanation tonometry and Doppler ultrasound can be used to obtain this measure and can be applied to population-based studies. It has been widely studied and is a reproducible measure (25,26). The carotid and femoral arteries are often used. PWV increases directly with increasing arterial stiffness. Increases in distending pressure increase PWV and, therefore, levels of blood pressure need to be taken into account when comparing groups. The effect of heart rate is less clear but may be a confounding factor as well (17).
Systolic contour analysis:
One of the predictable effects of an increase in PWV is augmentation of systolic pressure due to reflected pressure waves. A late systolic wave can be detected by contour analysis of a peripheral or carotid artery. Applanation tonometry with software algorithms has allowed widespread study of systolic contour. An augmentation index (AIx) is the ratio of the pulse pressure of the reflected wave divided by the overall pulse pressure. Karamanoglu et al (27) developed a method of studying peripheral arteries, using a generalized transfer function, a derived aortic waveform is generated. The SphygmoCor device (Atcor Medical, Australia) derives an AIx and can also calculate ejection duration (19). While the general transfer function has been validated (28), there is much controversy over the reliability of this measurement for the determination of arterial stiffness (29). The AIx is increased with increasing blood pressure and decreased heart rate and by vasoactive drugs (30). Wilkinson et al (31,32) were able to demonstrate in healthy subjects that NO inhibition increased the AIx, again suggesting functional regulation of large artery stiffness by the endothelium. Some of the limitations of the generalized transfer function can be overcome by measuring contour at the carotid artery (26,33).
Diastolic contour analysis:
Based on the Windkessel model of blood pressure, the decay of the reservoir pressure in part relates to peripheral resistance. An exponential decay curve represents capacitance (large) artery compliance and is referred to as C1. The C2 component provides a measure of small artery compliance (34,35). Again, peripheral arterial tonometry can be used to obtain the waveform for further calculations. Of the methods described, there is probably the most uncertainty in the reliability of this methodology.
Relationship of arterial stiffness to risk factors and their treatment
Since systolic pressure and pulse pressure increase with increasing age, it would be surprising if there were not a very important effect of age on measures of arterial stiffness (36). Indeed, this has been found in almost all reported studies. In a recent study of healthy Framingham participants, Mitchell et al (26) assessed PWV and carotid contour. Central arterial stiffness increased with increasing age to a greater degree than peripheral arterial stiffness (which is usually higher than aortic stiffness). Interestingly, reflected wave amplitude (AIx) was only weakly related to age. This suggests that central aortic stiffness and forward wave amplitude, as opposed to reflected waves, are the primary mechanisms for the increased central systolic and pulse pressure of aging. This increase in forward-going waves from the central aorta, not dampened by a concomitant increase in reflected waves to buffer the effect, could result in damaging levels of pressure in a variety of microvascular beds including the brain and kidney (37). Studies previous to this have shown a stronger relationship between the AIx and age (38).
The relationship between blood pressure and arterial stiffness is more difficult to ascertain because blood pressure is a major covariate in the various measurements of stiffness. That being said, PWV and the AIx have been shown to be predictors of cardiovascular events in hypertensive subjects (25). In the previously mentioned study by Mitchell et al (26), mean arterial pressure was a strong predictor of PWV and the AIx. Other factors associated with increased aortic stiffness include diabetes (39), hypercholesterolemia (40) and end stage renal disease (41). Recent studies have also demonstrated an association between PWV and C-reactive protein, independent of age and mean pressure (42).
The effect of cardiovascular drugs on measures of arterial stiffness has been extensively studied and reviewed (17,36). Many of the agents tested also lower blood pressure, and this effect must be differentiated from any effects on structural remodelling or improvement in endothelial function that would favourably affect arterial stiffness. Agents that decrease arterial stiffness include nitroglycerin (18,20), angiotensin-converting enzyme inhibitors and angiotensin receptor blockers (43), and calcium channel blockers (44). The data for beta-blockers are not nearly as clear. Drugs that have favourable effects on outcomes and endothelial function, such as statins, have been shown to decrease arterial stiffness, but further studies are needed in this area (45,46).
Prognostic implications of alterations in arterial stiffness
A biomarker can only truly elevate itself to the status of a surrogate marker when it has been shown to be an independent predictor of cardiovascular events. While measures of arterial stiffness are pertinent research end points, their use as surrogate markers require further study. That said, several longitudinal studies by research groups in France have suggested an independent prognostic importance of measures of arterial stiffness. In a cohort of 242 subjects with end stage renal disease, Blacher et al (47) measured PWV and the AIx (carotid artery). Both measures were shown to be independent predictors of mortality in this cohort. Age was the other major contributor to outcomes (33,41,47). The same group also followed a large cohort of hypertensive individuals (n=710). PWV was higher in a group with atherosclerosis, and at a particular age, PWV was the best predictor of cardiovascular mortality (25). In a second large hypertensive cohort, Boutouyrie et al (48) and Laurent et al (49,50) were able to show that PWV independent of age and pulse pressure was a predictor of coronary events, stroke and all-cause mortality. The RRs were between 1.4 and 2.1. In a recent cross-sectional study, Weber et al (51) demonstrated a relationship between the AIx and angiographically proven coronary disease. Finally, a study of elderly men (23) established a relationship between carotid plaque burden and all cause mortality. However, carotid arterial stiffness (Young’s modulus) added very little to risk predictions in this cohort. The data to date, while intriguing, certainly do not firmly establish an important prognostic role for measures of arterial stiffness. Ongoing observations from large randomized studies will be awaited with much interest.
ENDOTHELIAL DYSFUNCTION
The endothelium is a single-cell lining covering the internal surface of blood vessels. The strategic location of the endothelium allows it to sense changes in hemodynamic signals and respond by releasing a number of substances. A balance between endothelium-derived relaxing and contracting factors is critical in maintaining vascular homeostasis. When this balance is disrupted, it predisposes the vasculature to vasoconstriction, leukocyte adherence, platelet activation, mitogenesis, pro-oxidation, thrombosis, impaired coagulation, vascular inflammation and atherosclerosis (52).
NO is the key endothelium-derived relaxing factor, which plays a pivotal role in the maintenance of vascular tone and reactivity (53). In addition to being the main determinant of basal vascular smooth muscle tone, NO opposes the actions of potent endothelium-derived contracting factors such as angiotensin-II and endothelin-1. In addition, NO serves to inhibit platelet and white cell activation and maintain the vascular smooth muscle in a nonproliferative state. NO is synthesized from L-arginine under the influence of the enzyme NO synthase (NOS). NOS requires a critical cofactor, tetrahydrobiopterin, to facilitate NO production. Tetrahydrobiopterin deficiency leads to an ‘uncoupling of NOS’ with the resultant production of potent oxidants such as superoxide and hydrogen peroxide instead of NO. Superoxide inactivates NO to peroxynitrite, further decreasing NO activity in this uncoupled state. Cardiac risk factors in general lead to an increase in oxidative stress, attenuating net NO bioactivity and leading to endothelial dysfunction (54).
Methods of assessing endothelial function
Endothelial function refers to a physiological observation that is the result of stimulation of vasoactive substances released by or that interact with the vascular endothelium. Endothelium-dependent vasodilation can be assessed in the coronary and peripheral circulations in humans. In addition, measures of platelet function and inflammation or leukocyte activation are indirect measures of endothelial health. Most studies concentrate on vasomotor responses as a marker of endothelial function. There is no agreed on gold standard for the measurement of endothelial function.
Coronary circulation:
Quantitative coronary angiography can be used to examine the change in diameter to intracoronary infusions of the endothelium-dependent vasodilators such as acetylcholine. In healthy vessels, acetylcholine evokes an NO-mediated vasodilatory response; in patients with endothelial dysfunction, this effect is blunted, or paradoxical vasoconstriction may occur (55). Endothelial function of the coronary microvasculature (resistance vessels) can be assessed by employing intracoronary Doppler techniques and assessing coronary blood flow in response to pharmacological or physiological stimuli (56). Although considered by many to be the best assessment of endothelial function, this technique is limited by its invasive nature, expense and inaccessibility.
Peripheral circulation:
Celermajer et al (57) were the first to describe a noninvasive assessment of flow-mediated vasodilation (FMD) in the brachial or femoral artery. Brachial artery occlusion for 5 min resulted in reactive hyperemia after the cuff was released (blood flow increased five- to sevenfold), and this increase in shear stress resulted in FMD, a NO-dependent process. Normal arteries dilate 10% to 15% depending on the laboratory, position of the cuff and equipment used. The variability is acceptable (approximately 2% absolute on repeated measures, with a coefficient of variation of 20%) (58). While differences exist in the performance of the test among research laboratories, recent guidelines have suggested uniform methodology to reduce variability (59,60). Brachial artery FMD has been shown to correlate with measures of coronary endothelial function (61). This technique has been extensively used with very good reproducibility and low observer variability.
Resistance vessel function in the forearm is assessed by strain-gauge venous impedance plethysmography. This methodology examines the change in forearm blood flow in response to direct intra-arterial (brachial artery) administration of agonists (62). This technique is excellent for acute interventions with repeated measurements. The major drawbacks again are reproducibility and its more invasive nature than ultrasound. A new finger plethysmographic technique offers some promise as a reproducible noninvasive tool (63,64).
Relationship of endothelial function to risk factors and their treatment
Endothelial injury with resulting dysfunction appears to be the initiating event in atherosclerosis (65–67) and plays an important role in the ischemic manifestations of coronary disease (68). Ludmer et al (55) demonstrated that whereas acetylcholine induces epicardial coronary vasodilation in patients without atherosclerosis, paradoxical vasoconstriction occurs in patients with atherosclerosis. Atherosclerosis also impairs coronary resistance vessel function, despite the fact that resistance vessels are rarely affected by atherosclerosis (56,69). In addition, coronary atherosclerosis is characterized by peripheral vessel endothelial dysfunction (61).
Oxidative stress appears to play a pivotal role in the alteration of endothelial function associated with risk factors. Every traditional risk factor associated with accelerated atherosclerotic vascular disease has been associated with endothelial dysfunction in both animal and human models (70). Celermajer et al (57) demonstrated impaired endothelial function in children with familial hypercholesterolemia. This study was among the first in humans to demonstrate the important role of endothelial dysfunction early in the course of atherosclerosis. Similarly, abnormal vasomotion occurs in subjects with uncomplicated hypertension (71), diabetes (72–75), cigarette smoking (76,77) and low levels of high density lipoprotein (78,79). Oxidized low density lipoprotein plays an important role in abnormal endothelial vasorelaxation, leukocyte adhesion and endothelin production (80–83). In addition, oxygen free radicals impair endothelial function through direct inactivation of NO (84). While all traditional risk factors have been associated with peripheral endothelial dysfunction, a close quantitative relationship does not exist (85–88). This suggests that endothelial function measurements may add unique information not captured by Framingham risk scores. The potential effect of emerging risk factors on endothelial function needs to be fully explored. We have recently demonstrated no relationship between brachial FMD and C-reactive protein, for example (89).
We were among the first to demonstrate that low density lipoprotein reduction with an 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor improves coronary endothelium-dependent vasodilation (90). Numerous studies of lipid lowering have subsequently confirmed these results in a variety of patient populations (91–93). The subject of pharmacological treatment of endothelial dysfunction has been reviewed by myself and others recently (52,94,95). Suffice it to say, interventions associated with favourable cardiovascular outcomes have been associated with attenuation of endothelial dysfunction for the most part (96–101). The notable exception to this is hormone replacement therapy, which has been associated with improvement in endothelium-dependent vasomotion, but not cardiovascular outcome.
Prognostic implications of endothelial dysfunction
In the past four years, several retrospective studies have demonstrated an association between endothelial dysfunction and cardiovascular events (102). These studies have used coronary endothelial function testing (103–107), brachial impedance plethysmography (83,108) and brachial flow-mediated dilation (109–114). These studies in general are of small sample size (all less than 500 patients), retrospective in nature and included only individuals with documented vascular disease or hypertension. All of these recent studies showed an association between a measure of endothelial function and outcome, except for a recently reported Australian study of 444 patients that demonstrated that endothelial dysfunction was not predictive of mortality (111). Larger prospective studies are underway to more definitively address this question. These include a cohort from the Framingham study group (115) and a Canadian cohort of healthy middle-aged firefighters (Firefighters And Their Endothelium [FATE] Study) (116). These studies are required to clarify the prognostic significance of endothelial function testing.
INTERRELATIONSHIP BETWEEN ARTERIAL STIFFNESS AND ENDOTHELIAL DYSFUNCTION
Arterial stiffness and endothelial dysfunction represent different aspects of vascular disease. However, there is certainly some crosstalk between these two pathophysiological processes. NO is continuously released and has been shown to contribute to arterial compliance (20). Pharmacological agents that improve endothelial function, such as statins and angiotensin-converting enzyme inhibitors, also decrease arterial stiffness. This is related to favourable effects on endothelial-derived paracrine factors, structural remodelling and direct effects on blood pressure (117). Several authors have also described the use of pulse wave analysis to assess endothelial function. In these studies, the AIx was measured from a peripheral artery at baseline and then in response to an inhaled beta2-agonist (endothelium-dependent). A reduction in the AIx was noted and this was blocked by NOS inhibition. In addition, the response was blunted in subjects with coronary artery disease or hypercholesterolemia (118,119). The original description of this approach used photoplethysmography to assess digital volume pulse (120). Arterial stiffness has also been demonstrated to be related to brachial artery FMD vasodilation in a recent small study (121). And finally, a recently published study by Mitchell et al (122) has challenged our thinking about the effect of risk factors on brachial endothelial function. In a large cohort from Framingham, it was demonstrated that hyperemic-induced shear stress was an important predictor of brachial FMD. In addition, risk factors diminished the magnitude of shear stress, suggesting that a reduced stimulus explains the diminution in endothelial function in subjects with cardiovascular risk factors (122).
CONCLUSIONS
The study of vascular biomarkers has greatly enhanced our understanding of the underlying pathophysiology of atherosclerosis. This includes soluble biomarkers and physiological parameters such as measures of arterial stiffness and endothelial function. It is unlikely that any new marker will be as important a surrogate marker as blood pressure or cholesterol. Certainly, endothelial function and arterial compliance in theory could be important markers, but difficulty in defining the best measure, modest reproducibility and lack of convincing prognostic studies to date have limited their clinical utility thus far. Ongoing prospective studies will determine whether surrogate status will be obtained in the next decade for either of these markers. Until that time, they will remain important research tools to further our understanding of vascular biology and to assess the impact of new risk factors and their treatment on the vessel wall.
Figure 1).
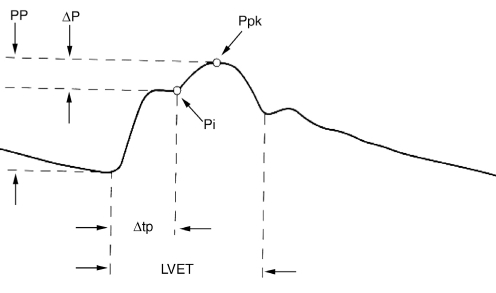
Carotid arterial contour. A reflected wave due to increased arterial stiffness generates a systolic wave (Ppk). The difference between this wave and the initial forward-going systolic wave (Pi) is then divided by the pulse pressure (systolic – diastolic pressure [ΔP]) to generate the augmentation index (AIx). AIx = ΔP/pulse pressure (PP). LVET Left ventricular ejection time; Δtp Time to Pi
Figure 2).
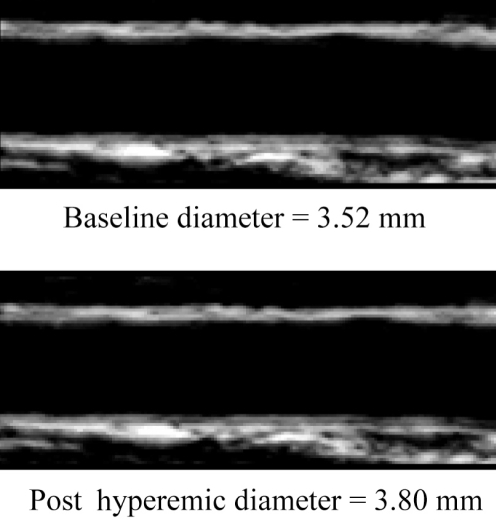
Ultrasound image of the brachial artery. At rest, the diameter is 3.52 mm, and between 60 s and 90 s postocclusion, the hyperemic diameter is 3.80 mm. Flow-mediated dilation = 3.80–3.52/3.52 ×100 = 8.0%
Figure 3).
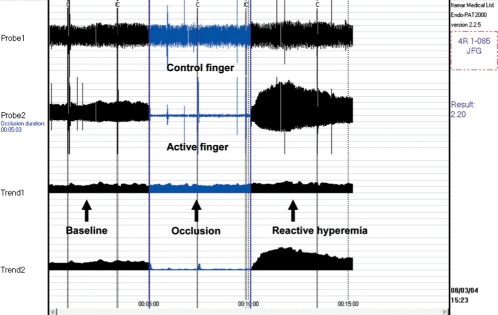
Determination of endothelial function by peripheral artery tonometry. This finger tip plethysmograph measures pulse volume amplitude (PVA) in the index finger of both hands at rest, during a 5 min arm occlusion and then postocclusion. The PVA index is the relative increase in PVA postocclusion in the active finger compared with the control finger. Values greater than approximately 1.4 are normal
Figure 4).
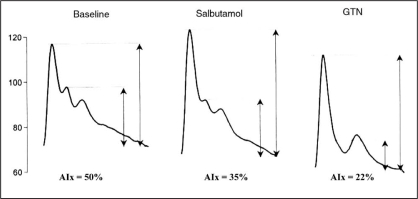
Use of systolic contour to assess endothelial function. The augmentation index (AIx) is measured from a peripheral artery without a generalized transfer function. Measurements are made at rest, in response to the endothelium-dependent stimulus (inhalation of a beta2-agonist) and in response to nitroglycerin (GTN). A fall in AIx with salbutamol is related to nitric oxide release and a decrease in arterial stiffness. Data from reference 118
TABLE 1.
Methods to determine arterial stiffness and endothelial function
| Artierial stiffness | Endothelial function |
|---|---|
|
|
Ach Acetylcholine; AIx Augmentation index; Large artery compliance; C1 C2 Small artery compliance; QCA Quantitative coronary angiography
TABLE 2.
Prognostic value of arterial stiffness measurements
| City, author, year (reference) | Patient status (n), measurement | Duration of study | Event | Conclusion |
|---|---|---|---|---|
| Paris, Blacher et al, 1999 (47) | 241 ESRD patients, PWV | 72 months | 73 deaths | Age and aortic PWV were predictors |
| Paris, London et al, 2001 (33) | Same 180 ESRD patients, PWV and AIx | 52 months | 70 deaths | Both PWV and AIx were predictors |
| Paris, Blacher et al, 2003 (41) | Same 242 ESRD patients, PWV | 78 months | 91 deaths, 58 CV deaths | Used PWV index – advanced age was predictor |
| Paris, Blacher et al, 1999 (25) | 710 hypertension patients, PWV | Less than two years | Not available | PWV related to FRS and CV mortality |
| Paris, Laurent et al, 2001 (49) | 1980 HT patients, PWV | 112 months | 107 deaths, 46 CV deaths | PWV was independent predictor |
| Paris, Boutouyrie et al, 2002 (48) | 1045 HT patients, PWV | 5.7 years | 97 CV events | PWV predicts Coronary events |
| Paris, Laurent et al, 2003 (50) | 1715 HT patients | 7.9 years | 25 fatal CVAs | Age and aortic PWV predicts stroke |
| Rotterdam, Stork et al, 2004 (23) | 367 men IMT, wall tracking United States | Four years | 70 deaths | Stiffness not independent of FRS |
| Paris, Meaume et al, 2001 (123) | 141 patients >70 years of age, PWV | Four years | 56 deaths; 27 CV deaths | OR 1.16 for PWV |
| Sydney, Weber et al, 2004 (51) | 465 men undergoing catheterization SphygmoCor (Altcor Medical, Australia) | Cross-sectional | Not applicable | AIx correlated with angiographic CAD |
AIx Augmentation index; CAD Coronary artery disease; CV Cardiovascular; CVA Cerebrovascular accident; ESRD End stage renal disease; FRS Framingham risk score; HT Hypertension; IMT Intima media thickening; PWV Pulse wave velocity
TABLE 3.
Prognostic significance of flow-mediated dilation
| Source (reference) | Patient status | Duration of study | Patient end points | Conclusion |
|---|---|---|---|---|
| University of Vienna, Austria (114) | 73 patients undergoing angiography | Five years | 27 CV events | Not related to events |
| Boston University, USA (112) | 187 elective vascular surgery patients | 30 days | 45 (25 TnI elevations) CV events | ED was independently predictive |
| University of Federico, Italy (109) | 131 PVD patients | 23 months | 39 CV events | ED and ABI were predictive |
| University of Modena, Italy (113) | 400 PM women with hypertension | 67 months | 32 CV events, change in ED to meds | No change in ED with BP medications was predictive |
| University of British Columbia British Columbia (110) | 152 CAD patients in cardiac rehabilitation | 34 months | 22 CV events | ED and carotid plaque were predictive |
| University of Queensland, Australia (111) | 444 at-risk patients | 24 months | 70 CV events | ED was not predictive |
ABI Ankle-brachial index; BP Blood pressure; CAD Coronary artery disease; CV Cardiovascular; ED Endothelial dysfunction; PM Postmenopausal; PVD Peripheral vascular disease; TnI Troponin I
REFERENCES
- 1.Grundy SM, Pasternak R, Greenland P, Smith S, Jr, Fuster V. Assessment of cardiovascular risk by use of multiple-risk-factor assessment equations: A statement for healthcare professionals from the American Heart Association and the American College of Cardiology. Circulation. 1999;100:1481–92. doi: 10.1161/01.cir.100.13.1481. [DOI] [PubMed] [Google Scholar]
- 2.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–47. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 3.Ridker PM. Evaluating novel cardiovascular risk factors: Can we better predict heart attacks. Ann Intern Med. 1999;130:933–7. doi: 10.7326/0003-4819-130-11-199906010-00018. [DOI] [PubMed] [Google Scholar]
- 4.Ridker PM, Brown NJ, Vaughan DE, Harrison DG, Mehta JL. Established and emerging plasma biomarkers in the prediction of first atherothrombotic events. Circulation. 2004;109:IV6–19. doi: 10.1161/01.CIR.0000133444.17867.56. [DOI] [PubMed] [Google Scholar]
- 5.Stampfer MJ, Ridker PM, Dzau VJ. Risk factor criteria. Circulation. 2004;109:IV3–5. doi: 10.1161/01.CIR.0000133446.69171.7d. [DOI] [PubMed] [Google Scholar]
- 6.Cohn JN. Introduction to surrogate markers. Circulation. 2004;109:IV20–1. doi: 10.1161/01.CIR.0000133441.05780.1d. [DOI] [PubMed] [Google Scholar]
- 7.Fleming TR, DeMets DL. Surrogate end points in clinical trials: Are we being misled? Ann Intern Med. 1996;125:605–13. doi: 10.7326/0003-4819-125-7-199610010-00011. [DOI] [PubMed] [Google Scholar]
- 8.Manolio T. Novel risk markers and clinical practice. N Engl J Med. 2003;349:1587–9. doi: 10.1056/NEJMp038136. [DOI] [PubMed] [Google Scholar]
- 9.Danesh J, Wheeler JG, Hirschfield GM, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–97. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 10.Ridker PM, Glynn RJ, Hennekens CH. C-reactive protein adds to the predictive value of total and HDL cholesterol in determining risk of first myocardial infarction. Circulation. 1998;97:2007–11. doi: 10.1161/01.cir.97.20.2007. [DOI] [PubMed] [Google Scholar]
- 11.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–65. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 12.Franklin SS, Gustin W, IV, Wong ND, et al. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation. 1997;96:308–15. doi: 10.1161/01.cir.96.1.308. [DOI] [PubMed] [Google Scholar]
- 13.Berger DS, Li JK, Laskey WK, Noordergraaf A. Repeated reflection of waves in the systemic arterial system. Am J Physiol. 1993;264:H269–81. doi: 10.1152/ajpheart.1993.264.1.H269. [DOI] [PubMed] [Google Scholar]
- 14.Karamanoglu M, Gallagher DE, Avolio AP, O’Rourke MF. Functional origin of reflected pressure waves in a multibranched model of the human arterial system. Am J Physiol. 1994;267:H1681–8. doi: 10.1152/ajpheart.1994.267.5.H1681. [DOI] [PubMed] [Google Scholar]
- 15.Fitchett DH. LV-arterial coupling: Interactive model to predict effect of wave reflections on LV energetics. Am J Physiol. 1991;261:H1026–33. doi: 10.1152/ajpheart.1991.261.4.H1026. [DOI] [PubMed] [Google Scholar]
- 16.Wang JJ, O’Brien AB, Shrive NG, Parker KH, Tyberg JV. Time-domain representation of ventricular-arterial coupling as a windkessel and wave system. Am J Physiol Heart Circ Physiol. 2003;284:H1358–68. doi: 10.1152/ajpheart.00175.2002. [DOI] [PubMed] [Google Scholar]
- 17.Oliver JJ, Webb DJ. Noninvasive assessment of arterial stiffness and risk of atherosclerotic events. Arterioscler Thromb Vasc Biol. 2003;23:554–66. doi: 10.1161/01.ATV.0000060460.52916.D6. [DOI] [PubMed] [Google Scholar]
- 18.Bank AJ, Kaiser DR, Rajala S, Cheng A. In vivo human brachial artery elastic mechanics: Effects of smooth muscle relaxation. Circulation. 1999;100:41–7. doi: 10.1161/01.cir.100.1.41. [DOI] [PubMed] [Google Scholar]
- 19.Nichols WW, O’Rourke MF. Therapeutic and monitoring strategies. In: Nichols WW, O’Rourke MF, editors. McDonald’s Blood Flow in Arteries: Theoretical, Experimental and Clinical Principles. 4th edn. London: Arnold; 1998. pp. 418–38. [Google Scholar]
- 20.Kinlay S, Creager MA, Fukumoto M, et al. Endothelium-derived nitric oxide regulates arterial elasticity in human arteries in vivo. Hypertension. 2001;38:1049–53. doi: 10.1161/hy1101.095329. [DOI] [PubMed] [Google Scholar]
- 21.O’Rourke M. Arterial stiffness, systolic blood pressure, and logical treatment of arterial hypertension. Hypertension. 1990;15:339–47. doi: 10.1161/01.hyp.15.4.339. [DOI] [PubMed] [Google Scholar]
- 22.Liao D, Arnett DK, Tyroler HA, et al. Arterial stiffness and the development of hypertension. The ARIC study. Hypertension. 1999;34:201–6. doi: 10.1161/01.hyp.34.2.201. [DOI] [PubMed] [Google Scholar]
- 23.Stork S, van den Beld AW, von Schacky C, et al. Carotid artery plaque burden, stiffness, and mortality risk in elderly men: A prospective, population-based cohort study. Circulation. 2004;110:344–8. doi: 10.1161/01.CIR.0000134966.10793.C9. [DOI] [PubMed] [Google Scholar]
- 24.Resnick LM, Militianu D, Cunnings AJ, Pipe JG, Evelhoch JL, Soulen RL. Direct magnetic resonance determination of aortic distensibility in essential hypertension: Relation to age, abdominal visceral fat, and in situ intracellular free magnesium. Hypertension. 1997;30:654–9. doi: 10.1161/01.hyp.30.3.654. [DOI] [PubMed] [Google Scholar]
- 25.Blacher J, Asmar R, Djane S, London GM, Safar ME. Aortic pulse wave velocity as a marker of cardiovascular risk in hypertensive patients. Hypertension. 1999;33:1111–7. doi: 10.1161/01.hyp.33.5.1111. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell GF, Parise H, Benjamin EJ, et al. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: The Framingham Heart Study. Hypertension. 2004;43:1239–45. doi: 10.1161/01.HYP.0000128420.01881.aa. [DOI] [PubMed] [Google Scholar]
- 27.Karamanoglu M, O’Rourke MF, Avolio AP, Kelly RP. An analysis of the relationship between central aortic and peripheral upper limb pressure waves in man. Eur Heart J. 1993;14:160–7. doi: 10.1093/eurheartj/14.2.160. [DOI] [PubMed] [Google Scholar]
- 28.Chen CH, Nevo E, Fetics B, et al. Estimation of central aortic pressure waveform by mathematical transformation of radial tonometry pressure. Validation of generalized transfer function. Circulation. 1997;95:1827–36. doi: 10.1161/01.cir.95.7.1827. [DOI] [PubMed] [Google Scholar]
- 29.Hope SA, Tay DB, Meredith IT, Cameron JD. Comparison of generalized and gender-specific transfer functions for the derivation of aortic waveforms. Am J Physiol Heart Circ Physiol. 2002;283:H1150–6. doi: 10.1152/ajpheart.00216.2002. [DOI] [PubMed] [Google Scholar]
- 30.Kelly RP, Millasseau SC, Ritter JM, Chowienczyk PJ. Vasoactive drugs influence aortic augmentation index independently of pulse-wave velocity in healthy men. Hypertension. 2001;37:1429–33. doi: 10.1161/01.hyp.37.6.1429. [DOI] [PubMed] [Google Scholar]
- 31.Wilkinson IB, Qasem A, McEniery CM, Webb DJ, Avolio AP, Cockcroft JR. Nitric oxide regulates local arterial distensibility in vivo. Circulation. 2002;105:213–7. doi: 10.1161/hc0202.101970. [DOI] [PubMed] [Google Scholar]
- 32.Wilkinson IB, MacCallum H, Cockcroft JR, Webb DJ. Inhibition of basal nitric oxide synthesis increases aortic augmentation index and pulse wave velocity in vivo. Br J Clin Pharmacol. 2002;53:189–92. doi: 10.1046/j.1365-2125.2002.1528adoc.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.London GM, Blacher J, Pannier B, Guerin AP, Marchais SJ, Safar ME. Arterial wave reflections and survival in end-stage renal failure. Hypertension. 2001;38:434–8. doi: 10.1161/01.hyp.38.3.434. [DOI] [PubMed] [Google Scholar]
- 34.Cohn JN, Finkelstein S, McVeigh GE, et al. Noninvasive pulse wave analysis for the early detection of vascular disease. Hypertension. 1995;26:503–8. doi: 10.1161/01.hyp.26.3.503. [DOI] [PubMed] [Google Scholar]
- 35.Manning TS, Shykoff BE, Izzo JL., Jr Validity and reliability of diastolic pulse contour analysis (windkessel model) in humans. Hypertension. 2002;39:963–8. doi: 10.1161/01.hyp.0000016920.96457.7c. [DOI] [PubMed] [Google Scholar]
- 36.Safar ME, Levy BI, Struijker-Boudier H. Current perspectives on arterial stiffness and pulse pressure in hypertension and cardiovascular diseases. Circulation. 2003;107:2864–9. doi: 10.1161/01.CIR.0000069826.36125.B4. [DOI] [PubMed] [Google Scholar]
- 37.Mitchell GF. Increased aortic stiffness: An unfavorable cardiorenal connection. Hypertension. 2004;43:151–3. doi: 10.1161/01.HYP.0000114581.77705.29. [DOI] [PubMed] [Google Scholar]
- 38.Kelly R, Hayward C, Avolio A, O’Rourke M. Noninvasive determination of age-related changes in human arterial pulse. Circulation. 1989;80:1652–9. doi: 10.1161/01.cir.80.6.1652. [DOI] [PubMed] [Google Scholar]
- 39.Wilkinson IB, MacCallum H, Rooijmans DF, et al. Increased augmentation index and systolic stress in type I diabetes mellitus. QJM. 2000;93:441–8. doi: 10.1093/qjmed/93.7.441. [DOI] [PubMed] [Google Scholar]
- 40.Wilkinson IB, Prasad K, Hall IR, et al. Increased central pulse pressure and augmentation index in subjects with hypercholesterolemia. J Am Coll Cardiol. 2002;39:1005–11. doi: 10.1016/s0735-1097(02)01723-0. [DOI] [PubMed] [Google Scholar]
- 41.Blacher J, Safar ME, Guerin AP, Pannier B, Marchais SJ, London GM. Aortic pulse wave velocity index and mortality in end-stage renal disease. Kidney Int. 2003;63:1852–60. doi: 10.1046/j.1523-1755.2003.00932.x. [DOI] [PubMed] [Google Scholar]
- 42.Yasmin, McEniery CM, Wallace S, Mackenzie IS, Cockcroft JR, Wilkinson IB. C-reactive protein is associated with arterial stiffness in apparently healthy individuals. Arterioscler Thromb Vasc Biol. 2004;24:969–74. doi: 10.1161/01.ATV.zhq0504.0173. [DOI] [PubMed] [Google Scholar]
- 43.Mahmud A, Feely J. Reduction in arterial stiffness with angiotensin II antagonist is comparable with and additive to ACE inhibition. Am J Hypertens. 2002;15:321–5. doi: 10.1016/s0895-7061(01)02313-5. [DOI] [PubMed] [Google Scholar]
- 44.Safar ME, London GM, Asmar RG, Hughes CJ, Laurent SA. An indirect approach for the study of the elastic modulus of the brachial artery in patients with essential hypertension. Cardiovasc Res. 1986;20:563–7. doi: 10.1093/cvr/20.8.563. [DOI] [PubMed] [Google Scholar]
- 45.Shige H, Dart A, Nestel P. Simvastatin improves arterial compliance in the lower limb but not in the aorta. Atherosclerosis. 2001;155:245–50. doi: 10.1016/s0021-9150(00)00558-x. [DOI] [PubMed] [Google Scholar]
- 46.Smilde TJ, van den Berkmortel FW, Wollersheim H, van Langen H, Kastelein JJ, Stalenhoef AF. The effect of cholesterol lowering on carotid and femoral artery wall stiffness and thickness in patients with familial hypercholesterolaemia. Eur J Clin Invest. 2000;30:473–80. doi: 10.1046/j.1365-2362.2000.00654.x. [DOI] [PubMed] [Google Scholar]
- 47.Blacher J, Guerin AP, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness on survival in end-stage renal disease. Circulation. 1999;99:2434–9. doi: 10.1161/01.cir.99.18.2434. [DOI] [PubMed] [Google Scholar]
- 48.Boutouyrie P, Tropeano AI, Asmar R, et al. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: A longitudinal study. Hypertension. 2002;39:10–5. doi: 10.1161/hy0102.099031. [DOI] [PubMed] [Google Scholar]
- 49.Laurent S, Boutouyrie P, Asmar R, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–41. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- 50.Laurent S, Katsahian S, Fassot C, et al. Aortic stiffness is an independent predictor of fatal stroke in essential hypertension. Stroke. 2003;34:1203–6. doi: 10.1161/01.STR.0000065428.03209.64. [DOI] [PubMed] [Google Scholar]
- 51.Weber T, Auer J, O’Rourke MF, et al. Arterial stiffness, wave reflections, and the risk of coronary artery disease. Circulation. 2004;109:184–9. doi: 10.1161/01.CIR.0000105767.94169.E3. [DOI] [PubMed] [Google Scholar]
- 52.Verma S, Anderson TJ. Fundamentals of endothelial function for the clinical cardiologist. Circulation. 2002;105:546–9. doi: 10.1161/hc0502.104540. [DOI] [PubMed] [Google Scholar]
- 53.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–6. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 54.Anderson TJ. Assessment and treatment of endothelial dysfunction in humans. J Am Coll Cardiol. 1999;34:631–8. doi: 10.1016/s0735-1097(99)00259-4. [DOI] [PubMed] [Google Scholar]
- 55.Ludmer PL, Selwyn AP, Shook TL, et al. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N Engl J Med. 1986;315:1046–51. doi: 10.1056/NEJM198610233151702. [DOI] [PubMed] [Google Scholar]
- 56.Zeiher AM, Drexler H, Wollschlager H, Just H. Endothelial dysfunction of the coronary microvasculature is associated with coronary blood flow regulation in patients with early atherosclerosis. Circulation. 1991;84:1984–92. doi: 10.1161/01.cir.84.5.1984. [DOI] [PubMed] [Google Scholar]
- 57.Celermajer DS, Sorensen KE, Gooch VM, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–5. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- 58.Sorensen KE, Celermajer DS, Spiegelhalter DJ, et al. Non-invasive measurement of human endothelium dependent arterial responses: Accuracy and reproducibility. Br Heart J. 1995;74:247–53. doi: 10.1136/hrt.74.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Coretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: A report of the International Brachial Artery Reactivity Task Force J Am Coll Cardiol 200239257–65.(Erratum 2002;39:1082) [DOI] [PubMed] [Google Scholar]
- 60.Mullen MJ, Kharbanda RK, Cross J, et al. Heterogenous nature of flow-mediated dilatation in human conduit arteries in vivo: Relevance to endothelial dysfunction in hypercholesterolemia. Circ Res. 2001;88:145–51. doi: 10.1161/01.res.88.2.145. [DOI] [PubMed] [Google Scholar]
- 61.Anderson TJ, Uehata A, Gerhard MD, et al. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol. 1995;26:1235–41. doi: 10.1016/0735-1097(95)00327-4. [DOI] [PubMed] [Google Scholar]
- 62.Creager MA, Cooke JP, Mendelsohn ME, et al. Impaired vasodilation of forearm resistance vessels in hypercholesterolemic humans. J Clin Invest. 1990;86:228–34. doi: 10.1172/JCI114688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bonetti PO, Pumper GM, Higano ST, Holmes DR, Jr, Kuvin JT, Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol. 2004;44:2137–41. doi: 10.1016/j.jacc.2004.08.062. [DOI] [PubMed] [Google Scholar]
- 64.Kuvin JT, Patel AR, Sliney KA, et al. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am Heart J. 2003;146:168–74. doi: 10.1016/S0002-8703(03)00094-2. [DOI] [PubMed] [Google Scholar]
- 65.Glass CK, Witztum JL. Atherosclerosis: The road ahead. Cell. 2001;104:503–16. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 66.Ross R. The pathogenesis of atherosclerosis: A perspective for the 1990s. Nature. 1993;362:801–9. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 67.Ross R. Atherosclerosis – an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 68.Meredith IT, Anderson TJ, Uehata A, Yeung AC, Selwyn AP, Ganz P. Role of endothelium in ischemic coronary syndromes. Am J Cardiol. 1993;72:27C–31C. doi: 10.1016/0002-9149(93)90252-8. [DOI] [PubMed] [Google Scholar]
- 69.Egashira K, Inou T, Hirooka Y, et al. Impaired coronary blood flow response to acetylcholine in patients with coronary risk factors and proximal atherosclerotic lesions. J Clin Invest. 1993;91:29–37. doi: 10.1172/JCI116183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Anderson TJ. Oxidative stress, endothelial function and coronary atherosclerosis. Cardiologia. 1997;42:701–14. [PubMed] [Google Scholar]
- 71.Panza JA, Quyyumi AA, Brush JE, Jr, Epstein SE. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N Engl J Med. 1990;323:22–7. doi: 10.1056/NEJM199007053230105. [DOI] [PubMed] [Google Scholar]
- 72.Johnstone M, Creager SJ, Scales KM, Cusco JA, Lee BK, Creager MA. Impaired endothelium-dependent vasodilation in patients with insulin-dependent diabetes mellitus. Circulation. 1993;88:2510–6. doi: 10.1161/01.cir.88.6.2510. [DOI] [PubMed] [Google Scholar]
- 73.Mullen MJ, Clarkson P, Donald AE, et al. Effect of enalapril on endothelial function in young insulin-dependent diabetic patients: A randomized, double-blind study. J Am Coll Cardiol. 1998;31:1330–5. doi: 10.1016/s0735-1097(98)00099-0. [DOI] [PubMed] [Google Scholar]
- 74.Williams SB, Cusco JA, Roddy MA, Johnstone MT, Creager MA. Impaired nitric oxide-mediated vasodilation in patients with non-insulin-dependent diabetes mellitus. J Am Coll Cardiol. 1996;27:567–74. doi: 10.1016/0735-1097(95)00522-6. [DOI] [PubMed] [Google Scholar]
- 75.Williams SB, Goldfine AB, Timimi FK, et al. Acute hyperglycemia attenuates endothelium-dependent vasodilation in humans in vivo. Circulation. 1998;97:1695–701. doi: 10.1161/01.cir.97.17.1695. [DOI] [PubMed] [Google Scholar]
- 76.Celermajer DS, Sorensen KE, Georgakopoulos D, et al. Cigarette smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilation in healthy young adults. Circulation. 1993;88:2149–55. doi: 10.1161/01.cir.88.5.2149. [DOI] [PubMed] [Google Scholar]
- 77.Celermajer DS, Adams MR, Clarkson P, et al. Passive smoking and impaired endothelium-dependent arterial dilation in healthy young adults. N Engl J Med. 1996;334:150–4. doi: 10.1056/NEJM199601183340303. [DOI] [PubMed] [Google Scholar]
- 78.Kuhn FE, Mohler ER, Satler LF, Reagan K, Lu DY, Rackley CE. Effects of high-density lipoprotein on acetylcholine-induced coronary vasoreactivity. Am J Cardiol. 1991;68:1425–30. doi: 10.1016/0002-9149(91)90274-o. [DOI] [PubMed] [Google Scholar]
- 79.O’Connell BJ, Genest J., Jr High-density lipoproteins and endothelial function. Circulation. 2001;104:1978–83. doi: 10.1161/hc3901.096667. [DOI] [PubMed] [Google Scholar]
- 80.Anderson TJ, Meredith IT, Charbonneau F, et al. Endothelium-dependent coronary vasomotion relates to the susceptibility of LDL to oxidation in humans. Circulation. 1996;93:1647–50. doi: 10.1161/01.cir.93.9.1647. [DOI] [PubMed] [Google Scholar]
- 81.Davi G, Romano M, Mezzetti A, et al. Increased levels of soluble P-selectin in hypercholesterolemic patients. Circulation. 1998;97:953–7. doi: 10.1161/01.cir.97.10.953. [DOI] [PubMed] [Google Scholar]
- 82.Heitzer T, Yla-Herttuala S, Luoma J, et al. Cigarette smoking potentiates endothelial dysfunction of forearm resistance vessels in patients with hypercholesterolemia. Role of oxidized LDL. Circulation. 1996;93:1346–53. doi: 10.1161/01.cir.93.7.1346. [DOI] [PubMed] [Google Scholar]
- 83.Heitzer T, Schlinzig T, Krohn K, Meinertz T, Munzel T.Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease Circulation 20011042673–8.(Erratum 2003;108:500) [DOI] [PubMed] [Google Scholar]
- 84.Rubanyi GM, Vanhoutte PM. Superoxide anions and hyperoxia inactivate endothelium-derived relaxing factor. Am J Physiol. 1986;250:H822–7. doi: 10.1152/ajpheart.1986.250.5.H822. [DOI] [PubMed] [Google Scholar]
- 85.Brevetti G, Silvestro A, Di Giacomo S, et al. Endothelial dysfunction in peripheral arterial disease is related to increase in plasma markers of inflammation and severity of peripheral circulatory impairment but not to classic risk factors and atherosclerotic burden. J Vasc Surg. 2003;38:374–9. doi: 10.1016/s0741-5214(03)00124-1. [DOI] [PubMed] [Google Scholar]
- 86.Celermajer DS, Sorensen KE, Bull C, Robinson J, Deanfield JE. Endothelium-dependent dilation in the systemic arteries of asymptomatic subjects relates to coronary risk factors and their interaction. J Am Coll Cardiol. 1994;24:1468–74. doi: 10.1016/0735-1097(94)90141-4. [DOI] [PubMed] [Google Scholar]
- 87.Hashimoto M, Kozaki K, Eto M, et al. Association of coronary risk factors and endothelium-dependent flow-mediated dilatation of the brachial artery. Hypertens Res. 2000;23:233–8. doi: 10.1291/hypres.23.233. [DOI] [PubMed] [Google Scholar]
- 88.Leeson P, Thorne S, Donald A, Mullen M, Clarkson P, Deanfield J. Non-invasive measurement of endothelial function: Effect on brachial artery dilatation of graded endothelial dependent and independent stimuli. Heart. 1997;78:22–7. doi: 10.1136/hrt.78.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Verma S, Wang CH, Lonn E, et al. Cross-sectional evaluation of brachial artery flow-mediated vasodilation and C-reactive protein in healthy individuals. Eur Heart J. 2004;25:1754–60. doi: 10.1016/j.ehj.2004.06.039. [DOI] [PubMed] [Google Scholar]
- 90.Anderson TJ, Meredith IT, Yeung AC, Frei B, Selwyn AP, Ganz P. The effect of cholesterol-lowering and antioxidant therapy on endothelium-dependent coronary vasomotion. N Engl J Med. 1995;332:488–93. doi: 10.1056/NEJM199502233320802. [DOI] [PubMed] [Google Scholar]
- 91.Dupuis J, Tardif JC, Cernacek P, Theroux P. Cholesterol reduction rapidly improves endothelial function after acute coronary syndromes. The RECIFE (reduction of cholesterol in ischemia and function of the endothelium) trial. Circulation. 1999;99:3227–33. doi: 10.1161/01.cir.99.25.3227. [DOI] [PubMed] [Google Scholar]
- 92.Tamai O, Matsuoka H, Itabe H, Wada Y, Kohno K, Imaizumi T. Single LDL apheresis improves endothelium-dependent vasodilatation in hypercholesterolemic humans. Circulation. 1997;95:76–82. doi: 10.1161/01.cir.95.1.76. [DOI] [PubMed] [Google Scholar]
- 93.Treasure CB, Klein JL, Weintraub WS, et al. Beneficial effects of cholesterol lowering therapy on the coronary endothelium in patients with coronary artery disease. N Engl J Med. 1995;332:481–7. doi: 10.1056/NEJM199502233320801. [DOI] [PubMed] [Google Scholar]
- 94.Hornig B, Drexler H. Reversal of endothelial dysfunction in humans. Coron Artery Dis. 2001;12:463–73. doi: 10.1097/00019501-200109000-00005. [DOI] [PubMed] [Google Scholar]
- 95.Verma S, Buchanan MR, Anderson TJ. Endothelial function testing as a biomarker of vascular disease. Circulation. 2003;108:2054–9. doi: 10.1161/01.CIR.0000089191.72957.ED. [DOI] [PubMed] [Google Scholar]
- 96.Anderson TJ, Elstein E, Haber H, Charbonneau F. Comparative study of ACE-inhibition, angiotensin II antagonism, and calcium channel blockade on flow-mediated vasodilation in patients with coronary disease (BANFF study) J Am Coll Cardiol. 2000;35:60–6. doi: 10.1016/s0735-1097(99)00537-9. [DOI] [PubMed] [Google Scholar]
- 97.Aymong ED, Curtis MJ, Youssef M, et al. Abciximab attenuates coronary microvascular endothelial dysfunction after coronary stenting. Circulation. 2002;105:2981–5. doi: 10.1161/01.cir.0000019905.18467.07. [DOI] [PubMed] [Google Scholar]
- 98.Husain S, Andrews NP, Mulcahy D, Panza JA, Quyyumi AA. Aspirin improves endothelial dysfunction in atherosclerosis. Circulation. 1998;97:716–20. doi: 10.1161/01.cir.97.8.716. [DOI] [PubMed] [Google Scholar]
- 99.Mancini GB, Henry GC, Macaya C, et al. Angiotensin-converting enzyme inhibition with quinapril improves endothelial vasomotor dysfunction in patients with coronary artery disease. The TREND (Trial on Reversing ENdothelial Dysfunction) study Circulation 199694258–65.(Erratum 1996;94:1490) [DOI] [PubMed] [Google Scholar]
- 100.Mather KJ, Verma S, Anderson TJ. Improved endothelial function with metformin in type 2 diabetes mellitus. J Am Coll Cardiol. 2001;37:1344–50. doi: 10.1016/s0735-1097(01)01129-9. [DOI] [PubMed] [Google Scholar]
- 101.Title LM, Cummings PM, Giddens K, Genest JJ, Jr, Nassar BA. Effect of folic acid and antioxidant vitamins on endothelial dysfunction in patients with coronary artery disease. J Am Coll Cardiol. 2000;36:758–65. doi: 10.1016/s0735-1097(00)00809-3. [DOI] [PubMed] [Google Scholar]
- 102.Mancini GB. Vascular structure versus function: Is endothelial dysfunction of independent prognostic importance or not? J Am Coll Cardiol. 2004;43:624–8. doi: 10.1016/j.jacc.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 103.Halcox JPJ, Schenke WH, Zalos G, et al. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 2002;106:653–8. doi: 10.1161/01.cir.0000025404.78001.d8. [DOI] [PubMed] [Google Scholar]
- 104.Hollenberg SM, Klein LW, Parrillo JE, et al. Coronary endothelial dysfunction after heart transplantation predicts allograft vasculopathy and cardiac death. Circulation. 2001;104:3091–6. doi: 10.1161/hc5001.100796. [DOI] [PubMed] [Google Scholar]
- 105.Schachinger V, Britten MB, Zeiher A. Impaired epicardial coronary vasoreactivity predicts for adverse cardiovascular events during long-term follow-up. Circulation. 2000;101:I902–7. [Google Scholar]
- 106.Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, Jr, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948–54. doi: 10.1161/01.cir.101.9.948. [DOI] [PubMed] [Google Scholar]
- 107.Targonski PV, Bonetti PO, Pumper GM, Higano ST, Holmes DR, Jr, Lerman A. Coronary endothelial dysfunction is associated with an increased risk of cerebrovascular events. Circulation. 2003;107:2805–9. doi: 10.1161/01.CIR.0000072765.93106.EE. [DOI] [PubMed] [Google Scholar]
- 108.Perticone F, Ceravolo R, Pujia A, et al. Prognostic significance of endothelial dysfunction in hypertensive patients. Circulation. 2001;104:191–6. doi: 10.1161/01.cir.104.2.191. [DOI] [PubMed] [Google Scholar]
- 109.Brevetti G, Silvestro A, Schiano V, Chiariello M. Endothelial dysfunction and cardiovascular risk prediction in peripheral arterial disease: Additive value of flow-mediated dilation to ankle-brachial pressure index. Circulation. 2003;108:2093–8. doi: 10.1161/01.CIR.0000095273.92468.D9. [DOI] [PubMed] [Google Scholar]
- 110.Chan SY, Mancini GB, Kuramoto L, Schulzer M, Frohlich J, Ignaszewski A. The prognostic importance of endothelial dysfunction and carotid atheroma burden in patients with coronary artery disease. J Am Coll Cardiol. 2003;42:1037–43. doi: 10.1016/s0735-1097(03)00927-6. [DOI] [PubMed] [Google Scholar]
- 111.Fathi R, Haluska B, Isbel N, Short L, Marwick TH. The relative importance of vascular structure and function in predicting cardiovascular events. J Am Coll Cardiol. 2004;43:616–23. doi: 10.1016/j.jacc.2003.09.042. [DOI] [PubMed] [Google Scholar]
- 112.Gokce N, Keaney JF, Jr, Hunter LM, Watkins MT, Menzoian JO, Vita JA. Risk stratification for postoperative cardiovascular events via noninvasive assessment of endothelial function: A prospective study. Circulation. 2002;105:1567–72. doi: 10.1161/01.cir.0000012543.55874.47. [DOI] [PubMed] [Google Scholar]
- 113.Modena MG, Bonetti L, Coppi F, Bursi F, Rossi R. Prognostic role of reversible endothelial dysfunction in hypertensive postmenopausal women. J Am Coll Cardiol. 2002;40:505–10. doi: 10.1016/s0735-1097(02)01976-9. [DOI] [PubMed] [Google Scholar]
- 114.Neunteufl T, Heher S, Katzenschlager R, et al. Late prognostic value of flow-mediated dilation in the brachial artery of patients with chest pain. Am J Cardiol. 2000;86:207–10. doi: 10.1016/s0002-9149(00)00857-2. [DOI] [PubMed] [Google Scholar]
- 115.Benjamin EJ, Larson MG, Keyes MJ, et al. Clinical correlates and heritability of flow-mediated dilation in the community: The Framingham Heart Study. Circulation. 2004;109:613–9. doi: 10.1161/01.CIR.0000112565.60887.1E. [DOI] [PubMed] [Google Scholar]
- 116.Anderson TJ, Roberts AC, Hildebrand K, et al. The fate of endothelial function testing: Rationale and design of the Firefighters And Their Endothelium (FATE) study. Can J Cardiol. 2003;19:61–6. [PubMed] [Google Scholar]
- 117.Schiffrin EL, Park JB, Intengan HD, Touyz RM. Correction of arterial structure and endothelial dysfunction in human essential hypertension by the angiotensin receptor antagonist losartan. Circulation. 2000;101:1653–9. doi: 10.1161/01.cir.101.14.1653. [DOI] [PubMed] [Google Scholar]
- 118.Hayward CS, Kraidly M, Webb CM, Collins P. Assessment of endothelial function using peripheral waveform analysis: A clinical application. J Am Coll Cardiol. 2002;40:521–8. doi: 10.1016/s0735-1097(02)01991-5. [DOI] [PubMed] [Google Scholar]
- 119.Wilkinson IB, Hall IR, MacCallum H, et al. Pulse-wave analysis: Clinical evaluation of a noninvasive, widely applicable method for assessing endothelial function. Arterioscler Thromb Vasc Biol. 2002;22:147–52. doi: 10.1161/hq0102.101770. [DOI] [PubMed] [Google Scholar]
- 120.Chowienczyk PJ, Kelly RP, MacCallum H, et al. Photoplethysmographic assessment of pulse wave reflection: Blunted response to endothelium-dependent beta2-adrenergic vasodilation in type II diabetes mellitus. J Am Coll Cardiol. 1999;34:2007–14. doi: 10.1016/s0735-1097(99)00441-6. [DOI] [PubMed] [Google Scholar]
- 121.Nigam A, Mitchell GF, Lambert J, Tardif JC. Relation between conduit vessel stiffness (assessed by tonometry) and endothelial function (assessed by flow-mediated dilatation) in patients with and without coronary heart disease. Am J Cardiol. 2003;92:395–9. doi: 10.1016/s0002-9149(03)00656-8. [DOI] [PubMed] [Google Scholar]
- 122.Mitchell GF, Parise H, Vita JA, et al. Local shear stress and brachial artery flow-mediated dilation: The Framingham Heart Study Hypertension 200444134–9.(Erratum 2005;45:e9) [DOI] [PubMed] [Google Scholar]
- 123.Meaume S, Benetos A, Henry OF, Rudnichi A, Safar ME. Aortic pulse wave velocity predicts cardiovascular mortality in subjects >70 years of age. Arterioscler Thromb Vasc Biol. 2001;21:2046–50. doi: 10.1161/hq1201.100226. [DOI] [PubMed] [Google Scholar]