Abstract
While complement is the most important component of humoral autoimmunity, and inflammation plays a key role in atherosclerosis, relatively few studies have looked at complement implications in atherosclerosis and its complications. C-reactive protein is a marker of inflammation and is also involved in atherosclerosis; it activates complement and colocalizes with activated complement proteins within the infarcting myocardium and the active atherosclerotic plaques. As new agents capable of modulating complement activity are being developed, new targets for the management of atherosclerosis are emerging that are related to autoimmunity and inflammation.
The present paper reviews the putative roles of the various complement activation pathways in the development of atherosclerosis, in ST segment elevation and non-ST segment elevation acute coronary syndromes, and in coronary artery bypass graft surgery. It also provides a perspective on new therapeutic interventions being developed to modulate complement activity. These interventions include the C1 esterase inhibitor, which may be consumed in some inflammatory states resulting in the loss of one of the mechanisms inhibiting activation of the classical and lectin pathways; TP10, a recombinant protein of the soluble complement receptor type 1 (sCR1) which inhibits the C3 and C5 convertases of the common pathway by binding C3b and C4b; a truncated version of the soluble complement receptor type 1 CRI lacking the C4b binding site which selectively inhibits the alternative pathway; and pexelizumab, a monoclonal antibody selectively blocking C5 to prevent the activation of the terminal pathway that is involved in excessive inflammation and autoimmune responses.
Keywords: Acute coronary syndromes, Atherosclerosis, Complement, Complement inhibitors, Coronary artery bypass grafting
Abstract
Tandis que le complément est le principal élément de l’auto-immunité humorale et que l’inflammation joue un rôle important dans l’athérosclérose, relativement peu d’études ont porté sur les répercussions du complément dans l’athérosclérose et ses complications. La protéine C-réactive est un marqueur de l’inflammation et participe également à l’athérosclérose. Elle active le complément et les colocalités par des protéines de complément activées dans le myocarde infarci et les plaques athéroscléreuses actives. À mesure que de nouveaux agents capables de moduler l’activité du complément sont mis au point, de nouvelles cibles de prise en charge de l’athérosclérose émergent, reliées à l’auto-immunité et à l’inflammation.
Le présent article analyse les rôles putatifs des diverses voies d’activation du complément dans l’apparition de l’athérosclérose, des syndromes coronariens aigus avec ou sans surélévation du segment ST et des pontages aortocoronariens. Il offre également une perspective sur les nouvelles interventions thérapeutiques mises au point pour moduler l’activité du complément. Ces interventions incluent l’inhibiteur de la C1 estérase, qui peut être consommé dans certains états inflammatoires et entraîner la perte de l’un des mécanismes qui inhibent l’activation des voies classiques et de l’adhésine; le TP10, une protéine recombinante du récepteur de complément soluble de type 1 qui inhibe les convertases C3 et C5 de la voie courante en liant le C3b et le C4b; une version tronquée du récepteur de complément soluble de type 1 sans le site de liaison C4b, qui peut inhiber sélectivement la voie alternative; et le pexclizumab (Alexion Pharmaceuticals, États-Unis), un anticorps monoclonal bloquant sélectivement le C5, qui prévient l’activation de la voie terminale qui participe à une inflammation excessive et aux réponses auto-immunes.
While the role of complement in innate humoral immunity and of inflammation in atherosclerosis was already recognized in the 18th century, the links between complement and atherosclerosis have only been recently established. In 1856, Rudolph Virchow (1) attributed atherosclerosis to an injury to the vessel wall. In 1899, Jules Bordet (2) described a heat labile serum component that augmented the ability of antibodies to eliminate pathogenic bacteria; Paul Ehrlich (3) later introduced the term ‘complement’ to describe this component. The cellular mechanisms and mediators for inflammation in atherosclerosis were recently more extensively described by Ross (4), Libby (5) and others. The experimental data, combined with histopathological observations of the presence of inflammation cells and mediators within atherosclerotic plaques, stimulated interest in blood markers of inflammation as potential tools in diagnosing the disease, understanding its pathophysiology and evaluating the natural history of the disease and the impact of various therapeutic interventions.
Among these markers, C-reactive protein (CRP) has emerged as the most useful marker (6). First recognized as an acute phase protein and as a complement activator, CRP is now suspected to contribute to atherosclerosis (7,8). The importance of complement in cardiovascular disease was first documented in myocardial cell injury related to ischemia and reperfusion and, subsequently, in the pathology of atherosclerosis and its complications. The present paper provides an overview on the role of complement in atherosclerosis, acute coronary syndromes and cardiac surgery, and offers perspectives on potentially useful therapeutic interventions to modulate its activity.
THE COMPLEMENT CASCADE
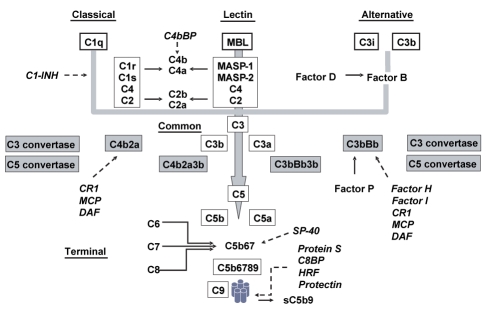
The complement system is composed of more than 30 proteins that act as activators, modulators or inhibitors. Various types of cells, mainly hepatocytes, but also monocytes, macrophages and gut epithelial cells, produce these proteins in various tissues. The proteins are usually inactive in blood but are readily activated by various triggers to fire millions of molecules that can deposit on invading microbes. The activation proceeds in a cascade through the effects of several proteases that generate biologically active products, and other proteases that lead to a more distal activation. Disrupting the chain reactions interrupts further progression. The full complement cascade is divided in five pathways including three proximal activation pathways that converge to form the common pathway which can further progress to the terminal pathway (Figure 1).
Figure 1).
Schematic representation of the complement cascade. The various activation pathways are underlined and the heavy lines and arrows showed the progression in the cascade of activation. There are three activation pathways that converge to a common pathway to further progress into a terminal pathway. The names of the various inhibitors are in italics with dotted arrows pointing to their site of action. C4bBP C4b-binding protein; C1-INH C1-esterase inhibitor; CR1 Complement receptor 1; DAF Decay-accelerating factor; Factor P Properdin; HRF Homologous restriction factor; MASP Mannan-binding lectin (MBL)-associated serine protease; MCP Membrane cofactor protein; Protein S Vitronectin; SP-40 Clusterin
The classical pathway proteins are labelled with a capital C followed by a number designating the various proteins following the order in which they were identified. When the proteins are cleaved, a lower case ‘a’ or ‘b’ is added to the number to describe the smallest cleavage component and the largest one, respectively – with the exception of C2b being smaller than C2a. The small ‘a’ fragments diffuse far from the activation site and can initiate local inflammatory responses at a distance by acting on specific cell receptors, whereas the large ‘b’ fragments directly bind ligands on cell membranes in vicinity to generate new proteases. The letter ‘i’ found before the C refers to cleaved fragments that are inactive. The alternative pathway proteins, often called factors, are simply described with a letter.
The classical pathway is antibody-mediated, although independent mechanisms may also occasionally act as initiators. It is activated through the binding of an antibody/antigen complex or of an immunoglobulin (Ig) G or IgM to C1q of the C1qrs complex. As a consequence, C1r is altered, and the altered form cleaves C1s, which in turn cleaves C4 into C4a and C4b, and C2 into C2a and C2b. C2a binds C4b on the cell membrane, forming the C3 convertase (C4bC2a). This pathway is calcium-dependent and is modulated by the C1-esterase inhibitor (C1-INH), which binds C1r and C1s to completely inhibit the proteolytic activity of the C1 qrs complex.
The lectin pathway results in the formation of the same C3 convertase as the classical pathway, but does if bypassing the need for an antibody and for the C1qrs complex. The equivalents of C1q, C1r and C1s that lead to cleavage of C2 and C4 are a mannan-binding lectin (MBL) protein and two MBL-associated serine proteases (MASP and MASP2, respectively).
The alternative pathway, also antibody-independent, is initiated through the binding of factor B, first cleaved to Bb by the activated plasma enzyme factor D, then to a C3b-like molecule (C3i) spontaneously generated by slow hydrolysis of native C3, to eventually form C3iBb. Factor C3iBb further amplifies the reaction by cleaving native C3 into C3a and C3b, the latter binding again fragment Bb to form the alternative pathway C3 convertase, C3bBb. Properdin stabilizes the C3bBb complex to delay its decay, whereas factor I and factor H act together to limit the formation of this complex.
The C3 convertase, whether generated by the classical or the alternative pathway, behaves very similarly in cleaving C3 into C3a and C3b. Although hundreds of C3 molecules can be cleaved by each convertase, leading to rapid complement activation, the process is closely controlled by mechanisms analogous in the two pathways. One of these mechanisms is the very short half-life of C3b due to rapid binding by red cells, and results in a limited period of time where it can bind factor B. Factor H further limits the reaction by displacing factor B from the C3 convertase, which interrupts the positive feedback loop, and by facilitating the natural property of factor I, which degrades C3b within the convertase. In the classical pathway, a C4b-binding protein plays the latter role, whereas C1-INH shuts down the proteolytic activity of C1r and C1s, restricting again the time window allowed for cleaving C4 and C2. When not inhibited, however, the C3 convertases continue to act on C3 to generate C3a and C3b; in extreme cases, C3 depletion will occur. C3a freely diffuses, while C3b binds the C3 convertases – C4bC2a in the classical and lectin pathways, and C3bBb in the alternative pathway – to form the C5 convertases C4bC2a3b and C3bBbC3b, respectively.
The terminal pathway is initiated upon the generation of one C5 convertase. The C5 convertases cleave C5 into C5a, which freely diffuses in surrounding fluids, and C5b, which serves as an anchor for the assembly of the membrane attack complex (MAC) by binding C6, and then C7 to form the C5b67 complex, which attaches itself to cell membranes. C8 binds this complex and promotes insertion of C9 molecules that create holes in the lipid layers of plasma membrane and result in cell lysis and death through a loss of the osmotic gradient and free entry of water into cells. The assembly of the terminal complex can be inhibited in cells that are not the primary targets through specific proteins, including protein S (vitronectin), the homologous restriction factor, SP-40 (clusterin), protectin (CD59) and a C8-binding protein. Protein S forms a complex with C5b9 to generate sC5b9, an inactive form that diffuses in the circulation.
COMPLEMENT ACTIVATION
Gram-negative bacteria, numerous viruses, immune complexes of the IgM and IgG type, ligand-bound CRP, apoptotic material, phospholipids and mitochondrial proteins can all activate the classical pathway. Carbohydrates with a mannose terminal activate the lectin pathway, while the alternative pathway may be activated by Gram-negative and several types of Gram-positive bacteria, fungi, viruses, parasites, tumour cells, heterologous red cells, aggregated IgA, proteases, clotting pathway products and the cobra venom factor.
BIOLOGICAL ACTIVITY
Complement is the most important component of the humoral autoimmune system. It possesses numerous functions related to host defense, plays a role in clearing immune complexes including antibody-coated bacteria and apoptotic cells, and forms interfaces between innate and adaptive immunity by recruiting and activating inflammatory cells. Most of the effects are mediated through complement receptors (CRs). The classical pathway acts through antibodies, while the alternative pathway provides a nonspecific first line of defense against numerous infectious agents. Complement activation is associated with producing several biologically active molecules that contribute to resistance, anaphylaxis and inflammation. C2b generated during the classical pathway activation is a prokinin that becomes biologically active following enzymatic alteration by plasmin. C3b and C4b act as opsonins on the cell surface of microorganisms, attaching CR1 on phagocytic cells to promote clearance of immune complexes and apoptotic material. C3a, C4a and C5a (in increasing order of potency) are anaphylotoxins inducing basophil/mast cell degranulation, smooth muscle contraction and platelet aggregation. C5a is chemotactic and a potent activator of neutrophils, basophils and macrophages; it promotes the expression of adhesion molecules on endothelial cells, and increases the expression of the CR1 and CR3 on neutrophils. Whereas the proximal complement pathways are usually beneficial, the terminal component can be associated with excess inflammation and autoimmune responses, and tissue demolition. Thus, complement can become chronically activated and elicits inflammation when immune complexes cannot be cleared from the body. Goodpasture’s disease is such an example of autoantibodies directed to the basement membranes of the renal glomeruli and lungs. Chronic infections can also perpetuate the formation of immune complexes, causing relentless activation and consumption of complement, and may occur during hepatitis C virus infection and bacterial endocarditis.
COMPLEMENT IN ATHEROSCLEROSIS
Whereas atherosclerosis can activate complement through many different mechanisms, a number of complement proteins can contribute to the formation and progression of atherosclerosis. There is a growing literature describing these interactions. Thus, ligand-bound CRP, immune complexes, enzymatically-modified low density lipoprotein bound to CRP, apoptotic material, phospholipids and mitochondrial proteins can all activate the classical pathway in atherosclerosis. Carbohydrates with the mannose terminal activate the lectin pathway, while oxidative stress promotes the adherence of these carbohydrates to the endothelial cells (9). C3b and coagulation proteases are activators of the alternative pathway.
Complement genes are expressed within human atherosclerosis (10). Anaphylatoxin receptors, mainly C3aR and C5aR, and complement components are found in young and old human atherosclerotic plaques (11). Numerous immunohistochemistry studies have showed colocalization of the terminal complex with CRP and apoptotic cells, supporting an important role of complement in mediating the inflammatory response in atherosclerosis and enabling self-autotoxicity within plaques (12). Sublytic assembly of C5b-9 induces smooth muscle cell and endothelial cell activation and proliferation, increases inter-leukin (IL)-8 and membrane cofactor protein (MCP-1) messenger RNA concentrations and protein secretion, and cytosolic to nuclear translocation of nuclear factor-kappa B (13). In rabbits fed with cholesterol, the terminal component is expressed in the intima in association with increased cholesterol content preceding the monocyte infiltration and foam cell formation (14).
COMPLEMENT IN MYOCARDIAL INFARCTION
Direct mediators of activation in infarcting tissues are immune complexes, antiheart autoantibodies, phospholipids and mitochondrial proteins released by ischemia and reperfusion, and CRP that directly bind C1q or the MBL. The tissue binding is facilitated by a lack of regulatory molecules in necrotic cells. Extensive activation is likely present, since proteins and messenger RNAs of complement proteins C1 to C9 are found in areas of recent and old myocardial infarctions along MAC deposits located on damaged cardiac myocytes (15). Infarcts that have evolved for more than 12 h and for up to five days contain more CRP, activated complement and CRP-complement complexes. More extensive CRP and complement depositions are found in patients with reinfarction and in those treated with reperfusion therapy, supporting the role of complement in reperfusion injury (16).
COMPLEMENT IN NON-ST SEGMENT ELEVATION ACUTE CORONARY SYNDROMES
Non-ST segment elevation syndromes are associated with no or minimal myocardial cell necrosis. Any complement activation in these syndromes may therefore reflect pathophysiology of the coronary plaques that trigger the unstable state rather than necrosis and reperfusion injury associated with cell necrosis in ST segment elevation myocardial infarction. These plaques are rich in lipids, have a thin cap and are infiltrated by inflammatory cells. Although immunochemistry studies have shown evidence of complement activation in these plaques (17), only a few studies have looked at the blood levels of complement proteins. Yasuda et al (18) described elevated sC5b-9 levels that correlated with infarct size in patients with cell necrosis but only a modest elevation of C3a, and not of sC5b-9, in those with unstable angina and no cell necrosis. The findings suggest strong complement activation in the former condition, and self-limited activation not progressing to activation of the terminal pathway in the latter (18). Another study reported an elevation of sC5b-9 levels in 47 patients with unstable angina and elevated CRP levels without, however, assessing the proximal components of the cascade (19). Preliminary results in the authors’ laboratory suggested an activation of the proximal, alternative and terminal pathways occurring independently of troponin T elevation, as shown by elevations in C4a, Bb, and C5a and sC5b-9 levels, respectively.
COMPLEMENT AND CORONARY ARTERY BYPASS GRAFTING
Cardiac surgery is associated with a systemic inflammation response that is accentuated when it is performed under extracorporeal circulation. Blood exposure to surfaces of the extracorporeal circuit, hypothermia, the surgical trauma, and tissue ischemia and reperfusion elicit this response, which is characterized by activation of the complement, leukocytes, platelets, the coagulation cascade and the production of cytokines. The systemic inflammation may cause many postoperative complications, including vital organ dysfunction, and eventually, multiorgan failure and death. All complement pathways appear to be involved in this reaction. Heparin and heparin-coated material were found to reduce complement activation. In one study, off-pump surgery was associated with less rapid production of C5a with less rapid and less profound formation of C5b-9 when compared with on-pump procedures (20). However, the administration of protamine to revert heparin effects at the end of these procedures resulted in further complement activation and production of C5a and C5b-9. This activation appears largely mediated through protamine-heparin complexes that bind C1q under the influence of CRP (21).
COMPLEMENT INHIBITION
As complement activity progresses through a cascade balanced by various activators and inhibitors, many junctures exist where pharmacological interventions may interrupt and/or modulate the cascade at turning points. The inhibition can be nonspecific, such as that produced by nafamostat mesilate (Futhan-175, BD Biosciences Pharmingen, USA), a large spectrum protease inhibitor, and by polyanions and polycations; high doses of immunoglobulin can inhibit C3, C4 and assembly of the terminal complex. Various agents can also more specifically inhibit proteins and/or activation pathways. Table 1 provides a listing of various targets and agents.
TABLE 1.
Targets and drugs to inhibit and/or modulate complement activity
| Targets | Agents |
|---|---|
| Nonspecific | Nafamostat mesilate |
| High doses of immunoglobulins | |
| Classical pathway | |
| C1q | C1q peptide |
| Lectin pathway | |
| Mannan-binding lectin | Anti-mannan-binding lectin mAb |
| Classical and lectin pathways | C1-INH |
| Alternative pathway | Anti-factor D mAb |
| sCR1 (des LHR-A) | |
| Common pathway | |
| C3 and C5 convertase | RsCR1 |
| C3 convertase | Compstatin |
| Terminal pathway | |
| C5 | Anti-C5 mAb (pexelizumab) |
| C9 assembly | Clusterin (CD59) conjugates |
| Anaphylatoxin | Anti-C5a mAb |
| Complement receptors | |
| C3aR | Peptide |
| C5aR | Peptide |
| Regulators of complement activation | |
| MCP (CD46) | sMCP |
| rMCP | |
| DAF (CD55) | Complement receptor related gene-gamma |
| CD59 | – |
DAF Decay-accelerating factor; C1-INH C1-esterase inhibitor; mAb Monoclonal antibody; MCP Membrane cofactor protein; sCR1 Soluble complement receptor 1
The classical pathway can be blocked at its origin by small peptides binding C1q. C1-INH is a naturally occurring regulator of the classical and lectin pathways and of the kallikreinkinin system, the latter effect preventing the formation of bradykinin. C1-INH derived from pooled human plasma is used in the prevention and treatment of congenital angioneurotic edema often associated with a deficiency of C1-INH. Evidence now exists suggesting that the administration of C1-INH could be useful in conditions associated with severe inflammation where it is consumed, such as burns, sepsis, cytokine-induced vascular leak syndrome, myocardial injury associated with acute myocardial infarction and coronary artery bypass grafting (CABG), and other diseases (22). The protective effects of C1-INH during ischemia/reperfusion appear, however, dose-dependent, since excess administration of C1-INH has been shown to possibly induce severe adverse effects. Doses of 40 IU/kg administered intravenously 5 min before reperfusion in a pig model of ischemia and reperfusion significantly reduced the area of necrosis predicted by the area at risk (22). They also suppressed local C3a and C5a generation, while reducing plasma concentrations of creatine kinase (CK) and troponin T. By contrast, no beneficial effects were observed in this model with doses of 100 IU/kg C1-INH, whereas doses of 200 IU/kg C1-INH provoked severe side effects and coagulation disorders. A recombinant formulation of C1-INH has been produced (23).
The alternative pathway and the lectin pathway are blocked by an antifactor D monoclonal antibody (mAb) and an anti-MBL mAb, respectively. The antifactor D mAb administered to baboons submitted to hypothermic extracorporeal circulation inhibited plasma Bb, C3a, sC5b-9, CD11b expression on neutrophils and monocytes, and reduced IL-6, CK-MB and lactic acid dehydrogenase concentrations compared with controls (24).
Various recombinant proteins of the soluble CR1 block the common pathways formed by the C3 and C5 convertases by binding C3b and C4b. These drugs can suppress ischemia/reperfusion injury, thermal trauma and immune complex-mediated inflammation. TP10 is an example of a recombinant sCR1 that is now under investigation in humans. A truncated version of sCR1 that lacks the C4b binding site, sCR1[desLHR-A], selectively inhibits the alternative pathway, preserving the clearance of immune complexes by the classical pathway. Compstatin is a small molecule that more specifically inhibits the cleavage of C3 to C3b and C3a. Two new therapeutic approaches are being developed: one is inhibition by specific peptides of CRs, such as C3aR and C5aR, while the other relates to the widely distributed cell surface glycoproteins, regrouped under the terminology of regulators of complement activity that prevent complement-mediated damage. The regulators of complement activity that have been studied the most are the membrane cofactor protein (soluble membrane cofactor protein, sCD46), the decay-accelerating factor (soluble decay-accelerating factor, sCD55), clusterin and protectin (sCD59), which are all soluble proteins.
The terminal pathway is a target for anti-C5 mAb that prevents formation of C5a and C5b and by CD59, and of a multifunctional glycoprotein (CD59) that inhibits formation of the MAC by blocking C9 coupling on C5b-8 and C5b-9, and the binding of C5b-7 to membrane of target cells. The murine mAb anti-C5 effectively blocks C5b-9-mediated cell lysis and C5a-induced chemotaxis of polymorphonuclear leukocytes. It was shown to be useful in preventing the induction and progression of experimentally collagen-induced rheumatoid arthritis, and in preventing hyperacute rejection in a presensitized mouse heart transplantation model, although it did not prevent late xenograft rejection caused by acute vascular rejection. When administered before myocardial ischemia and reperfusion, it significantly reduced left ventricular free wall polymorphonuclear leukocyte infiltration, infarct size and apoptosis measured by the TUNEL assay (25). Pexelizumab (Alexion Pharmaceuticals, USA) is a single-chain humanized fragment of the murine antibody that maintains the highly specific and potent inhibition level of C5 similar to that of the original antibody. The binding to C5 prevents its cleavage into C5a and C5b, abrogating the excessive inflammatory and autoimmune reactions associated with the terminal complex. At the same time, it spares the generation of C3a and C3b, maintaining the functions of opsonizing pathogenic microorganisms and immune complexes and, thus, preserving the host-defense mechanisms against infection.
The present review will focus on complement inhibitors that have been tested in cardiovascular diseases in humans. Based on strong experimental data showing that the inhibition of the various complement pathways control inflammation and prevent apoptosis and tissue destruction, the human investigation first was aimed at reducing infarct size in acute myocardial infarction, and myocardial cell protection in coronary artery bypass graft surgery. TP10 and pexelizumab are the two drugs being investigated. Current pilot studies are unveiling new objectives for the next generation of trials related to mechanisms of patient death and survival.
COMPLEMENT INHIBITION IN MYOCARDIAL INFARCTION
TP10, a C1-INH, and pexelizumab (an anti-C5 mAb), were studied. TP10 was administered at escalating loading doses followed by 48 h infusions in 22 patients, at least 6 h after onset of symptoms to prevent any interference with thrombolytic therapy (26). A dose-response was observed. The loading doses of 50 U/kg and 100 U/kg increased plasma C1-INH activity by approximately 100% and 200%, respectively, and these levels of activity were maintained for the duration of the infusion. The release of troponin T and CK-MB mass was reduced by 36% and 57% in the 13 patients who received thrombolysis and the 18 control patients, respectively (P=0.001). No drug-related adverse events were observed, and C4 fragments were reduced in a dose-dependent manner. However, higher doses were required in the test patients than in the healthy controls to reach target plasma levels of threefold above normal C1-INH values, suggesting an accelerated C1-INH consumption in myocardial infarction (27).
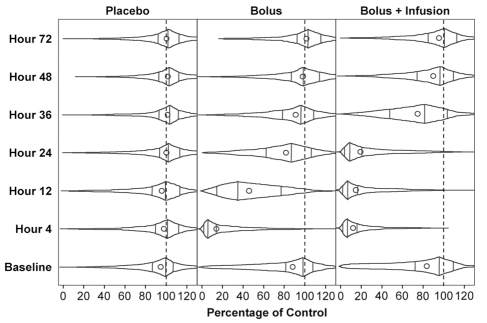
Pexelizumab was evaluated in two phase 2 studies, one involving 943 patients managed with fibrinolytic drugs, and the second involving 960 patients undergoing primary angioplasty (percutaneous coronary interventions) (28,29). In the two trials, patients were randomly assigned to receive placebo, pexelizumab 2.0 mg/kg bolus, or pexelizumab 2.0 mg/kg bolus and 0.05 mg/kg per infusion for 20 h. The bolus doses fully inhibited complement hemolytic activity for more than 4 h with a return toward baseline between 12 h and 24 h. In the infusion group, the inhibition remained complete beyond 24 h with a progressive recovery over the following 24 h (Figure 2). Both studies failed to show a reduction in the primary objective: infarct size assessed by the area under the curve of CK-MB release. Similarly, no reductions of the predefined composite end point of 90 day incidence of death, new or worsening congestive heart failure, shock or stroke were found. On the other hand, the COMplement inhibition in Myocardial infarction treated with Angioplasty (COMMA) (28) trial of patients undergoing primary angioplasty observed a markedly reduced 90-day mortality in the bolus and infusion arm group relative to the placebo group (1.8% versus 5.9%, respectively) (RR 0.30, 95% CI 0.11 to 0.81, P=0.014); an intermediate rate of 4.2% was seen in the bolus-only group (not significant). The occurrence of cardiogenic shock was 1.3% in the bolus and infusion group, 5.2% in the placebo group and 3.4% in the bolus-alone group (28).
Figure 2).
Complement inhibition as measured by hemolytic assays following placebo, a bolus of pexelizumab (Alexion Pharmaceuticals, USA) and a bolus followed by an infusion. Dots represent mean values, vertical lines represent median and interquartile ranges, and plot ends represent minimum and maximum values. Reproduced with permission from reference 26
Notably, the reduction of 70% in mortality and 60% in shock rate occurred with no observed differences in area at risk assessed with the extent of ST shifts; success of reperfusion therapy assessed by the thrombolysis in myocardial infarction (TIMI) grade flow after percutaneous coronary intervention and early ST segment resolution, nor in final infarct size evaluated by the QRS score at 24 h and the total amount of CK-MB released. A substudy of blood markers among COMMA patients showed that treatment was associated with a smaller elevation in CRP and IL-6 concentrations at the end of the infusion period compared with placebo, and that the blood levels of the markers at baseline, 24 h and 72 h were predictive of subsequent occurrence of death and cardiogenic shock. The data suggest a benefit of complement inhibition with pexelizumab through unconventional favourable effects that could be related to determinants of infarct recovery, such as inflammatory cytokines, apoptosis, inducible nitric oxide synthase and/or remodelling (30).
Based on these results, a large international study has been launched testing pexelizumab administered as a bolus and 24 h infusion in patients with ST segment elevation with planned primary angioplasty. The primary end point of this study is mortality.
COMPLEMENT INHIBITION IN CABG SURGERY
A study of C1-INH in 13 infants younger than 12 months of age who were undergoing extracorporeal circulation was interrupted prematurely due to frequent occurrence of vein thrombosis in all patients and death in nine of them (23). In a larger randomized, prospective, double-blind study of high-risk patients undergoing cardiac surgery, 564 patients (408 men and 156 women) received incremental doses of an intravenous bolus of TP10 (1 mg/kg, 3 mg/kg, 5 mg/kg or 10 mg/kg) or placebo immediately before initiating the extracorporeal circulation (31). The serum complement activity (CH50) was almost completely suppressed within 5 min to 10 min, only to recover progressively over the following 72 h. The generation of C3a and SC5b-9 associated with the extracorporeal circulation was also prevented. The primary composite end point of death, myocardial infarction, prolonged (24 h or more) intra-aortic balloon pump support, and prolonged intubation occurred in 31.4% of TP10 and 33.7% of placebo patients (P=0.31). This composite end point was significantly reduced in an analysis restricted to men only (P=0.025) as were the rates of death or MI by 36% (P=0.026). No significant excess of side effects occurred with treatment in this study (31).
Pexelizumab was administered before initiating the extracorporeal circulation in bolus doses of 0.2 mg/kg to 2.0 mg/kg in 35 patients (32). A significant dose-dependent inhibition of complement hemolytic activity persisted for up to 14 h, while a significant reduction in generation of sC5b-9 and in leukocyte activation, as measured by surface expression of CD11b, was observed with the doses of 1 mg/kg and 2 mg/kg. There was also a 40% reduction in myocardial injury assessed by CK-MB release. No side effects were reported with the drug, whereas a test of mental function (the mini mental state examination) suggested there were fewer new cognitive deficits with high doses (32). This benefit, however, was not seen with TP10 and was not reproduced in a larger trial with pexelizumab, the Pexelizumab for Reduction in Infarction and MOrtality (PRIMO)-CABG surgery trial (33). This randomized, double-blind, placebo-controlled trial included 3099 patients undergoing CABG surgery with or without valve surgery. Patients were randomly assigned to intravenous pexelizumab 2.0 mg/kg bolus followed by a perfusion of 0.05 mg/kg/h for 24 h or placebo, initiated 10 min before undergoing the procedure. The primary composite end point of 30-day rates of death or MI in patients with bypass grafting only occurred in 134 of 1373 (9.8%) patients receiving pexelizumab versus 161 of 1359 (11.8%) patients receiving placebo (RR 0.82; 95% CI 0.66 to 1.02; P=0.07). The secondary composite end point of death or MI at days 4 and 30 in all enrolled patients occurred in 178 of 1547 (11.5%) patients receiving pexelizumab versus 215 of 1535 (14.0%) receiving placebo (RR 0.82; 95% CI 0.68 to 0.99; P=0.03).
REFERENCES
- 1.Lowe GD. Virchow’s triad revisited: abnormal flow. Pathophysiol Haemost Thromb. 2003;33:455–7. doi: 10.1159/000083845. [DOI] [PubMed] [Google Scholar]
- 2.Bordet J. Sur l’agglutination et la dissolution des globules rouges par le serum d’animaux injectes de sang defibriné. Ann. De l’Inst. Pasteur. 1898;xii:688–695. [Google Scholar]
- 3.Ehrlich P. On Immunity, With Special Reference to Cell Life. The Croonian lecture. Proceedings of the Royal Society. 1900;66:424–448. [Google Scholar]
- 4.Ross R. Atherosclerosis – an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 5.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–74. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 6.Ridker PM, Koenig W, Fuster V. C-reactive protein and coronary heart disease. N Engl J Med. 2004;351:295–8. [PubMed] [Google Scholar]
- 7.Volanakis JE. Complement activation by C-reactive protein complexes. Ann N Y Acad Sci. 1982;389:235–50. doi: 10.1111/j.1749-6632.1982.tb22140.x. [DOI] [PubMed] [Google Scholar]
- 8.Verma S, Kuliszewski MA, Li SH, et al. C-reactive protein attenuates endothelial progenitor cell survival, differentiation, and function: Further evidence of a mechanistic link between C-reactive protein and cardiovascular disease. Circulation. 2004;109:2058–67. doi: 10.1161/01.CIR.0000127577.63323.24. [DOI] [PubMed] [Google Scholar]
- 9.Collard CD, Vakeva A, Morrissey MA, et al. Complement activation after oxidative stress: Role of the lectin complement pathway. Am J Pathol. 2000;156:1549–56. doi: 10.1016/S0002-9440(10)65026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yosajima K, Schwab K, McGeer EG, McGeer PL. Generation of C-reactive protein and complement components in atherosclerotic plaques. Am J Pathol. 2001;158:1039–51. doi: 10.1016/S0002-9440(10)64051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niculescu F, Rus HG, Vlaicu R. Activation of the human terminal complement pathway in atherosclerosis. Clin Immunol Immunopathol. 1987;45:147–55. doi: 10.1016/0090-1229(87)90029-8. [DOI] [PubMed] [Google Scholar]
- 12.Niculescu F, Niculescu T, Rus H. C5b-9 terminal complement complex assembly on apoptotic cells in human arterial wall with atherosclerosis. Exp Mol Pathol. 2004;76:17–23. doi: 10.1016/j.yexmp.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Kilgore KS, Schmid E, Shanley TP, et al. Sublytic concentrations of the membrane attack complex of complement induce endothelial interleukin-8 and monocyte chemoattractant protein-1 through nuclear factor-kappa B activation. Am J Pathol. 1997;150:2019–31. [PMC free article] [PubMed] [Google Scholar]
- 14.Seifert PS, Hugo F, Hansson GK, Bhakdi S. Prelesional complement activation in experimental atherosclerosis. Terminal C5b-9 complement deposition coincides with cholesterol accumulation in the aortic intima of hypercholesterolemic rabbits. Lab Invest. 1989;60:747–54. [PubMed] [Google Scholar]
- 15.Yasojima K, Schwab C, McGeer EG, McGeer PL. Human heart generates complement proteins that are upregulated and activated after myocardial infarction. Circ Res. 1998;83:860–9. doi: 10.1161/01.res.83.8.860. [DOI] [PubMed] [Google Scholar]
- 16.Nijmeijer R, Krijnen PA, Assink J, et al. C-reactive protein and complement depositions in human infarcted myocardium are more extensive in patients with reinfarction or upon treatment with reperfusion. Eur J Clin Invest. 2004;34:803–10. doi: 10.1111/j.1365-2362.2004.01425.x. [DOI] [PubMed] [Google Scholar]
- 17.Laine P, Pentikainen MO, Wurzner R, et al. Evidence for complement activation in ruptured coronary plaques in acute myocardial infarction. Am J Cardiol. 2002;90:404–8. doi: 10.1016/s0002-9149(02)02498-0. [DOI] [PubMed] [Google Scholar]
- 18.Yasuda M, Takeuchi K, Hiruma M, et al. The complement system in ischemic heart disease. Circulation. 1990;81:156–63. doi: 10.1161/01.cir.81.1.156. [DOI] [PubMed] [Google Scholar]
- 19.Hoffmeister HM, Ehlers R, Buttcher E, et al. Comparison of C-reactive protein and terminal complement complex in patients with unstable angina pectoris versus stable angina pectoris. Am J Cardiol. 2002;89:909–12. doi: 10.1016/s0002-9149(02)02237-3. [DOI] [PubMed] [Google Scholar]
- 20.Wehlin L, Vedin J, Vaage J, Lundahl J. Activation of complement and leukocyte receptors during on- and off pump coronary artery bypass surgery. Eur J Cardiothorac Surg. 2004;25:35–42. doi: 10.1016/s1010-7940(03)00652-3. [DOI] [PubMed] [Google Scholar]
- 21.Bruins P, te Velthuis H, Eerenberg-Belmer AJ, et al. Heparin-protamine complexes and C-reactive protein induce activation of the classical complement pathway: Studies in patients undergoing cardiac surgery and in vitro. Thromb Haemost. 2000;84:237–43. [PubMed] [Google Scholar]
- 22.Horstick G. C1-esterase inhibitor in ischemia and reperfusion. Immunobiology. 2002;205:552–62. doi: 10.1078/0171-2985-00154. [DOI] [PubMed] [Google Scholar]
- 23.Horstick G, Berg O, Heimann A, Gotze O, et al. Application of C1-esterase inhibitor during reperfusion of ischemic myocardium: Dose-related beneficial versus detrimental effects. Circulation. 2001;104:3125–31. doi: 10.1161/hc5001.100835. [DOI] [PubMed] [Google Scholar]
- 24.Undar A, Eichstaedt HC, Clubb FJ, Jr, et al. Novel anti-factor D monoclonal antibody inhibits complement and leukocyte activation in a baboon model of cardiopulmonary bypass. Ann Thorac Surg. 2002;74:355–62. doi: 10.1016/s0003-4975(02)03656-1. [DOI] [PubMed] [Google Scholar]
- 25.Vakeva AP, Agah A, Rollins SA, et al. Myocardial infarction and apoptosis after myocardial ischemia and reperfusion: Role of the terminal complement components and inhibition by anti-C5 therapy. Circulation. 1998;97:2259–67. doi: 10.1161/01.cir.97.22.2259. [DOI] [PubMed] [Google Scholar]
- 26.de Zwaan C, Kleine AH, Diris JH, et al. Continuous 48-h C1-inhibitor treatment, following reperfusion therapy, in patients with acute myocardial infarction. Eur Heart J. 2002;23:1670–7. doi: 10.1053/euhj.2002.3191. [DOI] [PubMed] [Google Scholar]
- 27.Diris JH, Hermens WT, Hemker PW, Lagrand WK, Hack CE, van Dieijen-Visser MP. Pharmacokinetics of C1-inhibitor protein in patients with acute myocardial infarction. Clin Pharmacol Ther. 2002;72:498–504. doi: 10.1067/mcp.2002.129320. [DOI] [PubMed] [Google Scholar]
- 28.Granger CB, Mahaffey KW, Weaver WD, et al. Pexelizumab, an anti-C5 complement antibody, as adjunctive therapy to primary percutaneous coronary intervention in acute myocardial infarction: The COMplement inhibition in Myocardial infarction treated with Angioplasty (COMMA) trial. Circulation. 2003;108:1184–90. doi: 10.1161/01.CIR.0000087447.12918.85. [DOI] [PubMed] [Google Scholar]
- 29.Mahaffey KW, Granger CB, Nicolau JC, et al. Effect of Pexelizumab, an anti-C5 complement antibody, as adjunctive therapy to fibrinolysis in acute myocardial infarction: The COMPlement inhibition in myocardial infarction treated with thromboLYtics (COMPLY) trial. Circulation. 2003;108:1176–83. doi: 10.1161/01.CIR.0000087404.53661.F8. [DOI] [PubMed] [Google Scholar]
- 30.Theroux P, Armstrong PW, Mahaffey KW, et al. Prognostic significance of blood markers of inflammation in patients with ST-segment elevation myocardial infarction undergoing primary angioplasty and effects of Pexelizumab, a C5 inhibitor: A substudy of the COMMA trial. Eur Heart J. 2005;26:1964–70. doi: 10.1093/eurheartj/ehi292. [DOI] [PubMed] [Google Scholar]
- 31.Lazar HL, Bokesch PM, van Lenta F, et al. Soluble human complement receptor 1 limits ischemic damage in cardiac surgery patients at high risk requiring cardiopulmonary bypass. Circulation. 2004;110:II274–9. doi: 10.1161/01.CIR.0000138315.99788.eb. [DOI] [PubMed] [Google Scholar]
- 32.Fitch JC, Rollins S, Matis L, et al. Pharmacology and biological efficacy of a recombinant, humanized, single-chain antibody C5 complement inhibitor in patients undergoing coronary artery bypass graft surgery with cardiopulmonary bypass. Circulation. 1999;100:2499–506. doi: 10.1161/01.cir.100.25.2499. [DOI] [PubMed] [Google Scholar]
- 33.Verrier ED, Shernan SK, Taylor KM, et al. Terminal complement blockade with Pexelizumab during coronary artery bypass graft surgery requiring cardiopulmonary bypass: A randomized trial. JAMA. 2004;291:2319–27. doi: 10.1001/jama.291.19.2319. [DOI] [PubMed] [Google Scholar]