Abstract
Experienced cardiac surgeons are aware that the intramyocardial portions of the coronary arteries are rarely affected by arteriosclerosis. This is a striking and reliable finding in the operating room that is present even when the preceding and subsequent segments of the arteries are diseased. The present review describes the published evidence of embryological, anatomical and physiological differences between intramyocardial coronary arteries and their epicardial counterparts. Possible mechanisms of these differences are explored, and hypotheses are advanced as to how one may capitalize on these differences to provide better diagnosis and treatment of coronary artery disease. The absence of vasa vasorum in the intramyocardial coronary arteries appears to play a major role in their protection from arteriosclerosis. Fully understanding this peculiar phenomenon – the sparing of the intramyocardial coronary arteries – would be one giant step closer to unlocking the remaining mysteries of arteriosclerosis in general.
Keywords: Arteriosclerosis, Coronary artery, Intramyocardial, Vasa vasorum
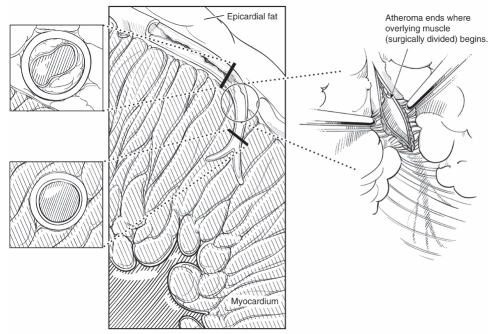
Any experienced cardiac surgeon knows that the intramyocardial portions of the coronary arteries are invariably spared from arteriosclerosis. This process is most striking in the circumflex and left anterior descending artery systems. In the circumflex marginal branches, the arteriosclerotic process ‘magically’ disappears as the vessel dives under a few muscle fibres (Figure 1). At this level, the vessel becomes soft, thin-walled and completely disease-free. This constant finding is underappreciated by those who do not handle coronary arteries on a daily basis, and it may have important implications in both the diagnosis and treatment of coronary artery disease. To understand why the intramyocardial coronary arteries are spared from arteriosclerosis, we must first explore the differences between intramyocardial and epicardial coronary arteries (Table 1). We will compare and contrast the respective segments of the arteries with regard to their embryology, internal environment, external environment, physical forces encountered and the morphology of the vessel wall. We will also suggest ways that these differences can be exploited for both the diagnosis and treatment of coronary artery disease.
Figure 1).
Transition from arteriosclerotic to normal coronary artery at the myocardial artery
TABLE 1.
Fundamental differences between epicardial and intramyocardial coronary arteries
| Characteristic |
Coronary arteries |
|
|---|---|---|
| Epicardial | Intramyocardial | |
| Investing tissues | Adipose | Muscular |
| External support | None | Substantial, so lower level of physical stress |
| Wall thickness | Thicker | Thinner (<0.035 mm) |
| Vasa vasorum | Yes | No |
| Embryology: Embryological isoform of smooth muscle myosin heavy chain (SMemb) | Present | Absent |
HISTORY
The first detailed study that described the intramyocardial portions of the coronary arteries was published by Geiringer in 1951 (1). It was in this paper that the term ‘mural’ coronary artery was coined. In this autopsy study, Geiringer clearly noted that the intramyocardial portions of the coronary arteries are less thick-walled than the epicardial vessels, and are free from arteriosclerosis. He also noted a lack of vasa vasorum within the mural coronary arteries. As described below, these findings may underlie the contrasting picture between the intramyocardial disease-resistant and the nearby epicardial disease-prone portions of the coronary arteries.
EMBRYOLOGY
The coronary arteries and their walls do not form as outgrowths from the aorta. It appears that the endothelium of the coronary arteries forms in situ, and the media and adventitia are derived locally from the pericardial mesoderm. These coverings then migrate inward to invest the coronary artery endothelium (2). If this migration is incomplete, the intramyocardial coronary arteries may have a different structural makeup. This may begin to explain the disparate behaviours of mural and epicardial coronary arteries.
To illustrate this point, Aikawa et al (3) measured the composition of three isoforms of vascular smooth muscle myosin heavy chains, termed SM1, SM2 and SMemb (the embryological form), within the walls of coronary arteries in various stages of development and arteriosclerosis. Paradoxically, the level of the embryological form (SMemb) tends to increase with advancing age and worsening arteriosclerosis. Interestingly, SMemb is absent from the intramyocardial portions of the coronary arteries. Thus, an embryological contribution to resistance from arteriosclerosis cannot be discounted. The other possibility, of course, is that as a coronary artery is exposed to stress, the smooth muscle fibres ‘regress’ to a more embryological state.
INTERNAL ENVIRONMENT
If the mural coronary arteries were exposed to different levels of blood concentration, oxygenation, pH, lipids or inflammatory mediators than their epicardial counterparts, it could explain the difference in propensity for arteriosclerosis. However, disease-free intramyocardial segments are often juxtaposed between two arteriosclerotic epicardial segments. Thus, this explanation is unlikely.
EXTERNAL ENVIRONMENT
The mural coronary arteries are, by necessity, surrounded by myocardium. The epicardial coronary arteries are surrounded largely by adipose tissue that recent evidence suggests may play either a pro- or anti-inflammatory role through paracrine signalling (4,5). Further work will be required to elucidate the specific role of this tissue.
PHYSICAL FORCES
Temperature
Significant temperature differences between mural and epicardial coronary arteries are unlikely to exist in the absence of inflammation, although this has not been well studied.
Pressure and stress
Here, significant differences are well established. Robicsek and Thubrikar (6) measured the intramyocardial pressure at different levels of the myocardium in a canine model. Using a modified version of the law of Laplace, they demonstrated that the intramyocardial coronary arteries undergo significantly lower levels of mural stress, in both the linear dimension (shear stress) and the circumferential dimension (hoop stress). They and other authors have clearly demonstrated a direct correlation between level of mural stress and development of arteriosclerosis (7).
THE VESSEL WALL
As demonstrated by Geiringer in 1951 (8), and reinforced on a daily basis by those who routinely perform coronary artery surgery, the intramyocardial coronary arteries have thinner walls than the epicardial coronary arteries. The most likely reason for this is the difference in stresses described above. It is probable this simple difference decreases the propensity for arteriosclerosis of the mural coronary arteries. Because the walls are so thin, oxygen and nutrients freely diffuse to supply the intima, media and adventitia. In coronary arteries, once a critical wall thickness of 0.035 mm is attained, the formation of vasa vasorum begins. These events rarely occur with mural coronary arteries (8).
Epicardial coronary arteries, on the other hand, very commonly attain this critical thickness. In normal epicardial coronary arteries, vasa vasorum are present in the outer media and adventitia. As endothelial stress occurs, the arteries become thicker and more metabolically active. The underperfused cells begin to elute inflammatory mediators, growth factors and proteases that increase the production of vasa vasorum (9).
The importance of vasa vasorum in the pathogenesis and complications of coronary artery disease cannot be overstated. As early as 1855, Rokitansky (10) suggested that vasa vasorum were important in the development of arteriosclerosis. In fact, subsequent evidence indicates that these new vessels are necessary for arteriosclerosis to exist (11). They are involved in the progression of arteriosclerosis (12) and prevention of their formation prevents arteriosclerosis (13).
Not only are vasa vasorum not normally present in such numbers and locations within the coronary artery walls, they are also abnormal vessels (14). They do not respond to regulatory signals in the same way that normal vessels respond (15). They are thin-walled and prone to rupture. A rupture creates a focal hematoma, leading to further inflammation and perpetuating the arteriosclerotic process, or causing plaque rupture and consequent vessel occlusion (16). Their thin walls may also make them excellent conduits for the transfer of lipids into an atherosclerotic plaque. They are also rich in leukocyte adhesion molecules, which promote extravasation of leukocytes into the plaque, thereby further promoting inflammation (17).
These coronary artery vasa vasorum are both harbingers and participants in the arteriosclerotic process, and as such, should be considered pathological. We therefore propose the term ‘pathological vasa vasorum (PVV)’ to describe them.
CLINICAL IMPLICATIONS
The intramyocardial coronary arteries serve as an example of how arteries can remain free from arteriosclerosis, even in the face of an aggressively arteriosclerotic milieu (cholesterol, smoking, family history, etc). For treatment of arteriosclerosis, work has begun toward limiting vasa vasorum production to slow the arteriosclerotic process (13). Early results have been encouraging. Work has also been performed to create a limited stress environment for vein grafts, similar to those that intramyocardial coronary arteries enjoy, by surrounding the grafts with external stents (18). This procedure has shown promising results and should be further pursued.
Finally, many modalities are being studied to identify pathological vasa vasorum in vivo (19,20). As yet, these studies have not reached the level of precision required to plan operative intervention; however, progress is being made. The ideal study would be safe, noninvasive (ie, noncatheter-based), rapid, relatively inexpensive and capable of demonstrating which arteriosclerotic lesions are most likely to require intervention and, based on objective data, what intervention will be required. To do so, the study must demonstrate the walls (including the level of neovascularization via vasa vasorum) and luminal details of coronary arteries, and be of surgical roadmap quality.
CONCLUSION
The immunity of intramyocardial coronary arteries to arteriosclerosis deserves additional directed study, which may lead to further elucidation of the mechanisms of arteriosclerosis. Understanding these mechanisms more fully may provide new opportunities for diagnosis and prevention of arteriosclerosis.
REFERENCES
- 1.Geiringer E. The mural coronary. Am Heart J. 1951;41:359–68. doi: 10.1016/0002-8703(51)90036-1. [DOI] [PubMed] [Google Scholar]
- 2.Mikawa T, Gourdie RG. Pericardial mesoderm generates a population of coronary smooth muscle cells migrating into the heart along with ingrowth of the epicardial organ. Dev Biol. 1996;174:221–32. doi: 10.1006/dbio.1996.0068. [DOI] [PubMed] [Google Scholar]
- 3.Aikawa M, Sivam PN, Kuro-o M, et al. Human smooth muscle myosin heavy chain isoforms as molecular markers for vascular development and atherosclerosis. Circ Res. 1993;73:1000–12. doi: 10.1161/01.res.73.6.1000. [DOI] [PubMed] [Google Scholar]
- 4.Iacobellis G, Barbaro G. The double role of epicardial adipose tissue as pro- and anti-inflammatory organ. Horm Metab Res. 2008;40:442–5. doi: 10.1055/s-2008-1062724. [DOI] [PubMed] [Google Scholar]
- 5.Eiras S, Teijeira-Fernández E, Shamagian LG, Fernandez AL, Vazquez-Boquete A, Gonzalez-Juanatey JR. Extension of coronary artery disease is associated with increased IL-6 and decreased adiponectin gene expression in epicardial adipose tissue. Cytokine. 2008;43:174–80. doi: 10.1016/j.cyto.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Robicsek F, Thubrikar MJ. The freedom from atherosclerosis of intramyocardial coronary arteries: Reduction of mural stress – a key factor. Eur J Cardiothorac Surg. 1994;8:228–35. doi: 10.1016/1010-7940(94)90151-1. [DOI] [PubMed] [Google Scholar]
- 7.Feldman CL, Stone PH. Intravascular hemodynamic factors responsible for progression of coronary atherosclerosis and development of vulnerable plaque. Curr Opin Cardiol. 2000;15:430–40. doi: 10.1097/00001573-200011000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Geiringer E. Intimal vascularization and atherosclerosis. J Pathol Bacteriol. 1951;63:201–11. doi: 10.1002/path.1700630204. [DOI] [PubMed] [Google Scholar]
- 9.Schaper W, Scholz D. Factors regulating arteriogenesis. Arterioscler Thromb Vasc Biol. 2003;23:1143–51. doi: 10.1161/01.ATV.0000069625.11230.96. [DOI] [PubMed] [Google Scholar]
- 10.Rokitansky C. A Manual of Pathological Anatomy. Philadelphia: Blanchard and Lea; 1855. p. 200. [Google Scholar]
- 11.Winternitz MC, Thomas RM, Le Compte PM, Thomas CC, editors. The Biology of Atherosclerosis. Springfield: Charles C Thomas; 1938. [Google Scholar]
- 12.Barger AC, Beeuwkes R, III, Lainey LL, Silverman KJ. Hypothesis: Vasa vasorum and neovascularization of human coronary arteries. A possible role in the pathophysiology of atherosclerosis. New Engl J Med. 1984;310:175–7. doi: 10.1056/NEJM198401193100307. [DOI] [PubMed] [Google Scholar]
- 13.Moulton KS, Heller E, Konerding MA, Flynn E, Palinski W, Folkman J. Angiogenesis inhibitors endostatin or TNP-470 reduce intimal neovascularization and plaque growth in apolipoprotein E-deficient mice. Circulation. 1999;99:1726–32. doi: 10.1161/01.cir.99.13.1726. [DOI] [PubMed] [Google Scholar]
- 14.Scotland RS, Vallance PJ, Ahluwalia A. Endogenous factors involved in regulation of tone of arterial vasa vasorum: Implications for conduit vessel physiology. Cardiovasc Res. 2000;46:403–11. doi: 10.1016/s0008-6363(00)00023-7. [DOI] [PubMed] [Google Scholar]
- 15.Scotland R, Vallance P, Ahluwalia A. Endothelin alters the reactivity of vasa vasorum: Mechanisms and implications for conduit vessel physiology and pathophysiology. Br J Pharmacol. 1999;128:1229–34. doi: 10.1038/sj.bjp.0702930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paterson JC. Heart disease and employment. Stress, intimal hemorrhage, and coronary occlusion. J Occup Med. 1961;3:59–63. [PubMed] [Google Scholar]
- 17.O’Brien KD, McDonald TO, Chait A, Allen MD, Alpers CE. Neovascular expression of E-selectin, intercellular adhesion molecule-1, and vascular cell adhesion molecule-1 in human atherosclerosis and their relation to intimal leukocyte content. Circulation. 1996;93:672–82. doi: 10.1161/01.cir.93.4.672. [DOI] [PubMed] [Google Scholar]
- 18.Angelini GD, Lloyd C, Bush R, Johnson J, Newby AC. An external, oversized, porous polyester stent reduces vein graft neointima formation, cholesterol concentration, and vascular cell adhesion molecule 1 expression in cholesterol-fed pigs. J Thorac Cardiovasc Surg. 2002;124:950–6. doi: 10.1067/mtc.2002.127004. [DOI] [PubMed] [Google Scholar]
- 19.Carlier S, Kakadiaris IA, Dib N, et al. Vasa vasorum imaging: A new window to the clinical detection of vulnerable atherosclerotic plaques. Curr Atheroscler Rep. 2005;7:164–9. doi: 10.1007/s11883-005-0040-2. [DOI] [PubMed] [Google Scholar]
- 20.Kaul S, Lindner JR. Visualizing coronary atherosclerosis in vivo: Thinking big, imaging small. J Am Coll Cardiol. 2004;43:461–3. doi: 10.1016/j.jacc.2003.11.010. [DOI] [PubMed] [Google Scholar]