Abstract
OBJECTIVE
Low-fat hypocaloric diets reduce insulin resistance and prevent type 2 diabetes in those at risk. Low-carbohydrate, high-fat diets are advocated as an alternative, but reciprocal increases in dietary fat may have detrimental effects on insulin resistance and offset the benefits of weight reduction.
RESEARCH DESIGN AND METHODS
We investigated a low-fat (20% fat, 60% carbohydrate) versus a low-carbohydrate (60% fat, 20% carbohydrate) weight reduction diet in 24 overweight/obese subjects ([mean ± SD] BMI 33.6 ± 3.7 kg/m2, aged 39 ± 10 years) in an 8-week randomized controlled trial. All food was weighed and distributed, and intake was calculated to produce a 500 kcal/day energy deficit. Insulin action was assessed by the euglycemic clamp and insulin secretion by meal tolerance test. Body composition, adipokine levels, and vascular compliance by pulse-wave analysis were also measured.
RESULTS
Significant weight loss occurred in both groups (P < 0.01), with no difference between groups (P = 0.40). Peripheral glucose uptake increased, but there was no difference between groups (P = 0.28), and suppression of endogenous glucose production was also similar between groups. Meal tolerance–related insulin secretion decreased with weight loss with no difference between groups (P = 0.71). The change in overall systemic arterial stiffness was, however, significantly different between diets (P = 0.04); this reflected a significant decrease in augmentation index following the low-fat diet, compared with a nonsignificant increase within the low-carbohydrate group.
CONCLUSIONS
This study demonstrates comparable effects on insulin resistance of low-fat and low-carbohydrate diets independent of macronutrient content. The difference in augmentation index may imply a negative effect of low-carbohydrate diets on vascular risk.
The worldwide pandemic of overweight/obesity is a major public health concern and is strongly linked to the rising prevalence of diabetes and cardiovascular disease (1,2). While excess caloric intake and positive energy balance are clearly associated with the development of overweight/obesity and its consequences, it is also possible that dietary macronutrient intake may be important, in particular increased levels of sugar or fat (3,4).
Insulin resistance is a key feature of the pathophysiology of obesity and diabetes and is also linked to the development of atherosclerosis (5). In addition to overall obesity, the anatomical distribution of adipose tissue influences insulin resistance and diabetes risk, with the highest risk in those with central or upper-body fat distribution characterized by a large waist circumference (6). Free fatty acids and adipocyte-derived proteins from omental fat may provide the molecular link between obesity and diabetes. Recently, retinol-binding protein 4 (RBP4) has been linked to insulin resistance, although there is little information regarding the effects of dietary modification (7). In a recent study, serum RBP4 was decreased following a carbohydrate-restricted diet but not a low-fat diet (8).
The U.S. Diabetes Prevention Program and the Finnish Diabetes Prevention Trial both demonstrated a powerful effect of lifestyle intervention to reduce diabetes development in high-risk subjects with impaired glucose tolerance (9,10). Relatively modest weight loss (5–7% of initial body weight) and moderate physical activity resulted in a 58% reduction in the incidence of type 2 diabetes, which was related to beneficial effects on insulin resistance. The lifestyle intervention achieved in these studies included a hypocaloric low-fat diet. However, outside such rigorous research studies, low-fat diets can be challenging for patients and hard to maintain (11).
Rising levels of overweight/obesity have resulted in a proliferation of weight loss diets, and there is currently a vigorous debate regarding the optimal dietary macronutrient composition that facilitates lasting and safe weight loss (12,13). In recent years, low-carbohydrate diets have attracted substantial media interest, presenting an attractive alternative to challenging lifestyle modifications (i.e., intentional caloric restriction and increased physical activity). These diets are portrayed as scientifically sound and limit carbohydrate amount and type to achieve unintentional calorie reduction through blunting appetite. Restriction of carbohydrate has also been shown to be effective in controlling blood glucose and insulin levels (14). As low-carbohydrate diets are generally associated with reciprocal increases in fat consumption, which has been linked to insulin resistance and lipid abnormalities, there are concerns that this may offset the benefits of weight reduction (15). It is also possible that different weight loss strategies exert varying effects on regional adiposity and adipokine levels, which may influence the metabolic effects of weight loss.
To investigate the effects of different weight loss strategies, we performed an 8-week randomized controlled trial to directly compare a low-carbohydrate hypocaloric diet (20% energy from carbohydrate, 60% energy from fat) with a low-fat hypocaloric diet (60% energy from carbohydrate, 20% energy from fat) in overweight/obese subjects. The primary outcome variable, insulin resistance, was assessed using the euglycemic-hyperinsulinemic clamp combined with isotope dilution techniques. Secondary outcomes included change in body composition, meal tolerance–related insulin secretion, vascular compliance, plasma leptin, and RBP4 levels.
RESEARCH DESIGN AND METHODS
Twenty-seven overweight or obese, but otherwise healthy, male and female volunteers (BMI ≥27 kg/m2) were recruited. Exclusion criteria included diabetes, use of a weight loss diet in the previous 6 months, pregnancy, or significant cardiac disease. Due to the use of radioisotopes as part of the assessments, women of childbearing age were excluded if they were not using effective contraception. All subjects gave written informed consent, and the protocol was approved by the research ethics committees of Northern Ireland and the Administration of Radioactive Substances Advisory Committee.
Habitual dietary intake was assessed at baseline using a 4-day food diary (including at least 1 weekend day). Thereafter, a parallel-group randomized controlled trial design was used to assign volunteers to an 8-week period of calorie restriction, during which they consumed one of two diets. Volunteers were randomized in blocks of four using a random number generator to ensure that equal numbers of volunteers received the low-carbohydrate and low-fat diets. Throughout the intervention, volunteers were advised to maintain their usual level of physical activity and keep other lifestyle factors unchanged. In addition, each volunteer took a standard multivitamin and mineral tablet.
Volunteers attended the Regional Centre for Endocrinology and Diabetes on two separate occasions (at 0745 h following an overnight fast) for baseline assessment. On the first occasion, anthropometric measurements and fasting blood samples were taken (for determination of plasma glucose, insulin, C-peptide, A1C, renal function, lipids, leptin, and RBP4). Arterial stiffness (by pulse-wave analysis) and insulin secretion (during a meal tolerance test) were also assessed. On the second occasion, insulin resistance was assessed by the euglycemic-hyperinsulinemic clamp combined with isotope dilution techniques. Whole-body dual-energy X-ray absorptiometry (DEXA) scanning was also performed (Lunar Prodigy scanner; GE Medical Systems, Madison, WI) in conjunction with Encore 2002 software. At the end of the 8-week dietary intervention, the baseline measurements were repeated.
Dietary intervention.
A 7-day cyclic menu was formulated for both diets using the dietary analysis program WISP (Weighed Intake Software Program; Tinuviel Software, Warrington, U.K.). The low-carbohydrate and low-fat diets were matched for protein (20% of energy) and fiber (18 g/day) but varied in carbohydrate (20 vs. 60% of total energy) and total fat content (60 vs. 20% of energy), respectively. The low-fat diet was also designed to derive an equivalent of 13% of energy from nonmilk extrinsic sugars, reflecting the average amount consumed by adults in the U.K. (16). Diets were designed and administered to produce a minimum weight loss of 0.5 kg per week. To attain this goal, volunteers were fed their assigned diet with a calorie level deficit of ∼500 kcal/day of estimated energy requirements (estimated by multiplying basal metabolic rate by an appropriate activity factor) (17). Total energy intakes were then modified (if required) on a weekly basis in 200-kcal decrements to maintain 0.5 kg weight loss per week, while still adhering to previously stated dietary profiles.
Volunteers attended on alternate days throughout the study and were supplied with all appropriate foodstuffs (preweighed into daily portions) for their particular diet. At each visit, compliance with the assigned diet was assessed by a nutritionist (M.S.) who asked questions concerning the palatability and acceptability of the diet. Foods that were “freely allowed” included noncaloric beverages (water, diet cola, tea, and coffee) and seasoning. Alcohol was not permitted. Representative menus for a single day on each diet are shown in Table 1.
TABLE 1.
Sample menus for a typical day on each intervention diet
| Meal | Low-carbohydrate diet (7.7 MJ) | Low-fat diet (7.6 MJ) |
|---|---|---|
| Breakfast | Scrambled eggs (40 g); bacon rasher, fat trimmed, grilled (20 g); hash brown, grilled (45 g) | Cornflakes (40 g), bran-based cereal (7 g), semiskimmed milk (200 g), pineapple juice (250 g) |
| Lunch | Wholemeal bread (72 g), cooked ham (30 g), mayonnaise (25 g) | Wholemeal bread (72 g); cooked ham (60 g); mayonnaise, reduced calorie (13 g); cheddar cheese, reduced fat (20 g) |
| Dinner | Potatoes, in skins, boiled (145 g); chicken breast in crumbs, baked (140 g); peas, boiled (95 g); carrots, boiled (70 g); gravy instant granules (4 g) | Potatoes, in skins, boiled (260 g); chicken breast in crumbs, baked (120 g); peas, boiled (50 g); gravy instant granules (5 g); |
| Additional foods | Peanuts, roasted and salted (40 g); Brazil nuts (30 g); butter (21 g); cheddar cheese (44 g); | Chocolate mousse, low fat (55 g); apple (153 g); jaffa cake (13 g); cola (150 g); chewy cereal bar (23 g); fruit yogurt, low fat (150 g); white bread, toasted (27g); strawberry jam (10 g); semiskimmed milk (300 g) |
Assessment of insulin action.
At the beginning and end of the dietary period, insulin sensitivity was assessed by the euglycemic-hyperinsulinemic clamp combined with infusion of [3-3H] glucose as previously described (18,19). A continuous insulin infusion was administered for 2 h at 2.0 mU · kg−1 · min−1 (0 time to 120 min).
Calculations.
The non–steady-state equations of Steele et al. (20), as modified by De Bodo et al. (21), were used to determine the glucose appearance (Ra) and disappearance (Rd), assuming a pool fraction of 0.65 and extracellular volume of 190 ml/kg. Rates of endogenous (hepatic) glucose production were then calculated by subtraction of the exogenous glucose infusion rates required to maintain euglycemia from isotopically determined rates of glucose appearance.
Analytical techniques.
Arterialized venous blood was used for all analyses in the clamp studies. Plasma for measurement of glucose-specific activity was deproteinized with barium hydroxide and zinc sulfate by the method of Somogyi (22). Aliquots of tracer infusate and labeled exogenous glucose infusion were spiked into nonradioactive plasma and processed in parallel to allow calculation of [3-3H] glucose infusion rates. Serum insulin was measured by enzyme-linked immunosorbent assay (ELISA) (Abbot IMx; Abbott Laboratories, Berkshire, U.K.). Glucose was measured using an automated glucose oxidase method using a Beckman Glucose Analyser 2. Commercial kits were used to measure C-peptide (Dako Diagnostics, Ely, U.K.) and nonesterified free fatty acids (Wako Chemicals, Neuss, Germany).
Adipokine measurement.
Plasma levels of leptin and RBP4 were determined using commercially available sandwich ELISAs, according to the manufacturer's instructions (DRG Instruments, Marburg, Germany). Leptin assays were carried out on the Triturus EIA Analyser system (Girfols, Barcelona, Spain). The interassay coefficients of variation (CVs) were 6.8 and 8.5% for leptin and RBP4, respectively.
Assessment of insulin secretion.
Insulin secretion was assessed by a meal tolerance test. After basal samples, volunteers consumed a liquid meal over 15 min (Ensure Plus; Abbott Nutrition, Kent, U.K.) standardized to body weight (10 kcal/kg). The meal provided ∼57.0% energy from carbohydrate, 28.2% energy from fat, and 14.8% energy from protein. Blood samples (for determination of insulin, C-peptide, glucose, and triglycerides) were taken every 30 min for the first hour and then every hour for the next 3 h.
Arterial stiffness.
Arterial stiffness was measured using pulse-wave analysis (model SCOR-Px; PWV Medical, Sydney, Australia) as described previously (18,23,24). The augmentation index (AIx, expressed as a percentage) was defined as the ratio of augmentation to pulse pressure and was used to estimate overall systemic arterial stiffness.
Statistical analysis.
Statistical analysis was performed using SPSS 13.0 for Windows (SPSS, Chicago, IL). Results are expressed as means ± SD. Independent-samples t tests were used to compare the two groups at baseline. Within-group changes in end points were assessed by using paired-samples t tests. Repeated-measures ANOVA with diet (i.e., low carbohydrate versus low fat) was set as a between-subject effect, and time (before diet versus after diet) as a within-subject effect was used to compare responses over time in both groups. The study (n = 12 per diet group) had a 90% power (with P < 0.05; two tailed) to detect a 3.5 μmol · kg−1 · min−1 (∼10%) difference in insulin action change between the two groups.
RESULTS
A total of 24 volunteers (15 female and 9 male subjects) successfully completed the intervention. The baseline clinical and anthropometric characteristics of the subjects completing the study (n = 12 assigned to the low-carbohydrate diet [7 female/5 male] and n = 12 [8 female/4 male] assigned to the low-fat diet) are given in Table 2. Volunteers were, on average, obese but were normotensive with normal fasting lipid profiles. The mean fasting plasma glucose for both groups was toward the upper limit of normal. Although glucose tolerance tests were not performed, the mean fasting plasma glucose of 5.6 mmol/l was in the pre-diabetes range, consistent with an increased risk for development of diabetes. Baseline clinical and anthropometric variables did not differ significantly between the two groups.
TABLE 2.
Baseline clinical and anthropometric characteristics of volunteers assigned to a low-carbohydrate (n = 12) or low-fat diet (n = 12)
| Low-carbohydrate diet | Low-fat diet | P* | |
|---|---|---|---|
| Age (years) | 37.1 ± 8.9 | 40.5 ± 10.4 | 0.40 |
| Height (m) | 1.68 ± 0.10 | 1.67 ± 0.12 | 0.25 |
| Weight (kg) | 97.7 ± 14.4 | 91.5 ± 11.1 | 0.78 |
| BMI (kg/m2) | 34.5 ± 4.2 | 32.8 ± 3.0 | 0.28 |
| Systolic blood pressure (mmHg) | 122 ± 12 | 127 ± 15 | 0.41 |
| Diastolic blood pressure (mmHg) | 70 ± 7 | 77 ± 9 | 0.06 |
| Waist circumference (cm) | 107.0 ± 11.1 | 105.1 ± 9.0 | 0.66 |
| Waist-to-hip ratio | 0.92 ± 0.08 | 0.92 ± 0.09 | 0.90 |
| Fasting plasma glucose (mmol/l) | 5.5 ± 0.8 | 5.6 ± 0.5 | 0.74 |
| Fasting insulin (mU/l) | 12.5 ± 6.2 | 11.4 ± 6.1 | 0.65 |
| A1C (%) | 5.3 ± 0.3 | 5.4 ± 0.3 | 0.79 |
| Total cholesterol (mmol/l) | 4.65 ± 1.06 | 5.00 ± 0.99 | 0.41 |
| LDL cholesterol (mmol/l) | 2.47 ± 1.08 | 2.85 ± 0.88 | 0.35 |
| HDL cholesterol (mmol/l) | 1.47 ± 0.40 | 1.40 ± 0.39 | 0.68 |
| Triglycerides (mmol/l) | 1.59 ± 0.55 | 1.55 ± 0.90 | 0.90 |
Data are means ± SD.
*Between-group comparisons analyzed by independent-samples t test.
Analysis of habitual dietary intake from 4-day food diaries collected at baseline (Table 3) showed that mean consumption of energy and nutrient intakes were similar between both dietary groups. The total dietary energy provided by carbohydrate and fat intake (at baseline) was ∼41 and 40%, respectively, suggesting that volunteers' diets were slightly lower in carbohydrate (by ∼8%) and higher in fat (by ∼4%) than average intakes reported for the adult population of the U.K. (16). Daily intakes of energy and nutrients consumed by volunteers during the intervention period for the low-carbohydrate and low-fat diets, respectively, are displayed in Table 4. Diets provided comparable amounts of energy and were matched for protein and fiber intake but in line with the study protocol were significantly different in terms of the profile of the other main macronutrients. Physical activity remained constant throughout the study.
TABLE 3.
Baseline daily energy and nutrient intakes of volunteers assigned to a low-carbohydrate (n = 12) or low-fat diet (n = 12)
| Low-carbohydrate diet | Low-fat diet | P* | |
|---|---|---|---|
| Energy (MJ/day) | 11.0 ± 2.5 | 9.5 ± 1.4 | 0.11 |
| Carbohydrate (total energy %) | 40 ± 7 | 41 ± 7 | 0.92 |
| Starch | 25 ± 3 | 24 ± 5 | 0.42 |
| Sugars | 15 ± 7 | 17 ± 4 | 0.46 |
| Nonmilk extrinsic sugar | 8 ± 6 | 11 ± 4 | 0.29 |
| Protein (total energy %) | 15 ± 2 | 16 ± 4 | 0.42 |
| Fat (total energy %) | 40 ± 8 | 39 ± 6 | 0.66 |
| Saturated fat | 15 ± 6 | 13 ± 3 | 0.34 |
| Monounsaturated fat | 13 ± 3 | 12 ± 3 | 0.69 |
| Polyunsaturated fat | 6 ± 2 | 7 ± 2 | 0.14 |
| Alcohol (total energy %) | 4 ± 2 | 4 ± 1 | 0.91 |
| Englyst fibre (g/day) | 18 ± 5 | 14 ± 4 | 0.71 |
Data are means ± SD.
*Between-group comparisons analyzed by independent-samples t test.
TABLE 4.
Intervention intakes of energy and nutrients consumed by subjects assigned to a low-carbohydrate (n = 12) or low-fat diet (n = 12)
| Low-carbohydrate diet | Low-fat diet | P* | |
|---|---|---|---|
| Energy (MJ/day) | 7.9 ± 1.8 | 7.1 ± 1.2 | 0.25 |
| Carbohydrate (total energy %) | 20 ± 0.1 | 60 ± 0.1 | <0.01 |
| Starch | 13 ± 1.9 | 35 ± 1.5 | <0.01 |
| Sugars | 7 ± 1.8 | 26 ± 1.4 | <0.01 |
| Nonmilk extrinsic sugar | 1 ± 0.8 | 16 ± 0.1 | <0.01 |
| Protein (total energy %) | 20 ± 0.1 | 20 ± 0.1 | 0.66 |
| Fat (total energy %) | 60 ± 0.1 | 20 ± 0.1 | <0.01 |
| Saturated fat | 21 ± 3.0 | 7 ± 0.7 | <0.01 |
| Monounsaturated fat | 21 ± 1.7 | 6 ± 0.5 | <0.01 |
| Polyunsaturated fat | 13 ± 2.3 | 3 ± 0.4 | <0.01 |
| Englyst fibre (g/day) | 18 ± 0.1 | 18 ± 0.1 | 0.13 |
Data are means ± SD.
*Between-group comparisons analyzed by independent-samples t test.
Weight loss and body composition.
The results in Table 5 indicate that the mean weight loss from baseline was similar between the two diet groups (P = 0.40). The mean weight loss following the low-carbohydrate and low-fat dietary interventions represented 7.6 and 7.1% of initial body weight, respectively (P < 0.01 within each group). There was no significant difference in the change in either waist circumference (reflecting central adiposity) or percentage body fat (determined by DEXA) between the diet groups (P = 0.63 and P = 0.77, respectively), although each significantly decreased compared with baseline (P < 0.05) following both dietary regimens.
TABLE 5.
Changes in anthropometric measurements, body composition, and metabolic variables in response to a hypocaloric low-carbohydrate (n = 12) and low-fat diet (n = 12)
| Variable | Low-carbohydrate diet |
Low-fat diet |
Between-group P† | ||||
|---|---|---|---|---|---|---|---|
| Before diet | After diet | P* | Before diet | After diet | P* | ||
| Weight (kg) | 97.7 ± 14.4 | 90.3 ± 12.9 | <0.01 | 91.5 ± 11.1 | 85.0 ± 11.2 | <0.01 | 0.40 |
| BMI (kg/m2) | 34.5 ± 4.2 | 31.9 ± 3.9 | <0.01 | 32.8 ± 3.0 | 30.5 ± 3.0 | <0.01 | 0.51 |
| Waist circumference (cm) | 107.0 ± 11.1 | 102.4 ± 10.4 | <0.01 | 105.1 ± 9.0 | 100.0 ± 8.5 | <0.01 | 0.63 |
| DEXA fat body mass (kg) | 38.8 ± 8.5 | 34.9 ± 9.0 | <0.01 | 37.3 ± 6.5 | 33.5 ± 6.6 | <0.01 | 0.89 |
| DEXA lean body mass (kg) | 54.4 ± 11.2 | 51.6 ± 9.8 | <0.01 | 50.3 ± 9.9 | 48.6 ± 9.8 | <0.01 | 0.19 |
| DEXA % body fat | 40.5 ± 6.6 | 38.9 ± 7.2 | 0.03 | 41.5 ± 6.4 | 39.7 ± 7.1 | <0.01 | 0.77 |
| Fasting plasma glucose (mmol/l) | 5.5 ± 0.8 | 5.4 ± 0.7 | 0.78 | 5.6 ± 0.5 | 5.4 ± 0.4 | 0.32 | 0.69 |
| Fasting serum insulin (mU/l) | 12.5 ± 6.2 | 7.4 ± 3.8 | 0.01 | 11.4 ± 6.1 | 9.0 ± 4.4 | 0.02 | 0.17 |
| A1C (%) | 5.3 ± 0.3 | 5.2 ± 0.003 | <0.01 | 5.4 ± 0.3 | 5.3 ± 0.4 | 0.41 | 0.36 |
| GIR (μmol · kg−1 · min−1) | 23.5 ± 7.4 | 28.3 ± 6.9 | 0.02 | 28.3 ± 9.5 | 30.2 ± 8.5 | 0.39 | 0.28 |
| Total cholesterol (mmol/l) | 4.65 ± 1.06 | 4.05 ± 0.82 | 0.06 | 5.00 ± 0.99 | 4.12 ± 1.00 | <0.01 | 0.43 |
| LDL cholesterol (mmol/l) | 2.46 ± 1.08 | 2.1 ± 0.78 | 0.05 | 2.85 ± 0.88 | 2.41 ± 0.76 | 0.01 | 0.64 |
| HDL cholesterol (mmol/l) | 1.47 ± 0.40 | 1.35 ± 0.32 | 0.11 | 1.40 ± 0.39 | 1.15 ± 0.30 | <0.01 | 0.12 |
| Triglycerides (mmol/l) | 1.59 ± 0.55 | 0.91 ± 0.33 | <0.01 | 1.55 ± 0.90 | 1.43 ± 0.57 | 0.44 | 0.01 |
| Leptin (ng/ml) | 35.6 ± 20.5 | 18.9 ± 18.3 | <0.01 | 36.1 ± 18.7 | 22.6 ± 15.5 | <0.01 | 0.30 |
| Retinol binding protein 4 (μg/ml) | 30.6 ± 4.6 | 26.5 ± 10.7 | 0.18 | 30.2 ± 14.6 | 24.0 ± 5.2 | 0.11 | 0.63 |
Data are means ± SD.
*Within-group comparisons analyzed by paired-samples t test.
†Between-group comparisons analyzed by ANOVA. GIR, glucose infusion rate during euglycemic clamp.
Clamp studies.
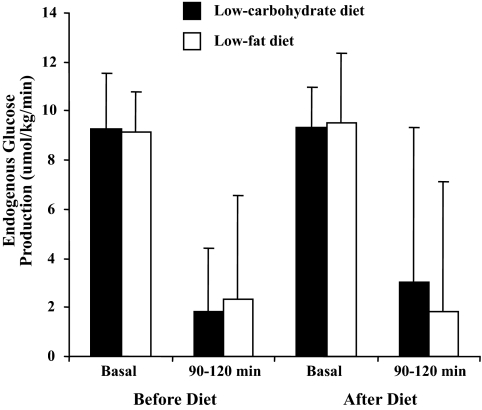
Plasma glucose was maintained at a constant level by exogenous glucose infusion with a CV <5% as the plateau for each clamp. There was no difference in the change in glucose infusion rate from baseline between the two diets (P = 0.28). Within the low-carbohydrate diet group, the glucose infusion rate significantly increased from baseline (23.5 ± 7.4 to 28.3 ± 6.9 μmol · kg−1 · min−1 postintervention, P = 0.02) (Table 5). In the low-fat diet group, no significant pre- to postdifference was observed in the glucose infusion rate (28.3 ± 9.5 μmol · kg−1 · min−1 at baseline to 30.2 ± 8.5 μmol · kg−1 · min−1 postintervention, P = 0.39). Fasting (basal) endogenous glucose production was similar following both diets and suppressed to a comparable degree during hyperinsulinemia (90–120 min) (Fig. 1). There was no difference in serum nonesterified fatty acid suppression when comparing baseline and end studies within the diet groups (low-carbohydrate group P = 0.73 and low-fat group P = 0.47). On comparison, between the groups there was no difference in suppression at the end of the study (P = 0.72) (Table 6).
FIG. 1.
Endogenous glucose production during clamp studies.
TABLE 6.
Changes in serum nonesterified fatty acid levels at basal state (−30 to 0 min) and during insulin infusion (90–120 min) in response to a hypocaloric low-carbohydrate (n = 12) and low-fat diet (n = 12)
| Variable | Low-carbohydrate diet |
Low-fat diet |
Between-group P† | ||||
|---|---|---|---|---|---|---|---|
| Before diet | After diet | P* | Before diet | After diet | P* | ||
| Nonesterified fatty acids (μmol/l) at basal state (−30 to 0 min)‡ | 706 ± 160 | 715 ± 194 | 0.89 | 698 ± 190 | 796 ± 209 | 0.09 | 0.33 |
| Nonesterified fatty acids (μmol/l) during insulin infusion (90–120 min)‡ | 180 ± 58 | 213 ± 82 | 0.10 | 244 ± 144 | 265 ± 210 | 0.34 | 0.78 |
Data are means ± SD.
*Within-group comparisons analyzed by paired-samples t test.
†Between-group comparisons analyzed by ANOVA.
‡Measured in clamp assessment.
Metabolic profiles.
Changes in fasting plasma glucose, A1C, and fasting serum insulin were not significantly different when the diets were compared (P = 0.69, 0.36, and 0.17, respectively). Within the low-carbohydrate and low-fat diet group, fasting serum insulin significantly decreased (P = 0.01 and P = 0.02, respectively), whereas fasting plasma glucose remained unchanged (P = 0.78 and P = 0.32, respectively). The change in A1C was only significant within the low-carbohydrate diet group (P < 0.01) (Table 5).
The only significant difference in lipid profiles between the two diets was the change in triglycerides (P = 0.01). This difference reflected a significant reduction in triglycerides after the low-carbohydrate diet (1.59 ± 0.55 to 0.91 ± 0.33 mmol/l postintervention, P < 0.01). Within the low-fat diet group, total, LDL, and HDL cholesterol did, however, decrease significantly following the intervention (P < 0.05).
Meal tolerance tests.
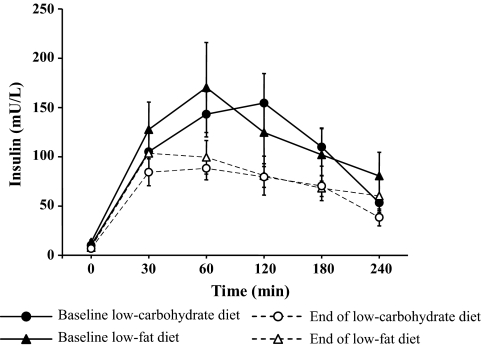
There was no significant difference in the change in insulin secretion from baseline between diets (P = 0.71). There was a significant reduction in meal-related insulin secretion following the low-carbohydrate diet (P = 0.05), whereas following the low-fat diet there was a similar trend toward a reduction, which was not statistically significant (P = 0.10). There was no change in glucose levels from baseline with either the low-fat or low-carbohydrate diet (P = 0.53 and P = 0.23, respectively, data not shown); consequently, there was no difference in the change from baseline in glucose levels between diets (P = 0.98). Likewise, there was no difference in change between the diets for postprandial triglyceride levels (P = 0.97, data not shown) (Fig. 2 and the online appendix [available at http://diabetes.diabetesjournals.org/cgi/content/full/db09-0098/DC1]).
FIG. 2.
Serum insulin levels during meal tolerance tests at baseline and following the hypocaloric low-carbohydrate and low-fat diets.
Adipokines.
Plasma leptin levels decreased significantly within each diet group (P < 0.01), but changes between groups were not significant (P = 0.30). Changes in plasma RBP4 were not significant within (low-carbohydrate group P = 0.18 and low-fat group P = 0.11) or between diet groups (P = 0.63) (Table 5).
Hemodynamic studies.
Improvements in systolic and diastolic blood pressure (both P < 0.05) were similar between the two diet groups (P = 0.91 and 0.73, respectively). The change in AIx was, however, significantly different (P = 0.04) when the two diets were directly compared. This difference related to a significant decrease in AIx following the low-fat diet (mean change in AIx at the end of 8 weeks 3.75 ± 5.53%, P = 0.04) compared with a nonsignificant increase within the low-carbohydrate group (mean change in AIx at the end of 8 weeks = 2.17 ± 7.22%, P = 0.32). Other indexes of arterial stiffness (including augmentation, time-to-wave reflection, and brachial pulse-wave velocity) were not significantly different between or within diet groups (Table 7).
TABLE 7.
Changes in hemodynamic variables in response to a hypocaloric low-carbohydrate (n = 12) and low-fat diet (n = 12)
| Variable | Low-carbohydrate diet |
Low-fat diet |
Between-group P† | ||||
|---|---|---|---|---|---|---|---|
| Before diet | After diet | P* | Before diet | After diet | P* | ||
| Systolic blood pressure (mmHg) | 122 ± 12 | 114 ± 10 | <0.01 | 127 ± 15 | 119 ± 11 | 0.03 | 0.91 |
| Diastolic blood pressure (mmHg) | 70 ± 7 | 65 ± 8 | 0.03 | 77 ± 9 | 71 ± 7 | <0.01 | 0.73 |
| Augmentation (mm/Hg) | 7.4 ± 7.1 | 8.4 ± 5.4 | 0.44 | 9.2 ± 7.4 | 8.3 ± 7.8 | 0.37 | 0.24 |
| Aortic augmentation index (%) | 12.3 ± 12.2 | 14.5 ± 11.9 | 0.32 | 17.0 ± 14.4 | 13.3 ± 16.3 | 0.04 | 0.04 |
| Time-to-wave reflection (ms) | 151 ± 20 | 156 ± 25 | 0.50 | 152 ± 23 | 161 ± 29 | 0.05 | 0.55 |
| Brachial pulse-wave velocity (ms) | 8.3 ± 0.6 | 8.2 ± 0.7 | 0.61 | 8.1 ± 1.7 | 8.1 ± 1.5 | 1.00 | 1.00 |
Data are means ± SD.
*Within-group comparisons analyzed by paired-samples t test.
†Between-group comparisons analyzed by ANOVA.
DISCUSSION
This study demonstrates that under hypocaloric conditions, a low-carbohydrate diet (60% fat, 20% carbohydrate) and a low-fat diet (60% carbohydrate, 20% fat) were equally effective in producing weight loss in overweight/obese adults. The observed weight loss (>7%) with both hypocaloric diets is comparable to that achieved in both the Diabetes Prevention Program and the Finnish Diabetes Prevention Trial (8,9). In these trials, relatively modest weight loss (5–7% of initial body weight) and moderate physical activity resulted in a 58% reduction in the 4-year incidence of type 2 diabetes (in high-risk individuals with impaired glucose tolerance). The current study demonstrated no difference between a low-fat and a low-carbohydrate weight reduction diet in the effect on insulin sensitivity, which suggests a comparable effect on prevention of diabetes, independent of dietary macronutrient composition. However, it should be noted that subjects enrolled in the current study had mean fasting plasma glucose at the upper end of the normal range (consistent with pre-diabetes and hence increased risk for development of diabetes), a distinction from the diabetes prevention trials that enrolled subjects with impaired glucose tolerance.
Previous studies (25,26) have demonstrated more rapid weight loss with low-carbohydrate diets. In this study, we did not restrict carbohydrate sufficiently to induce ketogenesis, and carbohydrate intake was maintained constant throughout the study, rather than the gradual increase in carbohydrate intake advocated by the Atkins diet. Furthermore, the caloric deficit was the same for both diets, and this may explain the comparable weight loss profiles.
A strength of the present study is the randomized controlled design and rigorous dietetic supervision. Menu plans were individually formulated to each volunteer's like/dislikes, and all meals were provided, ensuring that the intended composition was supplied to all volunteers. Although it is impossible to measure compliance with the diets under study, volunteers were reviewed every 2–3 days throughout the study and questioned regarding palatability and compliance with food provided. The intended weight loss was achieved, and this is further evidence of careful nutritional supervision and planning.
The primary outcome measure of insulin sensitivity was assessed using the gold standard hyperinsulinemic-euglycemic clamp combined with isotope dilution techniques. The high-dose insulin infusion used during the euglycemic clamp results in maximally stimulated glucose uptake and reflects skeletal muscle or peripheral insulin sensitivity. In our study group as a whole (n = 24), peripheral insulin sensitivity significantly improved (P = 0.03), but the change was only significant within the low-carbohydrate diet (P = 0.02), and there was no significant difference between groups (P = 0.28). The study was powered to exclude a 10% difference in insulin action, which is a level assumed to have a clinically relevant impact.
There was also no significant effect of altering macronutrient content on either fasting hepatic glucose production or its suppression during the clamp studies, which are both measures of hepatic insulin resistance, which is recognized as an early abnormality in type 2 diabetes (20). Furthermore, we found no difference in either fasting levels of nonesterified fatty acid concentrations or their suppression during hyperinsulinemia, indicating no differential effect on adipose tissue insulin action and suppression of lipolysis.
Previous studies suggest that improvement in insulin sensitivity after consumption of a low-carbohydrate diet is similar to that seen on a low-fat diet (when associated with weight loss) (25,26). However, this is based on suboptimal methods such as the quantitative insulin sensitivity check index (QUICKI). Diets high in total fat and saturated fat (relative to monounsaturated fat) have been shown to impair insulin sensitivity (15,27). The mechanism of this effect is not clearly understood, although it is possible that dietary modification modulates changes in the fatty acid composition of cell membranes, thus influencing insulin receptor binding/activity as well as ion permeability and cell signaling (28). However, no appreciable differences were found between diet groups in the current study using the reference standard technique to assess insulin sensitivity. It is possible that the effects of weight loss overcame any lesser effect of dietary macronutrient intake.
Abnormalities of insulin secretion may also contribute to the development of diabetes (29). We demonstrated a comparable effect of the two diets on both fasting and meal tolerance–related insulin secretion. Given the similar effects on insulin resistance, we conclude that both diets exert equivalent effects on prevention of diabetes, primarily related to the degree of weight reduction. The comparable reduction in BMI was largely attributable to a decrease in fat mass, mainly from the central body area, as demonstrated by both DEXA scanning and a reduction in waist circumference. Increased central body fat is associated with insulin resistance and the metabolic syndrome, and the reduction in central adiposity was related to the improvement in insulin sensitivity, which in turn might be expected to reduce the risk of type 2 diabetes and also cardiovascular risk (30).
As expected, the low-fat diet decreased both LDL and HDL cholesterol. Although the low-carbohydrate diet did not decrease LDL cholesterol, it was not associated with a significant decline in HDL cholesterol. Given the established evidence that LDL lowering reduces the risk of coronary heart disease, the lack of a decrease may be of concern. In contrast to the lack of a change in LDL and HDL in response to the low-carbohydrate diet, there was a significant reduction in triglycerides within this group compared with no significant change within the low-fat group. This response has been consistently reported in other studies comparing a low-carbohydrate and low-fat weight reduction diet (12). It has been speculated that this result is due to a combination of a decrease in the VLDL production rate and an increase in triglyceride removal from the blood (31). Previous studies (32,33) indicate that increased triglycerides are an independent risk factor for cardiovascular disease, although it is impossible to predict the overall effect of the lipid changes with the low-carbohydrate diet. Further examination of the lipid subfraction profile may help elucidate the effects of the dietary regimens on lipid metabolism. Indeed, previous studies have suggested that low-carbohydrate diets increase LDL particle size and decrease small dense LDL particles (34).
A major concern associated with low-carbohydrate diets is that the reciprocal increase in dietary fat intake, particularly if this includes saturated and trans fat, may have detrimental effects on cardiovascular risk. In addition to examining cardiovascular risk factors, we also assessed arterial stiffness, which is increasingly recognized as an important determinant of cardiovascular risk (35). Stiffening of the arterial tree increases the velocity and amplitude of the reflected pulse waves from the periphery, with the result that larger waves return to the aorta earlier. This augments central systolic pressure, which increases left ventricular workload and myocardial oxygen demand. In the present study, the change in AIx (a measure of overall systemic stiffness) was significantly more favorable with the low-fat diet, with a significant and favorable reduction in AIx compared with a nonsignificant increase in the low-carbohydrate diet group. A post hoc power analysis revealed that the study had 90% power to detect a between-diet difference of 9% for augmentation index. Although an isolated finding in the present study, this is consistent with a recent report that demonstrated a reduction in flow-mediated dilation following a low-carbohydrate diet compared with an increase after a low-fat diet (36). It is possible that the high fat content of a low-carbohydrate diet exerts detrimental effects on endothelial function, which raises concerns regarding the long-term safety and efficacy of low-carbohydrate diets.
Changes in leptin levels were comparable in both diets and related to weight loss rather than any specific effect of dietary macronutrient composition (37). Recently, RBP4 has been proposed as an adipocyte-derived factor that may regulate insulin sensitivity (7). Diet-induced changes in RBP4 were not responsible for the change in insulin sensitivity in the current study, as neither the low-fat nor the low-carbohydrate weight loss regimen had any significant effect on circulating concentrations. This is in keeping with one previous study (38), but in another study (7) changes in RBP4 were associated with an improvement in insulin sensitivity. Differences in study design may explain these conflicting findings (e.g., the latter study utilized an exercise intervention and examined both normal subjects and those with type 2 diabetes).
One limitation of this study is that the subjects were of white Western European origin and did not have significant baseline abnormalities. These factors can alter baseline insulin sensitivity and may influence interventional responses. In addition, to allow conclusions to be drawn about varying the carbohydrate and fat content of hypocaloric diets, overall calorie intake was controlled rather than allowing ad libitum consumption, thus allowing protein intake and fiber to be accurately matched in both diets. Furthermore, the type of fat in a low-carbohydrate diet (i.e., saturated/trans fat versus mono/polyunsaturated fat) may be important (39). The conclusions from these data must therefore be limited to the described overweight/obese group consuming a weight loss diet in a carefully controlled situation.
In conclusion, under the conditions of this study, a low-carbohydrate hypocaloric diet was as effective as a low-fat hypocaloric diet in achieving significant weight loss during an 8-week period. The 7% weight loss with both diets is comparable with the magnitude seen in diabetes prevention studies and is significant in terms of disease prevention (9,10). Both diets promoted weight loss from the central body region and were associated with comparable effects on insulin sensitivity. There was, however, a significant difference in AIx, a measure of vascular compliance, between the two diets that was not explained by changes in conventional vascular risk factors. This observation is of concern and, if confirmed, would suggest a potentially negative effect of a low-carbohydrate diet on long-term vascular health. Currently, supported by evidence from long-term trials, we believe that a low-fat diet should remain the preferred diet for diabetes prevention.
Supplementary Material
Acknowledgments
This study was supported by RRG 5.42 (principal investigator S.J.H.) from the Northern Ireland Department of Health and Social Services Research and Development Office and by an unrestricted research grant from The Sugar Bureau (U.K.).
No potential conflicts of interest relevant to this article were reported.
We are grateful to Dr. C. Patterson, The Queen's University of Belfast, for statistical advice; to Dr. C. Mercer, School of Medicine, The Queen's University of Belfast, for carrying out nonesterified fatty acid assays; to C. McMaster, School of Medicine, the Queen's University of Belfast, for carrying out adipokine assays; and to Brian Sheridan and the staff at Belfast Link Laboratories for carrying out insulin and C-peptide assays.
Footnotes
Clinical trial reg. no. ISRCTN85769730.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Klein S, Sheard NF, Pi-Sunyer X, Daly A, Wylie-Rosett J, Kulkarni K, Clark NG: Weight management through lifestyle modification for the prevention and management of type 2 diabetes: rationale and strategies. Diabetes Care 2004; 28: 2067– 2073 [DOI] [PubMed] [Google Scholar]
- 2.Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RHthe American Heart Association, Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation 2006; 113: 898– 918 [DOI] [PubMed] [Google Scholar]
- 3.Malik VS, Schulze MB, Hu FB: Intake of sugar-sweetened beverages and weight gain: a systematic review. Am J Clin Nutr 2006; 84: 274– 288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organisation Diet, Nutrition and the Prevention of Chronic Diseases: Report of a Joint WHO/FAO Expert Consultation Geneva, World Health Org., 2002. ( Tech. rep. ser. no. 916) [Google Scholar]
- 5.Taylor R: Pathogenesis of type 2 diabetes: tracing the reverse route from cure to cause. Diabetologia 2008; 51: 1781– 1789 [DOI] [PubMed] [Google Scholar]
- 6.Bonora E: Relationship between regional fat distribution and insulin resistance. Internat J Obes Relat Metab Disord 2000; 24( Suppl. 2): S32– S35 [DOI] [PubMed] [Google Scholar]
- 7.Graham TE, Yang Q, Bluher M, Hammarstedt A, Ciaraldi TP, Henry RR, Wason CJ, Oberbach A, Jansson P, Smith U, Kahn B: Retinol binding protein 4 and insulin resistance in lean, obese and diabetic subjects. N Engl J Med 2006; 354: 2552– 2563 [DOI] [PubMed] [Google Scholar]
- 8.Volek JS, Phinney SD, Forsythe CE, Quann EE, Wood RJ, Puglisi MJ, Kraemer WJ, Bibus DM, Fernandez ML, Feinman RD: Carbohydrate restriction has a more favorable impact on the metabolic syndrome than a low fat diet. Lipids 2009; 44: 297– 309 [DOI] [PubMed] [Google Scholar]
- 9.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DMthe Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346: 393– 403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa Mthe Finnish Diabetes Prevention Study Group Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001; 344: 1343– 1350 [DOI] [PubMed] [Google Scholar]
- 11.Kearney JM, McElhone S: Perceived barriers in trying to eat healthier-results of a pan-EU consumer attitudinal survey. Br J Nutr 1999; 81( Suppl. 2): S133– S137 [DOI] [PubMed] [Google Scholar]
- 12.Bravata DM, Sanders L, Huang J, Krumholz HM, Olkin I, Gardner CD, Bravata DM: Efficacy and safety of low carbohydrate diets; a systematic review. JAMA 2003; 289: 1837– 1850 [DOI] [PubMed] [Google Scholar]
- 13.Lara-Castro C, Garvey WT: Diet, insulin resistance, and obesity: zoning in on data for Atkins dieters living in South Beach. J Clin Endocrinol Metab 2004; 89: 4197– 4205 [DOI] [PubMed] [Google Scholar]
- 14.Volek JS, Fernandez ML, Feinman RD, Phinney SD: Dietary carbohydrate restriction induces a unique metabolic state positively affecting atherogenic dyslipidemia, fatty acid partitioning, and metabolic syndrome. Prog Lipid Res 2008; 47: 307– 318 [DOI] [PubMed] [Google Scholar]
- 15.Parillo M, Riccardi G: Diet composition and the risk of type 2 diabetes: epidemiological and clinical evidence. Br J Nutr 2004; 92: 7– 19 [DOI] [PubMed] [Google Scholar]
- 16.Henderson L, Gregory J, Irving K, Swan G: The National Diet and Nutrition Survey: Adults aged 19 to 64. Volume 2: Energy, Protein, Carbohydrate, Fat and Alcohol Intake London, The Stationery Office, 2003 [Google Scholar]
- 17.Schofield WN: Predicting basal metabolic rate, new standards and review of previous work. Hum Nutr Clin Nutr 1985; 39( Suppl. 1): 5– 41 [PubMed] [Google Scholar]
- 18.Black RN, Spence M, McMahon RO, Cuskelly GJ, Ennis CE, McCance DR, Young IS, Bell PM, Hunter SJ: Effect of eucaloric high- and low-sucrose diets with identical macronutrient profile on insulin resistance and vascular risk: a randomized controlled trial. Diabetes 2006; 55: 3566– 3572 [DOI] [PubMed] [Google Scholar]
- 19.Hunter SJ, Boyd AC, O'Harte FP, McKillop AM, Wiggam MI, Mooney MH, McCluskey JT, Lindsay JR, Ennis CN, Gamble R, Sheridan B, Barnett CR, McNulty H, Bell PM, Flatt PR: Demonstration of glycated insulin in human diabetic plasma and decreased biological activity assessed by euglycemic-hyperinsulinemic clamp technique in humans. Diabetes 2003; 52: 492– 498 [DOI] [PubMed] [Google Scholar]
- 20.Steele R, Wall JS, De Bodo RC, Altszuler N: Measurement of size and turnover rate of body glucose pool by the isotope dilution method. Am J Physiol 1956; 187: 15– 24 [DOI] [PubMed] [Google Scholar]
- 21.Debodo RC, Steele R, Altszuler N, Dunn A, Bishop JS: On the hormonal regulation of carbohydrate metabolism; studies with C14 glucose. Recent Prog in Horm Res 1963; 19: 445– 488 [PubMed] [Google Scholar]
- 22.Somogyi M: Determination of blood sugar. J Biol Chem 1945; 160: 69– 73 [Google Scholar]
- 23.Wilkinson IB, Fuchs SA, Jansen IM, Spratt JC, Murray GD, Cockcroft JR, Webb DJ: Reproducibility of pulse wave velocity and augmentation index measured by pulse wave analysis. J Hypertens 1998; 16: 2079– 2084 [DOI] [PubMed] [Google Scholar]
- 24.Mullan BA, Ennis CN, Fee HJ, Young IS, McCance DR: Protective effects of ascorbic acid on arterial haemodynamics during acute hyperglycemia. Am J Physiol Heart Circ Physiol 2004; 287: H1262– H1268 [DOI] [PubMed] [Google Scholar]
- 25.Foster GD, Wyatt HR, Hill JO, McGuckin BG, Brill C, Mohammed BS, Szapary PO, Rader DJ, Edman JS, Klein S: A Randomized trial of a low-carbohydrate diet for obesity. N Engl J Med 2003; 348: 2082– 2090 [DOI] [PubMed] [Google Scholar]
- 26.Samaha FF, Iqbal N, Seshadri P, Chicano KL, Daily DA, McGrory J, Williams T, Williams M, Gracely EJ, Stern L: A low-carbohydrate as compared with a low-fat diet in severe obesity. N Engl J Med 2003; 348: 2074– 2081 [DOI] [PubMed] [Google Scholar]
- 27.Vessby B, Unsitupa M, Hermansen K, Riccardi G, Rivellese AA, Tapsell LC, Nalsen C, Berglund L, Louheranta A, Rasmussen BM, Calvert GD, Maffetone A, Pedersen E, Gustafsson IB, Storlien LH: Substituting dietary saturated for monounsaturated fat impairs insulin sensitivity in healthy men and women: the KANWU study. Diabetologia 2001; 44: 312– 319 [DOI] [PubMed] [Google Scholar]
- 28.Pan DA, Lillioja S, Milner MR, Kriketos AD, Baur LA, Bogardus C, Storlien LH: Skeletal muscle membrane lipid composition is related to adiposity and insulin action. J Clin Invest 1995; 96: 2802– 2808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferranninni E: Insulin resistance versus insulin deficiency in non-insulin dependent diabetes mellitus problems and prospects. Endocr Rev 1988; 19: 477– 490 [DOI] [PubMed] [Google Scholar]
- 30.Haffner SM: Abdominal adiposity and cardiometabolic risk: do we have all the answers? Am J Med 2007; 120: S10– S16 [DOI] [PubMed] [Google Scholar]
- 31.Adam-Perrot A, Clifton P, Brouns F: Low-carbohydrate diets: nutritional and physiological aspects. Obes Rev 2006; 7: 49– 58 [DOI] [PubMed] [Google Scholar]
- 32.Austin MA, Hokanson JE, Edwards KL: Hypertriglyceridemia as a cardiovascular risk factor. Am J Cardiol 1998; 81: 7B– 12B [DOI] [PubMed] [Google Scholar]
- 33.Assmann G, Schulte H, Funke H, von Eckardstein A: The emergence of triglycerides as a significant independent risk factor in coronary artery disease. Eur Heart J 1998; 19( Suppl. M): M8– M14 [PubMed] [Google Scholar]
- 34.Dreon DM, Fernstrom HA, Williams PT, Krauss RM: A very low-fat diet is not associated with improved lipoprotein profiles in men with a predominance of large, low-density lipoproteins. Am J Clin Nutr 1999; 69: 411– 418 [DOI] [PubMed] [Google Scholar]
- 35.Arnett DK, Evans GW, Riley WA: Arterial stiffness: a new cardiovascular risk factor? Am J Epidemiol 1994; 140: 669– 682 [DOI] [PubMed] [Google Scholar]
- 36.Phillips SA, Jurva WJ, Syed Q, Syed AQ, Kulinski JP, Pleuss J, Hoffmann G, Gutterman DD: Benefit of a low-fat over a low-carbohydrate diet on endothelial health in obesity. Hypertension 2008; 51: 376– 382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shai I, Schwarzfuchs D, Henkin Y, Shahar DR, Witkow S, Greenberg I, Golan R, Fraser D, Bolotin A, Vardi H, Tangi-Rozental O, Zuk-Ramot R, Sarusi B, Brickner D, Schwartz Z, Sheiner E, Marko R, Katorza E, Thiery J, Fiedler GM, Bluher M, Stumvoll M, Stampfer MJ: Weight loss with a low-carbohydrate, mediterranean or low-fat diet. N Engl J Med 2008; 359: 229– 241 [DOI] [PubMed] [Google Scholar]
- 38.Haider DG, Schindler K, Prager G, Bohdjalian A, Luger A, Wolzt M, Ludvik B: Serum retinol binding protein 4 is reduced after weight loss in morbidly obese subjects. J Clin Endocrinol Metab 2007; 92: 1168– 1171 [DOI] [PubMed] [Google Scholar]
- 39.Halton TL, Willett WC, Liu S, Manson JE, Albert CM, Rexrode K, Hu FB: Low carbohydrate diet score and the risk of coronary heart disease in women. N Engl J Med 2006; 355: 1991– 2002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.