Abstract
OBJECTIVE
Insulin represses the expression of gluconeogenic genes at the mRNA level, but the hormone appears to have only weak inhibitory effects in vivo. The aims of this study were 1) to determine the maximal physiologic effect of insulin, 2) to determine the relative importance of its effects on gluconeogenic regulatory sites, and 3) to correlate those changes with alterations at the cellular level.
RESEARCH DESIGN AND METHODS
Conscious 60-h fasted canines were studied at three insulin levels (near basal, 4×, or 16×) during a 5-h euglycemic clamp. Pancreatic hormones were controlled using somatostatin with portal insulin and glucagon infusions. Glucose metabolism was assessed using the arteriovenous difference technique, and molecular signals were assessed.
RESULTS
Insulin reduced gluconeogenic flux to glucose-6-phosphate (G6P) but only at the near-maximal physiological level (16× basal). The effect was modest compared with its inhibitory effect on net hepatic glycogenolysis, occurred within 30 min, and was associated with a marked decrease in hepatic fat oxidation, increased liver fructose 2,6-bisphosphate level, and reductions in lactate, glycerol, and amino acid extraction. No further diminution in gluconeogenic flux to G6P occurred over the remaining 4.5 h of the study, despite a marked decrease in PEPCK content, suggesting poor control strength for this enzyme in gluconeogenic regulation in canines.
CONCLUSIONS
Gluconeogenic flux can be rapidly inhibited by high insulin levels in canines. Initially decreased hepatic lactate extraction is important, and later reduced gluconeogenic precursor availability plays a role. Changes in PEPCK appear to have little or no acute effect on gluconeogenic flux.
Hepatic glycogen metabolism in vivo is extremely sensitive to the effects of insulin. In the healthy state, small increases (twofold) in the plasma insulin level can result in near-complete inhibition of the net contribution of glycogen to hepatic glucose production (HGP) (1). This effect has been ascribed to activation of glycogen synthesis (2). On the other hand, the effects of insulin on hepatic gluconeogenesis are less potent, are more complex, and occur through multiple mechanisms. The direct inhibitory effect of insulin on the transcription and activity of key hepatic gluconeogenic enzymes through forkhead box class O-1 (FOXO1) phosphorylation, including phosphoenolpyruvate carboxykinase (PEPCK) (3,4), is well established. Further, insulin inhibits the secretion of glucagon, a known activator of gluconeogenesis (5), thereby bringing about an indirect inhibitory effect on the process in the liver. In addition, insulin inhibits lipolysis (6), which reduces circulating glycerol and nonesterified free fatty acid (NEFA) levels. Glycerol is an important gluconeogenic (GNG) precursor, and NEFAs provide energy for gluconeogenesis (7). Net hepatic lactate uptake is reduced by insulin through several mechanisms. First, the hormone influences substrate cycling through the glycolytic/GNG pathways via allosteric and phosphorylation-mediated changes in the activity of key enzymes (e.g., bifunctional enzyme and pyruvate kinase) (4,8). Second, hepatic lactate flux is regulated by insulin secondary to its inhibitory effect on lipolysis (e.g., via reduced citrate, which results in increased phosphofructokinase-1 [PFK-1] activity) (4,8,9). Insulin decreases net amino acid release from muscle via inhibition of proteolysis and potentially by increased protein synthesis (10), although the effect of reduced GNG amino acid precursor availability is offset to some degree by an increase in hepatic amino acid transport (11). Finally, evidence in rodents suggests that hepatic gluconeogenesis may also be inhibited as the result of insulin action in the hypothalamus. It is postulated that brain insulin action increases vagal transmission, thereby increasing the phosphorylation of signal transducer and activator of transcription 3 (STAT3) in the liver and in turn reducing PEPCK and glucose-6-phosphatase (G6Pase) transcription (12–14).
Assessment of gluconeogenesis in vivo is complicated by the sensitive effects of insulin on hepatic glycogen metabolism. Because higher insulin concentrations are required to suppress gluconeogenesis than to inhibit glycogenolysis or increase glycogen synthesis (15,16), gluconeogenically derived glucose-6-phosphate (G6P) can be diverted into hepatic glycogen even during mild hyperinsulinemia. As a result, redirection of the product of GNG flux into hepatic glycogen can decrease gluconeogenesis, per se, in the absence of a fall in GNG flux to G6P (1,17).
Despite the numerous mechanisms by which insulin can inhibit the GNG process, acute physiologic increases in insulin have minimal impact on GNG flux in humans and large animals (1,15,17–20). Much of what is known about the regulation of gluconeogenesis is derived from in vitro studies on tissues or cells that lack important inputs for GNG control (e.g., GNG precursor load, NEFA availability, neuronal transmission, glycogen, hormonal milieu). Likewise, many studies of GNG regulation have been performed in rodents, and it is not clear how control of the process may differ in those species compared with larger animals (e.g., basal rates of gluconeogenesis are 5–10 times higher in rats and mice than in humans or canines; glycogen stores are completely depleted during fasting in rodents but not in humans or canines).
Given the difficulty in detecting an inhibitory effect of insulin on GNG flux in vivo, conditions were optimized in the present study to allow such an effect to be seen. First, canines were fasted for 60 h to increase the percentage contribution of gluconeogenesis to HGP. In addition, because we previously failed to observe any suppression of GNG flux in overnight fasted canines when the level of insulin was increased 4-fold (20), we examined both 4- and 16-fold increases in insulin. Finally, insulin was elevated for 5 h to provide sufficient time for changes in insulin signaling to affect gene transcription and for the latter to translate into substantial effects on GNG enzyme levels and activities. To focus on the effects of insulin, glucagon and glucose were clamped at basal levels. In addition, liver biopsies were taken at the end of each study to allow the effects of insulin on molecular signaling and gene transcription to be correlated with alterations in whole-body metabolic flux rates.
RESEARCH DESIGN AND METHODS
Animal care and surgical procedures.
Sixteen conscious mongrel canines of either sex were studied after a 60-h fast. Housing and diet have been described previously (1). The surgical facility met the standards published by the American Association for the Accreditation of Laboratory Animal Care, and the protocols were approved by the Vanderbilt University Medical Center Animal Care Committee. All canines underwent a laparotomy 2 weeks before the experiment to implant portal vein infusion catheters into the jejunal and splenic veins, sampling catheters into the femoral artery and portal and hepatic veins, and ultrasonic flow probes (Transonic Systems, Ithaca, NY) around the hepatic artery and portal vein, as described elsewhere (1). All canines studied were healthy, as indicated by 1) leukocyte count <18,000/mm3, 2) hematocrit >35%, and 3) good appetite and normal stools.
Experimental design.
Animals were allowed to rest quietly in a Pavlov harness for 60 min before the experiments started. Each study consisted of a basal period (−40 to 0 min) and an experimental period (0–300 min). Somatostatin (0.8 μg · kg−1 · min−1; Bachem, Torrance, CA) was infused (0–300 min) to inhibit endogenous pancreatic hormone secretion. During the same period, intraportal infusions of glucagon (0.5 ng · kg−1 · min−1; Lilly, Indianapolis, IN) and insulin (300, 1,200, or 5,000 μU · kg−1 · min−1; Lilly) were given in the control (n = 5), 4× (n = 5), or 16× (n = 6) groups, respectively. Previously, when the plasma glucose level was maintained at basal levels by titration of intraportal insulin in 60-h fasted canines, the insulin requirement was 215 ± 24 μU · kg−1 · min−1 (21). Therefore, in the present study, insulin was infused in the control group at a rate slightly above basal. Glucose was infused intravenously to maintain euglycemia.
Immediately after the final sampling time, each animal was anesthetized and three sections of liver lobes were freeze clamped in situ and stored at −70°C as previously described (1). All animals were then killed, and the correct positions of the catheter tips were confirmed.
Analytical procedures.
Hematocrit; plasma glucose, glucagon, insulin, cortisol, and NEFA; and blood alanine, glycine, serine, threonine, lactate, glutamine, glutamate, glycerol, and β-hydroxybutyrate concentrations were determined as previously described (1). RNA extraction, cDNA synthesis, real-time PCR, SDS-PAGE, and Western blotting procedures were performed by standard methods, details of which are provided in the online appendix at http://diabetes.diabetesjournals.org/content/suppl/2009/08/25/db09-0328.DC1/DB09-0328_online_appendix.pdf. Fructose 2,6-bisphosphate (F2,6P2) levels and pyruvate kinase activity were determined as described in the online appendix.
Calculations.
Net hepatic substrate balances were calculated with the arteriovenous (A-V) difference method using the following formula: net hepatic balance = loadout – loadin, where loadout = [H] × HF and loadin = [A] × AF + [P] × PF and where [H], [A], and [P] are the substrate concentrations in hepatic vein, femoral artery, and portal vein blood or plasma, respectively, and HF, AF, and PF are the blood or plasma flow in the hepatic vein, hepatic artery, and portal vein, respectively, as determined by the ultrasonic flow probes. With this calculation, a positive value represents net output by the liver, whereas a negative value represents net hepatic uptake. Plasma glucose values were multiplied by 0.73 to convert them to blood glucose values as validated elsewhere (20). Net hepatic fractional extraction was calculated by dividing net hepatic substrate balance by hepatic substrate load. Nonhepatic glucose uptake was calculated as the glucose infusion rate plus net hepatic glucose balance, with changes in the glucose mass accounted for when deviations from steady state were present. The approximate insulin and glucagon levels in plasma entering the liver sinusoids were calculated using the formula [A] × %AF + [P] × %PF, where [A] and [P] are arterial and portal vein hormone concentrations, respectively, and %AF and %PF are the respective fractional contributions of arterial and portal flow to total hepatic blood flow.
Gluconeogenesis is the synthesis and subsequent hepatic release of glucose from noncarbohydrate precursors. Because carbon produced from flux through the GNG pathway can also be stored in glycogen, we make a distinction between gluconeogenesis and GNG flux to G6P. Hepatic GNG flux to G6P was determined by summing net hepatic uptake rates of GNG precursors (alanine, glycine, serine, threonine, glutamine, glutamate, glycerol, lactate, and pyruvate); these rates were divided by two to account for the incorporation of three carbon precursors into the six-carbon glucose molecule. Glycolytic flux was estimated by summing the net hepatic output rates (when such occurred) of the substrates noted above (in glucose equivalents) and hepatic glucose oxidation. In earlier studies, glucose oxidation was 0.2 ± 0.1 mg · kg−1 · min−1 even when the concentrations of circulating insulin, glucose, and NEFAs varied widely (22,23). Because glucose oxidation did not change appreciably under conditions similar to the present study, glucose oxidation was assumed to be constant (0.2 mg · kg−1 · min−1). Net hepatic glycogenolytic flux was estimated by subtracting GNG flux from the sum of net hepatic glucose balance and glycolytic flux. A positive number therefore represents net glycogen breakdown, whereas a negative number indicates net glycogen synthesis.
The assumptions related to using the A-V difference technique for assessing GNG flux to G6P should be considered. Ideally, GNG flux would be calculated using unidirectional hepatic uptake and output rates for each substrate, but this would be difficult because it would require the simultaneous use of multiple stable isotopes that could themselves induce a mild perturbation of the metabolic state; therefore, net hepatic balance was used instead. There is little or no production of GNG amino acids or glycerol by the liver, so in this case the compromise is of little consequence. This may not be the case for lactate, however. The estimate of GNG flux to G6P will be quantitatively accurate only if lactate flux is unidirectional at a given moment (i.e., either in or out of the liver). In a given cell, this is a reasonable assumption in light of the reciprocal control of gluconeogenesis and glycolysis (4). Spatial separation of metabolic pathways may exist, however, so that predominantly GNG periportal and glycolytic perivenous hepatocytes (24) could simultaneously take up and release lactate, respectively. Total GNG flux will be underestimated to the extent that this occurs and to the extent that intrahepatic precursors (GNG amino acids) contribute to the process. The method also assumes that there is 100% conversion of GNG precursors taken up by the liver into G6P (they are not oxidized or used in the synthesis of proteins or fatty acids). The errors due to these assumptions are difficult to assess but appear to be small and in fact are offsetting. Results obtained using the A-V difference technique described here were previously compared with results obtained with independent GNG measurements that are not subject to these assumptions, and they were similar (1,25), suggesting that these assumptions appear reasonable.
It should be noted that our estimates of gluconeogenesis and glycogenolysis relate solely to the liver. In a previous study, net renal glucose balance was close to zero in 60-h fasted canines, indicating that in a net sense the liver is the only source of glucose in such animals (21). To the extent that the kidney makes an absolute contribution to whole-body glucose production, we would slightly underestimate whole-body GNG flux.
Statistical analysis.
Statistical comparisons were carried out using two-way repeated measure ANOVA (group × time) (SigmaStat). One-way ANOVA comparison tests were used post hoc when significant F ratios were obtained. Significance was determined as P < 0.05.
RESULTS
Hormone levels.
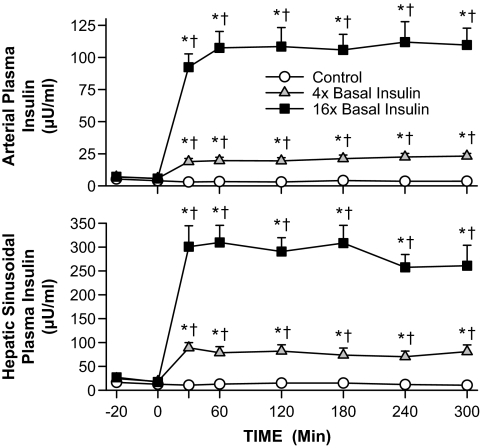
Plasma insulin levels did not increase appreciably in the control group, whereas they increased an average of 4- and 16-fold in the 4× and 16× groups, respectively (Fig. 1). The plasma arterial and hepatic sinusoidal glucagon and arterial cortisol levels remained basal and were similar over time and between groups (not shown).
FIG. 1.
Arterial and hepatic sinusoidal plasma insulin and glucagon in 60-h fasted conscious canines during the basal (−40 to 0 min) and experimental (0–300 min) periods (means ± SEM; n = 5, 5, and 6 in control, 4×, and 16× groups, respectively; *P < 0.05 vs. control group; †P < 0.05 vs. basal period).
Fat metabolism.
Hepatic NEFA uptake and oxidation play a role in the regulation of hepatic gluconeogenesis in vivo, as does the load of glycerol reaching the liver. In the control group, the arterial plasma NEFA level fell by ∼25% during the experimental period, whereas the glycerol level did not change significantly (↓∼10%; Table 1). There was a rapid fall in the arterial NEFA levels in the 4× and 16× groups (reduced by 58 and 75%, respectively, at 30 min; ↓85 and 94%, respectively, during the last hour). Glycerol levels also decreased in the hyperinsulinemic groups (↓45 and 43% at 30 min, respectively; ↓40 and 65%, respectively, during the last hour). These changes indicate rapid inhibition of lipolysis and increased re-esterification of fatty acids. In the control group, net hepatic NEFA uptake tended to decrease slowly over time (↓30% by the last hour), whereas in the 4× and 16× groups, net hepatic NEFA uptake decreased quickly (↓61 and 81%, respectively, at 30 min) and was markedly reduced (↓85 and 95%, respectively) by the last hour of the study (Table 1). Hepatic NEFA oxidation was also reduced by hyperinsulinemia, as indicated by marked reductions in β-hydroxybutyrate production in both hyperinsulinemic groups (Table 1).
Table 1.
Arterial plasma NEFA, blood glycerol, and β-hydroxybutyrate levels (μmol/l) and net hepatic balances (μmol · kg−1 · min−1) in 60-h fasted conscious canines during the basal (−40 to 0 min) and experimental (0–300 min) periods
| Basal period | Experimental period (min) |
||||||
|---|---|---|---|---|---|---|---|
| 30 | 60 | 120 | 180 | 240 | 300 | ||
| Plasma NEFA level (μmol/l) | |||||||
| Control | 863 ± 110 | 613 ± 121† | 579 ± 121† | 656 ± 121† | 656 ± 74† | 734 ± 121† | 688 ± 118† |
| 4× Basal Ins | 813 ± 134 | 345 ± 66*† | 179 ± 43*† | 169 ± 43*† | 148 ± 25*† | 151 ± 35*† | 99 ± 26*† |
| 16× Basal Ins | 1,151 ± 54* | 287 ± 61*† | 159 ± 42*† | 116 ± 33*† | 71 ± 26*† | 74 ± 38*† | 63 ± 37*† |
| Net hepatic NEFA uptake (μmol · kg−1 · min−1) | |||||||
| Control | 1.9 ± 0.5 | 1.7 ± 0.4 | 1.6 ± 0.4 | 1.8 ± 0.4 | 2.1 ± 0.4 | 1.4 ± 0.5 | 1.2 ± 0.3 |
| 4× Basal Ins | 2.2 ± 0.6 | 0.9 ± 0.3*† | 0.4 ± 0.2*† | 0.5 ± 0.3*† | 0.2 ± 0.2*† | 0.4 ± 0.1*† | 0.3 ± 0.2*† |
| 16× Basal Ins | 3.1 ± 0.5 | 0.6 ± 0.2*† | 0.4 ± 0.2*† | 0.2 ± 0.1*† | 0.2 ± 0.1*† | 0.2 ± 0.1*† | 0.1 ± 0.1*† |
| Plasma glycerol level (μmol/l) | |||||||
| Control | 68 ± 13 | 58 ± 12 | 54 ± 6 | 60 ± 9 | 60 ± 8 | 65 ± 10 | 57 ± 10 |
| 4× Basal Ins | 88 ± 14 | 48 ± 11† | 49 ± 16† | 51 ± 18† | 49 ± 15† | 58 ± 24† | 46 ± 10† |
| 16× Basal Ins | 103 ± 8 | 59 ± 11† | 45 ± 10† | 42 ± 8† | 31 ± 4† | 33 ± 11† | 38 ± 10† |
| Net hepatic glycerol uptake (μmol · kg−1 · min−1) | |||||||
| Control | 1.8 ± 0.3 | 1.2 ± 0.4 | 1.2 ± 0.2 | 1.1 ± 0.3 | 1.3 ± 0.3 | 1.2 ± 0.4 | 1.4 ± 0.3 |
| 4× Basal Ins | 1.3 ± 0.3 | 0.6 ± 0.1† | 0.7 ± 0.2 | 0.8 ± 0.3 | 0.7 ± 0.2 | 0.8 ± 0.3 | 0.8 ± 0.3 |
| 16× Basal Ins | 1.9 ± 0.4 | 0.9 ± 0.3† | 0.7 ± 0.3† | 0.8 ± 0.2† | 0.5 ± 0.1† | 0.5 ± 0.1† | 0.7 ± 0.1† |
| Plasma β-hydroxybutyrate level (μmol/l) | |||||||
| Control | 72 ± 18 | 51 ± 16 | 47 ± 16 | 51 ± 19 | 61 ± 21 | 58 ± 16 | 65 ± 16 |
| 4× Basal Ins | 75 ± 28 | 26 ± 6† | 14 ± 1† | 12 ± 2*† | 12 ± 2*† | 12 ± 2*† | 14 ± 2*† |
| 16× Basal Ins | 79 ± 17 | 21 ± 4† | 14 ± 2† | 10 ± 2*† | 10 ± 3*† | 8 ± 2*† | 11 ± 3*† |
| Net hepatic β-hydroxybutyrate output (μmol · kg−1 · min−1) | |||||||
| Control | 2.3 ± 0.5 | 1.5 ± 0.5 | 1.1 ± 0.4 | 1.4 ± 0.5 | 1.4 ± 0.4 | 1.5 ± 0.4 | 1.5 ± 0.4 |
| 4× Basal Ins | 1.6 ± 0.8 | 0.4 ± 0.3*† | 0.1 ± 0.1† | 0.1 ± 0.1*† | 0.1 ± 0.0*† | 0.1 ± 0.1*† | 0.0 ± 0.0*† |
| 16× Basal Ins | 2.7 ± 0.7 | 0.4 ± 0.2*† | 0.0 ± 0.1† | 0.1 ± 0.1*† | 0.0 ± 0.0*† | 0.0 ± 0.0*† | 0.0 ± 0.0*† |
Data are means ± SEM; n = 5, 5, and 6 in the control, 4×, and 16× insulin (Ins) groups, respectively;
*P < 0.05 vs. control group;
†P < 0.05 vs. basal period. Somatostatin and portal insulin and glucagon were infused at 0 min to control hormone levels, and glucose was infused to maintain euglycemia.
GNG precursor availability and hepatic uptake.
Lactate, glycerol, and alanine make the greatest quantitative contributions to GNG flux to G6P (52, 12, and 20%, respectively, during the basal state), with the other amino acids accounting for the remainder. In the control group, there were no changes in the level, net hepatic fractional extraction (NHFX), or net hepatic uptake of lactate over time (Table 2). There were rapid and sustained decreases in the NHFX of lactate in the 4× and 16× groups (↓40 and 50%, respectively, at 30 min) that persisted until the end of the study. Arterial lactate levels tended to rise in the 4× group and increased even more substantially in the 16× group (↑16 and 84%, respectively, by the last hour). Therefore, as a result of these somewhat offsetting effects, net hepatic lactate uptake was reduced by ∼50% (Δ−3 to −4 μmol · kg−1 · min−1) at 30 min in both groups but was reduced by only 33 and 23%, respectively, during the last hour.
TABLE 2.
Arterial blood lactate, alanine, and GNG amino acid (alanine, serine, glycine, threonine, glutamate, and glutamine) levels (μmol/l), net hepatic fractional extraction and uptake rates (μmol · kg−1 · min−1), and GNG precursor uptake (mg · kg−1 · min−1) in 60-h fasted conscious canines during the basal (−40 to 0 min) and experimental (0–300 min) periods
| Basal period | Experimental period (min) |
||||||
|---|---|---|---|---|---|---|---|
| 30 | 60 | 120 | 180 | 240 | 300 | ||
| Blood lactate level (μmol/l) | |||||||
| Control | 409 ± 103 | 488 ± 122 | 425 ± 126 | 429 ± 134 | 422 ± 121 | 399 ± 96 | 352 ± 71 |
| 4× Basal Ins | 474 ± 121 | 447 ± 82 | 539 ± 93 | 480 ± 107 | 483 ± 122 | 545 ± 124 | 555 ± 107 |
| 16× Basal Ins | 393 ± 49 | 513 ± 59 | 776 ± 107*† | 849 ± 180*† | 744 ± 127† | 741 ± 85† | 705 ± 49*† |
| Net hepatic lactate fractional extraction | |||||||
| Control | 0.49 ± 0.06 | 0.52 ± 0.09 | 0.50 ± 0.08 | 0.50 ± 0.07 | 0.56 ± 0.10 | 0.56 ± 0.05 | 0.56 ± 0.04 |
| 4× Basal Ins | 0.47 ± 0.10 | 0.28 ± 0.16* | 0.15 ± 0.12*† | 0.27 ± 0.06* | 0.30 ± 0.06* | 0.32 ± 0.06* | 0.25 ± 0.01* |
| 16× Basal Ins | 0.59 ± 0.04 | 0.28 ± 0.13*† | 0.24 ± 0.12*† | 0.22 ± 0.03*† | 0.29 ± 0.03*† | 0.27 ± 0.04*† | 0.32 ± 0.04*† |
| Net hepatic lactate uptake (μmol · kg−1 · min−1) | |||||||
| Control | 7.5 ± 0.9 | 7.2 ± 1.0 | 6.3 ± 0.4 | 6.7 ± 0.7 | 6.7 ± 0.6 | 7.7 ± 1.0 | 7.3 ± 0.7 |
| 4× Basal Ins | 6.3 ± 1.4 | 3.3 ± 1.8 | 1.6 ± 1.4*† | 3.4 ± 0.8 | 3.8 ± 0.3 | 4.4 ± 0.8 | 4.1 ± 0.9 |
| 16× Basal Ins | 8.0 ± 1.7 | 3.6 ± 1.8*† | 3.8 ± 1.6† | 5.0 ± 1.7 | 6.2 ± 1.9 | 5.6 ± 1.4 | 6.7 ± 1.5 |
| Blood alanine level (μmol/l) | |||||||
| Control | 255 ± 33 | 256 ± 31 | 243 ± 36 | 225 ± 39 | 219 ± 26 | 228 ± 22 | 233 ± 24 |
| 4× Basal Ins | 275 ± 44 | 262 ± 26 | 248 ± 16 | 203 ± 17† | 175 ± 18† | 179 ± 22† | 164 ± 24*† |
| 16× Basal Ins | 266 ± 16 | 232 ± 14† | 231 ± 12† | 192 ± 18† | 167 ± 16† | 141 ± 13*† | 129 ± 10*† |
| Net hepatic alanine fractional extraction | |||||||
| Control | 0.24 ± 0.04 | 0.24 ± 0.03 | 0.32 ± 0.03 | 0.37 ± 0.02† | 0.37 ± 0.03† | 0.33 ± 0.03 | 0.30 ± 0.05 |
| 4× Basal Ins | 0.28 ± 0.04 | 0.34 ± 0.03 | 0.37 ± 0.03 | 0.45 ± 0.02† | 0.46 ± 0.02*† | 0.42 ± 0.02*† | 0.46 ± 0.04*† |
| 16× Basal Ins | 0.33 ± 0.03 | 0.34 ± 0.04 | 0.41 ± 0.04 | 0.39 ± 0.04 | 0.46 ± 0.02† | 0.45 ± 0.03*† | 0.46 ± 0.03*† |
| Net hepatic alanine uptake (μmol · kg−1 · min−1) | |||||||
| Control | 2.6 ± 0.07 | 2.2 ± 0.5 | 2.6 ± 0.4 | 2.7 ± 0.4 | 2.4 ± 0.1 | 2.6 ± 0.3 | 2.5 ± 0.5 |
| 4× Basal Ins | 2.6 ± 0.7 | 2.5 ± 0.5 | 2.5 ± 0.3 | 2.6 ± 0.4 | 2.5 ± 0.4 | 2.3 ± 0.4 | 2.5 ± 0.6 |
| 16× Basal Ins | 3.0 ± 0.5 | 2.2 ± 0.4 | 2.3 ± 0.2 | 2.1 ± 0.3 | 2.3 ± 0.2 | 2.0 ± 0.2 | 1.9 ± 0.2 |
| Blood GNG amino acid level (μmol/l) | |||||||
| Control | 1,884 ± 161 | 1,810 ± 125 | 1,728 ± 142† | 1,591 ± 133† | 1,581 ± 123† | 1,632 ± 116† | 1,536 ± 171† |
| 4× Basal Ins | 1,839 ± 87 | 1,754 ± 78 | 1,536 ± 58† | 1,336 ± 76† | 1,199 ± 69*† | 1,199 ± 91*† | 1,170 ± 118*† |
| 16× Basal Ins | 1,575 ± 60 | 1,460 ± 89 | 1,247 ± 79*† | 1,072 ± 51*† | 995 ± 45*† | 915 ± 72*† | 852 ± 55*† |
| Net hepatic GNG amino acid fractional extraction | |||||||
| Control | 0.07 ± 0.02 | 0.09 ± 0.02 | 0.14 ± 0.02 | 0.15 ± 0.03 | 0.17 ± 0.03† | 0.14 ± 0.03 | 0.16 ± 0.04† |
| 4× Basal Ins | 0.08 ± 0.01 | 0.15 ± 0.02† | 0.17 ± 0.03† | 0.20 ± 0.01† | 0.15 ± 0.02† | 0.20 ± 0.02† | 0.20 ± 0.01† |
| 16× Basal Ins | 0.14 ± 0.03 | 0.15 ± 0.03 | 0.11 ± 0.03 | 0.12 ± 0.02 | 0.17 ± 0.02 | 0.15 ± 0.01 | 0.15 ± 0.03 |
| Net hepatic GNG amino acid uptake (μmol · kg−1 · min−1) | |||||||
| Control | 5.5 ± 2.3 | 5.1 ± 1.7 | 6.4 ± 1.2 | 6.3 ± 1.2 | 7.2 ± 1.5 | 6.1 ± 1.2 | 7.6 ± 0.8 |
| 4× Basal Ins | 4.3 ± 1.0 | 6.2 ± 0.5 | 6.3 ± 0.9 | 6.6 ± 0.3 | 4.9 ± 1.0 | 5.9 ± 1.1 | 6.0 ± 1.1 |
| 16× Basal Ins | 5.6 ± 1.0 | 4.9 ± 1.0 | 3.5 ± 0.4 | 3.2 ± 0.4* | 3.8 ± 0.4* | 3.2 ± 0.4 | 3.0 ± 0.4* |
| GNG precursor uptake in glucose equivalents (mg · kg−1 · min−1) | |||||||
| Control | 1.4 ± 0.3 | 1.3 ± 0.2 | 1.3 ± 0.1 | 1.3 ± 0.1 | 1.4 ± 0.1 | 1.4 ± 0.1 | 1.5 ± 0.1 |
| 4× Basal Ins | 1.1 ± 0.2 | 1.0 ± 0.2 | 0.8 ± 0.1 | 1.0 ± 0.1 | 0.9 ± 0.1 | 1.0 ± 0.2 | 1.0 ± 0.2 |
| 16× Basal Ins | 1.5 ± 0.3 | 0.9 ± 0.2† | 0.8 ± 0.1† | 0.9 ± 0.2† | 1.0 ± 0.2† | 0.9 ± 0.1† | 1.0 ± 0.1† |
Data are means ± SEM; n = 5, 5, and 6 in the control, 4×, and 16× groups, respectively;
*P < 0.05 vs. control group;
†P < 0.05 vs. basal period. Somatostatin and portal insulin and glucagon were infused at 0 min to control hormone levels, and glucose was infused to maintain euglycemia.
Net hepatic glycerol uptake fell rapidly (↓31, 57, and 54% [Δ−0.7 to −1 μmol · kg−1 · min−1) by 30 min in the control, 4×, and 16× groups, respectively, in parallel with changes in glycerol availability (Table 1). By the last hour of the study, rates of net hepatic glycerol uptake were reduced by 25, 42, and 68%, respectively.
In the control and 4× groups, there were decreases in blood alanine level that offset increases in alanine NHFX so that net hepatic alanine uptake did not change significantly over time (Table 3). In the 16× group, there was a progressive decrease in the alanine level (↓50% by the end of the study), but the NHFX of alanine did not increase enough to offset this fall; therefore, net hepatic alanine uptake was reduced by one-third during the last hour of the study. The average summed GNG amino acid (alanine, serine, glycine, threonine, glutamate, and glutamine) levels followed a similar pattern of decline as alanine (reduced by 16, 36, and 44% in the control, 4×, and 16× groups, respectively) (Table 2). The NHFX of GNG amino acids increased from basal in the control and 4× groups (2.1- and 2.4-fold, respectively, during the last hour) but did not change in the 16× group. As a result, net hepatic GNG amino acid uptake increased by 26 and 38% (Δ1.4–1.7 μmol · kg−1 · min−1) in the control and 4× groups, respectively, whereas in the 16× group there was a slow decline (↓44% by the last hour; Δ−2.5 μmol · kg−1 · min−1) that paralleled amino acid availability.
TABLE 3.
Glucose infusion rate and nonhepatic glucose uptake in 60-h fasted conscious canines during the basal (−40 to 0 min) and experimental (0–300 min) periods
| Basal period | Experimental period (min) |
||||||
|---|---|---|---|---|---|---|---|
| 30 | 60 | 120 | 180 | 240 | 300 | ||
| Glucose infusion rate (mg · kg−1 · min−1) | |||||||
| Control | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.1 ± 0.1 | 0.3 ± 0.1 | 0.6 ± 0.3 | 0.5 ± 0.2 |
| 4× Basal Ins | 0.0 ± 0.0 | 0.7 ± 0.1 | 2.7 ± 0.6*† | 4.1 ± 1.0*† | 4.9 ± 1.0*† | 6.1 ± 0.9*† | 7.2 ± 1.2*† |
| 16× Basal Ins | 0.0 ± 0.0 | 3.2 ± 1.1*† | 7.5 ± 1.2*† | 11.7 ± 1.4*† | 12.5 ± 1.1*† | 14.5 ± 1.3*† | 16.6 ± 1.7*† |
| Nonhepatic glucose uptake (mg · kg−1 · min−1) | |||||||
| Control | 1.8 ± 0.3 | 1.9 ± 0.3 | 1.5 ± 0.2 | 1.6 ± 0.2 | 1.5 ± 0.2 | 1.8 ± 0.4 | 1.7 ± 0.3 |
| 4× Basal Ins | 1.6 ± 0.2 | 1.9 ± 0.3 | 3.1 ± 0.6 | 4.6 ± 0.9*† | 5.0 ± 0.9*† | 6.3 ± 0.9*† | 6.4 ± 1.2*† |
| 16× Basal Ins | 2.0 ± 0.3 | 4.5 ± 1.3*† | 7.9 ± 1.4*† | 11.4 ± 1.4*† | 12.0 ± 1.4*† | 13.8 ± 1.6*† | 15.7 ± 1.8*† |
Data are means ± SEM; n = 5, 5, and 6 in the control, 4×, and 16× groups, respectively;
*P < 0.05 vs. control group;
†P < 0.05 vs. basal period. Somatostatin and portal insulin and glucagon were infused at 0 min to control hormone levels, and glucose was infused to maintain euglycemia.
Liver and nonhepatic glucose fluxes.
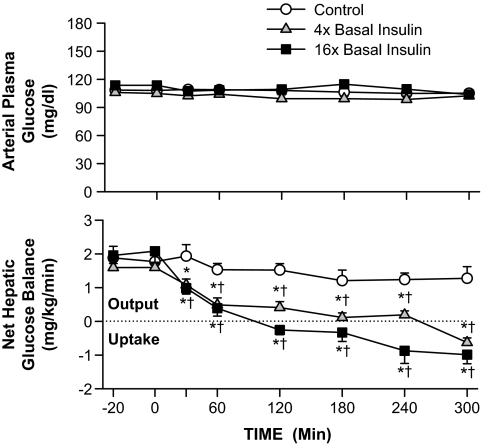
Net hepatic glucose output (NHGO) was similar during the basal period in the three groups (1.6–2.0 mg · kg−1 · min−1; Fig. 2). By the last hour, NHGO was 1.3 ± 0.2 mg · kg−1 · min−1 in the control group. In the 4× and 16× groups, hyperinsulinemia had suppressed NHGO by 50% at 30 min and by the last hour had switched the liver to net hepatic glucose uptake (0.2 ± 0.1 and 0.9 ± 0.3 mg · kg−1 · min−1, respectively). Euglycemia was maintained by glucose infusion (reaching 0.5 ± 0.2, 6.6 ± 1.0, and 15.5 ± 1.5 mg · kg−1 · min−1 in the control, 4×, and 16× groups, respectively, during the last hour; Table 3). The majority of the increase in glucose requirement in the 4× and 16× groups related to increases in muscle glucose uptake (nonhepatic glucose uptake was 1.8 ± 0.3, 6.4 ± 1, and 14.7 ± 1.6 mg · kg−1 · min−1, respectively, during the last hour; Table 3).
FIG. 2.
Arterial plasma glucose level and net hepatic balance in 60-h fasted conscious canines during the basal (−40 to 0 min) and experimental (0–300 min) periods (means ± SEM; n = 5, 5, and 6 in control, 4×, and 16× groups, respectively; *P < 0.05 vs. control group; †P < 0.05 vs. basal period).
GNG and glycogenolytic flux rates.
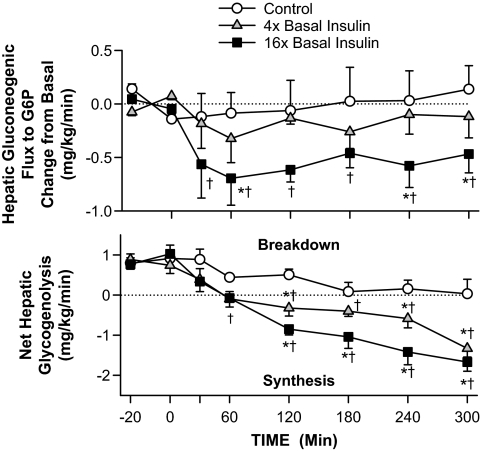
GNG flux to G6P did not change from basal in the control and 4× groups, whereas in the 16× group there was a rapid (30 min) and sustained decrease (↓35%; Δ−0.5 mg · kg−1 · min−1; Table 2; Fig. 3). In the control group, net hepatic glycogenolysis decreased over time until it was almost completely inhibited by the end of the experiment. Net hepatic glycogenolysis decreased more rapidly in the 4× and 16× groups, and by the last hour of the study the liver had switched to net hepatic glycogen deposition (1.0 ± 0.2 and 1.5 ± 0.2 mg · kg−1 · min−1, respectively; Fig. 3).
FIG. 3.
Change from basal hepatic gluconeogenic flux to G6P and net hepatic glycogenolytic flux in 60-h fasted conscious canines during the basal (−40 to 0 min) and experimental (0–300 min) periods (means ± SEM; n = 5, 5, and 6 in control, 4×, and 16× groups, respectively; *P < 0.05 vs. control group; †P < 0.05 vs. basal period). Basal rates of GNG flux were 1.40 ± 0.26, 1.14 ± 0.16, and 1.56 ± 0.26 mg · kg−1 · min−1, respectively.
Molecular effects of insulin.
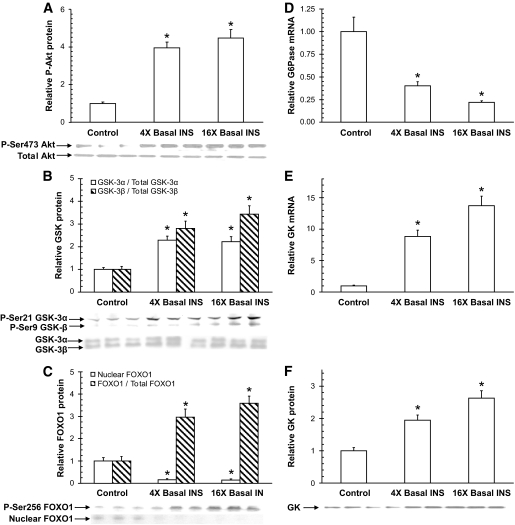
The effects of insulin treatment on targets of insulin receptor signaling were assessed in biopsies taken at the end of each experiment. Akt, a marker of activation of the phosphatidylinositol 3 kinase insulin-signaling pathway, was similarly activated in both the 4× and 16× groups, with the ratio of phosphorylated P-Ser473 Akt to total Akt increased 3.9- and 4.5-fold, respectively, compared with the control group (Fig. 4A). In line with the changes in glycogen flux, the ratio of P-Ser9 glycogen synthase kinase 3α (GSK-3α) to total GSK-3α increased 2.3- and 2.2-fold in the 4× and 16× groups, respectively, compared with control, and the ratio of P-Ser21 GSK-3β to total GSK-3β increased 2.8- and 3.4-fold, respectively (Fig. 4B). The ratio of P-Ser256 FOXO1 to total FOXO1 (a transcription factor involved in the regulation of GNG enzymes, including G6Pase and PEPCK) increased 3.0- and 3.6-fold in the 4× and 16× groups, respectively (Fig. 4C), and analysis of total FOXO1 levels in nuclear-enriched fractions detected reductions of 84 and 85%, respectively, compared with the control group (Fig. 4C). Although there were no significant differences between the 4× and 16× groups for Akt, GSK-3, or FOXO1 phosphorylation, activation in the 16× group always tended to be slightly greater compared with the 4× group.
FIG. 4.
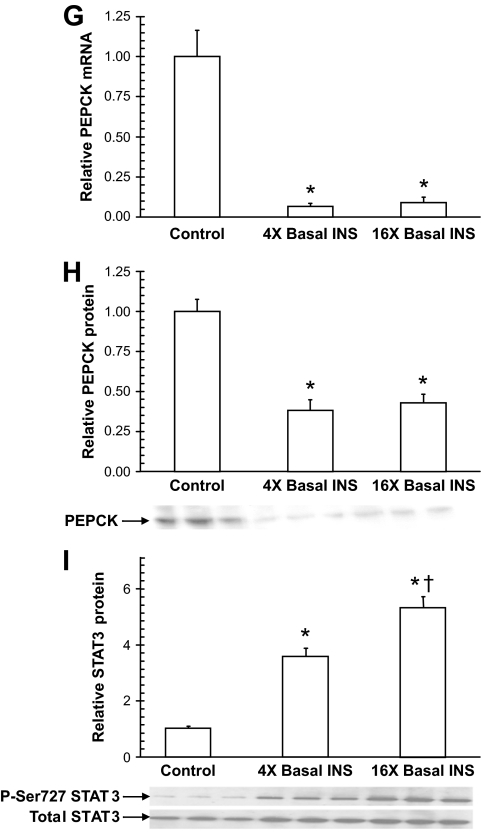
Analysis of liver taken in 60-h fasted conscious canines after 300 min of treatment. Insulin increased the phosphorylation of Akt (A), GSK-3α and GSK-3β (B), and FOXO1 (C), which translocated out of the nucleus. G6Pase mRNA expression was reduced by insulin (D), and glucokinase mRNA (E), and protein (F) expression increased. PEPCK mRNA (G) and protein (H) expression decreased. The increase in phosphorylation of STAT3 (I) relative to total STAT3 was insulin dose dependent. Each graph represents means ± SEM; n = 5, 5, and 6 in the control, 4×, and 16× groups, respectively; three representative blots are shown for each; *P < 0.05 versus control group; †P < 0.05 versus 4× group.
G6Pase and glucokinase serve as “gatekeepers” for hepatic glucose uptake and release. G6Pase mRNA expression was decreased by 60 and 80% in the 4× and 16× groups, respectively, compared with the control group (Fig. 4D). Both glucokinase mRNA and protein expression increased in a stepwise manner with increasing hyperinsulinemia: mRNA levels increased 8.8- and 13.7-fold, whereas protein levels in cytoplasmic-enriched fractions increased 1.9- and 2.6-fold, respectively (Fig. 4E and F). Analysis of GNG markers revealed that hyperinsulinemia decreased PEPCK mRNA and protein levels by 90 and 60%, respectively, with no differences between the 4× and 16× groups (Fig. 4G and H). Hepatic STAT3 phosphorylation (shown to be activated in the liver in response to central insulin action [12]) was increased 3.5- and 5.2-fold, respectively.
F2,6P2 is a regulator of the glycolytic and GNG pathways, and pyruvate kinase catalyzes the conversion of phosphoenolpyruvate into pyruvate. At the end of the study, the hepatic concentrations of F2,6P2 were 4.6 ± 1.1, 11.6 ± 1.7, and 26.9 ± 3.3 nmol/g in the control, 4×, and 16× groups, respectively (Table 4). Pyruvate kinase activities were 0.46 ± 0.06, 0.70 ± 0.09, and 0.82 ± 0.08 units/mg protein, respectively (Table 4).
TABLE 4.
Hepatic F2,6P2 levels and pyruvate kinase activities at the end of the study in 60-h fasted conscious canines
| Control group | 4× group | 16× group | |
|---|---|---|---|
| F2,6P2 (nmol/g) | 4.6 ± 1.1 | 11.6 ± 1.7* | 26.9 ± 3.3* |
| Pyruvate kinase activity (U/mg protein) | 0.46 ± 0.06 | 0.70 ± 0.09* | 0.82 ± 0.08* |
Data are means ± SEM; n = 5, 5, and 6 in the control, 4×, and 16× groups, respectively;
*P < 0.05 vs. control group. Somatostatin and portal insulin and glucagon were infused at 0 min to control hormone levels and glucose was infused to maintain euglycemia.
DISCUSSION
The in vivo regulation of GNG flux by insulin is complex and not fully understood. In this study, conditions were optimized to increase the likelihood of observing an acute effect of insulin on the process. Canines were fasted for 60 h to enhance gluconeogenesis, insulin was elevated for 5 h to allow sufficient time for transcriptional and translational changes to occur, and two insulin doses were administered (the highest of which produced a maximal physiologic level). In agreement with previous studies (20,26), GNG flux to G6P did not change significantly over time in the control or 4× groups. At the highest level of insulin, however, GNG flux to G6P was reduced, but only by one-third. Further, this change occurred rapidly (within 30 min), presumably before an insulin-induced decrease in PEPCK activity was possible. Thereafter, no further change in GNG flux occurred, despite an eventual marked decrease in PEPCK protein.
As expected, insulin signaling pathways were activated with hyperinsulinemia, as indicated by increased phosphorylation of Akt, GSK-3, and FOXO1. The balance between the activities of G6Pase and glucokinase determines glucose flux into and out of the liver. Insulin reduced G6Pase mRNA (and presumably the protein level) and increased glucokinase mRNA and glucokinase protein levels so that the ratio of G6Pase to glucokinase expression in the three groups corresponded inversely with circulating insulin levels, reflecting the shift toward net hepatic glucose uptake.
Net hepatic glycogenolysis accounted for one-third of the glucose produced by the liver in the basal period (versus two-thirds after an 18-h fast [1]), in agreement with previous findings in 66-h fasted canines in which glycogenolysis was estimated to account for 41% of basal HGP using an independent technique and 23% using the A-V difference method (25). The decrease in HGP that occurred during hyperinsulinemia in the present study was due primarily to reduced net glycogenolysis. Insulin increased the phosphorylation of GSK-3, thereby inactivating it and facilitating activation of glycogen synthase (27). The rapid effect on glycogen metabolism occurred in a dose-dependent manner, such that net glycogen synthesis was clearly evident after 5 h of hyperinsulinemia (Δ2.5 mg · kg−1 · min−1) in the 16× group, even under euglycemic conditions. In the control group, an insulin infusion rate that was only modestly (∼40%) above the basal endogenous insulin secretion rate (21) completely eliminated net glycogen breakdown by 5 h, demonstrating the exquisite sensitivity of liver glycogen metabolism to small increments in insulin.
GNG flux to G6P was rapidly reduced in the 16× group, decreasing near maximally by 30 min, although the effect was modest (Δ from baseline 0.5 mg · kg−1 · min−1). This initial decrease was primarily the result of a fall in net hepatic lactate uptake and was associated with the insulin-induced inhibition of lipolysis. Human (28,29) and canine (30) experiments have shown that plasma NEFAs stimulate hepatic gluconeogenesis and that a fall in circulating NEFAs causes a redirection of carbon flow within the liver from glucose to lactate output (9). It is likely that this results from increased glycolytic flux through PFK-1 due to reduced inhibition of the enzyme by citrate, a product of fat oxidation (4,8). In the present study, hyperinsulinemia led to robust, rapid, and dose-dependent effects on fat metabolism (75% reduction in NEFA levels and net hepatic uptake, and almost complete inhibition of hepatic fat oxidation in the 16× group at 30 min). In addition to NEFA-mediated effects, the rapid effect of insulin on GNG flux to G6P was also probably explained by allosteric and covalent regulation of GNG and glycolytic enzymes. Insulin stimulates dephosphorylation of the bifunctional enzyme, activating the kinase and inactivating the phosphatase, thereby increasing F2,6P2 (4,8). This leads to increased glycolytic flux through PFK-1 and to a lesser degree decreased GNG flux through fructose 1,6-bisphosphatase (4,8). In addition, insulin activates pyruvate kinase by cAMP-dependent and -independent mechanisms and possibly by phosphorylation (4,31), which allows for increased lactate formation from pyruvate. Indeed, in the 4× and 16× groups, respectively, we observed 2.5- and 5.8-fold increases in F2,6P2 and 1.5- and 1.8-fold elevations in pyruvate kinase activity, although the time course of these changes is not known. Thus, the rapid decrease in GNG flux to G6P, and in particular net lactate uptake, which can be explained by the direct and indirect effects of insulin.
The hepatic GNG precursor load and extraction are also determinants of GNG flux. In the 4× and 16× groups, inhibition of lipolysis resulted in decreased glycerol levels, and therefore the contribution of glycerol to GNG flux decreased. The inhibition of net hepatic lactate fractional extraction remained constant during hyperinsulinemia (∼50%); however, circulating lactate levels increased, especially in the 16× group (↑85%), as a result of reduced liver lactate uptake (32) and increased muscle and fat lactate production (33,34). Net hepatic GNG amino acid fractional extraction increased quickly in the 4× group, to nearly twice the basal rate at 30 min, most likely due to insulin stimulation of hepatic amino acid transport (35). Therefore, despite decreased amino acid availability (probably due to reduced muscle protein turnover [10]), net hepatic GNG amino acid uptake increased enough to offset the reductions in net lactate and glycerol uptake in the 4× group, and thus GNG flux did not change. In the 16× group, although hepatic extraction of alanine increased by 40% during hyperinsulinemia, a collective increase in GNG amino acid fractional extraction did not occur. Thus, decreased net hepatic uptake of lactate, glycerol, and GNG amino acids all contributed to the fall in GNG flux in this group. The fall in lactate uptake predominated early, whereas reduced amino acid uptake became more important by the end of the study.
PEPCK is considered to be a key GNG enzyme, and its mRNA levels are frequently used as an index of gluconeogenesis. PEPCK mRNA and protein expression were measured at the end of the study to determine their correspondence with GNG flux. Despite marked and equal suppression of PEPCK by insulin in the 4× and 16× groups (mRNA decreased by 90% and protein by 60%), GNG flux did not change in the 4× group, and there was only a 33% decrease in the 16× group. In addition, inhibition of GNG flux in the 16× group occurred within 30 min of the rise in insulin, and the degree of inhibition remained unchanged over the subsequent 4.5 h of hyperinsulinemia. Although PEPCK transcription can be reduced by insulin in vitro within minutes (36,37), the enzyme's activity is determined by its protein levels, which change much more slowly. Therefore, a decrease in PEPCK activity is unlikely to explain the rapid changes in GNG flux that we observed. Thus, although PEPCK transcription and translation were clearly regulated by insulin, the enzyme did not appear to be rate limiting for gluconeogenesis. Some studies in rats have demonstrated control of gluconeogenesis and glucose production via inhibition of PEPCK with 3-mercaptopicolinate (38,39), and it has been concluded that PEPCK is the rate-controlling enzyme for gluconeogenesis (40). On the other hand, studies using metabolic control analysis in isolated hepatocytes have suggested that a number of enzymes play important roles in controlling GNG flux and that PEPCK displays weak control strength (41–43). Our results agree with those from studies in transgenic mice where a 70% reduction in PEPCK content resulted in only a 20% reduction in GNG flux (44) and from a very recent study in patients with type 2 diabetes where increased transcriptional expression of PEPCK did not account for increased gluconeogenesis (45). Thus, at least in canines and humans, PEPCK appears to contribute little to the hormonal regulation of gluconeogenesis.
Insulin action in the hypothalamus may also control hepatic gluconeogenesis (12–14), although this has been demonstrated only in rodents. Activation of brain insulin receptors was shown to increase hepatic STAT3 phosphorylation, resulting in inhibition of PEPCK and G6Pase transcription in mice (12). In the present study, insulin increased phosphorylation of STAT3 in the liver in a dose-dependent fashion, but the time course of the activation is not known. We cannot say whether insulin action in the brain was partially responsible for the observed changes in PEPCK because the insulin levels in the liver itself rose markedly. Nevertheless, using the same logic as noted above, it is unlikely that a STAT3-mediated change in the activity of the GNG enzymes had any effect on GNG flux to G6P.
In summary, this study demonstrates that a large, but still physiologic, rise in plasma insulin can reduce GNG flux to G6P acutely in the conscious canine. However, this effect required a near-maximal physiological level of the hormone and was modest compared with the effect on glycogenolysis, even in 60-h fasted animals. The decrease in GNG flux occurred within 30 min and was associated with marked inhibition of hepatic fat oxidation, increases in hepatic F2,6P2 level and pyruvate kinase activity, and reductions in hepatic lactate, glycerol, and amino acid extraction. No further diminution in GNG flux occurred over the remaining 4.5 h of the study, despite a marked decrease in PEPCK content, suggesting poor control strength for this enzyme in GNG regulation in canines.
Supplementary Material
Acknowledgments
This research was supported in part by National Institutes of Health Grant R37-DK-18243 and the Diabetes Research and Training Center Grant SP-60-AM20593. D.S.E. was supported by the Vanderbilt Molecular Endocrinology Training Program 5T32-DK-07563–12. A.D.C. was supported by the Jacquelyn A. Turner and Dr. Dorothy J. Turner Chair in Diabetes Research.
No potential conflicts of interest relevant to this article were reported.
We thank Dr. Masakazu Shiota, Dr. Rob Hall, Jon Hastings, Angelina Penaloza, Wanda Snead, Patrick Donahue, and Suzan Vaughan (Vanderbilt University) for excellent technical support. We are grateful to Dr. Alex Lange (University of Minnesota) for providing reagents and expertise.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Edgerton DS, Cardin S, Emshwiller M, Neal D, Chandramouli V, Schumann WC, Landau BR, Rossetti L, Cherrington AD: Small increases in insulin inhibit hepatic glucose production solely caused by an effect on glycogen metabolism. Diabetes 2001; 50: 1872– 1882 [DOI] [PubMed] [Google Scholar]
- 2.Petersen KF, Laurent D, Rothman DL, Cline GW, Shulman GI: Mechanism by which glucose and insulin inhibit net hepatic glycogenolysis in humans. J Clin Invest 1998; 101: 1203– 1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kahn CR, Lauris V, Koch S, Crettaz M, Granner DK: Acute and chronic regulation of phosphoenolpyruvate carboxykinase mRNA by insulin and glucose. Mol Endocrinol 1989; 3: 840– 845 [DOI] [PubMed] [Google Scholar]
- 4.Pilkis SJ, Granner DK: Molecular physiology of the regulation of hepatic gluconeogenesis and glycolysis. Annu Rev Physiol 1992; 54: 885– 909 [DOI] [PubMed] [Google Scholar]
- 5.Young A: Inhibition of glucagon secretion. Adv Pharmacol 2005; 52: 151– 171 [DOI] [PubMed] [Google Scholar]
- 6.Duncan RE, Ahmadian M, Jaworski K, Sarkadi-Nagy E, Sul HS: Regulation of lipolysis in adipocytes. Annu Rev Nutr 2007; 27: 79– 101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boden G: Free fatty acids-the link between obesity and insulin resistance. Endocr Pract 2001; 7: 44– 51 [DOI] [PubMed] [Google Scholar]
- 8.Hers HG, Hue L: Gluconeogenesis and related aspects of glycolysis. Annu Rev Biochem 1983; 52: 617– 653 [DOI] [PubMed] [Google Scholar]
- 9.Sindelar DK, Chu CA, Rohlie M, Neal DW, Swift LL, Cherrington AD: The role of fatty acids in mediating the effects of peripheral insulin on hepatic glucose production in the conscious dog. Diabetes 1997; 46: 187– 196 [DOI] [PubMed] [Google Scholar]
- 10.Pozefsky T, Felig P, Tobin JD, Soeldner JS, Cahill GF, Jr: Amino acid balance across tissues of the forearm in postabsorptive man. Effects of insulin at two dose levels. J Clin Invest 1969; 48: 2273– 2282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kilberg MS, Barber EF, Handlogten ME: Characteristics and hormonal regulation of amino acid transport system A in isolated rat hepatocytes. Curr Top Cell Regul 1985; 25: 133– 163 [DOI] [PubMed] [Google Scholar]
- 12.Inoue H, Ogawa W, Asakawa A, Okamoto Y, Nishizawa A, Matsumoto M, Teshigawara K, Matsuki Y, Watanabe E, Hiramatsu R, Notohara K, Katayose K, Okamura H, Kahn CR, Noda T, Takeda K, Akira S, Inui A, Kasuga M: Role of hepatic STAT3 in brain-insulin action on hepatic glucose production. Cell Metab 2006; 3: 267– 275 [DOI] [PubMed] [Google Scholar]
- 13.Obici S, Zhang BB, Karkanias G, Rossetti L: Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med 2002; 8: 1376– 1382 [DOI] [PubMed] [Google Scholar]
- 14.Pocai A, Lam TK, Gutierrez-Juarez R, Obici S, Schwartz GJ, Bryan J, Aguilar-Bryan L, Rossetti L: Hypothalamic K(ATP) channels control hepatic glucose production. Nature 2005; 434: 1026– 1031 [DOI] [PubMed] [Google Scholar]
- 15.Adkins A, Basu R, Persson M, Dicke B, Shah P, Vella A, Schwenk WF, Rizza R: Higher insulin concentrations are required to suppress gluconeogenesis than glycogenolysis in nondiabetic humans. Diabetes 2003; 52: 2213– 2220 [DOI] [PubMed] [Google Scholar]
- 16.Gastaldelli A, Toschi E, Pettiti M, Frascerra S, Quinones-Galvan A, Sironi AM, Natali A, Ferrannini E: Effect of physiological hyperinsulinemia on gluconeogenesis in nondiabetic subjects and in type 2 diabetic patients. Diabetes 2001; 50: 1807– 1812 [DOI] [PubMed] [Google Scholar]
- 17.Chiasson JL, Liljenquist JE, Finger FE, Lacy WW: Differential sensitivity of glycogenolysis and gluconeogenesis to insulin infusions in dogs. Diabetes 1976; 25: 283– 291 [DOI] [PubMed] [Google Scholar]
- 18.Boden G, Cheung P, Homko C: Effects of acute insulin excess and deficiency on gluconeogenesis and glycogenolysis in type 1 diabetes. Diabetes 2003; 52: 133– 137 [DOI] [PubMed] [Google Scholar]
- 19.Chiasson JL, Atkinson RL, Cherrington AD, Keller U, Sinclair-Smith BC, Lacy WW, Liljenquist JE: Effects of insulin at two dose levels on gluconeogenesis from alanine in fasting man. Metabolism 1980; 29: 810– 818 [DOI] [PubMed] [Google Scholar]
- 20.Edgerton DS, Cardin S, Pan C, Neal D, Farmer B, Converse M, Cherrington AD: Effects of insulin deficiency or excess on hepatic gluconeogenic flux during glycogenolytic inhibition in the conscious dog. Diabetes 2002; 51: 3151– 3162 [DOI] [PubMed] [Google Scholar]
- 21.Edgerton DS, Jacobson PB, Opgenorth TJ, Zinker B, Beno D, von Geldern T, Ohman L, Scott M, Neal D, Cherrington AD: Selective antagonism of the hepatic glucocorticoid receptor reduces hepatic glucose production. Metabolism 2006; 55: 1255– 1262 [DOI] [PubMed] [Google Scholar]
- 22.Moore MC, Satake S, Lautz M, Soleimanpour SA, Neal DW, Smith M, Cherrington AD: Nonesterified fatty acids and hepatic glucose metabolism in the conscious dog. Diabetes 2004; 53: 32– 40 [DOI] [PubMed] [Google Scholar]
- 23.Satake S, Moore MC, Igawa K, Converse M, Farmer B, Neal DW, Cherrington AD: Direct and indirect effects of insulin on glucose uptake and storage by the liver. Diabetes 2002; 51: 1663– 1671 [DOI] [PubMed] [Google Scholar]
- 24.Jungermann K, Katz N: Functional specialization of different hepatocyte populations. Physiol Rev 1989; 69: 708– 764 [DOI] [PubMed] [Google Scholar]
- 25.Goldstein RE, Rossetti L, Palmer BA, Liu R, Massillon D, Scott M, Neal D, Williams P, Peeler B, Cherrington AD: Effects of fasting and glucocorticoids on hepatic gluconeogenesis assessed using two independent methods in vivo. Am J Physiol Endocrinol Metab 2002; 283: E946– E957 [DOI] [PubMed] [Google Scholar]
- 26.Edgerton DS, Cardin S, Neal D, Farmer B, Lautz M, Pan C, Cherrington AD: Effects of hyperglycemia on hepatic gluconeogenic flux during glycogen phosphorylase inhibition in the conscious dog. Am J Physiol Endocrinol Metab 2004; 286: E510– E522 [DOI] [PubMed] [Google Scholar]
- 27.Lee J, Kim MS: The role of GSK3 in glucose homeostasis and the development of insulin resistance. Diabetes Res Clin Pract 77 Suppl 2007; 1: S49– S57 [DOI] [PubMed] [Google Scholar]
- 28.Boden G, Chen X, Capulong E, Mozzoli M: Effects of free fatty acids on gluconeogenesis and autoregulation of glucose production in type 2 diabetes. Diabetes 2001; 50: 810– 816 [DOI] [PubMed] [Google Scholar]
- 29.Staehr P, Hother-Nielsen O, Landau BR, Chandramouli V, Holst JJ, Beck-Nielsen H: Effects of free fatty acids per se on glucose production, gluconeogenesis, and glycogenolysis. Diabetes 2003; 52: 260– 267 [DOI] [PubMed] [Google Scholar]
- 30.Chu CA, Sherck SM, Igawa K, Sindelar DK, Neal DW, Emshwiller M, Cherrington AD: Effects of free fatty acids on hepatic glycogenolysis and gluconeogenesis in conscious dogs. Am J Physiol Endocrinol Metab 2002; 282: E402– E411 [DOI] [PubMed] [Google Scholar]
- 31.Claus TH, El-Maghrabi MR, Pilkis SJ: Modulation of the phosphorylation state of rat liver pyruvate kinase by allosteric effectors and insulin. J Biol Chem 1979; 254: 7855– 7864 [PubMed] [Google Scholar]
- 32.Metcalfe HK, Monson JP, Cohen RD, Padgham C: Enhanced carrier-mediated lactate entry into isolated hepatocytes from starved and diabetic rats. J Biol Chem 1988; 263: 19505– 19509 [PubMed] [Google Scholar]
- 33.Hagstrom-Toft E, Enoksson S, Moberg E, Bolinder J, Arner P: Absolute concentrations of glycerol and lactate in human skeletal muscle, adipose tissue, and blood. Am J Physiol 1997; 273: E584– E592 [DOI] [PubMed] [Google Scholar]
- 34.Qvisth V, Hagstrom-Toft E, Moberg E, Sjoberg S, Bolinder J: Lactate release from adipose tissue and skeletal muscle in vivo: defective insulin regulation in insulin-resistant obese women. Am J Physiol Endocrinol Metab 2007; 292: E709– E714 [DOI] [PubMed] [Google Scholar]
- 35.Gebhardt R, Kleemann E: Hormonal regulation of amino acid transport system N in primary cultures of rat hepatocytes. Eur J Biochem 1987; 166: 339– 344 [DOI] [PubMed] [Google Scholar]
- 36.Granner D, Andreone T, Sasaki K, Beale E: Inhibition of transcription of the phosphoenolpyruvate carboxykinase gene by insulin. Nature 1983; 305: 549– 551 [DOI] [PubMed] [Google Scholar]
- 37.Sasaki K, Cripe TP, Koch SR, Andreone TL, Petersen DD, Beale EG, Granner DK: Multihormonal regulation of phosphoenolpyruvate carboxykinase gene transcription. The dominant role of insulin. J Biol Chem 1984; 259: 15242– 15251 [PubMed] [Google Scholar]
- 38.Blackshear PJ, Holloway PA, Aberti KG: The effects of inhibition of gluconeogenesis on ketogenesis in starved and diabetic rats. Biochem J 1975; 148: 353– 362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferre P, Pegorier JP, Girard J: The effects of inhibition of gluconeogenesis in suckling newborn rats. Biochem J 1977; 162: 209– 212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rognstad R: Rate-limiting steps in metabolic pathways. J Biol Chem 1979; 254: 1875– 1878 [PubMed] [Google Scholar]
- 41.Argaud D, Halimi S, Catelloni F, Leverve XM: Inhibition of gluconeogenesis in isolated rat hepatocytes after chronic treatment with phenobarbital. Biochem J 1991; 280( pt 3): 663– 669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Groen AK, van Roermund CW, Vervoorn RC, Tager JM: Control of gluconeogenesis in rat liver cells. Flux control coefficients of the enzymes in the gluconeogenic pathway in the absence and presence of glucagon. Biochem J 1986; 237: 379– 389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rigoulet M, Leverve XM, Plomp PJ, Meijer AJ: Stimulation by glucose of gluconeogenesis in hepatocytes isolated from starved rats. Biochem J 1987; 245: 661– 668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burgess SC, He T, Yan Z, Lindner J, Sherry AD, Malloy CR, Browning JD, Magnuson MA: Cytosolic phosphoenolpyruvate carboxykinase does not solely control the rate of hepatic gluconeogenesis in the intact mouse liver. Cell Metab 2007; 5: 313– 320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Samuel VT, Beddow SA, Iwasaki T, Zhang XM, Chu X, Still CD, Gerhard GS, Shulman GI: Fasting hyperglycemia is not associated with increased expression of PEPCK or G6Pc in patients with type 2 diabetes. Proc Natl Acad Sci U S A 2009; 106: 12121– 12126 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.