Emerging evidence suggests that HDL function is not always accurately predicted by HDL cholesterol levels. The functions of HDL include reverse cholesterol transport and modulation of inflammation. These functions appear to have evolved as part of the innate immune system. In healthy individuals, in the absence of systemic oxidative stress and inflammation, HDL is anti-inflammatory. However, in those with chronic illnesses such as diabetes that are characterized by systemic oxidative stress and inflammation, HDL may actually promote the inflammatory response (i.e., it may become proinflammatory). HDL may be thought of as a shuttle. The size of the shuttle can be estimated by HDL cholesterol levels. The shuttle's cargo can change dramatically from one that efficiently promotes reverse cholesterol transport and is anti-inflammatory to one that is less effective in promoting reverse cholesterol transport and is also proinflammatory without any change in the size of the shuttle (i.e., these changes in HDL cargo can occur without any change in HDL cholesterol levels). Understanding these issues may lead to improved use of HDL as a biomarker and may also lead to new therapeutic targets and therapies.
HDL can modulate LDL oxidation.
Lipoproteins evolved to facilitate the extracellular transport of lipids in multicellular organisms. The major protein in LDL is apolipoprotein (apo)-B. This protein contains a binding domain that causes LDL to be deposited in the extracellular matrix of many tissues, particularly in arteries that are predisposed to atherosclerosis. As a result of the binding of apoB-containing proteins to extracellular matrix molecules, the concentration of apoB in the subendothelial space of even normal arteries is twofold higher than in plasma (1). The deposition of LDL in the extracellular matrix of the subendothelial space predisposes it to oxidation. The oxidized lipids that result evoke a tissue response similar to that which occurs in response to a Mycobacterium (2,3).
Unlike LDL, HDLs do not normally bind to extracellular matrix molecules, and the concentration of the main protein in HDL (apoA-I) in the subendothelial space of normal arteries is only one-fifth the concentration found in plasma (1). Adding LDL to an artery wall model constructed from cultured human aortic endothelial and smooth muscle resulted in LDL being deposited in the subendothelial space where the cells oxidized the LDL lipids. This caused the cells to synthesize and secrete monocyte chemoattractant protein (MCP)-1, which evokes a potent inflammatory response of the type seen in atherosclerosis. Addition of normal HDL abolished this process, indicating that normal HDL is capable of preventing LDL oxidation and the inflammatory response induced by LDL (4).
The acute-phase response changes HDL's ability to inhibit LDL oxidation.
Van Lenten et al. (5) were the first to report that HDL loses its ability to inhibit LDL oxidation during the acute-phase response. HDL from normal rabbits and humans prior to elective surgery prevented LDL oxidation and prevented LDL-induced MCP-1 production (measured by a bioassay) in cultures of human artery wall cells. In contrast, HDL from the same rabbits or humans, isolated at the peak of an acute-phase response, was less effective in inhibiting LDL oxidation and actually increased LDL-induced MCP-1 production (5). This change in HDL was paralleled by changes in HDL composition. Among the changes noted at the peak of the acute-phase response was a decrease in activity of two HDL-associated enzymes, paraoxonase-1 (PON1) and platelet-activating factor acetylhydrolase (PAF-AH). Upon resolution of the acute-phase response, these HDL-associated enzyme activities returned toward baseline and the anti-inflammatory properties of the HDL were restored (5). Subsequently, it was found that these enzymes were partly responsible for the ability of normal HDL to inhibit proinflammatory LDL-derived oxidized lipids (6–8). The ability of HDL to prevent the formation of LDL-derived oxidized lipids or to inactivate them was determined to be a major factor in identifying the anti-inflammatory properties of HDL (9,10).
The chronic acute-phase response.
Gabay and Kushner (11) noted that many chronic disease states are associated with a chronic acute-phase response defined by the persistent presence of acute-phase reactants in the plasma. By this definition, diseases characterized by persistent elevations of acute-phase reactants such as C-reactive protein (CRP) are examples of a chronic acute-phase response. The important clinical implications of the persistent elevations of these plasma biomarkers in chronic disease states have been extensively studied (12–14).
HDL in diabetes, the metabolic syndrome, obesity, and familial hypercholesterolemia.
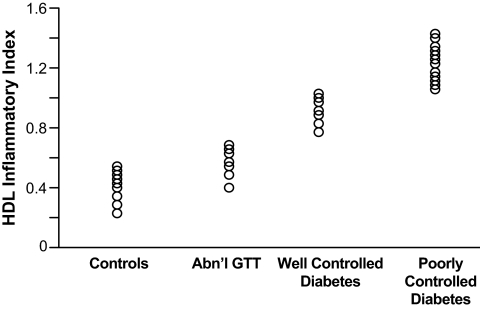
Hedrick et al. (15) found that glycation of HDL by incubation under hyperglycemic conditions caused the HDL to lose its ability to inhibit monocyte adhesion to human aortic endothelial cells exposed to oxidized LDL. Glycation of the HDL-associated enzyme PON1 also prevented its ability to inhibit monocyte adhesion to the endothelial cells. In subjects with type 2 diabetes and documented coronary artery disease, PON1 activity was reduced by 40% (P < 0.0001) compared with nondiabetic subjects (15). Figure 1 shows a progressive loss of the ability of HDL to inhibit LDL oxidation and LDL-induced MCP-1 in subjects with an abnormal glucose tolerance test, subjects with well-controlled diabetes, and subjects with poorly controlled diabetes compared with normal control subjects. HDL cholesterol levels were not significantly different for any of the groups (data not shown).
FIG. 1.
HII in diabetes. Abn'l GTT, abnormal oral glucose tolerance test.
Another example of the inability of HDL cholesterol levels to predict HDL inflammatory properties comes from the article by Roberts et al. (16). HDL inflammatory properties improved despite a fall in HDL cholesterol levels in obese men with characteristics of the metabolic syndrome who were treated with a 3-week residential program of diet and daily aerobic exercise (16). In South Asian immigrants with a high incidence of metabolic syndrome and a high risk for coronary heart disease (CHD), the inflammatory properties of HDL significantly correlated with carotid intima-media thickness (17).
Visceral abdominal obesity is now recognized as a chronic inflammatory state. Perségol et al. (18) found that HDL taken from subjects with abdominal obesity was defective in reversing the effects of oxidized LDL on vascular relaxation compared with HDL from normal subjects. They also found that HDL from both type 1 and type 2 diabetic subjects was similarly dysfunctional (19,20). These results also suggest that although the etiologies of type 1 and type 2 diabetes are different, the abnormalities in HDL function that result from type 1 and type 2 diabetes are similar. Cancello et al. (21) found that omental adipose tissue in morbidly obese humans contained significantly more macrophages than the subcutaneous adipose tissue. Moreover, the increased macrophage accumulation in omental adipose tissue was correlated with fasting glucose and insulin levels and insulin sensitivity and was also closely associated with hepatic fibroinflammatory lesions (21). These results are consistent with macrophage cytokines in omental fat playing a role in insulin resistance as well as the changes in HDL that have been observed in patients with abdominal obesity.
The impact of LDL levels on the inflammatory properties of HDL was demonstrated by the significant improvement in HDL inflammatory properties that occurred after LDL apheresis in patients with familial hypercholesterolemia, despite the decrease in HDL cholesterol levels caused by the treatment (22).
HDL in CHD.
HDL from 27 patients with documented CHD, with normal lipid levels, and without diabetes failed to inhibit LDL oxidation and LDL-induced MCP-1 production in cultures of human artery wall cells, whereas HDL from 31 age- and sex-matched control subjects did not demonstrate this failure (23).
Ansell et al. (24) compared the ability of LDL from a normal control to induce MCP-1 in cultures of human aortic wall cells or to induce fluorescence in a cell-free assay in the absence of HDL and in the presence of a test HDL. The values in the presence of the control LDL with the absence of HDL were normalized to 1.0. The values obtained in the presence of both the control LDL and the test HDL were divided by the values obtained in the presence of the control LDL with the absence of HDL to obtain the HDL inflammatory index (HII). If HII was <1.0, the test HDL was classified as anti-inflammatory. If the addition of the test HDL together with the control LDL resulted in HII >1.0, the test HDL was classified as proinflammatory. Blood was taken from 26 subjects with normal plasma lipid levels but with CHD or CHD equivalents defined by the Cholesterol Education Program Adult Treatment Panel III criteria (25), and the patients were then started on 40 mg simvastatin daily for 6 weeks. HII for the patients prior to simvastatin treatment was means ± SD 1.38 ± 0.91 compared with 0.38 ± 0.14 for age- and sex-matched control subjects (P = 1.5 × 10−5). After 6 weeks of treatment, HII for the patients was 1.08 ± 0.71, which was a significant improvement (P = 0.002) but still, on average, proinflammatory (24). Ansell et al. (24) also studied a group of 20 patients with high HDL cholesterol levels (95 ± 14 mg/dl) and normal LDL and triglyceride levels but documented CHD. HII for this group was 1.28 ± 0.29. Ansell et al. (24) concluded that the inflammatory/anti-inflammatory properties of HDL distinguished patients from control subjects better than HDL cholesterol levels.
HDL in rheumatic diseases.
Systemic lupus erythematosus (SLE) is an autoimmune disease with chronic inflammation and episodes of acute inflammation. Women with SLE have a dramatically increased risk of CHD (7- to 50-fold greater than normal). Proinflammatory HDL was found in 44.7% of women with SLE, 20.1% of women with rheumatoid arthritis, and only 4.1% of healthy women, even though all three groups had normal HDL cholesterol levels (26). Administering 80 mg atorvastatin daily to rheumatoid arthritis patients significantly improved HDL inflammatory properties, which significantly worsened in a group given placebo (27). Scleroderma is a chronic illness associated with abnormal capillaries and Reynaud's phenomenon. Weihrauch et al. (28) reported that scleroderma patients had both low HDL cholesterol levels and proinflammatory HDL.
HDL in other diseases with a chronic acute-phase response.
Chronic renal disease is recognized as a condition associated with chronic inflammation. HII was determined in 189 patients on hemodialysis who were followed prospectively for 30 months. Subjects with HII >1.0 had significantly more comorbid conditions and worse quality of life (short-form 36 [SF36] health survey questionnaire) than patients with HII <1.0. Despite no difference in total cholesterol levels, LDL cholesterol levels, triglyceride levels, or HDL cholesterol levels, after 30 months of follow-up, the patients with HII >1.0 had a significantly higher mortality, determined by Kaplan-Meier curves adjusted for case-mix variables, than patients with HII <1.0 (29). Crohn's disease is another chronic illness associated with persistent elevations in acute-phase reactants. HDL from Crohn's disease patients was found to be dysfunctional and was associated with increased carotid intima-media thickness (30).
HDL in leprosy.
Cruz et al. (31) reported that normal HDL strongly promoted the conversion of monocytes into CD1b+ dendritic cells. In many respects, the cellular events in leprosy are similar to those in atherosclerosis. Cruz et al. found that HDL from leprosy patients was proinflammatory (i.e., HII >1.0) and was also less effective in promoting the conversion of monocytes into CD1b+ dendritic cells than anti-inflammatory HDL from control subjects.
HDL in inbred strains of mice and in rabbits.
Inbred strains of mice have been useful in dissecting genetic traits associated with various diseases. Navab et al. (32) found that inbred strains of mice susceptible to atherosclerosis all had anti-inflammatory HDL, whereas inbred strains resistant to atherosclerosis all had proinflammatory HDL. Additionally, Navab et al. found that human HDL that was proinflammatory was less able to promote cholesterol efflux from human macrophages than was HDL that was anti-inflammatory. HDL cholesterol levels failed to predict lesion area in cholesterol-fed rabbits, but levels of the acute-phase reactant serum amyloid A (SAA) and HII values both accurately predicted lesion area and were significantly correlated with each other (33).
Oxidative stress and the acute-phase response as modifiers of HDL content.
As noted above, PON1 is an HDL-associated enzyme. Bhattacharyya et al. (34) found that the PON1 genotype predicted a dose-dependent association with PON1 activity and with indexes of systemic oxidative stress. The oxidative enzyme myeloperoxidase, which is present in increased concentrations at sites of inflammation, was found to preferentially associate with HDL and cause oxidative damage to apoA-I, resulting in impaired ability of the apoA-I to promote cholesterol efflux (35). A specific tyrosine residue (residue 166) in apoA-I that is required for activation of lecithin cholesterol acyltransferase (LCAT) was found to be the preferred target for oxidative modification (36). Compared with that from normal subjects, HDL from patients with CHD is enriched with apoE and acute-phase proteins (37). Combined treatment with a statin and niacin reduced the apoE content of HDL from CHD patients and resulted in an HDL proteome more similar to that in healthy individuals (38).
The connection between oxidized lipids and the induction of an acute-phase response and the generation of proinflammatory HDL was shown by the injection of oxidized phospholipids into atherosclerosis-susceptible and -resistant mice (39). The injection of oxidized phospholipids into the atherosclerosis-susceptible strain resulted in a significant increase in the HDL-associated acute-phase reactant apoJ and a significant decrease in the HDL-associated enzyme PON1, although there was no change in the strain that is resistant to atherosclerosis (39).
The role of Hb and haptoglobin in determining the inflammatory properties of HDL.
In mice and humans, there is always a small amount of Hb in the plasma that is outside of erythrocytes (RBCs). In the absence of clinical hemolysis, the concentration of Hb in the plasma outside RBCs is ∼10 μmol/l, whereas the concentration in RBCs is >1 mol/l. Watanabe et al. (40) reported that feeding an atherogenic diet to mice that are genetically susceptible to atherosclerosis did not increase the concentration of plasma Hb outside of RBCs but resulted in the association of the Hb specifically with HDL. HDL containing Hb was found to be dysfunctional and proinflammatory in mice and humans (40,41).
The major protein for binding Hb outside of RBCs is haptoglobin (Hp), which is a positive acute-phase protein (i.e., it increases during an acute-phase response), and Hp is known to be associated with HDL. In humans, there are two alleles (1 and 2) for Hp yielding three genotypes: Hp 1-1, Hp 2-1, and Hp 2-2. Diabetic subjects with the Hp 2-2 genotype (∼40% of diabetic subjects) are at increased risk for CHD (42). Although vitamin E has failed to alter outcomes in large studies of patients with atherosclerosis, in a subgroup of middle-aged individuals with both type 2 diabetes and the Hp 2-2 genotype, vitamin E supplementation in a double-blinded clinical trial significantly reduced cardiovascular events (43,44). Mice only have the Hp 1-1 genotype. Levy and colleagues (45) genetically engineered mice to express the Hp 2-2 genotype and found that these mice had impaired reverse cholesterol transport in vivo compared with Hp 1-1 mice. Additionally, serum from Hp 2-1 or Hp 2-2 diabetic humans was inferior to serum from Hp 1-1 subjects in promoting cholesterol efflux from macrophages in vitro (45).
The Hp 1-1 genotype produces an Hp monomer that is monovalent and, consequently, can only associate with one other Hp molecule to create dimers. The Hp 2-2 genotype results in a bivalent molecule that can associate with two different Hp monomers to form cyclic polymers. Levy and colleagues (46) found that in Hp 2-2 diabetic humans and Hp 2-2 diabetic mice, Hb and lipid peroxides associated with HDL were increased and the HDL was dysfunctional in its ability to promote cholesterol efflux from macrophages compared with Hp 1-1 subjects. In a crossover placebo-controlled study, vitamin E treatment decreased oxidative modification of HDL and improved HDL function in Hp 2-2 subjects with diabetes but not Hp 1-1 diabetic subjects (46). Interestingly, on an atherogenic diet, Hb does not associate with HDL in mice lacking Hp, and these mice do not develop proinflammatory HDL, although mice that are wild type for Hp do (41).
HDL cholesterol as a predictor of risk.
HDL cholesterol has long been known to be a powerful predictor of risk for clinical events due to atherosclerosis in large populations (47). However, in all of the clinical studies published, many of the clinical events occurred in subjects with perfectly normal HDL cholesterol levels (32). Additionally, Briel et al. (48) recently published a large meta-regression analysis indicating that simply increasing the amount of circulating HDL cholesterol does not reduce the risk of CHD events, CHD deaths, or total deaths. An obvious example of the dissociation between HDL cholesterol levels and risk for CHD can be found in subjects with apoA-IMilano (49). These individuals have a mutant apoA-I protein that causes low HDL cholesterol levels, but these subjects do not appear to have increased risk for CHD (49). The studies cited above indicate that the composition, functionality, and inflammatory properties of HDL may be as important as HDL cholesterol levels in determining risk for CHD.
HDL as a potential therapy and therapeutic target.
In animal models, HDL and apoA-I have been highly efficacious in the treatment of atherosclerosis (50,51). In pilot studies in humans, apoA-I has also shown promise, including in patients with diabetes (52,53). The initial pilot study (52) suggested that weekly intravenous administration for 5–6 weeks might be adequate. However, larger subsequent trials have not shown sufficient attainment of desired goals after such short treatment periods (54).
The potential benefit of an HDL-based strategy in diabetes was recently demonstrated in a study showing that infusion of recombinant HDL particles increased AMP-activated protein kinase in skeletal muscle, increased plasma insulin levels, and decreased plasma glucose levels in type 2 diabetic subjects (55). ApoA-I is a relatively large protein with 243 amino acid residues, making its large-scale production for clinical use a significant challenge. Given the likely cost and need for prolonged treatments requiring intravenous administration, it is not likely that such a therapy would be used for millions of patients with atherosclerosis and diabetes.
The search for apoA-I mimetic peptides.
More than 2 decades ago, J.P. Segrest and A.M. Anantharamaiah designed a peptide with only 18 amino acid residues that lacked sequence homology with apoA-I but contained a class A amphipathic helix, like that found in apoA-I (56–58). The original peptide was named 18A because of its 18 amino acid residues and the class A amphipathic helix. Addition of blocking groups gave the peptide increased helical stability and increased ability to bind nonoxidized lipids. The resulting peptide was named 2F because of the two phenylalanine residues on the hydrophobic face. Although this peptide bound nonoxidized lipids similarly to apoA-I, it failed to improve atherosclerosis in a mouse model (59). Based on the assumption that oxidized lipids derived from LDL were likely to play a more important role than nonoxidized lipids in the development of atherosclerosis, a series of peptides in which amino acids were conservatively substituted in 2F was tested for the ability to block LDL from inducing MCP-1 in cultures of human artery wall cells. The 2F peptide was found to be relatively weak in this regard compared with peptides with 4–6 phenylalanine residues on the hydrophobic face (59). A peptide with 5 phenylalanine residues on the hydrophobic face (5F) significantly reduced lesions in a mouse model of atherosclerosis (60). A peptide with 4 phenylalanine residues on the hydrophobic face (4F) was also found to be efficacious in mouse models of atherosclerosis (61). Subsequent studies indicated that the 4F peptide improved a variety of inflammation-based diseases in animal models including influenza A pneumonia (62), hyperlipidemia- and sickle cell–mediated vascular dysfunction (63,64), scleroderma (28), type 1 diabetes (65,66), obesity and type 2 diabetes (67,68), hepatic fibrosis (69), vascular dementia (70), arthritis (71), hyperlipidemia-mediated renal inflammation (72), accelerated vein graft atherosclerosis (73), and Alzheimer's disease (74). Additionally, the 4F peptide inhibited the inflammatory response and improved survival in septic rats (75). The 4F peptide was also found to synergize with statins in a mouse model of atherosclerosis, causing regression of lesions (76), and in vitro, it improved the anti-inflammatory properties of HDL from patients with end-stage renal disease (77).
In all of the animal models in which the anti-inflammatory properties of HDL were studied, HII significantly improved after peptide treatment (60–62,64,71,76). In humans with CHD or CHD equivalents, a single oral dose of the 4F peptide synthesized from all D-amino acids significantly improved the HII (78). The efficacy of the 4F peptide, when given by injection, was the same whether the peptide was synthesized from all D- or all L-amino acids (33). It was initially thought that only peptides synthesized from D-amino acids could be given orally because the peptides synthesized from all L-amino acids were rapidly degraded by intestinal proteases (61). However, more recently, it was found that oral administration of the 4F peptide synthesized from all L-amino acids was efficacious if given with niclosamide, a drug that has been used for decades in the treatment of parasitic infections and has low toxicity for mammals. At an acidic pH, niclosamide appears to form a complex with 4F, protecting the peptide from degradation by intestinal proteases and thus allowing dramatically increased bioactivity after absorption (79).
The plasma concentrations of the 4F peptide after oral administration to animal models or in human studies were very low. In a mouse model of atherosclerosis, the maximal plasma concentration was ∼130 nmol/l (80), and in humans, it was ∼4 nmol/l (78). Because 4F is an apoA-I mimetic peptide and the concentration of apoA-I in mouse models of atherosclerosis and in humans is ∼35 μmol/l, it was difficult to understand how the peptide could be bioactive. The answer to this conundrum was found to be that the original peptide 2F was selected for its ability to bind nonoxidized lipids similar to apoA-I, whereas the 4F peptide was selected for its ability to inhibit LDL oxidation by human artery wall cells. Direct comparison of the ability of human apoA-I and the 4F peptide to bind nonoxidized lipids versus oxidized lipids showed that apoA-I and 4F bound nonoxidized lipids similarly, but the 4F peptide bound oxidized lipids as much as 5 million–fold better than apoA-I (81). It was concluded that the mechanism of action of the 4F peptide relates to this remarkable ability to bind oxidized lipids (82,83). Consistent with this conclusion was the finding that, in vivo, the 4F peptide specifically removed oxidized lipids from inflamed tissue and that the removal of these oxidized lipids was associated with resolution of the inflammatory changes (72).
Summary of the present state of the art and potential for future innovations.
Measuring HDL cholesterol levels may not accurately predict the composition, functionality, and anti-inflammatory properties of HDL. HDL in diseases associated with a chronic acute-phase response has been found to be dysfunctional and proinflammatory. Currently, there are no tests widely available for measuring the composition, functionality, and inflammatory properties of HDL in clinical practice. However, it appears that the composition, functionality, and inflammatory properties of HDL are directly related to the presence or absence of conditions that are known to induce a chronic acute-phase response. These conditions are clinically recognizable (e.g., diabetes, visceral obesity, CHD, rheumatic diseases, chronic inflammatory gastrointestinal conditions, chronic renal disease, and chronic infections). Studies using HDL and HDL mimetics as therapeutic agents are all in early-phase clinical trials. Studies in animals and in the early clinical trials are encouraging, but it likely will be some time before the outcome of definitive studies is known. In the meantime, our therapeutic approach must continue to emphasize lifestyle modification, control of diabetes, obesity, hyperlipidemia, and hypertension as well as the appropriate use of aspirin, statins, ACE inhibitors, and β-blockers for patients with CHD.
Acknowledgments
This work was supported in part by U.S. Public Health Service Grants HL-30568 and HL-34343 and the Laubisch, Castera, and M.K. Gray Funds at the University of California Los Angeles.
M.N., G.M.A., S.T.R., and A.M.F. are principals in Bruin Pharma, and A.M.F. is an officer in Bruin Pharma. No other potential conflicts of interest relevant to this article were reported.
REFERENCES
- 1.Navab M, Berliner JA, Watson AD, Hama SY, Territo MC, Lusis AJ, Shih DM, Van Lenten BJ, Frank JS, Demer LL, Edwards PA, Fogelman AM: The yin and yang of oxidation in the development of the fatty streak: a review based on the 1994 George Lyman Duff Memorial Lecture. Arterioscler Thromb Vasc Biol 1996; 16: 831– 842 [DOI] [PubMed] [Google Scholar]
- 2.Navab M, Berliner JA, Subbanagounder G, Hama S, Lusis AJ, Castellani LW, Reddy S, Shih D, Shi W, Watson AD, Van Lenten BJ, Vora D, Fogelman AM: HDL and the inflammatory responses induced by LDL-derived oxidized phospholipids. Arterioscler Thromb Vasc Biol 2001; 21: 481– 488 [DOI] [PubMed] [Google Scholar]
- 3.Berliner JA, Watson AD: A role for oxidized phospholipids in atherosclerosis. N Engl J Med 2005; 353: 9– 11 [DOI] [PubMed] [Google Scholar]
- 4.Navab M, Imes SS, Hama SY, Hough GP, Ross LA, Bork RW, Valente AJ, Berliner JA, Drinkwater DC, Laks H: Monocyte transmigration induced by modification of low density lipoprotein in cocultures of human aortic wall cells is due to induction of monocyte chemotactic protein 1 synthesis and is abolished by high density lipoprotein. J Clin Invest 1991; 88: 2039– 2046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Lenten BJ, Hama SY, de Beer FC, Stafforini DM, McIntyre TM, Prescott SM, La Du BN, Fogelman AM, Navab M: Anti-inflammatory HDL becomes pro-inflammatory during the acute phase response: loss of protective effect of HDL against LDL oxidation in aortic wall cell cocultures. J Clin Invest 1995; 96: 2758– 2767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watson AD, Navab M, Hama SY, Sevanian A, Prescott SM, Stafforini DM, McIntyre TM, Du BN, Fogelman AM, Berliner JA: Effect of platelet activating factor-acetylhydrolase on the formation and action of minimally oxidized low density lipoprotein. J Clin Invest 1995; 95: 774– 782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watson AD, Berliner JA, Hama SY, La Du BN, Faull KF, Fogelman AM, Navab M: Protective effect of high density lipoprotein associated paraoxonase: inhibition of the biological activity of minimally oxidized low density lipoprotein. J Clin Invest 1995; 96: 2882– 2891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watson AD, Leitinger N, Navab M, Faull KF, Hörkkö S, Witztum JL, Palinski W, Schwenke D, Salomon RG, Sha W, Subbanagounder G, Fogelman AM, Berliner JA: Structural identification by mass spectrometry of oxidized phospholipids in minimally oxidized low density lipoprotein that induce monocyte/endothelial interactions and evidence for their presence in vivo. J Biol Chem 1997; 272: 13597– 13607 [DOI] [PubMed] [Google Scholar]
- 9.Navab M, Hama SY, Cooke CJ, Anantharamaiah GM, Chaddha M, Jin L, Subbanagounder G, Faull KF, Reddy ST, Miller NE, Fogelman AM: Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: step 1. J Lipid Res 2000; 41: 1481– 1494 [PubMed] [Google Scholar]
- 10.Navab M, Hama SY, Anantharamaiah GM, Hassan K, Hough GP, Watson AD, Reddy ST, Sevanian A, Fonarow GC, Fogelman AM: Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: steps 2 and 3. J Lipid Res 2000; 41: 1495– 1508 [PubMed] [Google Scholar]
- 11.Gabay C, Kushner I: Acute-phase proteins and other systemic responses to inflammation. N Engl J Med 1999; 340: 448– 454 [DOI] [PubMed] [Google Scholar]
- 12.Ridker PM: Inflammatory biomarkers and risks of myocardial infarction, stroke, diabetes, and total mortality: implications for longevity. Nutr Rev 2007; 65: S253– S259 [DOI] [PubMed] [Google Scholar]
- 13.Bruno G, Fornengo P, Novelli G, Panero F, Perotto M, Segre O, Zucco C, Deambroqio P, Bargero G, Perin PC: C-reactive protein and 5-year survival in type 2 diabetes: the Casale Monferrato Study. Diabetes 2009; 58: 926– 933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ridker PM: C-reactive protein: eighty years from discovery to emergence as a major risk marker for cardiovascular disease. Clin Chem 2009; 55: 209– 215 [DOI] [PubMed] [Google Scholar]
- 15.Hedrick CC, Thorpe SR, Fu MX, Harper CM, Yoo J, Kim SM, Wong H, Peters AL: Glycation impairs high-density lipoprotein function. Diabetologia 2000; 43: 312– 320 [DOI] [PubMed] [Google Scholar]
- 16.Roberts CK, Ng C, Hama S, Eliseo AJ, Barnard RJ: Effect of a short-term diet and exercise intervention on inflammatory/anti-inflammatory properties of HDL in overweight/obese men with cardiovascular risk factors. J Appl Physiol 2006; 101: 1727– 1732 [DOI] [PubMed] [Google Scholar]
- 17.Dodani S, Kaur R, Reddy S, Reed GL, Navab M, George V: Can dysfunctional HDL explain high coronary artery disease risk in South Asians? Int J Cardiol 2008; 129: 125– 132 [DOI] [PubMed] [Google Scholar]
- 18.Perségol L, Vergès B, Gambert P, Duvillard L: Inability of HDL from abdominally obese subjects to counteract the inhibitory effect of oxidized LDL on vasorelaxation. J Lipid Res 2007; 48: 1396– 1401 [DOI] [PubMed] [Google Scholar]
- 19.Perségol L, Foissac M, Lagrost L, Athias A, Gambert P, Vergès B, Duvillard L: HDL particles from type 1 diabetic patients are unable to reverse the inhibitory effect of oxidised LDL on endothelium-dependent vasorelaxation. Diabetologia 2007; 50: 2384– 2387 [DOI] [PubMed] [Google Scholar]
- 20.Perségol L, Vergès B, Foissac M, Gambert P, Duvillard L: Inability of HDL from type 2 diabetic patients to counteract the inhibitory effect of oxidized LDL on endothelium-dependent vasorelaxation. Diabetologia 2006; 49: 1380– 1386 [DOI] [PubMed] [Google Scholar]
- 21.Cancello R, Tordjman J, Poitou C, Guilhem G, Bouillot JL, Hugol D, Coussieu C, Basdevant A, Bar Hen A, Bedossa P, Guerre-Millo M, Clément K: Increased infiltration of macrophages in omental adipose tissue is associated with marked hepatic lesions in morbid human obesity. Diabetes 2006; 55: 1554– 1561 [DOI] [PubMed] [Google Scholar]
- 22.Opole IO, Belmont JM, Kumar A, Moriarty PM: Effect of low-density lipoprotein apheresis on inflammatory and noninflammatory high-density lipoprotein cholesterol. Am J Cardiol 2007; 100: 1416– 1418 [DOI] [PubMed] [Google Scholar]
- 23.Navab M, Hama SY, Hough GP, Subbanagounder G, Reddy ST, Fogelman AM: A cell-free assay for detecting HDL that is dysfunctional in preventing the formation of or inactivating oxidized phospholipids. J Lipid Res 2001; 42: 1308– 1317 [PubMed] [Google Scholar]
- 24.Ansell BJ, Navab M, Hama S, Kamranpour N, Fonarow G, Hough G, Rahmani S, Mottahedeh R, Dave R, Reddy ST, Fogelman AM: Inflammatory/antiinflammatory properties of high-density lipoprotein distinguish patients from control subjects better than high-density lipoprotein cholesterol levels and are favorably affected by simvastatin treatment. Circulation 2003; 108: 2751– 2756 [DOI] [PubMed] [Google Scholar]
- 25.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001; 285: 2486– 2497 [DOI] [PubMed] [Google Scholar]
- 26.McMahon M, Grossman J, FitzGerald J, Dahlin-Lee E, Wallace DJ, Thong BY, Badsha H, Kalunian K, Charles C, Navab M, Fogelman AM, Hahn BH: Proinflammatory high-density lipoprotein as a biomarker for atherosclerosis in patients with systemic lupus erythematosus and rheumatoid arthritis. Arthritis Rheum 2006; 54: 2541– 2549 [DOI] [PubMed] [Google Scholar]
- 27.Charles-Schoeman C, Khanna D, Furst DE, McMahon M, Reddy ST, Fogelman AM, Paulus HE, Park GS, Gong T, Ansell BJ: Effects of high-dose atorvastatin on antiinflammatory properties of high density lipoprotein in patients with rheumatoid arthritis: a pilot study. J Rheumatol 2007; 34: 1459– 1464 [PubMed] [Google Scholar]
- 28.Weihrauch D, Xu H, Shi Y, Wang J, Brien J, Jones DW, Kaul S, Komorowski RA, Csuka ME, Oldham KT, Pritchard KA: Effects of D-4F on vasodilation, oxidative stress, angiostatin, myocardial inflammation, and angiogenic potential in tight-skin mice. Am J Physiol Heart Circ Physiol 2007; 293: H1432– H1441 [DOI] [PubMed] [Google Scholar]
- 29.Kalantar-Zadeh K, Kopple JD, Kamranpour N, Fogelman AM, Navab M: HDL-inflammatory index correlates with poor outcome in hemodialysis patients. Kidney Int 2007; 72: 1149– 1156 [DOI] [PubMed] [Google Scholar]
- 30.van Leuven SI, Hezemans R, Levels JH, Snoek S, Stokkers PC, Hovingh GK, Kastelein JJ, Stroes ES, de Groot E, Hommes DW: Enhanced atherogenesis and altered high density lipoprotein in patients with Crohn's disease. J Lipid Res 2007; 48: 2640– 2646 [DOI] [PubMed] [Google Scholar]
- 31.Cruz D, Watson AD, Miller CS, Montoya D, Ochoa MT, Sieling PA, Gutierrez MA, Navab M, Reddy ST, Witztum JL, Fogelman AM, Rea TH, Eisenberg D, Berliner J, Modlin RL: Host-derived oxidized phospholipids and HDL regulate innate immunity in human leprosy. J Clin Invest 2008; 118: 2917– 2928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Navab M, Ananthramaiah GM, Reddy ST, Van Lenten BJ, Ansell BJ, Hama S, Hough G, Bachini E, Grijalva VR, Wagner AC, Shaposhnik Z, Fogelman AM: The double jeopardy of HDL. Ann Med 2005; 37: 173– 178 [DOI] [PubMed] [Google Scholar]
- 33.Van Lenten BJ, Wagner AC, Navab M, Anantharamaiah GM, Hama S, Reddy ST, Fogelman AM: Lipoprotein inflammatory properties and serum amyloid A levels but not cholesterol levels predict lesion area in cholesterol-fed rabbits. J Lipid Res 2007; 48: 2344– 2353 [DOI] [PubMed] [Google Scholar]
- 34.Bhattacharyya T, Nicholls SJ, Topol EJ, Zhang R, Yang X, Schmitt D, Fu X, Shao M, Brennan DM, Ellis SG, Brennan ML, Allayee H, Lusis AJ, Hazen SL: Relationship of paraoxonase 1 (PON1) gene polymorphisms and functional activity with systemic oxidative stress and cardiovascular risk. JAMA 2008; 299: 1265– 1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nicholls SJ, Zheng L, Hazen SL: Formation of dysfunctional high-density lipoprotein by myeloperoxidase. Trends Cardiovasc Med 2005; 15: 212– 219 [DOI] [PubMed] [Google Scholar]
- 36.Wu Z, Wagner MA, Zheng L, Parks JS, Shy JM, 3rd, Smith JD, Gogonea V, Hazen SL: The refined structure of nascent HDL reveals a key functional domain for particle maturation and dysfunction. Nat Struct Mol Biol 2007; 14: 861– 868 [DOI] [PubMed] [Google Scholar]
- 37.Vaisar T, Pennathur S, Green PS, Gharib SA, Hoofnagle AN, Cheung MC, Byun J, Vuletic S, Kassim S, Singh P, Chea H, Knopp RH, Brunzell J, Geary R, Chait A, Zhao XQ, Elkon K, Marcovina S, Ridker P, Oram JF, Heinecke JW: Shotgun proteomics implicates protease inhibition and complement activation in the antiinflammatory properties of HDL. J Clin Invest 2007; 117: 746– 756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Green PS, Vaisar T, Pennathur S, Kulstad JJ, Moore AB, Marcovina S, Brunzell J, Knopp RH, Zhao XQ, Heinecke JW: Combined statin and niacin therapy remodels high-density lipoprotein proteome. Circulation 2008; 118: 1259– 1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Navab M, Hama-Levy S, Van Lenten BJ, Fonarow GC, Cardinez CJ, Castellani LW, Brennan ML, Lusis AJ, Fogelman AM, La Du BN: Mildly oxidized LDL induces an increased apolipoprotein J/paraoxonase ratio. J Clin Invest 1997; 99: 2005– 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Watanabe J, Chou KJ, Liao JC, Miao Y, Meng HH, Ge H, Grijalva V, Hama S, Kozak K, Buga G, Whitelegge JP, Lee TD, Farias-Eisner R, Navab M, Fogelman AM, Reddy ST: Differential association of hemoglobin with proinflammatory high density lipoproteins in atherogenic/hyperlipidemic mice: a novel biomarker of atherosclerosis. J Biol Chem 2007; 282: 23698– 23707 [DOI] [PubMed] [Google Scholar]
- 41.Watanabe J, Grijalva V, Hama S, Barbour K, Berger FG, Navab M, Fogelman AM, Reddy ST: Hemoglobin and its scavenger protein haptoglobin associate with apoA-1-containing particles and influence the inflammatory properties and function of high density lipoprotein. J Biol Chem 2009; 284: 18292– 18301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levy AP, Hochberg I, Jablonski K, Resnick HE, Lee ET, Best L, Howard BVStrong Heart Study Haptoglobin phenotype is an independent risk factor for cardiovascular disease in individuals with diabetes: The Strong Heart Study. J Am Coll Cardiol 2002; 40: 1984– 1990 [DOI] [PubMed] [Google Scholar]
- 43.Milman U, Blum S, Shapira C, Aronson D, Miller-Lotan R, Anbinder Y, Alshiek J, Bennett L, Kostenko M, Landau M, Keidar S, Levy Y, Khemlin A, Radan A, Levy AP: Vitamin E supplementation reduces cardiovascular events in a subgroup of middle-aged individuals with both type 2 diabetes mellitus and the haptoglobin 2-2 genotype: a prospective double-blinded clinical trial. Arterioscler Thromb Vasc Biol 2008; 28: 341– 347 [DOI] [PubMed] [Google Scholar]
- 44.Blum S, Milman U, Shapira C, Miller-Lotan R, Bennett L, Kostenko M, Landau M, Keidar S, Levy Y, Khemlin A, Radan A, Levy AP: Dual therapy with statins and antioxidants is superior to statins alone in decreasing the risk of cardiovascular disease in a subgroup of middle-aged individuals with both diabetes mellitus and the haptoglobin 2-2 genotype. Arterioscler Thromb Vasc Biol 2008; 28: e18– e20 [DOI] [PubMed] [Google Scholar]
- 45.Asleh R, Miller-Lotan R, Aviram M, Hayek T, Yulish M, Levy JE, Miller B, Blum S, Milman U, Shapira C, Levy AP: Haptoglobin genotype is a regulator of reverse cholesterol transport in vitro and in vivo. Circ Res 2006; 99: 1419– 1425 [DOI] [PubMed] [Google Scholar]
- 46.Asleh R, Blum S, Kalet-Litman S, Alshiek J, Miller-Lotan R, Asaf R, Rock W, Aviram M, Milman U, Shapira C, Abassi Z, Levy AP: Correction of HDL dysfunction in individuals with diabetes and the haptoglobin 2-2 genotype. Diabetes 2008; 57: 2794– 2800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR: High density lipoprotein as a protective factor against coronary heart disease: The Framingham Study. Am J Med 1977; 62: 707– 714 [DOI] [PubMed] [Google Scholar]
- 48.Briel M, Ferreira-Gonzalez I, You JJ, Karanicolas PJ, Akl EA, Wu P, Belchacz B, Bassler D, Wei X, Sharman A, Whitt I, da Silva SA, Khalid Z, Nordmann AJ, Zhou Q, Walter SD, Vale N, Bhatnagar N, O'Regan C, Mills EJ, Bucher HC, Montori VM, Guyatt GH: Association between change in high density lipoprotein cholesterol and cardiovascular disease morbidity and mortality: systematic review and meta-regression analysis. BMJ 2009; 338: b92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alexander ET, Tanaka M, Kono M, Saito H, Rader DJ, Phillips MC: Structural and functional consequences of the Milano mutation (R173C) in human apolipoprotein A-I. J Lipid Res 2009; 50: 1409– 1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Badimon JJ, Badimon L, Fuster V: Regression of atherosclerotic lesions by high density lipoprotein plasma fraction in the cholesterol-fed rabbit. J Clin Invest 1990; 85: 1234– 1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Plump AS, Scott CJ, Breslow JL: Human apolipoprotein A-I gene expression increases high density lipoprotein and suppresses atherosclerosis in the apolipoprotein E-deficient mouse. Proc Natl Acad Sci U S A 1994; 91: 9607– 9611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nissen SE, Tsunoda T, Tuzcu EM, Schoenhagen P, Cooper CJ, Yasin M, Eaton GM, Lauer MA, Sheldon WS, Grines CL, Halpern S, Crowe T, Blankenship JC, Kerensky R: Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA 2003; 290: 2292– 22300 [DOI] [PubMed] [Google Scholar]
- 53.Patel S, Drew BG, Nakhla S, Duffy SJ, Murphy AJ, Barter PJ, Rye KA, Chin-Dusting J, Hoang A, Sviridov D, Celermajer DS, Kingwell BA: Reconstituted high-density lipoprotein increases plasma high-density lipoprotein anti-inflammatory properties and cholesterol efflux capacity in patients with type 2 diabetes. J Am Coll Cardiol 2009; 53: 962– 971 [DOI] [PubMed] [Google Scholar]
- 54.Tardif JC, Grégoire J, L'Allier PL, Ibrahim R, Lespérance J, Heinonen TM, Kouz S, Berry C, Basser R, Lavoie MA, Guertin MC, Rodés-Cabau JEffect of rHDL on Atherosclerosis-Safety and Efficacy (ERASE) Investigators Effects of reconstituted high-density lipoprotein infusions on coronary atherosclerosis: a randomized controlled trial. JAMA 2007; 297: 1675– 1682 [DOI] [PubMed] [Google Scholar]
- 55.Drew BG, Duffy SJ, Formosa MF, Natoli AK, Henstridge DC, Penfold SA, Thomas WG, Mukhamedova N, de Courten B, Forbes JM, Yap FY, Kaye DM, van Hall G, Febbraio MA, Kemp BE, Sviridov D, Steinberg GR, Kingwell BA: High-density lipoprotein modulates glucose metabolism in patients with type 2 diabetes mellitus. Circulation 2009; 119: 2103– 2111 [DOI] [PubMed] [Google Scholar]
- 56.Anantharamaiah GM, Jones JL, Brouillette CG, Schmidt CF, Chung BH, Hughes TA, Bhown AS, Segrest JP: Studies of synthetic peptide analogs of amphipathic helix: structure of complexes with dimyristoyl phosphatidylcholine. J Biol Chem 1985; 260: 10248– 10255 [PubMed] [Google Scholar]
- 57.Venkatachalapathi YV, Phillips MC, Epand RM, Epand RF, Tytler EM, Segrest JP, Anantharamaiah GM: Effect of end group blockage on the properties of a class A amphipathic helical peptide. Proteins 1993; 15: 349– 359 [DOI] [PubMed] [Google Scholar]
- 58.Yancey PG, Bielicki JK, Johnson WJ, Lund-Katz S, Palgunachari MN, Anantharamaiah GM, Segrest JP, Phillips MC, Rothblat GH: Efflux of cellular cholesterol and phospholipid to lipid-free apolipoproteins and class A amphipathic peptides. Biochemistry 1995; 34: 7955– 7965 [DOI] [PubMed] [Google Scholar]
- 59.Datta G, Chaddha M, Hama S, Navab M, Fogelman AM, Garber DW, Mishra VK, Epand RM, Epand RF, Lund-Katz S, Phillips MC, Segrest JP, Anantharamaiah GM: Effects of increasing hydrophobicity on the physical-chemical and biological properties of a class A amphipathic helical peptide. J Lipid Res 2001; 42: 1096– 1104 [PubMed] [Google Scholar]
- 60.Garber DW, Datta G, Chaddha M, Palgunachari MN, Hama SY, Navab M, Fogelman AM, Segrest JP, Anantharamaiah GM: A new synthetic class A amphipathic peptide analogue protects mice from diet-induced atherosclerosis. J Lipid Res 2001; 42: 545– 552 [PubMed] [Google Scholar]
- 61.Navab M, Anantharamaiah GM, Hama S, Garber DW, Chaddha M, Hough G, Lallone R, Fogelman AM: Oral administration of an Apo A-I mimetic peptide synthesized from D-amino acids dramatically reduces atherosclerosis in mice independent of plasma cholesterol. Circulation 2002; 105: 290– 292 [DOI] [PubMed] [Google Scholar]
- 62.Van Lenten BJ, Wagner AC, Anantharamaiah GM, Garber DW, Fishbein MC, Adhikary L, Nayak DP, Hama S, Navab M, Fogelman AM: Influenza infection promotes macrophage traffic into arteries of mice that is prevented by D-4F, an apolipoprotein A-I mimetic peptide. Circulation 2002; 106: 1127– 1132 [DOI] [PubMed] [Google Scholar]
- 63.Ou J, Ou Z, Jones DW, Holzhauer S, Hatoum OA, Ackerman AW, Weihrauch DW, Gutterman DD, Guice K, Oldham KT, Hillery CA, Pritchard KA, Jr: L-4F, an apolipoprotein A-1 mimetic, dramatically improves vasodilation in hypercholesterolemia and sickle cell disease. Circulation 2003; 107: 2337– 2341 [DOI] [PubMed] [Google Scholar]
- 64.Ou J, Wang J, Xu H, Ou Z, Sorci-Thomas MG, Jones DW, Signorino P, Densmore JC, Kaul S, Oldham KT, Pritchard KA, Jr: Effects of D-4F on vasodilation and vessel wall thickness in hypercholesterolemic LDL receptor-null and LDL receptor/apolipoprotein A-I double-knockout mice on Western diet. Circ Res 2005; 97: 1190– 1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kruger AL, Peterson S, Turkseven S, Kaminski PM, Zhang FF, Quan S, Wolin MS, Abraham NG: D-4F induces heme oxygenase-1 and extracellular superoxide dismutase, decreases endothelial cell sloughing, and improves vascular reactivity in rat model of diabetes. Circulation 2005; 111: 3126– 3134 [DOI] [PubMed] [Google Scholar]
- 66.Peterson SJ, Husney D, Kruger AL, Olszanecki R, Ricci F, Rodella LF, Stacchiotti A, Rezzani R, McClung JA, Aronow WS, Ikehara S, Abraham NG: Long-term treatment with the apolipoprotein A1 mimetic peptide increases antioxidants and vascular repair in type I diabetic rats. J Pharmacol Exp Ther 2007; 322: 514– 520 [DOI] [PubMed] [Google Scholar]
- 67.Peterson SJ, Drummond G, Kim DH, Li M, Kruger AL, Ikehara S, Abraham NG: L-4F treatment reduces adiposity, increases adiponectin levels, and improves insulin sensitivity in obese mice. J Lipid Res 2008; 49: 1658– 1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Peterson SJ, Kim DH, Li M, Positano V, Vanella L, Rodella LF, Piccolomini F, Puri N, Gastaldelli A, Kusmic C, L'Abbate A, Abraham NG: The L-4F mimetic peptide prevents insulin resistance through increased levels of HO-1, pAMPK, and pAKT in obese mice. J Lipid Res 2009; 50: 1293– 1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.DeLeve LD, Wang X, Kanel GC, Atkinson RD, McCuskey RS: Prevention of hepatic fibrosis in a murine model of metabolic syndrome with nonalcoholic steatohepatitis. Am J Pathol 2008; 173: 993– 1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Buga GM, Frank JS, Mottino GA, Hendizadeh M, Hakhamian A, Tillisch JH, Reddy ST, Navab M, Anantharamaiah GM, Ignarro LJ, Fogelman AM: D-4F decreases brain arteriole inflammation and improves cognitive performance in LDL receptor-null mice on a Western diet. J Lipid Res 2006; 47: 2148– 2160 [DOI] [PubMed] [Google Scholar]
- 71.Charles-Schoeman C, Banquerigo ML, Hama S, Navab M, Park GS, Van Lenten BJ, Wagner AC, Fogelman AM, Brahn E: Treatment with an apolipoprotein A-1 mimetic peptide in combination with pravastatin inhibits collagen-induced arthritis. Clin Immunol 2008; 127: 234– 244 [DOI] [PubMed] [Google Scholar]
- 72.Buga GM, Frank JS, Mottino GA, Hakhamian A, Narasimha A, Watson AD, Yekta B, Navab M, Reddy ST, Anantharamaiah GM, Fogelman AM: D-4F reduces EO6 immunoreactivity, SREBP-1c mRNA levels, and renal inflammation in LDL receptor-null mice fed a Western diet. J Lipid Res 2008; 49: 192– 205 [DOI] [PubMed] [Google Scholar]
- 73.Li X, Chyu KY, Faria Neto JR, Yano J, Nathwani N, Ferreira C, Dimayuga PC, Cercek B, Kaul S, Shah PK: Differential effects of apolipoprotein A-I-mimetic peptide on evolving and established atherosclerosis in apolipoprotein E-null mice. Circulation 2004; 110: 1701– 1705 [DOI] [PubMed] [Google Scholar]
- 74.Handattu SP, Garber DW, Monroe CE, van Groen T, Kadish I, Nayyar G, Cao D, Palgunachari MN, Li L, Anantharamaiah GM: Oral apolipoprotein A-I mimetic peptide improves cognitive function and reduces amyloid burden in a mouse model of Alzheimer's disease. Neurobiol Dis 2009; 34: 525– 534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang Z, Datta G, Zhang Y, Miller AP, Mochon P, Chen YF, Chatham JC, Anantharamaiah GM, White CR: Apolipoprotein A-I mimetic peptide treatment inhibits inflammatory responses and improves survival in septic rats. Am J Physiol Heart Circ Physiol 2009; 297: H866– H873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Navab M, Anantharamaiah GM, Hama S, Hough G, Reddy ST, Frank JS, Garber DW, Handattu S, Fogelman AM: D-4F and statins synergize to render HDL antiinflammatory in mice and monkeys and cause lesion regression in old apolipoprotein E-null mice. Arterioscler Thromb Vasc Biol 2005; 25: 1426– 1432 [DOI] [PubMed] [Google Scholar]
- 77.Vaziri ND, Moradi H, Pahl MV, Fogelman AM, Navab M: In vitro stimulation of HDL anti-inflammatory activity and inhibition of LDL pro-inflammatory activity in the plasma of patients with end-stage renal disease by an apoA-1 mimetic peptide. Kidney Int 2009; 76: 437– 444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bloedon LT, Dunbar R, Duffy D, Pinell-Salles P, Norris R, DeGroot BJ, Movva R, Navab M, Fogelman AM, Rader DJ: Safety, pharmacokinetics, and pharmacodynamics of oral apoA-I mimetic peptide D-4F in high-risk cardiovascular patients. J Lipid Res 2008; 49: 1344– 1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Navab M, Ruchala P, Waring AJ, Lehrer RI, Hama S, Hough G, Palgunachari MN, Anantharamaiah GM, Fogelman AM: A novel method for oral delivery of apolipoprotein mimetic peptides synthesized from all L-amino acids. J Lipid Res 2009; 50: 1538– 1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Navab M, Anantharamaiah GM, Reddy ST, Hama S, Hough G, Grijalva VR, Wagner AC, Frank JS, Datta G, Garber D, Fogelman AM: Oral D-4F causes formation of pre-β high-density lipoprotein and improves high-density lipoprotein-mediated cholesterol efflux and reverse cholesterol transport from macrophages in apolipoprotein E-null mice. Circulation 2004; 109: 3215– 3220 [DOI] [PubMed] [Google Scholar]
- 81.Van Lenten BJ, Wagner AC, Jung CL, Ruchala P, Waring AJ, Lehrer RI, Watson AD, Hama S, Navab M, Anantharamaiah GM, Fogelman AM: Anti-inflammatory apoA-I-mimetic peptides bind oxidized lipids with much higher affinity than human apoA-I. J Lipid Res 2008; 49: 2302– 2311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Epand RF, Mishra VK, Palgunachari MN, Anantharamaiah GM, Epand RM: Anti-inflammatory peptides grab on to the whiskers of atherogenic oxidized lipids. Biochim Biophys Acta 2009; 1788: 1967– 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Navab M, Shechter I, Anantharamaiah GM, Reddy ST, Van Lenten BJ, Fogelman AM: Structure and function of HDL mimetics. Arterioscler Thromb Vasc Biol 16July2009. [ Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]