Abstract
OBJECTIVE
The aim of this study was to investigate the impact of 9 days of bed rest on insulin secretion, insulin action, and whole-body glucose and fat metabolism in first-degree relative (FDR) and matched control (CON) subjects.
RESEARCH DESIGN AND METHODS
A total of 13 FDR and 20 CON subjects participated in the study. All were studied before and after 9 days of bed rest using the clamp technique combined with indirect calorimetry preceded by an intravenous glucose tolerance test. Glucose and glycerol turnover rates were studied using stable isotope kinetics.
RESULTS
Bed rest caused a significant decrease in whole-body insulin sensitivity in both groups. Hepatic insulin resistance was elevated in FDR subjects prior to bed rest and was significantly augmented by bed rest in FDR (P < 0.01) but not in CON (P = NS) subjects. The rate of whole-body lipolysis decreased during bed rest in both FDR and CON subjects, with no significant differences between the groups. Insulin resistance induced by bed rest was fully accounted for by the impairment of nonoxidative glucose metabolism in both groups (overall P < 0.001).
CONCLUSIONS
Whole-body insulin action in both insulin-resistant FDR and healthy CON subjects deteriorates with 9 days of bed rest, converging toward similar degrees of whole-body insulin resistance. FDR subjects exhibit hepatic insulin resistance (HIR), which, in contrast to CON subjects, deteriorates in response to physical inactivity. FDR subjects exhibit reduced insulin secretion when seen in relation to their degree of HIR but not peripheral insulin resistance.
Type 2 diabetes is caused by a complicated interplay between genetic and environmental factors that influence defects of peripheral and hepatic insulin action, insulin secretion, adipose tissue metabolism and lipolysis, and possibly a range of additional metabolic defects in various other organs (1). First-degree relatives (FDR subjects) of patients with type 2 diabetes have been characterized by insulin resistance and β-cell dysfunction (2,3).
The habitual degree of physical activity is a moderator of glucose and fat metabolism, including insulin action (4,5). Physical inactivity is associated with increased morbidity and mortality (6,7) and has negative effects on lipid metabolism and insulin sensitivity (8–10). Studies of the regulatory mechanisms influencing skeletal muscle lipoprotein lipase (LPL) activity provided proof of the principle that the cellular and molecular mechanisms influencing LPL activity, and therefore fatty acid metabolism, during physical inactivity are distinct from the cellular events influencing LPL during exercise training. Indeed, reducing normal physical activity level has a much greater effect on LPL regulation than adding vigorous exercise training on top of the normal level of nonexercise activity (11). Thus, there are reasons to believe that exercise training versus physical inactivity influences additional molecular mechanisms and metabolic pathways relevant to metabolic health and risk of type 2 diabetes in a differential manner in humans.
Previous studies (12,13) documented the detrimental effect of inactivity on insulin action in healthy individuals. The Dallas Bedrest and Training Study showed that 3 weeks of bed rest caused a fall in Vo2max comparable to 30 years of aging (14). Previous studies have demonstrated reduced Vo2max in healthy FDR subjects (15). It has been estimated that a sedentary lifestyle accounts for at least 25% of type 2 diabetes incidence (16), and sedentary FDR subjects have about three times the risk of developing type 2 diabetes (17).
Although muscle insulin resistance and defective pancreatic insulin secretion may represent the most prominent defects of metabolism in FDR subjects (18,19), defects of metabolism in other organs, including liver (3,20,21), are important for elevating plasma glucose levels in type 2 diabetic patients. Little is known about the response of muscle, liver, pancreas, and adipose tissue metabolism in FDR subjects when exposed to physical inactivity, and there is a need to understand the impact of physical inactivity on mechanisms involved in the development of type 2 diabetes (22).
In the present study, we investigated the effects of 9 days of bed rest on in vivo metabolism in FDR and control (CON) subjects. We hypothesized that FDR subjects may be more sensitive to physical inactivity than CON subjects as a result of their well-known defects of insulin action and secretion and their a priori increased risk of developing type 2 diabetes.
RESEARCH DESIGN AND METHODS
The data presented in this article are part of a larger study on the influence of physical inactivity in humans. This work was initiated and is funded by the European Union Framework VI, EXGENESIS project.
Thirty-three young Caucasian men were recruited to the study. All study subjects were born at term with normal birth weight to mothers with no record of gestational diabetes. FDR subjects were recruited via their parents (n = 10), who attended Steno Diabetes Center, Denmark, and via advertisements in local newspapers (n = 3).
Inclusion criteria were the presence of at least one parent with type 2 diabetes and one additional family member with type 2 diabetes. Seven subjects had more than one second-degree relative with type 2 diabetes. Three subjects from the CON group declined their consent during the study and were excluded from all analyses. The two groups were similar with respect to age and BMI. All subjects had a normal level of fasting glucose measured before entering the study.
Ethics approval.
The study was approved by the regional ethics committee (ref. no. 01-262546), and all procedures were performed in accordance with the guidelines of the Declaration of Helsinki.
Experimental protocol.
The experimental protocol is presented in detail in Fig. 1.
FIG. 1.
Study outline. The experimental protocol presented as a figure showing activities undertaken during the study.
Control period.
Subjects were requested to abstain from strenuous physical activity for 3 days before examination. To ensure standardized conditions, all subjects were provided with a standardized diet 3 days before the first study day and one with adjusted caloric content during bed rest to ensure weight stability. Body composition was determined by a dual-energy X-ray absorptiometry scan (Lunar Prodigy Advance; GE Healthcare). Vo2max was measured on a bicycle ergometer with a stepwise incremental test using the leveling-off criterion (Jaeger Instruments, Höchberg, Germany).
Bed rest challenge studies.
All subjects were admitted to Steno Diabetes Center for 9 days and were not permitted to deviate from a half-recumbent position during this period. Toilet visits, limited to 15 min per day, were allowed. Blood samples for measurements of fasting plasma insulin and C-peptide were taken in the morning of days 1, 2, 3, 5, 7, and 9 of bed rest.
Hyperinsulinemic-euglycemic clamp(s) combined with stable isotope infusion and indirect calorimetry.
Identical in vivo experiments were performed before and after bed rest. The clamp procedure was initiated at 7:00 a.m. after a 10-h overnight fast. A polyethylene catheter was placed in the antecubital vein for blood sampling. The hand was kept in a heated Plexiglas box to ensure arterialization of the venous blood (23,24). A second catheter was placed in the antecubital vein of the contralateral arm for test infusions. Immediately after taking the background samples, a primed constant infusion of [6,6-2H5] glucose (priming bolus of 20 μmol/kg; continuous infusion rate of 0.220 μmol · min−1 · kg−1) and [1,1,2,3,3-5H2] glycerol (priming bolus of 1.5 μmol/kg; continuous infusion rate of 0.1 μmol · min−1 · kg−1) was started (0 min) and maintained for 150 min to determine glucose and glycerol kinetics in the basal state. Blood samples for measuring plasma glucose and glycerol enrichments were drawn at baseline (0 min) and in the first steady-state period (120, 135, and 150 min, respectively). All isotopes were purchased from Cambridge Isotope Laboratories (Andover, MA). Blood samples for measuring plasma glycerol and lactate were drawn at baseline and in the first (120 and 150 min, respectively) and second (330 and 360 min, respectively) steady-state periods. The first steady-state period was defined as the last 30 min of the 150-min basal period, when the tracer equilibria of [2H5] glucose and [5H2] glycerol were expected; the second steady-state period was defined as the last 30 min of the insulin clamp period. Blood samples for determining free fatty acids (FFAs); A1C; total, HDL, LDL, and VLDL cholesterol; and triglycerides were drawn at baseline, and blood samples for measuring FFAs in the insulin-stimulated state were drawn at 360 min.
To determine β-cell function, an intravenous glucose tolerance test (IVGTT) was initiated after the first steady-state period. A glucose bolus of 0.3 g/kg body wt was infused over 1 min at 150 min. Plasma samples for glucose, insulin, and C-peptide were collected at 150, 152, 154, 156, 158, 160, 165, 170, and 180 min. Following the IVGTT, a primed-continuous insulin infusion was initiated and fixed at 80 mU/m2 per min through the 180-min clamp (180–360 min). A variable infusion of unlabeled glucose (180 g/l) was used to maintain euglycemia during insulin infusion. Plasma glucose concentration was monitored every 5 min during clamp using a OneTouch (LifeScan, Milpitas, CA) blood glucose meter. The precision expressed as the coefficient of variation (CV) of the OneTouch meter in 20 replicate assays of venous blood samples was 3.4%. The CV of the glucose infusion rate at the steady state were 15.6% in CON subjects and 15.5% in FDR subjects before bed rest and 21.1% in CON subjects and 22.5% in FDR subjects after bed rest.
The target blood glucose concentration was 5 mmol/l. Samples for determining plasma insulin and C-peptide were drawn at 0, 120, 240, 270, 300, 330, and 360 min. Urine samples were collected at 0 min and 360 min. Oxygen consumption (Vo2) and carbon dioxide production (VCO2) were measured during steady state using indirect calorimetry with a flow-through canopy gas analyzer system (Deltatrac; Datex, Helsinki, Finland) as previously described (25).
Biochemical and tracer analyses.
Blood samples for plasma insulin, C-peptide, FFAs, and triglycerides and blood samples for glucose and glycerol enrichment determination were centrifuged immediately at 4°C, and plasma samples were stored at −80°C. Plasma insulin and C-peptide concentrations were determined by AutoDELPHIA time-resolved fluoroimmunoassay(PerkinElmer Wallac Oy, Turku, Finland). FFAs were quantified by an enzymatic colorimetric method (Wako, Richmond, VA). A1C was measured by high-performance liquid chromatography on a Bio-Rad Variant (Bio-Rad Laboratories, Hercules, CA). Plasma triglyceride concentration was determined with Triglyceride GPO-PAP (Roche Diagnostics, Mannheim, Germany). Total and HDL cholesterol were analyzed with an enzymatic colorimetric test (Roche Diagnostics). LDL cholesterol was calculated from the Friedewald formula (26), and VLDL cholesterol was calculated as plasma triglycerides divided by 2.2. Plasma was analyzed enzymatically for glycerol and lactate (FA-C kit; Wako Chemical, Neuss, Germany) on an automatic analyzer (Cobas Fara; Roche, Basel, Switzerland). Stable isotope enrichments were measured as previously described (27).
Calculations: IVGTT and β-cell test.
The area under the curve (AUC) was calculated using a trapezoidal method for glucose and insulin during the first-phase insulin response (FPIR), 0–10 min of the IVGTT. PHI1 (ö ratio) was calculated as (AUCinsulin [0–10 min]/AUCglucose [0–10 min]) and the incremental FPIR during the IVGTT as (AUCinsulin [0–10 min] − AUCbasal [ins 0 × 10 min]). The insulin secretion disposition index expressing the inverse hyperbolic relationship between insulin secretion and insulin action may be a better estimate of the “true” in vivo pancreatic β-cell insulin secretion capacity. The peripheral insulin secretion disposition index (Di-peripheral.) was calculated as FPIR × M. Furthermore, we calculated the hepatic insulin secretion disposition index (Di-hepatic) as FPIR/HIR, where HIR is the hepatic insulin resistance index, which was calculated as the product of mean fasting plasma insulin concentration and basal hepatic glucose production (28). The HIR as well as the peripheral and hepatic insulin secretion disposition indexes have been described in more detail and validated in previous studies (28–30).
Hyperinsulinemic-euglycemic clamp: glucose infusion rates and indirect calorimetry.
Glucose infusion rates were calculated as the mean of steady-state glucose infusion rates during the predefined insulin-stimulated steady-state period from 150 to 180 min. Basal and insulin-stimulated glucose and lipid oxidation were calculated according to the methods of Frayn (31).
Stable isotope tracer calculations.
The total rate of glucose/glycerol appearance was calculated as Ra (endogenous) = Rd = Ftotal/Eglucose, where Ra and Rd are the respective rates of appearance and disappearance (μmol · kg fat-free mass [FFM]−1 · min−1), and Ftotal is the total infusion rate of glucose/glycerol tracer (μmol · kg FFM−1 · min−1). Eglucose/glycerol is the enrichment of glucose/glycerol in plasma expressed as tracer-to-tracee ratio (TTR). The Ra of glucose is a measure of endogenous glucose production and represents hepatic glucose production in the basal state, and Ra of glycerol is a measure of whole-body lipolysis in the basal state (32).
Statistics.
Statistical analysis was performed with the SAS statistical analysis package (version 9.1; SAS Institute, Cary, NC). One-way ANOVAs were performed to test for differences between groups before and after bed rest. Paired-sample t tests were used to detect statistically significant differences within groups in response to bed rest. The Kolmogorov-Smirnov test was used to test whether data were normally distributed before and/or after logarithmic transformation of nonnormally distributed data. Correlations were calculated using Pearson or Spearman correlation coefficient. Values of P < 0.05 were considered significant. Data are presented as means ± SD.
RESULTS
Clinical characteristics.
The groups were matched for age and BMI (Table 1). FDR subjects were characterized by a significantly higher total fat mass and fat percentage than CON subjects before and after bed rest. FDR subjects had a greater trunk fat mass (FM) (g)–to–total FM (g) ratio, a lower leg FM (g)–to–total FM (g) ratio, and a higher percentage trunk FM–to–leg FM ratio (P < 0.0001) before and after the bed rest intervention compared with CON subjects (P < 0.0001). We showed significantly higher plasma LDL and VLDL cholesterol levels and a lower Vo2max in FDR subjects after bed rest (Table 1) as well as a borderline significant higher levels of plasma triglycerides and total cholesterol in FDR subjects after bed rest (all P = 0.05).
TABLE 1.
Clinical characteristics of male study participants before and after bed rest
| Before bed rest |
After bed rest |
|||
|---|---|---|---|---|
| FDR group | CON group | FDR group | CON group | |
| n | 13 | 20 | 13 | 20 |
| Age (years) | 26.4 ± 4.4 | 25.0 ± 1.0 | 26.4 ± 4.4 | 25.0 ± 1.0 |
| Weight (kg) | 84.0 ± 11.7 | 82.5 ± 10.1 | 83.6 ± 11.6 | 82.2 ± 10.4 |
| Height (m) | 1.84 ± 0.06 | 1.85 ± 0.05 | 1.84 ± 0.06 | 1.85 ± 0.05 |
| FFM (kg) | 58.6 ± 5.4 | 63.8 ± 4.7 | 58.4 ± 5.5 | 63.6 ± 4.8† |
| BMI (kg/m2) | 24.9 ± 3.1 | 24.1 ± 2.3 | 24.8 ± 3.1 | 23.9 ± 2.4 |
| Vo2max (ml · min−1 · kg−1) | 39.1 ± 6.7 | 43.5 ± 6.0 | 37.5 ± 6.6 | 42.8 ± 4.9† |
| Systolic blood pressure (mmHg) | 126 ± 11 | 128 ± 11 | 128 ± 8 | 126 ± 9 |
| Diastolic blood pressure (mmHg) | 71 ± 10 | 68 ± 3 | 71 ± 8 | 70 ± 7 |
| Waist-to-hip ratio | 0.88 ± 0.06 | 0.85 ± 0.04 | 0.87 ± 0.06 | 0.86 ± 0.04 |
| Total FM (kg) | 21.7 ± 9.1 | 14.3 ± 7.5* | 21.5 ± 9.6 | 14.5 ± 7.8† |
| Whole-body fat percentage (%) | 25.0 ± 8.3 | 16.9 ± 7.0* | 24.9 ± 9.0 | 17.1 ± 7.3† |
| Trunk FM–to–total FM ratio | 0.58 ± 0.03 | 0.48 ± 0.04* | 0.58 ± 0.04 | 0.49 ± 0.04† |
| Leg FM–to–total FM ratio‡ | 0.29 ± 0.04 | 0.37 ± 0.02* | 0.29 ± 0.04 | 0.37 ± 0.03† |
| % trunk FM–to–leg FM ratio | 2.06 ± 0.37 | 1.31 ± 0.18* | 2.07 ± 0.45 | 1.33 ± 0.22† |
| Triglycerides (mmol/l) | 1.1 ± 0.4 | 0.9 ± 0.4 | 1.3 ± 0.5 | 1.0 ± 0.5§ |
| Total cholesterol (mmol/l) | 4.5 ± 1.0 | 3.9 ± 0.8 | 4.4 ± 0.8 | 3.7 ± 0.9§ |
| HDL cholesterol (mmol/l) | 1.2 ± 0.3 | 1.2 ± 0.3 | 1.1 ± 0.3‖ | 1.1 ± 0.3‖ |
| LDL cholesterol (mmol/l) | 2.8 ± 0.3 | 2.2 ± 0.7 | 2.7 ± 0.7 | 2.0 ± 0.5† |
| VLDL cholesterol (mmol/l) | 0.5 ± 0.2 | 0.4 ± 0.2 | 0.6 ± 0.2‖ | 0.4 ± 0.2† |
| A1C (%) | 5.1 ± 0.3 | 5.1 ± 0.2 | NM | NM |
Data are means ± SD.
*Significant difference between FDR and CON groups before bed rest, P < 0.05.
†Significant difference between FDR and CON groups after bed rest, P < 0.05.
‡Log-transformed data.
§P = 0.05 between FDR and CON groups after bed rest.
‖Significant difference before vs. after bed rest; P < 0.05. NM, not measured.
We assessed the habitual degree of physical activity before the bed rest experiments using the International Physical Activity Questionnaire (33). No difference of habitual physical activity was seen between groups with time spent sitting before bed rest 7.2 ± 0.6 h/day in CON subjects versus 6.5 ± 0.9 h/day in FDR subjects.
Impact of bed rest on insulin sensitivity.
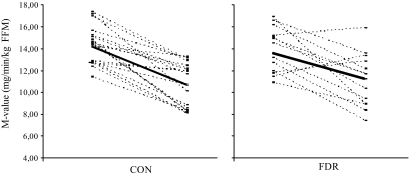
FDR and CON subjects responded to bed rest with a decrease in whole-body insulin sensitivity (P < 0.01), as measured by the hyperinsulinemic-euglycemic clamp technique (Fig. 2). FDR subjects became more insulin resistant after bed rest compared with CON subjects, as determined by the homeostasis model assessment index (P < 0.05) (Table 3). Data from the hyperinsulinemic-euglycemic clamps indicated lower glucose uptake rates before the intervention in FDR subjects compared with CON subjects (P < 0.05) (Table 2). However, this difference was not statistically significant when insulin action was expressed in relation to FFM (Table 2). Fasting blood glucose levels were not significantly different between groups, whereas plasma insulin levels tended to be higher in FDR subjects than in CON subjects after bed rest (P = 0.058).
FIG. 2.
The average glucose infusion rate (M value) in response to 9 days of bed rest in CON and FDR subjects, respectively. The solid line represents the average M value change in CON and FDR subjects in response to bed rest.
TABLE 3.
Results from homeostasis model assessment of insulin resistance, peripheral and hepatic insulin secretion disposition index (Di-peripheral and Di-hepatic)
| Before bed rest |
After bed rest |
|||
|---|---|---|---|---|
| FDR group | CON group | FDR group | CON group | |
| n | 13 | 20 | 13 | 20 |
| Basal HOMA-IR* | 1.0 ± 0.3 | 0.8 ± 0.3 | 1.6 ± 0.7† | 1.1 ± 0.5†‡ |
| Di-peripheral (10−3 × pmol · l−1 · min−1 · mg−1 · min−1 · kg FFM−1)* | 28.9 ± 20.9 | 27.4 ± 15.9 | 34.8 ± 33.5 | 32.7 ± 17.6 |
| Di-hepatic (pmol · l−1 · min−1 · μmol−1 · min−1 · kg FFM−1 · pmol−1 · l−1)* | 4.1 ± 1.8 | 11.5 ± 16.9§ | 4.5 ± 2.9 | 9.6 ± 6.3‡ |
Data are means ± SD.
*Log-transformed data.
†Significant difference before vs. after bed rest; P < 0.05.
‡Significant difference between FDR and CON groups after bed rest, P < 0.05.
§Significant difference between FDR and CON groups before bed rest, P < 0.05. Di-hepatic, measure for hepatic insulin action; Di-peripheral, peripheral disposition index, measure for peripheral insulin action; HOMA-IR, homeostasis model assessment of insulin resistance.
TABLE 2.
Results of IVGTT, hyperinsulinemic-euglycemic clamp, and indirect calorimetry in male study participants before and after bed rest
| Before bed rest |
After bed rest |
|||
|---|---|---|---|---|
| FDR group | CON group | FDR group | CON group | |
| n | 13 | 20 | 13 | 20 |
| Fasting plasma glucose (mmol/l) | ||||
| Basal | 4.8 ± 0.4 | 4.6 ± 0.4 | 4.8 ± 0.3 | 4.6 ± 0.4 |
| Insulin-stimulated state | 5.2 ± 0.4 | 5.2 ± 0.3 | 5.2 ± 0.3 | 5.1 ± 0.3 |
| Fasting plasma insulin (pmol/l)* | ||||
| Basal | 32 ± 10 | 28 ± 10 | 51 ± 22† | 37 ± 18† |
| Insulin-stimulated state | 777 ± 126 | 769 ± 176 | 833 ± 160 | 832 ± 160 |
| Fasting plasma C-peptide (pmol/l) | ||||
| Basal | 522 ± 117 | 376 ± 168‡ | 678 ± 201† | 472 ± 187†§ |
| Insulin-stimulated state | 495 ± 156 | 323 ± 182‡ | 641 ± 238† | 412 ± 212†§ |
| Fasting plasma glycerol (μmol/l)* | ||||
| Basal | 65 ± 17 | 89 ± 35‡ | 67 ± 18 | 65 ± 28† |
| Insulin-stimulated state | 26 ± 8 | 27 ± 16 | 28 ± 11 | 27 ± 10 |
| Fasting plasma lactate (mmol/l)* | ||||
| Basal | 0.6 ± 0.1 | 0.7 ± 0.3 | 0.8 ± 0.3† | 0.7 ± 0.2 |
| Insulin-stimulated state | 1.1 ± 0.2 | 1.2 ± 0.4 | 1.0 ± 0.4 | 1.1 ± 0.2† |
| Fasting plasma free fatty acids (μmol/l) | ||||
| Basal | 366 ± 76 | 461 ± 227 | 283 ± 128 | 258 ± 131† |
| Insulin-stimulated state | 10.8 ± 6.5 | 8.8 ± 4.2 | 11.5 ± 5.6 | 9.2 ± 4.5 |
| M value (mg · min−1 · kg body wt−1) | ||||
| Insulin-stimulated state | 9.9 ± 2.0 | 11.3 ± 1.5‡ | 8.0 ± 2.2† | 8.4 ± 1.7† |
| M value (mg · min−1 · FFM−1) | ||||
| Insulin-stimulated state | 13.9 ± 2.1 | 14.4 ± 1.7 | 11.1 ± 2.5† | 10.7 ± 2.0† |
| Glucose oxidation rate (mg · min−1 · FFM−1) | ||||
| Basal | 1.8 ± 0.4 | 1.6 ± 0.5 | 2.5 ± 0.7† | 2.5 ± 0.9† |
| Insulin-stimulated state | 4.7 ± 0.7 | 4.5 ± 0.6 | 4.5 ± 0.7 | 4.3 ± 0.7 |
| Fat oxidation rate (mg · min−1 · FFM−1) | ||||
| Basal | 0.9 ± 0.2 | 1.0 ± 0.3 | 0.6 ± 0.3† | 0.6 ± 0.4† |
| Insulin-stimulated state | 0.01 ± 0.24 | 0.05 ± 0.28 | -0.03 ± 0.28 | 0.05 ± 0.26 |
| Nonoxidative glucose metabolism (mg · min−1 · FFM−1) | ||||
| Insulin-stimulated state | 9.2 ± 1.8 | 9.9 ± 1.7 | 6.6 ± 2.3† | 6.5 ± 2.0† |
| Respiration quotient | 0.83 ± 0.02 | 0.82 ± 0.03 | 0.87 ± 0.04† | 0.86 ± 0.05† |
| Basal | ||||
| Insulin-stimulated state | 0.96 ± 0.03 | 0.95 ± 0.03 | 0.96 ± 0.04 | 0.95 ± 0.03 |
| FPIR (pmol · l−1 · min−1)* | 2,173 ± 1,704 | 1,866 ± 932 | 3,368 ± 4,047† | 3,023 ± 1,702† |
| AUC10min (pmol · l−1 · min−1) | 2,440 ± 1,734 | 2,132 ± 967 | 3,766 ± 4,134† | 3,355 ± 1,765† |
| PHI1* | 18.0 ± 12.7 | 17.2 ± 7.2 | 27.6 ± 24.2† | 27.3 ± 13.5† |
Data are means ± SD. Respiration quotient = VCO2/Vo2; FPIR = incremental area; AUC10min = total area under the IVGTT curve in the first 10 min; PHI1 = AUCinsulin/AUCglucose.
*Log-transformed data.
†Significant difference before versus after bed rest; P < 0.05.
‡Significant difference between FDR and CON groups before bed rest, P < 0.05.
§Significant difference between FDR and CON groups after bed rest, P < 0.05.
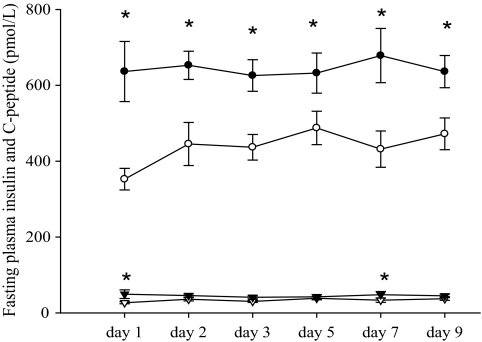
We found significant differences in plasma C-peptide levels between the two groups before and after bed rest (Table 2) as well as significantly higher levels of fasting plasma insulin (P < 0.05) in FDR compared with CON subjects on days 1 and 7 of bed rest and significantly higher levels of fasting plasma C-peptide on days 1, 2, 3, 5, 7, and 9 of bed rest (Fig. 3).
FIG. 3.
Fasting plasma insulin during 9 days of bed rest. *P < 0.05. Data are presented as means ± SE in FDR (▾) and CON (▿) subjects. Fasting plasma C-peptide during 9 days of bed rest. *P < 0.05. Data are presented as means ± SE in FDR (●) and CON (○) subjects.
Impact of bed rest on insulin secretion during IVGTT.
No significant differences were detected in FPIR, total AUC, or PHI1 between the groups, either before or after the intervention. Insulin secretion expressed in relation to the degree of muscle insulin resistance (Di-peripheral) was similar in both groups before and after bed rest (Table 3). When insulin secretion was calculated with respect to the degree of HIR (Di-hepatic), we found lower Di-hepatic in FDR subjects compared with CON subjects before and after bed rest (P < 0.001), indicating a disproportionality between insulin secretion and action.
Impact of bed rest on gaseous exchange measurements.
Basal glucose oxidation increased and basal fat oxidation decreased (P < 0.05) in both groups in response to bed rest (Table 2). The two groups also exhibited a significant bed rest–induced decrease in insulin-stimulated nonoxidative glucose metabolism (P < 0.01). In contrast to the basal substrate turnover rates, the insulin-stimulated glucose and fat oxidation rates were not significantly affected by bed rest. Basal respiration quotient rates were significantly increased, and insulin-stimulated respiration quotient rates were similar in both groups in response to bed rest. FDR subjects demonstrated lower p-glycerol levels in the basal state before the intervention than did CON subjects (P < 0.05) (Table 2).
Stable isotope tracer kinetics.
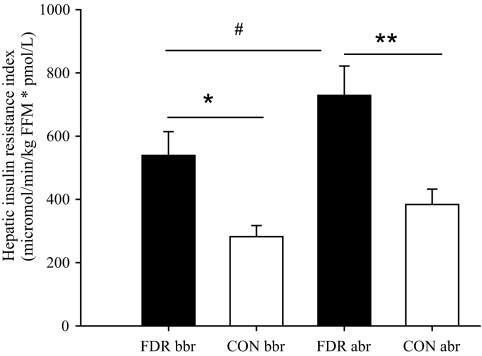
FDR subjects exhibited a significantly increased HIR compared with CON subjects before and after bed rest (P < 0.01) (Fig. 4). The absolute rate of endogenous glucose production (Ra glucose) was significantly elevated in FDR before and after bed rest (Table 4). There were no significant differences in basal whole-body lipolysis rate between the groups either before or after bed rest (Table 4). However, we found a tendency toward a decreased rate of whole-body lipolysis from 2.8 ± 1.4 to 2.2 ± 1.3 μmol · min−1 · kg FFM−1 in response to bed rest when the two groups were combined (P = 0.07).
FIG. 4.
HIR index. *P < 0.01; **P < 0.001; #significant increase of HIR in response to bed rest. Data are presented as means ± SEM in FDR (■) and CON (□) subjects; abr, after bed rest; bbr, before bed rest.
TABLE 4.
Tracer kinetics in basal state in male study participants before and after bed rest
| Before bed rest |
After bed rest |
|||
|---|---|---|---|---|
| FDR group | CON group | FDR group | CON group | |
| n | 13 | 20 | 13 | 20 |
| Ra glucose (μmol · min−1 · kg FFM−1) | 16.0 ± 4.7 | 9.7 ± 4.1* | 15.0 ± 6.0 | 10.4 ± 3.7† |
| Ra glycerol (μmol · min−1 · kg FFM−1) | 2.7 ± 1.4 | 2.8 ± 1.4 | 2.1 ± 1.3 | 2.2 ± 1.3 |
| HIR index (μmol · min−1 · kg FFM−1 · pmol−1 · l−1) | 539 ± 272 | 282 ± 158* | 729 ± 335‡ | 384 ± 217§ |
Data are means ± SD.
*Significant difference between FDR and CON groups before bed rest, P < 0.05.
†Significant difference between FDR and CON groups after bed rest, P < 0.05.
‡Significant difference before versus after bed rest; P < 0.05.
§Log-transformed data.
DISCUSSION
We have shown that bed rest causes a severe and similar degree of whole-body insulin resistance in FDRs of patients with type 2 diabetes and matched CON subjects. FDR subjects exhibit HIR, which, in contrast to healthy CON subjects, deteriorates in response to physical inactivity. FDR subjects exhibit reduced insulin secretion in relation to their degree of HIR but not peripheral insulin resistance.
The finding of a similar degree of whole-body insulin resistance after bed rest does to some extent refute our hypothesis that FDR subjects are more sensitive to the deleterious effects of physical inactivity on metabolism. However, the data suggest that a lower limit for whole-body insulin action may have been reached in both groups after exposure to bed rest, confirming the serious adverse effects of physical inactivity on whole-body insulin action in both groups. Although the CON group, in accordance with previous studies (21), tended to be more insulin sensitive than the FDR subjects prior to bed rest, neither the absolute nor relative decline of peripheral insulin action was significantly greater in CON than in FDR subjects, and so we cannot conclude that physical inactivity is more detrimental in CON than in FDR subjects. Also, the greater impairment of hepatic insulin action in FDR compared with CON subjects in response to bed rest could be taken as supporting the opposite conclusion. The fact that whole-body insulin resistance reached statistical significance only when expressed as the M value in mg · min−1 · kg body wt−1, but not when expressed in relation to degree of lean body mass (Table 2), may be due to the increased fat mass in FDR subjects and, to some extent, the more limited statistical power of this compared with other studies (2).
The physical activity questionnaires revealed no significant difference in daily physical activity level between FDR and CON subjects. However, more detailed and objective measurements of the daily physical activity level are required to determine the extent to which insulin resistance in FDR is due to a relatively lower level of habitual physical activity.
The finding that insulin resistance due to physical inactivity is fully explained by an impairment of nonoxidative glucose metabolism in both study groups is consistent with a major defect of muscle glycogen storage rate in response to physical inactivity (34), which in turn may be due to reduced muscle GLUT-4 content and activity and, thus, an impairment of glucose transport into the cell (35,36).
Using stable glucose isotopes, we found a higher rate of hepatic glucose production (HGP) in nondiabetic FDR subjects compared with CON subjects, which, in the presence of fasting hyperinsulinemia, is interpreted as HIR (Table 4). The disproportionately increased HGP in FDR subjects is consistent with one other study (37), although most previous studies (2,38,39) reported normal hepatic glucose production in nondiabetic FDR. However, these studies used radioactive (tritiated)-labeled glucose as tracer, and the ambient and commonly elevated fasting plasma insulin levels were not taken into account when calculating hepatic insulin action (2). The nondiabetic carriers of two of the most significant recently identified type 2 diabetes susceptibility genes, TCF7L2 and FTO, are characterized by a disproportionately elevated HGP (40–42). Our findings from the present study of a significant accentuation of HIR by bed rest in FDR subjects, which was not seen in CON subjects, indicates that FDR subjects may be more sensitive to physical inactivity at the site of hepatic glucose metabolism and insulin action. While fasting plasma insulin and C-peptide levels were similar on the day before the bed rest study began (Table 2), significant differences of plasma insulin and C-peptide levels were observed between groups already on day 1 during the bed rest challenges (Fig. 3). The fact that these differences did not become more pronounced during the 9-day bed rest periods suggests that the effect of bed rest on hepatic insulin action in FDR subjects was already present from day 1.
Fat accumulation in the liver has been proposed as one mechanism controlling insulin resistance in obesity and type 2 diabetes (43–46). FDR subjects in this study were characterized by altered regional fat distribution, with more fat located in the upper body (e.g., abdomen) than in the lower body (e.g., leg), in accordance with previous studies (3,47). In support of an influence of total and regional fat mass on HGP, the difference in the absolute rate of HGP between the groups before and after bed rest disappeared after correction for the significant contribution of total and abdominal fat masses. However, HIR was elevated in FDR subjects even after correction for abdominal and total fat content, indicating that factors other than fat mass and distribution, including fasting plasma insulin, may contribute to the elevated HIR in FDR subjects.
We are unaware of any previous studies demonstrating development of HIR by physical inactivity, and the data in this study suggest that this feature is primarily seen in subjects with preexisting visceral obesity and/or a positive family history of type 2 diabetes. The extent to which the mechanism by which bed rest accentuates HIR in FDR subjects may be explained by excessive hepatic fat accumulation is unknown and requires exact determinations of hepatic fat content. However, the idea that lipogenesis and hepatic fat content may increase disproportionately more in FDR than in CON subjects in response to bed rest is supported by the finding of significantly higher levels of plasma triglycerides, LDL, and VLDL cholesterol in the FDR subjects after bed rest (Table 1), which, in turn, theoretically may be due to a disproportionately reduced LPL activity in the FDR subjects. To this end, insulin, per se, stimulates hepatic lipogenesis (48), and we speculate that the sequence of events may be that plasma insulin levels increase primarily to compensate for whole-body insulin resistance in response to physical inactivity. Subsequently, elevation of endogenous plasma insulin levels promotes increased hepatic triglyceride synthesis and fat accumulation, leading to a greater rate of gluconeogenesis and HIR, which is predominantly seen in the FDR subjects with elevated visceral fat accumulation and whole-body insulin resistance as well as HIR.
The absolute rate of appearance of glycerol was similar in the two groups before and after bed rest, so the lower plasma glycerol levels in the FDR subjects before bed rest may reflect an increased rate of hepatic uptake of glycerol and gluconeogenesis in the FDR. The rate of whole-body lipolysis and basal fat oxidation decreased to a similar extent in FDR and CON subjects in response to bed rest, which may explain why no differences were observed in plasma glycerol levels after bed rest. Differences in utilization of other gluconeogenetic substrates and/or differences in glycogenolysis may explain the increased HGP in FDR subjects after bed rest.
Defective insulin secretion, either as an absolute measure or when calculated as the disposition index (Di), has been reported in previous studies of nondiabetic individuals with a genetic predisposition to type 2 diabetes (49,50), including nondiabetic carriers of the type 2 diabetes risk alleles of the TCF7L2 genotype (40,41). In this study, the Di was lower in FDR subjects when calculated in relation to the degree of hepatic, but not peripheral, insulin action. Despite the impaired insulin secretion relative to hepatic insulin action, this did not result in overt hyperglycemia in the FDR subjects after bed rest. Accordingly, overt hyperglycemia and type 2 diabetes may not develop until insulin secretion is significantly reduced when seen in relation also to the degree of impairment of whole-body insulin action and nonoxidative glucose metabolism in FDR subjects. Finally, this study documents that insulin secretion increases significantly in FDR and healthy CON subjects in response to bed rest (Table 3).
In conclusion, 9 days of bed rest causes severe whole-body insulin resistance and a compensatory increase of insulin secretion in healthy young men with and without a positive family history of type 2 diabetes. While whole-body insulin resistance converged toward similar levels in both groups during bed rest, HIR was aggravated in FDR subjects only in response to bed rest, which in turn may be related to the presence of visceral obesity. FDR subjects exhibit reduced insulin secretion when seen in relation to their degree of HIR but not peripheral insulin resistance. The results underscore the importance of avoiding physical inactivity even for relatively short periods in healthy subjects with and without a positive family history of diabetes.
Acknowledgments
This study was funded by the European Union 6th Framework EXGENESIS Grant and supported by the Danish Strategic Research Council. A.C.A. was in receipt of a PhD scholarship from the Academy of Muscle Biology, Exercise, and Health Research and the Ministry of Science, Technology, and Innovation, Copenhagen, Denmark.
No potential conflicts of interest relevant to this article were reported.
The authors thank Lars Sander Koch, Marianne Modest, and Nina Pluszek for eminent and dedicated technical assistance during the experiments and in the analysis of samples. The authors thank the volunteers who participated as subjects in the study.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Vaag A: On the pathophysiology of late onset non-insulin dependent diabetes mellitus: current controversies and new insights. Dan Med Bull 1999; 46: 197– 234 [PubMed] [Google Scholar]
- 2.Vaag A, Henriksen JE, Beck-Nielsen H: Decreased insulin activation of glycogen synthase in skeletal muscles in young nonobese Caucasian first-degree relatives of patients with non-insulin-dependent diabetes mellitus. J Clin Invest 1992; 89: 782– 788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nyholm B, Nielsen MF, Kristensen K, Nielsen S, Ostergard T, Pedersen SB, Christiansen T, Richelsen B, Jensen MD, Schmitz O: Evidence of increased visceral obesity and reduced physical fitness in healthy insulin-resistant first-degree relatives of type 2 diabetic patients. Eur J Endocrinol 2004; 150: 207– 214 [DOI] [PubMed] [Google Scholar]
- 4.Helmrich SP, Ragland DR, Leung RW, Paffenbarger RS, Jr: Physical activity and reduced occurrence of non-insulin-dependent diabetes mellitus. N Engl J Med 1991; 325: 147– 152 [DOI] [PubMed] [Google Scholar]
- 5.Eriksson J, Lindstrøm J, Valle T, Aunola S, Hamalanen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Lauhkonen M, Lehto P, Lehtonen A, Louheranta A, Mannelin M, Martikkala V, Rastas M, Sundvall J, Turpeinen A, Viljanen T, Uusitupa M, Tuomilehto J: Prevention of type II diabetes in subjects with impaired glucose tolerance: the Diabetes Prevention Study (DPS) in Finland: study design and 1-year interim report on the feasibility of the lifestyle intervention programme. Diabetologia 1999; 42: 793– 801 [DOI] [PubMed] [Google Scholar]
- 6.Booth FW, Chakravarthy MV, Gordon SE, Spangenburg EE: Waging war on physical inactivity: using modern molecular ammunition against an ancient enemy. J Appl Physiol 2002; 93: 3– 30 [DOI] [PubMed] [Google Scholar]
- 7.Lees SJ, Booth FW: Physical inactivity is a disease. World Rev Nutr Diet 2005; 95: 73– 79 [DOI] [PubMed] [Google Scholar]
- 8.Slentz CA, Houmard JA, Johnson JL, Bateman LA, Tanner CJ, McCartney JS, Duscha BD, Kraus WE: Inactivity, exercise training and detraining, and plasma lipoproteins. STRRIDE: a randomized, controlled study of exercise intensity and amount. J Appl Physiol 2007; 103: 432– 442 [DOI] [PubMed] [Google Scholar]
- 9.Petibois C, Cassaigne A, Gin H, Deleris G: Lipid profile disorders induced by long-term cessation of physical activity in previously highly endurance-trained subjects. J Clin Endocrinol Metab 2004; 89: 3377– 3384 [DOI] [PubMed] [Google Scholar]
- 10.Zderic TW, Hamilton MT: Physical inactivity amplifies the sensitivity of skeletal muscle to the lipid-induced downregulation of lipoprotein lipase activity. J Appl Physiol 2006; 100: 249– 257 [DOI] [PubMed] [Google Scholar]
- 11.Hamilton MT, Hamilton DG, Zderic TW: Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes 2007; 56: 2655– 2667 [DOI] [PubMed] [Google Scholar]
- 12.King DS, Dalsky GP, Clutter WE, Young DA, Staten MA, Cryer PE, Holloszy JO: Effects of lack of exercise on insulin secretion and action in trained subjects. Am J Physiol 1988; 254: E537– E542 [DOI] [PubMed] [Google Scholar]
- 13.McCoy M, Proietto J, Hargreves M: Effect of detraining on GLUT-4 protein in human skeletal muscle. J Appl Physiol 1994; 77: 1532– 1536 [DOI] [PubMed] [Google Scholar]
- 14.McGuire DK, Levine BD, Williamson JW, Snell PG, Blomqvist CG, Saltin B, Mitchell JH: A 30-year follow-up of the Dallas Bedrest and Training Study: II. effect of age on cardiovascular adaptation to exercise training. Circulation 2001; 104: 1358– 1366 [PubMed] [Google Scholar]
- 15.Berntorp K, Lindgarde F: Impaired physical fitness and insulin secretion in normoglycaemic subjects with familial aggregation of type 2 diabetes mellitus. Diabetes Res 1985; 2: 151– 156 [PubMed] [Google Scholar]
- 16.Manson JE, Nathan DM, Krolewski AS, Stampfer MJ, Willett WC, Hennekens CH: A prospective study of exercise and incidence of diabetes among US male physicians. JAMA 1992; 268: 63– 67 [PubMed] [Google Scholar]
- 17.Ohlson LO, Larsson B, Bjorntorp P, Eriksson H, Svardsudd K, Welin L, Tibblin G, Wilhelmsen L: Risk factors for type 2 (non-insulin-dependent) diabetes mellitus: thirteen and one-half years of follow-up of the participants in a study of Swedish men born in 1913. Diabetologia 1988; 31: 798– 805 [DOI] [PubMed] [Google Scholar]
- 18.Vaag A, Alford F, Henriksen FL, Christopher M, Beck-Nielsen H: Multiple defects of both hepatic and peripheral intracellular glucose processing contribute to the hyperglycaemia of NIDDM. Diabetologia 1995; 38: 326– 336 [DOI] [PubMed] [Google Scholar]
- 19.Beck-Nielsen H, Henriksen JE, Vaag A, Hother-Nielsen O: Pathophysiology of non-insulin-dependent diabetes mellitus (NIDDM). Diabetes Res Clin Pract 1995; 28( Suppl.): S13– S25 [DOI] [PubMed] [Google Scholar]
- 20.Kriketos AD, Greenfield JR, Peake PW, Furler SM, Denyer GS, Charlesworth JA, Campbell LV: Inflammation, insulin resistance, and adiposity: a study of first-degree relatives of type 2 diabetic subjects. Diabetes Care 2004; 27: 2033– 2040 [DOI] [PubMed] [Google Scholar]
- 21.Perseghin G, Ghosh S, Gerow K, Shulman GI: Metabolic defects in lean nondiabetic offspring of NIDDM parents: a cross-sectional study. Diabetes 1997; 46: 1001– 1009 [DOI] [PubMed] [Google Scholar]
- 22.Kannel WB, Wilson P, Blair SN: Epidemiological assessment of the role of physical activity and fitness in development of cardiovascular disease. Am Heart J 1985; 109: 876– 885 [DOI] [PubMed] [Google Scholar]
- 23.Liu D, Moberg E, Kollind M, Lins PE, Adamson U, Macdonald IA: Arterial, arterialized venous, venous and capillary blood glucose measurements in normal man during hyperinsulinaemic euglycaemia and hypoglycaemia. Diabetologia 1992; 35: 287– 290 [DOI] [PubMed] [Google Scholar]
- 24.Nauck MA, Blietz RW, Qualmann C: Comparison of hyperinsulinaemic clamp experiments using venous, ‘arterialized’ venous or capillary euglycaemia. Clin Physiol 1996; 16: 589– 602 [DOI] [PubMed] [Google Scholar]
- 25.Ferrannini E: The theoretical bases of indirect calorimetry: a review. Metabolism 1988; 37: 287– 301 [DOI] [PubMed] [Google Scholar]
- 26.Johnson R, McNutt P, MacMahon S, Robson R: Use of the Friedewald formula to estimate LDL-cholesterol in patients with chronic renal failure on dialysis. Clin Chem 1997; 43: 2183– 2184 [PubMed] [Google Scholar]
- 27.van Hall G, Sacchetti M, Radegran G, Saltin B: Human skeletal muscle fatty acid and glycerol metabolism during rest, exercise and recovery. J Physiol 2002; 543: 1047– 1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bajaj M, Suraamornkul S, Romanelli A, Cline GW, Mandarino LJ, Shulman GI, DeFronzo RA: Effect of a sustained reduction in plasma free fatty acid concentration on intramuscular long-chain fatty Acyl-CoAs and insulin action in type 2 diabetic patients. Diabetes 2005; 54: 3148– 3153 [DOI] [PubMed] [Google Scholar]
- 29.Abdul-Ghani MA, Matsuda M, DeFronzo RA: Strong association between insulin resistance in liver and skeletal muscle in non-diabetic subjects. Diabet Med 2008; 25: 1289– 1294 [DOI] [PubMed] [Google Scholar]
- 30.Groop LC, Bonadonna RC, DelPrato S, Ratheiser K, Zyck K, Ferrannini E, DeFronzo RA: Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus: evidence for multiple sites of insulin resistance. J Clin Invest 1989; 84: 205– 213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frayn KN: Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol 1983; 55: 628– 634 [DOI] [PubMed] [Google Scholar]
- 32.Wolfe RR, Chinkes D: Isotope Tracers in Metabolic Research: Principles and Practice of Kinetic Analysis Hoboken, NJ, Willey and Sons, 2005 [Google Scholar]
- 33.Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, Oja P: International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003; 35: 1381– 1395 [DOI] [PubMed] [Google Scholar]
- 34.Mikines KJ, Richter EA, Dela F, Galbo H: Seven days of bed rest decrease insulin action on glucose uptake in leg and whole body. J Appl Physiol 1991; 70: 1245– 1254 [DOI] [PubMed] [Google Scholar]
- 35.Han XX, Fernando PK, Bonen A: Denervation provokes greater reductions in insulin-stimulated glucose transport in muscle than severe diabetes. Mol Cell Biochem 2000; 210: 81– 89 [DOI] [PubMed] [Google Scholar]
- 36.Handberg A, Megeney LA, McCullagh KJ, Kayser L, Han XX, Bonen A: Reciprocal GLUT-1 and GLUT-4 expression and glucose transport in denervated muscles. Am J Physiol 1996; 271: E50– E57 [DOI] [PubMed] [Google Scholar]
- 37.Osei K: Increased basal glucose production and utilization in nondiabetic first-degree relatives of patients with NIDDM. Diabetes 1990; 39: 597– 601 [DOI] [PubMed] [Google Scholar]
- 38.Eriksson J, Franssila-Kallunki A, Ekstrand A, Saloranta C, Widen E, Schalin C, Groop L: Early metabolic defects in persons at increased risk for non-insulin-dependent diabetes mellitus. N Engl J Med 1989; 321: 337– 343 [DOI] [PubMed] [Google Scholar]
- 39.Nielsen MF, Nyholm B, Caumo A, Chandramouli V, Schumann WC, Cobelli C, Landau BR, Rizza RA, Schmitz O: Prandial glucose effectiveness and fasting gluconeogenesis in insulin-resistant first-degree relatives of patients with type 2 diabetes. Diabetes 2000; 49: 2135– 2141 [DOI] [PubMed] [Google Scholar]
- 40.Lyssenko V, Lupi R, Marchetti P, Del GS, Orho-Melander M, Almgren P, Sjogren M, Ling C, Eriksson KF, Lethagen AL, Mancarella R, Berglund G, Tuomi T, Nilsson P, Del PS, Groop L: Mechanisms by which common variants in the TCF7L2 gene increase risk of type 2 diabetes. J Clin Invest 2007; 117: 2155– 2163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wegner L, Hussain MS, Pilgaard K, Hansen T, Pedersen O, Vaag A, Poulsen P: Impact of TCF7L2 rs7903146 on insulin secretion and action in young and elderly Danish twins. J Clin Endocrinol Metab 2008; 93: 4013– 4019 [DOI] [PubMed] [Google Scholar]
- 42.Grunnet LG, Brons C, Jacobsen S, Nilsson E, Astrup A, Hansen T, Pedersen O, Poulsen P, Quistorff B, Vaag A: Increased recovery rates of phosphocreatine and inorganic phosphate after isometric contraction in oxidative muscle fibers and elevated hepatic insulin resistance in homozygous carriers of the A-allele of FTO rs9939609. J Clin Endocrinol Metab 2009; 94: 596– 602 [DOI] [PubMed] [Google Scholar]
- 43.Pietilainen KH, Kaprio J, Borg P, Plasqui G, Yki-Jarvinen H, Kujala UM, Rose RJ, Westerterp KR, Rissanen A: Physical inactivity and obesity: a vicious circle. Obesity (Silver Spring) 2008; 16: 409– 414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yki-Jarvinen H: Fat in the liver and insulin resistance. Ann Med 2005; 37: 347– 356 [DOI] [PubMed] [Google Scholar]
- 45.Pedersen SB, Borglum JD, Schmitz O, Bak JF, Sorensen NS, Richelsen B: Abdominal obesity is associated with insulin resistance and reduced glycogen synthetase activity in skeletal muscle. Metabolism 1993; 42: 998– 1005 [DOI] [PubMed] [Google Scholar]
- 46.Wajchenberg BL: Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev 2000; 21: 697– 738 [DOI] [PubMed] [Google Scholar]
- 47.Barwell ND, Malkova D, Moran CN, Cleland SJ, Packard CJ, Zammit VA, Gill JM: Exercise training has greater effects on insulin sensitivity in daughters of patients with type 2 diabetes than in women with no family history of diabetes. Diabetologia 2008; 51: 1912– 1919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Petersen KF, Dufour S, Savage DB, Bilz S, Solomon G, Yonemitsu S, Cline GW, Befroy D, Zemany L, Kahn BB, Papademetris X, Rothman DL, Shulman GI: The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proc Natl Acad Sci U S A 2007; 104: 12587– 12594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beck-Nielsen H, Groop LC: Metabolic and genetic characterization of prediabetic states. Sequence of events leading to non-insulin-dependent diabetes mellitus. J Clin Invest 1994; 94: 1714– 1721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vaag A, Henriksen JE, Madsbad S, Holm N, Beck-Nielsen H: Insulin secretion, insulin action, and hepatic glucose production in identical twins discordant for non-insulin-dependent diabetes mellitus. J Clin Invest 1995; 95: 690– 698 [DOI] [PMC free article] [PubMed] [Google Scholar]