Abstract
OBJECTIVE
Fibroblast growth factor-21 (FGF-21) is a potent metabolic regulator, which in animal models has been shown to improve glucose metabolism and insulin sensitivity. Recently, FGF-21 was shown to be expressed and secreted from murine muscle cells in response to insulin stimulation.
RESEARCH DESIGN AND METHODS
We studied muscular FGF-21 expression and plasma FGF-21 after acute insulin stimulation in young healthy men during a hyperinsulinemic- euglycemic clamp. Furthermore, we investigated systemic levels and muscle FGF-21 expression in humans with or without insulin resistance and chronic elevated insulin.
RESULTS
FGF-21 was barely detectable in young healthy men before insulin infusion. After 3 or 4 h of insulin infusion during a hyperinsulinemic-euglycemic clamp, muscular FGF-21 expression increased significantly. Plasma FGF-21 followed the same pattern. In individuals with chronic elevated insulin, muscular FGF-21 expression was associated with hyperinsulinemia in men but not in women. In plasma, hyperinsulinemia and fasting glucose were positively associated with plasma FGF-21 while plasma FGF-21 correlated negatively with HDL cholesterol. No associations between muscle and plasma FGF-21 were found in the individuals with chronic hyperinsulinemia.
CONCLUSIONS
FGF-21 is expressed in human skeletal muscle in response to insulin stimulation, suggesting that FGF-21 is an insulin-regulated myokine. In support, we found an association between chronic hyperinsulinemia and levels of FGF-21.
Fibroblast growth factor-21 (FGF-21), which is a member of the FGF super family, has recently been described as playing important roles in controlling glucose and lipid metabolism (1,2). Therapeutic administration of FGF-21 in rodents and nonhuman primates exerts metabolic control by protecting the animals from diet-induced obesity and lowering fasting plasma glucose, triglycerides, and insulin and glucagon levels in diabetic animal models (3–5). FGF-21 also improves the lipoprotein profiles by decreasing LDL and total cholesterol and increasing HDL cholesterol (4). FGF-21 transgenic mice exhibit similar metabolic characteristics, namely reduced adiposity and resistance to diet-induced metabolic disturbances (3).
Mechanistic animal studies focusing on the role of FGF-21 during fasting conditions have shown that FGF-21 is a downstream mediator of peroxisome proliferator–activated receptor-α (PPAR-α) (6,7), and PPAR-α–induced FGF-21 release leads to 1) stimulation of lipolysis in adipose tissue for the release of fatty acids, 2) direct stimulation of hepatic ketogenesis, and 3) induction of torpor in rodents.
In contrast to the animal studies, little information is available on the functional role of FGF-21 in humans. In healthy humans there is a large interindividual variation in serum FGF-21 with no correlation to age, sex, BMI, or plasma glucose (8). Two cross-sectional studies on Chinese individuals showed that plasma FGF-21 is elevated in both lean individuals diagnosed with type 2 diabetes (9) and in obese subjects (10) compared with healthy control subjects; accordingly, circulating FGF-21 was shown to be positively correlated with fasting insulin. Recently, two contradicting studies showed that plasma FGF-21 either increased during a hyperinsulinemic-euglycemic clamp (11) or showed no correlations to parameters of the hyperinsulinemic-euglycemic clamp (12).
Muscle tissue has received attention for being an important secretory organ able to secrete metabolic regulatory proteins (myokines). The main example is interleukin-6, which is released systemically in response to exercise and modulates metabolic functions in adipose tissue (13). Recently it was shown that FGF-21 is expressed and released from muscle tissue in Akt-overexpressing mice (14). Murine muscle cell culture studies supported these findings showing FGF-21 release in response to insulin stimulation and activated Akt.
Thus, we hypothesize that FGF-21 is expressed and secreted from muscle tissue in humans in response to insulin. We investigated 1) FGF-21 expression in plasma and muscle from 23 healthy subjects during a hyperinsulinemic-euglycemic clamp and 2) FGF-21 expression in muscle and plasma from 198 individuals with or without insulin resistance.
RESEARCH DESIGN AND METHODS
Protocol for clinical studies.
We studied FGF-21 expression and plasma levels in 1) healthy young males during a hyperinsulinemic-euglycemic clamp and 2) individuals with or without type 2 diabetes. All studies were approved by the Ethics Committee of Copenhagen and Frederiksberg Council, Denmark. All participants were given both oral and written information about the experimental procedures before giving their written informed consent. Before the experiment day, all subjects underwent a thorough clinical examination and blood samples for evaluation of renal, hepatic, and thyroid function; hemoglobin; white blood cell counts; thrombocytes; electrolytes; plasma coagulation factors; and plasma glucose were collected.
Human study 1.
Fifteen subjects received a 3-h hyperinsulinemic- euglycemic clamp with blood sampling and muscle biopsies before and after the clamp, while additionally eight subjects underwent a 6-h clamp with muscle biopsies taken at 0, 2, 4, and 6 h during the clamp, as previously published (15). All subjects had peripheral catheters placed in an antecubital vein for infusion of insulin, glucose, and potassium and one catheter in a contralateral hand vein for blood sampling. Insulin (Actrapid, Novo Nordisk; 100 units/ml) was infused continuously at an infusion rate of 0.08 units/min per m2. Glucose (200 g/1,000 ml) was infused at rates adjusted to maintain blood glucose at 5 mmol/l. To maintain potassium at normal values, isotonic saline with potassium was infused if needed. Arterialized blood was analyzed at intervals of 10 min for glucose and potassium concentrations.
Human study 2.
Individuals with type 2 diabetes were recruited, and healthy control subjects were matched by BMI. The group has previously been described in the study by Nielsen et al. (16). Using a cross-sectional case-control design, participants (n = 198) were divided into three distinct groups according to the result of an oral glucose tolerance test (normal glucose tolerance [NGT], impaired glucose tolerance [IGT], or type 2 diabetes). Exclusion criteria were treatment with insulin, recent or ongoing infection, history of malignant disease, or treatment with anti-inflammatory drugs. Data from 198 subjects were complete, and only those subjects were included in the ongoing analysis.
Participants reported in the laboratory between 8:00 and 10:00 a.m. after an overnight fast. Blood samples were drawn from the antecubital vein, and a muscle biopsy was obtained. In addition, an oral glucose tolerance test was performed.
Plasma ELISA analysis.
Plasma concentrations of FGF-21 were measured using a commercial ELISA kit (Human FGF-21; Biovendor, Germany). Plasma samples were diluted 1:1 and analyzed according to the manufacturer's instructions. All samples were run as duplicates and were within the range of the standard curve. The results were in the lower range of the standard curve; therefore, a coefficient of variation within the duplicates of 30% was accepted. The interassay coefficients of variation were validated within our work and were 27%.
RNA extraction and quantitative PCR analysis.
RNA was extracted from 20–30 mg of skeletal muscle biopsies using TriZol (Invitrogen, Carlsbad, CA) and reverse transcribed using random hexamer primers (TaqMan reverse transcription reagents; Applied Biosystems). Real-time PCR was performed on an ABI PRISM 7900 Sequence Detection System (PE Biosystems) using TaqMan reagents (Applied Biosystems). Sequence-specific FGF-21 primer/probe sets were used for FGF-21 amplification, while predeveloped household TaqMan primer/probe sets (Applied Biosystems) were used for 18s and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) detection. The relative expression of FGF-21 was normalized to the endogenous control and expressed as FGF-21 expression per 18s or GAPDH expression. The levels of 18s or GAPDH mRNA in the skeletal muscle were not influenced by the treatment (data not shown).
Statistics.
All statistical analyses were conducted using SPSS for Windows, release 15.0 (SPSS, Chicago, IL). The assumption of normality was tested using a Kolmogorov-Smirnov test. If data did not reach normality, a logarithmic transformation was performed. P < 0.05 was considered as significant. In the experimental clamp study, the differences between prevalues and 3-h values in plasma and muscle FGF-21 were analyzed using paired t test. In the epidemiological study, unless otherwise stated, analyses were performed with sexes separated. Differences between different groups were tested by ANOVA. If ANOVA revealed significant differences between the glycemic groups, a Tukey post hoc test was added. The association of plasma or muscle FGF-21 and variables related to metabolic syndrome was tested using linear regression analysis. Plasma or muscle FGF-21 was set as the dependent variable and variables related to metabolic syndrome as independent variables with adjustments for age and BMI.
RESULTS
The effect of acute hyperinsulinemia on muscle FGF-21 mRNA and plasma FGF-2.
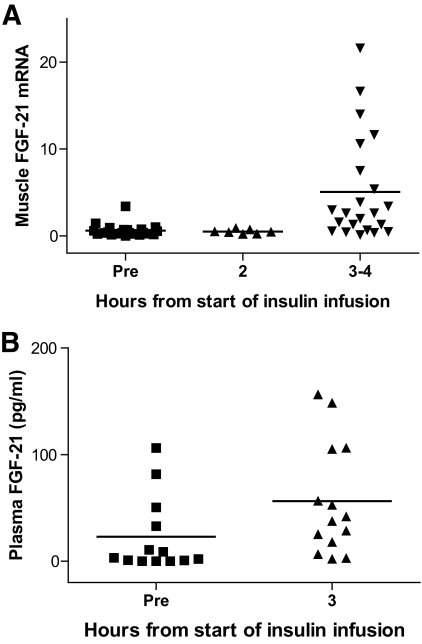
Muscle biopsies were obtained before and during hyperinsulinemic- euglycemic clamp from healthy young men (Fig. 1A). Before insulin infusion, FGF-21 was expressed at very low levels. After 3–4 h of insulin infusion, muscular FGF-21 mRNA increased significantly (P = 0.001) and was detectable in 21 of 23 subjects. Plasma FGF-21 followed the same pattern (Fig. 1B). Before insulin infusion, plasma FGF-21 could only be detected in 4 of 14 subjects. After the 3 h of insulin infusion, FGF-21 was detectable in 11 of 14 subjects with general increases in all subjects (P = 0.006).
FIG. 1.
Muscle and plasma FGF-21 during acute hyperinsulinemia. Healthy young men underwent a hyperinsulinemic-euglycemic insulin clamp. Fifteen subjects had muscle biopsies and blood samples before and after 3 h, while additionally eight subjects had muscle biopsies before and 2 and 4 h into the clamp. Data are presented as FGF-21 expression normalized to 18s expression. A: Muscle FGF-21 expression was significantly increased by 3–4 h (P = 0.001, paired t test). B: Plasma FGF-21 increased significantly after 3 h (P = 0.006, paired t test).
Muscle FGF-21 and plasma FGF-21 during chronic hyperinsulinemia.
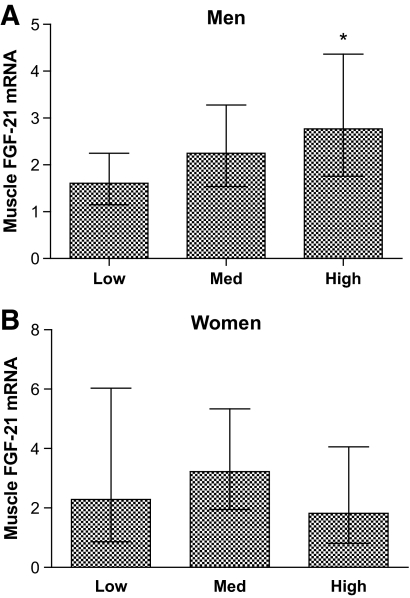
Muscle biopsies were obtained from individuals with either NGT, IGT, or type 2 diabetes, and groups were matched for BMI. The characteristics of the groups are presented in Table 1. Dividing the subjects into quartiles according to fasting insulin levels showed that the 25% with the highest fasting insulin levels had significantly higher FGF-21 expression than the lowest 25% (Fig. 2). This association was only present in men, not women. Because an association between age and muscle FGF-21 was demonstrated (P = 0.001), further adjustments for age and BMI were performed in a multivariate regression analysis. We found a positive association with fasting insulin and homeostasis model assessment (HOMA) score (Table 2). Moreover, muscle FGF-21 mRNA was negatively associated with HDL cholesterol (data not shown). In general, plasma FGF-21 followed the same pattern as that found for muscle FGF-21 mRNA (Table 2). A positive relationship was demonstrated between plasma FGF-21 and fasting plasma insulin (β = 0.172, R2 = 0.022, and P = 0.04), although the association did not remain significant when individuals were divided according to sex. Plasma FGF-21 was positively correlated with fasting glucose, HOMA, and triglycerides in men and negatively correlated with HDL cholesterol in women when analyzed separately (Table 2). Furthermore, plasma FGF-21 was significantly increased in type 2 diabetic compared with healthy control subjects 146.2 ± 2.5 pg/ml versus 102.2 ± 2.6 pg/ml (P = 0.008). No associations between muscle and plasma FGF-21 were found.
Table 1.
Characteristic of subjects with NGT, IGT, and type 2 diabetes
| Men |
Women |
|||||||
|---|---|---|---|---|---|---|---|---|
| Mean | 95% CI |
P | Mean | 95% CI |
P | |||
| Low | High | Low | High | |||||
| Age (years) | ||||||||
| NGT | 55.7 | 52.4 | 59.0 | 48.7 | 44.5 | 52.8 | ||
| IGT | 58.9 | 51.9 | 65.9 | 60.5* | 53.2 | 67.8 | ||
| T2D | 60.7 | 58.3 | 63.2 | 0.06 | 57.0* | 52.4 | 61.8 | 0.002 |
| BMI (kg/m2) | ||||||||
| NGT | 29.7 | 28.3 | 31.1 | 30.3 | 26.8 | 33.7 | ||
| IGT | 33.0 | 30.5 | 35.4 | 31.3 | 27.1 | 35.4 | ||
| T2D | 30.4 | 29.2 | 31.7 | 0.06 | 31.8 | 28.3 | 35.3 | 0.81 |
| Waist circumference (cm) | ||||||||
| NGT | 106.2 | 102.7 | 109.8 | 95.3 | 86.7 | 104.0 | ||
| IGT | 114.7* | 109.0 | 120.4 | 99.2 | 92.4 | 106.0 | ||
| T2D | 108.7 | 105.5 | 111.8 | 0.05 | 100.2 | 93.0 | 107.4 | 0.64 |
| Waist-to-hip ratio | ||||||||
| NGT | 0.99 | 0.97 | 1.01 | 0.87 | 0.82 | 0.91 | ||
| IGT | 1.02 | 0.99 | 1.05 | 0.86 | 0.83 | 0.88 | ||
| T2D | 1.01 | 1.00 | 1.03 | 0.07 | 0.88 | 0.85 | 0.91 | 0.69 |
| Glucose (mmol/l)† | ||||||||
| NGT | 5.23 | 5.11 | 5.35 | 5.00 | 4.85 | 5.22 | ||
| IGT | 5.83 | 5.55 | 6.12 | 5.97* | 5.61 | 6.35 | ||
| T2D | 9.39*‡ | 8.54 | 10.31 | <0.001 | 7.93*‡ | 6.60 | 9.53 | <0.001 |
| Insulin (pmol/l)† | ||||||||
| NGT | 46.5 | 40.4 | 53.5 | 39.5 | 30.1 | 51.7 | ||
| IGT | 95.8* | 73.7 | 124.5 | 63.4 | 48.6 | 82.9 | ||
| T2D | 71.2* | 58.1 | 87.2 | 0.001 | 69.8* | 48.8 | 100.0 | 0.01 |
| A1C (%)† | ||||||||
| NGT | 5.51 | 5.44 | 5.57 | 5.57 | 5.44 | 5.71 | ||
| IGT | 5.90 | 5.78 | 6.02 | 6.02 | 5.84 | 6.20 | ||
| T2D | 7.26*‡ | 6.90 | 7.64 | <0.001 | 6.71*‡ | 6.20 | 7.25 | <0.001 |
| HOMA score† | ||||||||
| NGT | 0.88 | 0.76 | 1.01 | 0.74 | 0.57 | 0.96 | ||
| IGT | 1.83* | 1.42 | 2.37 | 1.23 | 1.06 | 1.62 | ||
| T2D | 1.63* | 1.32 | 2.01 | <0.001 | 1.49* | 1.03 | 2.15 | 0.002 |
| FGF-21, plasma (pg/ml)† | ||||||||
| NGT | 103.3 | 79.6 | 134.2 | 99.6 | 78.2 | 126.9 | ||
| IGT | 143.3 | 116.9 | 175.8 | 151.0 | 105.2 | 216.6 | ||
| T2D | 149.0 | 119.8 | 185.4 | 0.07 | 136.0 | 98.9 | 186.9 | 0.09 |
| FGF-21, mRNA (expression/GAPDH)†‡§ | ||||||||
| NGT | 1.83 | 1.28 | 2.63 | 2.14 | 1.23 | 3.73 | ||
| IGT | 1.83 | 0.84 | 3.97 | 3.68 | 1.67 | 8.10 | ||
| T2D | 2.62 | 1.85 | 3.71 | 0.33 | 4.28 | 1.07 | 6.18 | 0.54 |
For men, n = 59 NGT, 18 IGT, and 65 type 2 diabetic (T2D); for women, n = 27 NGT, 12 IGT, and 17 type 2 diabetic. P value is shown for one-way ANOVA.
*Statistical significant difference from NGT (P < 0.05) after Tukey post hoc test.
†Log-transformed before analysis; data shown as geometric mean.
‡Statistical significant difference from IGT (P < 0.05) after Tukey post hoc test.
§Number of subjects: men is 56 NGT/14 IGT/52 type 2 diabetic and women 24 NGT/11 IGT/13 type 2 diabetic.
FIG. 2.
Muscle FGF-21 expression during chronic hyperinsulinemia. Men (A) and women (B) were divided into quartiles based on fasting insulin levels (low = 25% lowest, med = middle 50%, high = 25% highest). Data are presented as geometric mean and 95% CI. In men, the “high” quartile had significantly higher FGF-21 expression than the “low” quartile (P = 0.04). No significance was found in women. *P < 0.05.
Table 2.
Linear regression of muscle FGF-21 expression adjusted by GAPDH and plasma FGF-21 for men and women
| Muscle FGF-21 mRNA |
Plasma FGF-21 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| β Coefficients | 95% CI |
R2 | P | β Coefficients | 95% CI |
R2 | P | |||
| Low | High | Low | High | |||||||
| Men (n = 122 for muscle and n = 142 for plasma) | ||||||||||
| Body weight (kg) | 0.002 | −0.013 | 0.017 | 0.095 | 0.75 | <0.001 | −0.009 | 0.009 | 0.021 | 0.97 |
| Waist-to-hip ratio | −0.187 | −1.916 | 1.543 | 0.094 | 0.83 | 0.097 | −1.034 | 1.228 | 0.021 | 0.87 |
| Waist circumference (cm) | −0.002 | −0.023 | 0.019 | 0.094 | 0.85 | 0.003 | −0.010 | 0.016 | 0.023 | 0.66 |
| Glucose (mmol/l)* | 0.309 | −0.273 | 0.891 | 0.102 | 0.30 | 0.446 | 0.055 | 0.838 | 0.056 | 0.03 |
| Insulin (pmol/l)* | 0.465 | 0.100 | 0.829 | 0.140 | 0.01 | 0.194 | −0.048 | 0.437 | 0.039 | 0.12 |
| A1C (%)* | 0.428 | −0.799 | 1.654 | 0.097 | 0.49 | 0.646 | −0.134 | 1.425 | 0.040 | 0.10 |
| HOMA score* | 0.393 | 0.058 | 0.729 | 0.133 | 0.02 | 0.238 | 0.015 | 0.461 | 0.052 | 0.04 |
| HDL cholesterol* | −0.864 | −1.600 | −0.128 | 0.134 | 0.02 | −0.496 | −1.007 | 0.015 | 0.047 | 0.06 |
| FGF-21, plasma (pg/ml)* | 0.139 | −0.102 | 0.380 | 0.104 | 0.26 | |||||
| Women (n = 48 for muscle and n = 56 for plasma) | ||||||||||
| Body weight (kg) | <0.001 | −0.024 | 0.024 | 0.067 | 1.00 | 0.003 | −0.007 | 0.013 | 0.097 | 0.59 |
| Waist-to-hip ratio | 0.568 | −1.558 | 2.694 | 0.073 | 0.59 | −0.626 | −1.542 | 0.290 | 0.124 | 0.18 |
| Waist circumference (cm) | 0.010 | −0.014 | 0.034 | 0.082 | 0.41 | −0.009 | −0.019 | 0.001 | 0.147 | 0.07 |
| Glucose (mmol/l)* | 0.394 | −1.019 | 1.807 | 0.074 | 0.58 | 0.114 | −0.520 | 0.749 | 0.095 | 0.72 |
| Insulin (pmol/l)* | −0.253 | −0.914 | 0.408 | 0.080 | 0.45 | 0.010 | −0.285 | 0.305 | 0.092 | 0.95 |
| A1C (%)* | 0.695 | −2.606 | 3.996 | 0.071 | 0.67 | 0.321 | −1.165 | 1.807 | 0.096 | 0.67 |
| HOMA score* | −0.199 | −0.842 | 0.443 | 0.076 | 0.54 | 0.008 | −0.278 | 0.295 | 0.092 | 0.96 |
| HDL cholesterol* | 0.319 | −1.045 | 1.682 | 0.072 | 0.64 | −0.730 | 1.294 | −0.166 | 0.197 | 0.01 |
| FGF-21, plasma (pg/ml)* | −0.534 | −1.190 | 0.121 | 0.112 | 0.11 | |||||
Regressions adjusted by BMI and age.
*Log-transformed before analysis.
DISCUSSION
The present study shows that insulin stimulates the expression of FGF-21 in human skeletal muscle and induces an increase in circulating FGF-21 levels. In addition to the acute effect of insulin on muscular FGF-21 expression, we found a positive correlation between fasting insulin and muscle FGF-21 mRNA and plasma FGF-21 in individuals with a wide range of insulin sensitivity. This was particularly reflected in the acute in vivo insulin infusion study where unstimulated young muscles in most cases did not express FGF-21 at detectable levels. Moreover, we found a correlational relationship between muscle FGF-21 expression and age. In line with previous studies (9,10), we did not find associations between plasma FGF-21 and age.
The finding that muscular FGF-21 expression is regulated by insulin is in accordance with a recent report from Izumiya et al. (14), which found that insulin induced FGF-21 expression in murine muscle cells. In the latter study, FGF-21 was identified via microarray screening for genes that were upregulated in Akt-overexpressing mice. Thus, the pathway for regulation for FGF-21 in muscle seems completely different from regulation of FGF-21 expression in liver, where FGF-21 is highly expressed. Several animal studies have focused on the regulation of FGF-21 in liver (6,7,17). Here FGF-21 is controlled by PPAR-α, and PPAR-α agonists have been shown to mimic the induction of FGF-21. The liver expression of FGF-21 is closely associated with fasting/fed transition in mice as PPAR-α, and thus FGF-21, is induced by fasting (18). In the muscles, however, FGF-21 expression seems to by regulated by the insulin/Akt-signaling pathway.
In studying patients with chronic hyperinsulinemia, we found correlations between plasma and muscle FGF-21 and fasting insulin. Intriguingly, the association between muscle FGF-21 and insulin was only present in men. Human myocardial studies have shown a sex-dependent regulation of Akt with increased nuclear localization of phosphor-Akt in young women. This increased activity of Akt in women might explain the large muscular expression of FGF-21 in our female subjects with NGT (19). In addition, estrogen stimulates Akt phosphorylation and nuclear translocation. The women in our study were not controlled for estrogen and menstrual-cycle status, which can explain the large variation in FGF-21 expression that we find in our female subjects and thus the lack of associations to fasting insulin levels.
We were not able to determine any correlation between muscle FGF-21 mRNA and plasma FGF-21 even though both correlated with fasting insulin. This indicates that muscle tissue is not the primary source of FGF-21 during chronic hyperinsulinemia. Previous studies have shown that plasma FGF-21 correlated significantly with FGF-21 expression in adipose tissue, suggesting that the adipose tissue might be the primary source of plasma FGF-21 in humans (10). Thus, FGF-21 expression in muscles may have a paracrine role in the muscle tissue.
In conclusion, insulin stimulates muscular expression of FGF-21 and increases plasma FGF-21. Moreover, muscular FGF-21 expression is associated with hyperinsulinemia. This suggests that FGF-21 expression in muscles is regulated by insulin.
Acknowledgments
The Centre of Inflammation and Metabolism is supported by the Danish National Research Foundation (grant no. 02-512-55). This study was further supported by the Danish Medical Research Council and the Commission of the European Communities (contract no. LSHM-CT-2004-005272 EXGENESIS).
No potential conflicts of interest relevant to this article were reported.
We thank the patients for their participation. Ruth Rousing and Hanne Villumsen are acknowledged for their technical assistance.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Moore DD: Physiology: sister act. Science 2007; 316: 1436– 1438 [DOI] [PubMed] [Google Scholar]
- 2.Kharitonenkov A, Shanafelt AB: Fibroblast growth factor-21 as a therapeutic agent for metabolic diseases. Biodrugs 2008; 22: 37– 44 [DOI] [PubMed] [Google Scholar]
- 3.Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, Sandusky GE, Hammond LJ, Moyers JS, Owens RA, Gromada J, Brozinick JT, Hawkins ED, Wroblewski VJ, Li DS, Mehrbod F, Jaskunas SR, Shanafelt AB: FGF-21 as a novel metabolic regulator. J Clin Invest 2005; 115: 1627– 1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kharitonenkov A, Wroblewski VJ, Koester A, Chen YF, Clutinger CK, Tigno XT, Hansen BC, Shanafelt AB, Etgen GJ: The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology 2007; 148: 774– 781 [DOI] [PubMed] [Google Scholar]
- 5.Coskun T, Bina HA, Schneider MA, Dunbar JD, Hu CC, Chen Y, Moller DE, Kharitonenkov A: Fibroblast growth factor 21 corrects obesity in mice. Endocrinology 2008; 149: 6018– 6027 [DOI] [PubMed] [Google Scholar]
- 6.Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, Li Y, Goetz R, Mohammadi M, Esser V, Elmquist JK, Gerard RD, Burgess SC, Hammer RE, Mangelsdorf DJ, Kliewer SA: Endocrine regulation of the fasting response by PPAR alpha-mediated induction of fibroblast growth factor 21. Cell Metab 2007; 5: 415– 425 [DOI] [PubMed] [Google Scholar]
- 7.Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E: Hepatic fibroblast growth factor 21 is regulated by PPAR alpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab 2007; 5: 426– 437 [DOI] [PubMed] [Google Scholar]
- 8.Gälman C, Lundåsen T, Kharitonenkov A, Bina HA, Eriksson M, Hafström I, Dahlin M, Amark P, Angelin B, Rudling M: The circulating metabolic regulator FGF21 is induced by prolonged fasting and PPAR alpha activation in man. Cell Metab 2008; 8: 169– 174 [DOI] [PubMed] [Google Scholar]
- 9.Chen WW, Li L, Yang GY, Li K, Qi XY, Zhu W, Tang Y, Liu H, Boden G: Circulating FGF-21 levels in normal subjects and in newly diagnosed patients with type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes 2008; 116: 65– 68 [DOI] [PubMed] [Google Scholar]
- 10.Zhang X, Yeung DC, Karpisek M, Stejskal D, Zhou ZG, Liu F, Wong RL, Chow WS, Tso AW, Lam KS, Xu A: Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes 2008; 57: 1246– 1253 [DOI] [PubMed] [Google Scholar]
- 11.Mai K, Andres J, Biedasek K, Weicht J, Bobbert T, Sabath M, Meinus S, Reinecke F, Mohlig M, Weickert MO, Clemenz M, Pfeiffer AF, Kintscher U, Spuler S, Spranger J: Free fatty acids link metabolism and regulation of the insulin-sensitizing fibroblast growth factor-21. Diabetes 2009; 58: 1532– 1538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li H, Bao Y, Xu A, Pan X, Lu J, Wu H, Lu H, Xiang K, Jia W: Serum fibroblast growth factor 21 is associated with adverse lipid profiles and gamma-glutamyltransferase but not insulin sensitivity in Chinese subjects. J Clin Endocrinol Metab 2009; 94: 2151– 2156 [DOI] [PubMed] [Google Scholar]
- 13.Pedersen BK, Febbraio MA: Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev 2008; 88: 1379– 1406 [DOI] [PubMed] [Google Scholar]
- 14.Izumiya Y, Bina HA, Ouchi N, Akasaki Y, Kharitonenkov A, Walsh K: FGF21 is an Akt-regulated myokine. FEBS Lett 2008; 582: 3805– 3810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krogh-Madsen R, Møller K, Dela F, Kronborg G, Jauffred S, Pedersen BK: Effect of hyperglycemia and hyperinsulinemia on the response of IL-6, TNF-alpha, and FFAs to low-dose endotoxemia in humans. Am J Physiol Endocrinol Metab 2004; 286: E766– E772 [DOI] [PubMed] [Google Scholar]
- 16.Nielsen AR, Hojman P, Erikstrup C, Fischer C, Plomgaard P, Mounier R, Mortensen OH, Broholm C, Taudorf S, Krogh-Madsen R, Lindegaard B, Petersen AM, Gehl J, Pedersen BK: Association between interleukin-15 and obesity: interleukin-15 as a potential regulator of fat mass. J Clin Endocrinol Med 2008; 93: 4486– 4493 [DOI] [PubMed] [Google Scholar]
- 17.Lundåsen T, Hunt MC, Nilsson LM, Sanyal S, Angelin B, Alexson SE, Rudling M: PPAR alpha is a key regulator of hepatic FGF21. Biochem Biophys Res Commun 2007; 360: 437– 440 [DOI] [PubMed] [Google Scholar]
- 18.Reitman ML: FGF21: a missing link in the biology of fasting. Cell Metab 2007; 5: 405– 407 [DOI] [PubMed] [Google Scholar]
- 19.Camper-Kirby D, Welch S, Walker A, Shiraishi I, Setchell KD, Schaefer E, Kajstura J, Anversa P, Sussman MA: Myocardial Akt activation and gender: increased nuclear activity in females versus males. Circ Res 2001; 88: 1020– 1027 [DOI] [PubMed] [Google Scholar]