Abstract
OBJECTIVE
As data on the predictive characteristics of diabetes-associated autoantibodies for type 1 diabetes in the general population are scarce, we assessed the predictive performance of islet cell autoantibodies (ICAs) in combination with autoantibodies against insulin (IAAs), autoantibodies against GAD, and/or islet antigen 2 for type 1 diabetes in children with HLA-defined disease predisposition recruited from the general population.
RESEARCH DESIGN AND METHODS
We observed 7,410 children from birth (median 9.2 years) for β-cell autoimmunity and diabetes. If a child developed ICA positivity or diabetes, the three other antibodies were measured in all samples available from that individual. Persistent autoantibody positivity was defined as continued positivity in at least two sequential samples including the last available sample.
RESULTS
Pre-diabetic ICA positivity was observed in 1,173 subjects (15.8%), 155 of whom developed type 1 diabetes. With ICA screening, 86% of 180 progressors (median age at diagnosis 5.0 years) were identified. Positivity for four antibodies was associated with the highest disease sensitivity (54.4%) and negative predictive values (98.3%) and the lowest negative likelihood ratio (0.5). The combination of persistent ICA and IAA positivity resulted in the highest positive predictive value (91.7%), positive likelihood ratio (441.8), cumulative disease risk (100%), and specificity (100%). Young age at seroconversion, high ICA level, multipositivity, and persistent positivity for IAA were significant risk markers for type 1 diabetes.
CONCLUSIONS
Within the general population, the combination of HLA and autoantibody screening resulted in disease risks that are likely to be as high as those reported among autoantibody-positive siblings of children with type 1 diabetes.
Type 1 diabetes is an immune-mediated disease that leads to the destruction of the pancreatic β-cells and eventually to total dependence on exogenous insulin. The clinical manifestation of the disease is preceded by a preclinical phase, during which diabetes-associated autoantibodies (DAAs) can be detected in the peripheral circulation. The timing and the type of autoantibodies to appear have been used as predictive markers for type 1 diabetes among first-degree relatives of affected individuals (1), but data on the predictive value of DAAs in the background population, from whom ∼90% of new cases are derived, are scarce (2).
Today, type 1 diabetes is one of the most common severe chronic ailments of children and adolescents in developed countries (3–5), and its incidence is continuously increasing. In Finland, the incidence is highest in the world, reaching 64 new cases per 100,000 children ages <15 years in 2005 (6). In 1994, a birth cohort study aimed at predicting and preventing type 1 diabetes in the general population was launched. The current work represents data from the first 14 years of this study, assessing the predictive characteristics of DAAs in a population-derived cohort of children with HLA-conferred susceptibility to type 1 diabetes.
RESEARCH DESIGN AND METHODS
The Finnish Type 1 Diabetes Prediction and Prevention (DIPP) study was carried out in three university hospitals (Turku, Oulu, and Tampere). More than 90% of the 11,000 babies born annually in these centers take part in cord blood screening for type 1 diabetes–associated HLA genotypes (7). Infants carrying the high-risk genotype (HLA DQB1*02/0302) or the moderate-risk genotypes (HLA DQB1*0302/x; x≠*02, *0301, *0602, or *0603) are eligible for a prospective follow-up study in which participants are monitored for the appearance of DAAs and type 1 diabetes (supplementary Fig. 1 [available at http://diabetes.diabetesjournals.org/cgi/content/full/db08-1305/DC1]) (8,9). Information on the family history of type 1 diabetes is collected with structured questionnaires completed by the parents soon after the birth of the baby. In the study centers in Oulu and Tampere, the clinical follow-up visits take place at the age of 3, 6, 12, 18, and 24 months and, after that, annually. In Turku, the visits occur every 3 months until the age of 2 years and subsequently every 6 months, which makes it theoretically possible that children from Turku could seroconvert at a younger age than other DIPP children, but according to current analyses this was not the case. For the seroconverted subjects, the follow-up visits are organized every 3 months.
At the follow-up visits, venous blood samples are obtained, and for the current analyses, ICAs were used as the primary screening tool for β-cell autoimmunity. Subjects with transplacentally transferred maternal antibodies were regarded as seronegative as long as no de novo synthesis of antibodies was observed (10). Persistent autoantibody positivity (prefix “p”) was defined as positivity in at least two sequential samples, the last sample available being positive. The last pre-diabetic and/or the first diabetic samples were taken into account when defining the persistence of the autoantibody status. Participants with persistent positivity for at least two of the autoantibodies analyzed were eligible for a randomized, double-blinded, and placebo-controlled intervention trial with nasally administrated insulin. Codes for the intervention treatment were opened in November 2007, and the results showed that the intervention had no effect on the progression rate to clinical diabetes (11). The present study cohort comprised DIPP children who had remained in the follow-up study for at least 1 year or presented with diabetes before the age of 1 year (one case diagnosed at the age of 0.9 years) by the end of August 2004. The cohort included 7,410 children (3,897 male subjects [52.6%]), and 177 (2.4%) had a family member affected by type 1 diabetes at the time of birth. The closing time point for the data on autoantibodies and progression to type 1 diabetes was 31 December 2008.
Screening for HLA DQB1 genotypes was performed on cord blood samples by time-resolved triple-label hybridization (12). Autoantibodies were measured on serum samples: islet cell autoantibodies (ICAs) with immunofluorescence (13) and antibodies against insulin (IAAs), antibodies against GAD (GADAs), and islet antigen 2 (IA-2A) with specific radiobinding assays (14–16). The cut-off values for ICA, IAA, GADA, and IA-2A positivity were 2.5 Juvenile Diabetes Foundation units (JDFU), 3.48 relative units (RU), 14.13 World Health Organization (WHO) units/ml (= 5.36 RU), and 1.91 WHO units/ml (= 0.43 RU), respectively. The reference values for the IAA, GADA, and IA-2A assays were based on the 99th percentile of >370 nondiabetic Finnish children and adolescents. Samples with IAA, GADA, or IA-2A values between the 97th and 99.5th percentiles, as well as all the ICA-positive samples, were retested to confirm the antibody status. The disease sensitivity and specificity of the ICA assay were 100 and 98%, respectively, based on an international standardization workshop round (17). According to the 2005 Diabetes Autoantibody Standardization Program (DASP) workshop, the disease sensitivity of the IAA, GADA, and IA-2A assays were 58, 82, and 72%, respectively, while corresponding specificities were 98, 96, and 100%, respectively. The diagnosis of type 1 diabetes was based on WHO criteria (18) and the primary case ascertainment done by reviewing the local registers of children with newly diagnosed type 1 diabetes in each of the three participating university hospitals. The Finnish Pediatric Diabetes Register was used as the secondary source of ascertainment (19). The local ethics committees approved the protocol of the DIPP study, and written informed consent was obtained from the legal representatives of the participating children.
Data analysis.
To analyze the predictive characteristics of ICA-based autoantibody combinations, participants were categorized by their maximal autoantibody status by the end of December 2008. Each child could belong to one group only, except when the categories “two or more” and “three or more” DAAs were analyzed. For subjects with fluctuating autoantibodies, the maximal combination or, in the case of various combinations with similar numbers of positive autoantibodies, the first combination to appear was chosen. Disease sensitivity and specificity, as well as positive (PPV) and negative (NPV) predictive values and likelihood ratios (+LR and −LR), were determined for ICA alone and for ICA in combination with the other three autoantibodies. The Kaplan-Meier method with the log-rank test was used to analyze and compare the cumulative diabetes risks. Odds ratios (ORs) for the risk of type 1 diabetes were calculated. Distributions between groups were tested with the χ2 test and correlations analyzed with the Pearson's (r) or Spearman's methods (rs). CIs were given at 95% (95% CI), and statistical significance was set at P < 0.05 (two-tailed). Statistical analyses were performed with SPSS 16.0 for Windows (SPSS, Chicago, IL).
RESULTS
We observed from birth 7,410 children for positivity for DAAs and progression to type 1 diabetes over a median follow-up time of 9.2 years (range 0.9–14.2). The median follow-up time for subjects remaining unaffected by type 1 diabetes was 9.3 years (5.4–14.2), and the median age at diagnosis among 180 progressors (93 male subjects [2.4%]) was 5.0 years (0.9–12.5). The median age at the initial seroconversion was 4.2 years (0.2–13.7) among unaffected ICA-positive subjects, whereas among progressors, it was 1.5 years (0.3–9.6; P < 0.001). Progressors reached the maximal ICA-based autoantibody status by the median age of 2.2 years (0.5–10.1), while in unaffected subjects, the maximal autoantibody status was observed at the age of 5.1 years (0.5–13.7; P < 0.001). Delay from the initial seroconversion to maximal autoantibody positivity varied between 0.0 and 11.0 years, and among progressors and unaffected subjects, the median delays were 0.5 and 0.0 years (P < 0.001), respectively. The high-risk genotype (DQB1*02/0302) was carried by 1,575 children (21.3%) and the moderate-risk genotypes (DQB1*0302/x; x ≠ *02 or a protective allele) by 5,835 children (78.7%). The high-risk genotype was associated with higher risks for developing β-cell autoimmunity, positivity for multiple autoantibodies, persistent positivity, and type 1 diabetes (P < 0.001 for all comparisons) (supplementary Table 1). The corresponding ORs for the high- versus moderate-risk groups were 1.7 (95% CI 1.4–2.0), 2.4 (1.9–3.1), 1.7 (1.4–2.0), and 3.2 (2.1–4.8), respectively.
Altogether, 1,173 subjects (15.8%), 155 of whom were progressors, tested positive for ICAs during the follow-up. A majority (n = 967; 82.4%) of the first autoantibody-positive samples were ICA positive, while positivity for either ICAs or IAAs was seen in 93.3% (1,094 of 1,173) of the initially positive samples. Seventeen (68.0%) of 25 progressors who had remained ICA-negative during the pre-diabetic phase were positive for IAAs either before or at the time of the diagnosis (Table 1), and, in all, among the ICA-positive children, those who tested positive also for IAAs had a higher cumulative disease risk (59.6% [95% CI 49.9–69.3]) than those who remained IAA negative (10.8% [6.8–14.9]; P < 0.001). Twelve of 15 (80%) progressors without any signs of pre-diabetic β-cell autoimmunity did not adhere to the follow-up schedule of the DIPP study. Among these subjects, the median delay from the last sampling to diagnosis was 3.8 years (range 1.9–6.2). All previously seronegative progressors having samples available at diagnosis had developed β-cell autoimmunity by that time, and all but one tested positive for multiple autoantibodies.
Table 1.
Progressors remaining ICA negative during the pre-diabetic phase
| HLA-DQB1 | Age (years) |
Delay (years) |
DAA status |
|||
|---|---|---|---|---|---|---|
| Seroconversion | Last visit | Diagnosis | Last visit to diagnosis | Pre-diabetic | At diagnosis | |
| High risk (*02/*0302) | ||||||
| Female | 0.98 | 0.98 | 1.34 | 0.36 | IAA | ICA, IAA, GADA |
| Female | 1.00 | 1.40 | 0.40 | Negative | ICA, IAA, GADA | |
| Female | 0.95 | 1.51 | 1.69 | 0.18 | IAA, GADA | ICA, IAA, GADA |
| Female | 1.07 | 3.25 | 2.18 | Negative | ICA, IAA, GADA | |
| Female | 4.00 | 4.33 | 0.33 | Negative | Sample not available | |
| Female | 1.58 | 5.64 | 4.06 | Negative | All four DAA | |
| Female | 7.59 | 11.99 | 4.40 | Negative | ICA, GADA, IA-2A | |
| Male | 0.96 | 1.62 | 0.65 | Negative | ICA, IAA | |
| Male | 2.96 | 7.70 | 4.74 | Negative | ICA, GADA, IA-2A | |
| Male | 2.51 | 8.74 | 6.23 | Negative | All four DAA | |
| Moderate risk (*0302/x) | ||||||
| Female | 0.50 | 0.76 | 0.97 | 0.21 | IAA | IAA, GADA |
| Female | 0.77 | 0.77 | 1.08 | 0.31 | IAA, GADA | All four DAA |
| Female | 0.99 | 0.99 | 1.47 | 0.48 | IAA | ICA, IAA |
| Female | 0.25 | 2.13 | 1.88 | Negative | ICA, IAA, GADA | |
| Female | 0.27 | 3.06 | 2.79 | Negative | All four DAA | |
| Female | 2.47 | 5.96 | 3.49 | Negative | All four DAA | |
| Female | 3.08 | 6.40 | 3.32 | Negative | ICA, IAA, IA-2A | |
| Female | 3.02 | 6.57 | 3.55 | Negative | GADA | |
| Female | 4.01 | 4.01 | 8.18 | 4.17 | GADA | ICA, GADA, IA-2A |
| Female | 0.32 | 6.24 | 5.92 | Negative | ICA | |
| Male | 1.12 | 2.14 | 4.72 | 2.58 | IAA | ICA, IAA, IA-2A |
| Male | 1.02 | 1.02 | 5.41 | 4.39 | IAA | Sample not available |
| Male | 1.03 | 7.15 | 6.12 | Negative | ICA, IA-2A | |
| Male | 2.53 | 2.53 | 2.70 | 0.17 | IAA, IA-2A | ICA, IAA, IA-2A |
| Male | 0.79 | 0.79 | 0.87 | 0.08 | IAA, GADA | IAA, GADA |
x, nonprotective HLA allele.
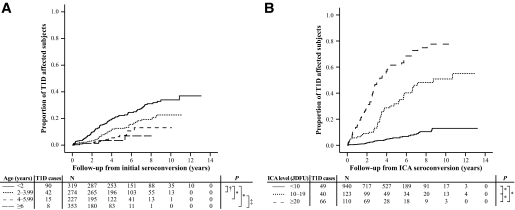
The age at which subjects seroconverted had a predictive role regarding the risk of type 1 diabetes. Among all ICA-positive subjects, those with seroconversion before the age of 2 years had the highest cumulative disease risk (36.9% [95% CI 28.5–45.3]) (Fig. 1A), and for that age-group, the OR for type 1 diabetes was 5.0 (95% CI 3.5–7.1) when compared with those who had seroconverted after the age of 2 years. Subjects seroconverting under the age of 2 years were also more often positive for multiple autoantibodies at first positive sampling (18.3 vs. 12.1% in subjects with seroconversion at or after the age of 2 years, P = 0.006). The median delay from the initial seroconversion to diagnosis was 2.8 years (range 0.02–10.9) among the ICA-positive progressors, and this delay did not correlate with the age at seroconversion (rs = 0.005, P = 0.95).
FIG. 1.
Effect of the seroconversion age on the diabetes-free survival (A) and progression to type 1 diabetes in relation to initial ICA titer (JDFU) (B). *P < 0.001; †P = 0.003; ‡P = 0.006. T1D, type 1 diabetes.
The median ICA level in the first ICA-positive samples was 5 JDFU (range 3–640) among nonprogressors and 15 JDFU (4–668) (P < 0.001) in progressors. The higher the initial ICA value, the higher the cumulative disease risk (Fig. 1B). The 5-year progression rate for those with a low initial ICA level (<10 JDFU) was 5.7% (95% CI 3.9–7.5), while the corresponding values for those with moderate (10–19 JDFU) and high (≥20 JDFU) ICA titers were 31.8% (21.8–41.8) and 61.2% (51.1–71.9), respectively. During the follow-up, the difference in ICA levels became more prominent between progressors and nonprogressors: the peak ICA titer among progressors reached 168 JDFU (range 5–2,620), while it remained at 5 JDFU (3–2,620) in nonprogressors (P < 0.001). Prospective observations from the time point at which the maximal ICA level was observed showed a 5-year cumulative disease risk of 2.3% (95% CI 0.3–4.3) among those with a low ICA (<10 JDFU) level at that time, whereas among those with moderate (10–19 JDFU) and high (≥20 JDFU) ICA levels, the risk estimates were 11.7% (2.9–20.5) and 76.5% (61.4–91.6) (P < 0.001 between all groups), respectively. The maximal ICA level correlated clearly with the number of detectable autoantibodies at sampling (rs = 0.68, P < 0.001), but the correlation between the ICA titer and type 1 diabetes remained significant, even after correcting for the number of positive autoantibodies (rs = 0.10, P < 0.001).
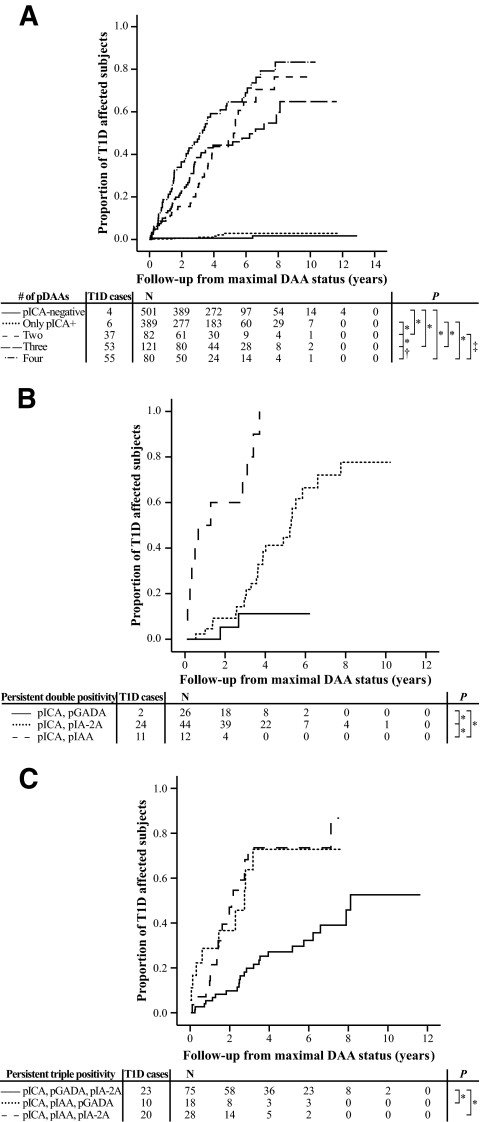
To further analyze the predictive role of ICAs in combination with the other three autoantibodies, ICA-positive subjects were categorized by their maximal autoantibody status. Frequencies and predictive characteristics (sensitivity, specificity, PPV, NPV, +LR, −LR, and cumulative disease risks) of the autoantibody combinations are presented in Table 2 and Figs. 2 and 3. Positivity for four antibodies was associated with the highest disease sensitivity (54.4%) and NPV (98.9%) and the lowest −LR (0.5). The combination of persistent ICA and IAA positivity resulted in the highest PPV (91.7%), +LR (441.8), cumulative disease risk (100%), and specificity (100%). The highest cumulative disease risks were associated with IAAs (Fig. 2B and C), whereas GADA positivity resulted in significantly lower disease risks. Especially, the combination of ICAs and GADAs resulted in a low progression rate, thus decreasing also the risk estimate of double positivity. Transient and persistent ICA positivity had different predictive characters, since only 1.7% (95% CI 0–4.0) of those with transient ICA-based positivity developed type 1 diabetes during follow-up, whereas among those with (at least) pICA positivity, the proportion was 41.2% (34.1–48.3) (P < 0.001). In the whole study population, the difference between the seronegative and transiently ICA-positive subjects remained small but significant (0.5% [95% CI 0.3–0.7] vs. 1.7%) (P = 0.008). The pICA-based combinations of multiple persistently positive autoantibodies had highly variable disease risks, the highest associating with the combination of pICAs and pIAAs (100%) and the lowest with pICAs and pGADAs (11.2%). Persistent IAA positivity seemed to distinguish those with a high disease risk and rapid progression to type 1 diabetes from those with a lower disease risk and slower progression rate. In this population, the pIAA positivity–associated 5-year disease risk was 70.7% (95% CI 62.2–79.1) compared with that of 11.9% (8.4–15.4) observed among the ICA-positive subjects lacking pIAA positivity. Since all children had been observed for at least 5 years, the 5-year predictive characteristics are presented in supplementary Table 2. The predictive characteristics in relation to HLA genotype are shown in supplementary Table 3, while the corresponding characteristics of those 7,077 children who had no affected first-degree relative are presented in supplementary Table 4.
TABLE 2.
Predictive characteristics (sensitivity, specificity, PPV, NPV, +LR, −LR, and cumulative disease risk) of the four diabetes-associated autoantibodies (DAAs, ICAs, IAAs, and GADAs, and IA-2A)
| Combinations of DAA | Type 1 diabetes/all | Sensitivity | Specificity | PPV | NPV | +LR | −LR | Cumulative risk |
|---|---|---|---|---|---|---|---|---|
| Total number of subjects (n) | 180/7,410 | |||||||
| ICA (the only positive DAA) | 1/782 | 0.6 (0–3.1) | 89.2 (88.5–89.9) | 0.1 (0.0–0.7) | 97.3 (96.9–97.7) | 0.1 (0.0–0.3) | 1.1 (1.1–1.1) | 0.3 (0–0.8) |
| Double positivity* | 14/128 | 7.8 (4.3–12.7) | 98.4 (98.1–98.7) | 10.9 (6.1–17.7) | 97.7 (97.4–98.1) | 4.9 (2.9–8.3) | 0.9 (0.9–1.0) | 16.0 (7.4–24.6) |
| ICA, IAA | 10/56 | 5.6 (2.7–10.0) | 99.4 (99.2–99.5) | 17.9 (8.9–30.4) | 97.7 (97.3–98.0) | 8.7 (4.5–16.7) | 1.0 (0.9–1.0) | 24.9 (9.8–40.0) |
| ICA, GADA | 2/60 | 1.1 (0.1–4.0) | 99.2 (99.0–99.4) | 3.3 (0.4–11.5) | 97.6 (97.2–97.9) | 1.4 (0.4–5.0) | 1.0 (1.0–1.0) | 4.4 (0–10.5) |
| ICA, IA-2A | 2/12 | 1.1 (0.1–4.0) | 99.9 (99.7–99.9) | 16.7 (2.1–48.4) | 97.6 (97.2–97.9) | 8.0 (2.0–32.3) | 1.0 (1.0–1.0) | 25.0 (0–55.0) |
| Triple positivity* | 42/87 | 23.3 (17.4–30.2) | 99.4 (99.2–99.5) | 48.3 (37.4–59.2) | 98.1 (97.8–98.4) | 37.5 (25.4–55.2) | 0.8 (0.7–0.8) | 66.7 (52.8–80.7) |
| ICA, IAA, GADA | 15/36 | 8.3 (4.7–13.4) | 99.7 (99.6–99.8) | 41.7 (25.5–59.2) | 97.8 (97.4–98.1) | 28.7 (15.2–54.1) | 0.9 (0.9–0.9) | 54.2 (33.3–75.1) |
| ICA, IAA, IA-2A | 20/27 | 11.1 (6.9–16.6) | 99.9 (99.8–100) | 74.1 (53.7–88.9) | 97.8 (97.5–98.2) | 114.8 (50.5–262.7) | 0.9 (0.9–0.9) | 84.7 (69.2–100) |
| ICA, GADA, IA-2A | 7/24 | 3.9 (1.6–7.8) | 99.8 (99.6–99.9) | 29.2 (12.6–51.1) | 97.7 (97.3–98.0) | 16.5 (7.1–38.3) | 1.0 (0.9–1.0) | 41.5 (17.4–65.5) |
| All four DAA positive | 98/176 | 54.4 (46.9–61.9) | 98.9 (98.7–99.1) | 55.7 (48.0–63.2) | 98.9 (98.6–99.1) | 50.5 (39.5–63.9) | 0.5 (0.4–0.5) | 76.5 (66.0–87.1) |
| Positive for at least ICA | 155/1,173 | 86.1 (80.2–90.8) | 85.9 (85.1–86.7) | 13.2 (11.3–15.3) | 99.6 (99.4–99.7) | 6.1 (5.7–6.5) | 0.2 (0.1–0.2) | 24.7 (20.1–29.3) |
| Two or more positive DAA* | 154/391 | 85.6 (79.6–90.3) | 96.7 (96.3–97.1) | 39.4 (34.5–44.4) | 99.6 (99.5–99.8) | 26.1 (23.4–28.3) | 0.1 (0.1–0.2) | 63.1 (54.2–72.0) |
| Three or more positive DAA* | 140/263 | 77.8 (71.0–83.6) | 98.3 (98.0–98.6) | 53.2 (47.0–59.4) | 99.4 (99.2–99.6) | 45.7 (39.0–52.5) | 0.2 (0.2–0.3) | 73.5 (64.9–82.1) |
| Only ICA persistently positive | 6/389 | 3.3 (1.2–7.1) | 94.7 (94.2–95.2) | 1.5 (0.6–3.3) | 97.5 (97.1–97.9) | 0.6 (0.3–1.3) | 1.0 (1.0–1.0) | 2.9 (0.5–5.3) |
| Persistent double positivity* | 37/82 | 20.6 (14.9–27.2) | 99.4 (99.2–99.5) | 45.1 (34.1–56.5) | 98.0 (97.7–98.4) | 33.0 (22.0–49.4) | 0.8 (0.8–0.8) | 76.4 (59.5–93.3) |
| pICA, pIAA | 11/12 | 6.1 (3.1–10.7) | 100 (99.9–100) | 91.7 (61.5–99.8) | 97.7 (97.3–98.0) | 441.8 (74.2–2659.9) | 0.9 (0.9–1.0) | 100 (100–100) |
| pICA, pGADA | 2/26 | 1.1 (0.1–4.0) | 99.7 (99.5–99.8) | 7.7 (0.9–25.1) | 97.6 (97.2–97.9) | 3.3 (0.9–12.6) | 1.0 (1.0–1.0) | 11.2 (0.0–25.8) |
| pICA, pIA-2A | 24/44 | 13.3 (8.7–19.2) | 99.7 (99.6–99.8) | 54.5 (38.8–69.9) | 97.9 (97.5–98.2) | 48.2 (27.3–85.0) | 0.9 (0.8–0.9) | 77.7 (60.3–95.0) |
| Persistent triple positivity* | 53/121 | 29.4 (22.9–36.7) | 99.1 (98.8–99.3) | 43.8 (34.8–53.1) | 98.3 (97.9–98.5) | 31.3 (22.6–43.0) | 0.7 (0.7–0.8) | 64.8 (49.5–80.1) |
| pICA, pIAA, pGADA | 10/18 | 5.6 (2.7–10.0) | 99.9 (99.8–100) | 55.6 (30.8–78.5) | 97.7 (97.3–98.0) | 50.2 (20.6–122.3) | 0.9 (0.9–1.0) | 72.8 (47.4–98.2) |
| pICA, pIAA, pIA-2A | 20/28 | 11.1 (6.9–16.6) | 99.9 (99.8–100) | 71.4 (51.3–86.8) | 97.8 (97.5–98.2) | 100.4 (45.9–221.2) | 0.9 (0.9–0.9) | 87.0 (66.9–100) |
| pICA, pGADA, pIA-2A | 23/75 | 12.8 (8.3–18.6) | 99.3 (99.1–99.5) | 30.7 (20.5–42.4) | 97.9 (97.5–98.2) | 17.8 (11.1–28.1) | 0.9 (0.8–0.9) | 52.6 (32.6–72.5) |
| All four DAA persistently positive | 55/80 | 30.6 (23.9–37.8) | 99.7 (99.5–99.8) | 68.8 (57.4–78.7) | 98.3 (98.0–98.6) | 88.4 (57.0–137.6) | 0.7 (0.7–0.7) | 83.4 (71.7–95.0) |
| Persistently positive for at least ICA | 151/672 | 83.9 (77.7–88.9) | 92.8 (92.2–93.4) | 22.5 (19.4–25.8) | 99.6 (99.4–99.7) | 11.6 (10.6–12.5) | 0.2 (0.1–0.2) | 41.2 (34.1–48.3) |
| ≥2 DAA persistently positive* | 145/283 | 80.6 (74.0–86.1) | 98.1 (97.7–98.4) | 51.2 (45.3–57.2) | 99.5 (99.3–99.7) | 42.2 (36.5–47.7) | 0.2 (0.1–0.3) | 73.2 (64.5–81.8) |
| ≥3 DAA persistently positive* | 108/201 | 60.0 (52.4–67.2) | 98.7 (98.4–99.0) | 53.7 (46.6–60.8) | 99.0 (98.7–99.2) | 46.6 (37.5–57.3) | 0.4 (0.3–0.5) | 73.0 (63.0–83.0) |
Data are % (95% CI) unless otherwise indicated. Type 1 diabetes/all progressors/all subjects positive for the given specificity; p, persistently positive autoantibody. In the case of defined autoantibody categories, each individual is included in only one category.
*ICA positivity-based categorization. The data in bold represent the highest or lowest (−LR) value for each characteristic.
FIG. 2.
Progression to type 1 diabetes (T1D) in relation to number of positive autoantibodies (A) and combinations of double (B) and triple (C) positivity further defined. *P < 0.001; †P = 0.02; ‡P = 0.04.
FIG. 3.
Progression to type 1 diabetes (T1D) in relation to number of persistently positive autoantibodies (A) and combinations of persistent double (B) and triple (C) positivity further defined. p, persistent positivity. *P < 0.001; †P = 0.006; ‡P = 0.03.
DISCUSSION
The virtues of the present work lie in the extensive series of children with HLA-conferred diabetes susceptibility derived from a background population with the highest incidence of type 1 diabetes in the world, and the fact that they were observed already from birth for the appearance of signs of β-cell autoimmunity and progression to overt type 1 diabetes. Because of the Finnish Pediatric Diabetes Register (19), it was also possible to trace the majority of progressors that had dropped out from the regular pre-diabetic follow-up and often to even get a blood sample for autoantibody analyses at the time of their diagnosis. Our main limitation was using ICAs as the only primary screening tool for β-cell autoimmunity, thus missing some ICA-negative subjects with positivity for molecular autoantibodies. The rationale for this approach was based on the knowledge available in 1994 at the initiation phase of the DIPP study. At that time, ICA represented the autoantibody reactivity with the most robust information on their predictive value (20,21).
The sensitivity of the current screening program would have increased from the observed 86% for ICAs to 97%, if IAAs had been added to the initial screening. This would have reduced the number of pre-diabetically seronegative progressors. According to our experience, however, the main reason for pre-diabetic seronegativity was discontinuation of the regular follow-up and, extremely rarely, the fact that no seroconversion had occurred. In the present series, there were three progressors having otherwise clinically obvious type 1 diabetes but no known detectable autoantibodies at the last follow-up visit 4–8 months before the diagnosis. Unfortunately, for one of these individuals, no autoantibody sample was obtained at the diagnosis of clinical disease, while the other two had developed at least ICA and IAA positivity by the time of their diagnosis. Twelve of 15 prediabetically seronegative subjects had dropped out from the regular follow-up, and in this group, the shortest delay from the last pre-diabetic sample to diagnosis was 1.9 years. With this observation in mind, one might suggest that in any screening program based on autoantibody detection in young children, the sampling interval should not exceed 2 years.
In the general childhood population selected for disease risk–related HLA genotypes, isolated low-level ICA positivity did not confer a significant increase in the disease risk when compared with autoantibody-negative children, but the risk of type 1 diabetes associated with this marker was related to its level as well as with multipositivity. These findings are similar to those observed among first-degree relatives of children with type 1 diabetes (22–24). However, the strong correlation between the ICA titer and the number of positive molecular autoantibodies indicated the latter phenomenon to be the true risk marker rather than the ICA titer. As previously reported among first-degree relatives of patients with type 1 diabetes, we also observed that the younger the child at the seroconversion, the higher the risk of presenting with clinical diabetes during the observation period (25). Part of this finding may result from the variation in the follow-up times among the subjects, since the DIPP study is still ongoing, but according to the analysis of diabetes-free survival, the group of the youngest seroconverters differed from the older ones already 2 years after the seroconversion (progression rate 9.7 vs. 1.4–3.3% in the older age-groups).
The degree of the variation in sensitivity, PPV, +LR, and cumulative disease risk estimates associated with the different combinations of multipositivity was conspicuously wide. Sensitivity of the categorized autoantibody combinations remained mainly modest, except for the quadruple positivity with a slightly higher value of 54%. Specificity was >98% for all other markers except isolated ICA and pICA positivity, and the NPV values were all >97%. Remarkably high PPV (92%), +LR (442), and disease risk (100%) were associated with the combination of persistently positive ICAs and IAAs. All persistent autoantibody combinations except the combination of pICAs and pGADAs had +LR values >10, indicating an increased disease risk (26). The predictive characteristics of the combined ICA and GADA positivity resembled single ICA positivity, and since some of the ICA positivity is derived from GADA reactivity, one may assume that the findings regarding this combination may be at least partly explained by overlapping antibody reactivity.
Observations from the current study confirm that in the general population the combination of HLA genotyping and autoantibody detection can provide predictive values similar to those reported among first-degree relatives of affected patients (27–29). For example, in the Childhood Diabetes in Finland (DiMe) study (29), among siblings of children with newly diagnosed type 1 diabetes, positivity for at least three autoantibodies was associated with a 5-year cumulative risk of ∼57%, while the corresponding risk was 62% in the present series. Positivity for multiple autoantibodies is, however, probably less frequent in the background population than among family members, even after screening for individuals with HLA-conferred disease susceptibility. In this study, the frequency of positivity for three or more DAAs was 3.5% (263 of 7,410), while the corresponding figure for DiMe siblings was 4.6%. Accordingly, to identify similar numbers of individuals at high risk (>50% over 5 years) of disease progression from the background population with HLA-defined diabetes susceptibility, and from siblings of affected children, one-third more children should be screened from the background population. Nevertheless, persistently multipositive children in the general population represent the majority of individuals at risk for type 1 diabetes, and without preventive measures covering this part of the population, only a minor proportion of future cases can be prevented. Given that the natural progression rate is extremely high in this group of children, especially among those developing persistent positivity for IAAs, these children might represent a subgroup in which more aggressive preventive treatments may be justified in the future. Altogether, our experience shows that it is feasible to observe children with HLA-defined diabetes susceptibility from birth and to identify individuals developing β-cell autoimmunity among them. As soon as effective preventive treatments are available, prevention programs based on HLA genotyping and regular autoantibody analyses may well be relevant in high-incidence countries, such as Finland.
Supplementary Material
Acknowledgments
This study was supported by special public grants for medical research at the Tampere, Oulu, Turku, and Helsinki University Hospitals; the Academy of Finland; the Juvenile Diabetes Research Foundation; the Novo Nordisk Foundation; the Foundation for Pediatric Research; and European Union Biomed 2 (BMH4-CT98-3314 and BMH4-CT98-3363). The funding sources did not participate in the design or conduct of the study; in the collection, analysis, or interpretation of the data; or in the preparation, review, or approval of the manuscript.
No potential conflicts of interest relevant to this article were reported.
Parts of the preliminary data were presented at the International Society for Pediatric and Adolescent Diabetes Annual Meeting, Krakow, Poland, 31 August–3 September 2005.
We thank the dedicated and talented staff of the DIPP study for their clinical, data, and laboratory support. We also thank all the children and their families who generously volunteered their time and knowledge.
Author contributions: H.T.A.S. and M.K. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: M.K., J.I., and O.S.; acquisition of data: H.T.A.S., S.S., A.H., J.L., P.V., and R.V.; analysis and interpretation of data: H.T.A.S., M.K., and R.V.; drafting of the manuscript: H.T.A.S. and M.K.; critical revision of the manuscript for important intellectual content: S.S., A.H., J.L., T.S., P.V., R.V., J.I., and O.S.; statistical analysis: H.T.A.S. and M.K.; obtained funding: H.T.A.S., T.S., R.V., J.I., M.K., O.S.; administrative, technical, or material support: T.S., S.S., A.H., J.L., and R.V.; and study supervision: M.K., J.I., and O.S.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bingley PJ, Bonifacio E, Ziegler A-G, Schatz DA, Atkinson MA, Eisenbarth GS.the Immunology of Diabetes Society. Proposed guidelines on screening for risk of type 1 diabetes. Diabetes Care 2001; 24: 398. [DOI] [PubMed] [Google Scholar]
- 2.Kupila A, Keskinen P, Simell T, Erkkilä S, Arvilommi P, Korhonen S, Kimpimäki T, Sjöroos M, Ronkainen M, Ilonen J, Knip M, Simell O: Genetic risk determines the emergence of diabetes-associated autoantibodies in young children. Diabetes 2001; 51: 646– 651 [DOI] [PubMed] [Google Scholar]
- 3.Onkamo P, Väänänen S, Karvonen M, Tuomilehto J: Worldwide increase in incidence of type I diabetes: the analysis of the data on published incidence trends. Diabetologia 1999; 42: 1395– 1403 [DOI] [PubMed] [Google Scholar]
- 4.Karvonen M, Viik-Kajander M, Moltchanova E, Libman I, LaPorte R, Tuomilehto J.the Diabetes Mondiale (DiaMond) Project Group. Incidence of childhood type 1 diabetes worldwide. Diabetes Care 2000; 23: 1516– 1526 [DOI] [PubMed] [Google Scholar]
- 5.Gale EAM: The rise of childhood type 1 diabetes in the 20th century. Diabetes 2002; 51: 3353– 3361 [DOI] [PubMed] [Google Scholar]
- 6.Harjutsalo V, Sjöberg L, Tuomilehto J: Time trends in the incidence of type 1 diabetes in Finnish children: a cohort study. Lancet 2008; 371: 1777– 1782 [DOI] [PubMed] [Google Scholar]
- 7.Kupila A, Muona P, Simell T, Arvilommi P, Savolainen H, Hämäläinen AM, Korhonen S, Kimpimäki T, Sjöroos M, Ilonen J, Knip M, Simell O.the Juvenile Diabetes Research Foundation Centre for the Prevention of Type 1 Diabetes in Finland. Feasibility of genetic and immunological prediction of type I diabetes in a population-based birth cohort. Diabetologia 2001; 44: 290– 297 [DOI] [PubMed] [Google Scholar]
- 8.Ilonen J, Reijonen H, Herva E, Sjöroos M, Iitiä A, Lövgren T, Veijola R, Knip M, Åkerblom HK: Rapid HLA-DQB1 genotyping for four alleles in the assessment of diabetes risk in the Finnish population. Diabetes Care 1996; 19: 795– 800 [DOI] [PubMed] [Google Scholar]
- 9.Hermann R, Turpeinen H, Laine AP, Veijola R, Knip M, Simell O, Sipilä I, Åkerblom HK, Ilonen J: HLA DR–DQ-encoded genetic determinants of childhood-onset type 1 diabetes in Finland: an analysis of 622 nuclear families. Tissue Antigens 2003; 62: 162– 169 [DOI] [PubMed] [Google Scholar]
- 10.Hämäläinen A-M, Ilonen J, Simell O, Savola K, Kulmala P, Kupila A, Simell T, Erkkola R, Koskela P, Knip M: Prevalence and fate of type 1 diabetes-associated autoantibodies in cord blood samples from newborn infants of non-diabetic mothers. Diabetes Metab Res Rev 2002; 18: 57– 63 [DOI] [PubMed] [Google Scholar]
- 11.Näntö-Salonen K, Kupila A, Simell S, Salonsaari T, Siljander H, Salonsaari T, Hekkala A, Korhonen S, Erkkola R, Sipilä JI, Haavisto L, Siltala M, Tuominen J, Hakalax J, Hyöty H, Ilonen J, Veijola R, Simell T, Knip M, Simell O: Can type 1 diabetes be prevented by a tolerisation strategy: nasal insulin in children with genetic risk and autoantibodies. Lancet 2008; 372: 1746– 1755 [DOI] [PubMed] [Google Scholar]
- 12.Sjöroos M, Iitiä A, Ilonen J, Reijonen H, Lövgren T: Triple-label hybridization assay for type 1 diabetes-related HLA alleles. Biotechniques 1995; 18: 870– 877 [PubMed] [Google Scholar]
- 13.Bottazzo GF, Florin-Christensen A, Doniach D: Islet cell antibodies in diabetes mellitus with autoimmune polyendocrine deficiencies. Lancet 1974; 2: 1279– 83 [DOI] [PubMed] [Google Scholar]
- 14.Williams AJK, Bingley PJ, Bonifacio E, Palmer JP, Gale EAM: A novel micro-assay for insulin autoantibodies. J Autoimmun 1997; 10: 473– 478 [DOI] [PubMed] [Google Scholar]
- 15.Savola K, Sabbah E, Kulmala P, Vähäsalo P, Ilonen J, Knip M: Autoantibodies associated with type I diabetes mellitus persist after diagnosis in children. Diabetologia 1998; 41: 1293– 1297 [DOI] [PubMed] [Google Scholar]
- 16.Savola K, Bonifacio E, Sabbah E, Kulmala P, Vähäsalo P, Karjalainen J, Tuomilehto-Wolf E, Meriläinen J, Åkerblom HK, Knip M.the Childhood Diabetes in Finland Study Group. IA-2 antibodies: a sensitive marker of IDDM with clinical onset in childhood and adolescence. Diabetologia 1998; 41: 424– 429 [DOI] [PubMed] [Google Scholar]
- 17.Greenbaum CJ, Palmer JP, Nagataki S, Yamaguchi Y, Molenaar JL, Van Beers WA, MacLaren NK, Lernmark Å: Improved specificity of ICA assay in the Fourth International Immunology of Diabetes Serum Exchange Workshop. Diabetes 1992; 41: 1570– 1574 [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization/Department of Noncommunicable Disease Surveillance Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications: Part 1: Diagnosis and Classification of Diabetes Mellitus. Geneva, World Health Org., 1999, p. 1– 49 [Google Scholar]
- 19.Mäkinen A, Härkönen T, Ilonen J, Knip Mthe Finnish Pediatric Diabetes Register Characterization of the humoral immune response to islet antigen 2 in children with newly diagnosed type 1 diabetes. Eur J Endocrinol 2008; 159: 19– 26 [DOI] [PubMed] [Google Scholar]
- 20.Riley WJ, Maclaren NK, Krischer J, Spillar RP, Silverstein JH, Schatz DA, Schwartz S, Malone J, Shah S, Vadheim C, et al. : A prospective study of the development of diabetes in relatives of patients with insulin-dependent diabetes. N Engl J Med 1990; 323: 1167– 1172 [DOI] [PubMed] [Google Scholar]
- 21.Knip M, Vähäsalo P, Karjalainen J, Lounamaa R, Åkerblom HK.the Study Group on Childhood Diabetes in Finland. Natural history of preclinical IDDM in high risk siblings. Diabetologia 1994; 37: 388– 393 [DOI] [PubMed] [Google Scholar]
- 22.Bonifacio E, Bingley PJ, Shattock M, Dean BM, Dunger D, Gale EA, Bottazzo GF: Quantification of islet-cell antibodies and prediction of insulin-dependent diabetes. Lancet 1990; 335: 147– 149 [DOI] [PubMed] [Google Scholar]
- 23.Bingley PJ.the ICARUS Group. Interactions of age, islet cell antibodies, insulin autoantibodies and first phase insulin response in predicting risk of progression to IDDM in relatives: the ICARUS dataset. Diabetes 1996; 45: 1720– 1728 [DOI] [PubMed] [Google Scholar]
- 24.Bingley PJ, Gale EAM.the European Nicotinamide Diabetes Intervention Trial (ENDIT) Group. Progression to type 1 diabetes in islet cell antibody-positive relatives in the European Nicotinamide Diabetes Intervention Trial: the role of additional immune, genetic and metabolic markers of risk. Diabetologia 2006; 49: 881– 890 [DOI] [PubMed] [Google Scholar]
- 25.Hummel M, Bonifacio E, Schmid S, Walter M, Knopff A, Ziegler A-G: Brief communication: early appearance of islet autoantibodies predicts childhood type 1 diabetes in offspring of diabetic patients. Ann Intern Med 2004; 140: 882– 886 [DOI] [PubMed] [Google Scholar]
- 26.McGee S: Simplifying likelihood ratios. J Gen Intern Med 2002; 17: 646– 649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bingley PJ, Christie MR, Bonifacio E, Bonfanti R, Shattock M, Fonte MT, Bottazzo GF, Gale EA: Combined analysis of autoantibodies improves prediction of IDDM in islet cell antibody-positive relatives. Diabetes 1994; 43: 1304– 1310 [DOI] [PubMed] [Google Scholar]
- 28.Verge CF, Gianani R, Kawasaki E, Yu L, Pietropaolo M, Jackson RA, Chase HP, Eisenbarth GS: Prediction of type 1 diabetes in first-degree relatives using a combination of insulin, GAD and ICA512bdc/IA-2 autoantibodies. Diabetes 1996; 45: 926– 933 [DOI] [PubMed] [Google Scholar]
- 29.Kulmala P, Savola K, Petersen JS, Vähäsalo P, Karjalainen J, Löppönen T, Dyrberg T, Åkerblom HK, Knip M: Prediction of insulin-dependent diabetes mellitus in siblings of diabetic children: a population-based study. J Clin Invest 1998; 101: 327– 336 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.