Abstract
BACKGROUND:
Fragmented QRS complexes in the electrocardiograms (ECGs) of patients with coronary artery disease are associated with adverse cardiac events. However, there are limited data on its predictive usefulness in patients with nonischemic dilated cardiomyopathy. Left ventricular dyssynchrony is common in heart failure patients who have wide QRS intervals, but its frequency in patients with narrow QRS intervals is uncertain.
OBJECTIVES:
To investigate the relationship between fragmented QRS complexes and intraventricular dyssynchrony in patients with nonischemic dilated cardiomyopathy in sinus rhythm.
METHODS:
Sixty nonischemic dilated cardiomyopathy patients with sinus rhythm and narrow QRS intervals were recruited. Forty patients had a fragmented QRS in their basal ECG, and 20 patients did not have a fragmented QRS. Patients were analyzed for correlation between fragmented QRS complexes and intraventricular dyssynchrony.
RESULTS:
The maximal difference in time to the peak myocardial systolic velocity between any two left ventricular segments (Max-ASE Sys), and maximal difference between Max-ASE Sys and the mean value of all segments (Max-ASE to Mean Sys) were significantly higher in patients with fragmented QRS complexes (P=0.001 and P=0.003, respectively). Seventy-two per cent of the patients with fragmented QRS complexes had significant left ventricular dyssynchrony; 15% of patients without fragmented QRS complexes had significant left ventricular dyssynchrony (P<0.0001). The presence of fragmented QRS complexes in leads corresponding to the specific ventricular segment in basal ECG was found to detect intraventricular dyssynchrony with 90.6% sensitivity (negative predictive value of 85%).
CONCLUSION:
Fragmentation in the resting ECG is associated with significant intraventricular dyssynchrony in patients with nonischemic cardiomyopathy, narrow QRS and sinus rhythm. Fragmentation in ECG might be useful in identifying patients who could benefit from cardiac resynchronization therapy.
Keywords: Cardiomyopathy, Dyssynchrony, Fragmented QRS complex
Abstract
HISTORIQUE :
Chez les patients souffrant de coronaropathie, on associe les complexes QRS fragmentés à l’électrocardiogramme (ÉCG) à des complications cardiaques. Or, on manque de données sur son utilité prédictive chez les patients qui souffrent de cardiomyopathie dilatée non ischémique. La dyssynchronie ventriculaire gauche s’observe fréquemment chez les insuffisants cardiaques qui présentent des QRS élargis, mais sa fréquence chez les patients dont les QRS sont étroits est mal connue.
OBJECTIF :
Analyser le lien entre les QRS fragmentés et la dyssynchronie intraventriculaire chez des patients souffrant de cardiomyopathie dilatée non ischémique dont le rythme est sinusal.
MÉTHODE :
Les auteurs ont recruté 60 patients atteints de cardiomyopathie dilatée non ischémique présentant un rythme sinusal et des QRS étroits. À l’ÉCG de base, 40 patients présentaient des QRS fragmentés et 20 patients, des QRS non fragmentés. On a soumis les patients à une analyse de corrélation entre les complexes QRS fragmentés et la dyssynchronie intraventriculaire.
RÉSULTATS :
La différence maximale pour ce qui est du temps d’atteinte du pic systolique de vélocité (Vmax) myocardique entre n’importe quelle paire de segments ventriculaires gauches et la différence maximale entre ce Vmax et la valeur moyenne de tous les segments (Vmax:moyenne des segments) ont été significativement plus marquées chez les patients qui avaient des complexes QRS fragmentés (p = 0,001 et p = 0,003, respectivement). Soixante-douze pour cent des patients dont les QRS étaient fragmentés présentaient une dyssynchronie ventriculaire gauche significative, contre 15 % des patients dont les QRS n’étaient pas fragmentés (p < 0,0001). La présence de QRS fragmentés aux dérivations correspondant aux segments ventriculaires spécifiques à l’ÉCG de base s’est révélée prédictive d’une dyssynchronie intraventriculaire avec une sensibilité de 90,6 % (valeur prédictive négative de 85 %).
CONCLUSION :
La fragmentation des QRS à l’ÉCG au repos est associée à une dyssynchronie intraventriculaire significative chez les patients souffrant de cardiomyopathie non ischémique en présence de QRS étroits et de rythme sinusal. La fragmentation à l’ÉCG pourrait servir à reconnaître les patients susceptibles de bénéficier d’un traitement de resynchronisation cardiaque.
Heart failure is a serious health problem that affects many people. (1–3). Left ventricular (LV) dyssynchrony is frequently encountered in heart failure patients, especially in those with wide QRS intervals (4). Intraventricular dyssynchrony has also been reported in patients with narrow QRS intervals (4–8). Cardiac resynchronization therapy (CRT) is a useful management strategy for patients with wide QRS intervals and symptomatic heart failure who are on maximal medical therapy. CRT has been associated with functional improvement and decreased mortality (9–12). Because intraventricular dyssynchrony is uncommon in patients with narrow QRS intervals compared with patients with wide QRS intervals, it is difficult to decide which patients with narrow QRS intervals should be referred for CRT (4,5,7). The presence of fragmented QRS (fQRS) complexes in patients with coronary artery disease has been associated with regional myocardial damage, increased cardiac adverse events and decreased event-free survival (13–16). fQRS complexes are also seen in patients with LV aneurysms (17,18). There are no data in patients with nonischemic dilated cardiomyopathy. We investigated the relationship between fQRS complexes and intraventricular dyssynchrony in patients with nonischemic dilated cardiomyopathy.
METHODS
Patient selection
The present study was conducted in the heart failure clinic at the Kosuyolu Heart Education and Research Hospital in Istanbul (Kartal, Turkey). Forty patients with an LV ejection fraction of less than 40% and sinus rhythm having narrow (less than 120 ms) and fQRS complexes were consecutively recruited. All patients had heart failure symptoms and were receiving beta-blockers, angiotensin-converting enzyme inhibitors, diuretics and digoxin. All patients included in the study were diagnosed with LV dysfunction for at least two years, were using the above-mentioned medications for at least six months, and were symptomatic for at least the previous six months. In addition, 20 nonischemic dilated cardiomyopathy patients with narrow QRS intervals who did not have fQRS complexes in their resting electrocardiogram (ECG) were prospectively included in the study. Patients with organic valvular heart disease, a history of myocardial infarction, ischemic ECG findings, angiographically significant coronary artery disease (more than 50% stenosis in any epicardial coronary artery), atrial fibrillation, chronic liver or kidney failure, as well as patients with permanent pacemakers were excluded from the study. The study protocol was approved by the institutional review board and the subjects gave written informed consent for their participation in the study.
ECG
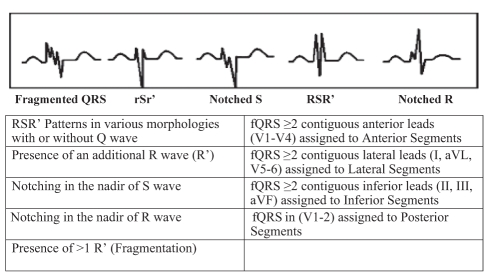
The resting 12-lead ECG (0.5 Hz to 150 Hz, 25 mm/s, 10 mm/mV) was analyzed by two independent clinicians who were blinded to echocardiographic data. There was a 99.5% concordance for ECG signs. In case of disagreement, the final diagnosis was achieved by mutual agreement. The fQRS included various RSR′ patterns and was defined by the presence of an additional R wave (R′), notching in the nadir of the S wave, notching of the R wave, or the presence of more than one R′ (fragmentation) in two contiguous leads corresponding to a major myocardial segment (Figure 1). The presence of fQRS in two or more contiguous anterior leads (V1 to V5) corresponded to anterior myocardial segments, the presence of fQRS in two or more lateral leads (I, aVL and V5,V6) corresponded to the lateral myocardial segments, the presence of fQRS in two or more inferior leads (II, III and aVF) corresponded to the inferior myocardial segments, and the presence of fQRS in V1 and V2 corresponded to the posterior myocardial segments. The fQRS was also seen in more than one myocardial segment in some patients.
Figure 1).
The fragmented QRS (fQRS) on a 12-lead electrocardiogram is defined as the presence of slurred QRS complexes with various RSR′ patterns (≥1 R′ or notching of S wave or R wave) without typical bundle branch block in two contiguous leads corresponding to a major coronary artery territory
Echocardiography
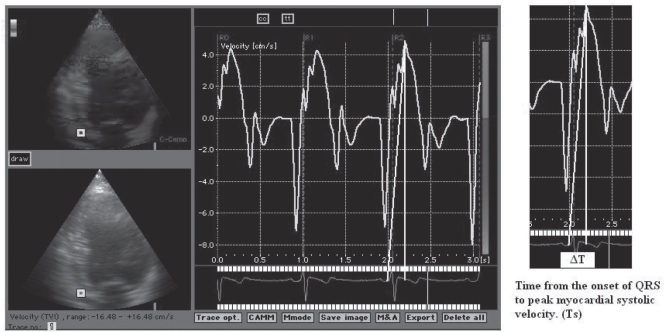
Standard echocardiography with Doppler studies were performed by using a commercially available system (System 5; VingMed-General Electric, Norway). Two echocardiographers who were unaware of the study performed the examinations, and they were blinded to the ECGs and clinical status of each patient. LV dimension and ejection fraction were measured by two-dimensional guided M-mode echocardiography according to the guidelines of the American Society of Echocardiography (19). The maximal rate of LV systolic pressure increase was used as an index of LV systolic performance and was estimated from the steepest increasing segment of the continuous-wave Doppler image of the mitral regurgitation velocity spectrum (20). The effective orifice area and the regurgitant volume of the functional mitral regurgitation jet were calculated by the ‘proximal isovelocity surface area’ method (21). Pulmonary artery systolic pressure was estimated from the tricuspid regurgitation jet by continuous wave Doppler. Tissue Doppler imaging was performed in the apical views (four-chamber, two-chamber and long-axis) for the long-axis motion of the left ventricle. Two-dimensional echocardiography with tissue Doppler colour imaging was performed with a 2.5 MHz phase array transducer. The system was set by bypassing the high-pass filter, while the low-frequency Doppler shifts were input directly into an autocorrelator (22). Gain settings, filtres and pulse repetitive frequency were adjusted to optimize colour saturation, and a colour Doppler frame scanning rate of 100 Hz to 140 Hz was used. At least three consecutive beats were stored, and the images were digitized and analyzed off-line by a computer (EchoPac 6.3; VingMed-General Electric). Myocardial regional velocity curves were constructed from the digitized images (23). For a detailed assessment of regional myocardial function, 7 mm × 7 mm of sampling window was placed at the myocardial segment of interest. In each view, both the basal and middle segments were assessed. Therefore, septal, anteroseptal, anterior, lateral, inferior and posterior segments were interrogated at both basal and middle levels. For the measurement of timing, the beginning of the QRS complex was used as the reference point, where the times to peak myocardial sustained systolic (Figure 2) and early diastolic velocities were quantified (24). The estimated interobserver and intraobserver variabilities were 4.3% and 3.7%, respectively. For the assessment of synchronicity, the maximal difference in sustained systolic and early diastolic velocities between any two of the LV segments (Max-ASE Sys and Max-ASE Dias, respectively) and the maximal difference between the mean value of all segments and each segment (Max-ASE to Mean Sys and Max-ASE to Mean Dias) were calculated. To assess global cardiac function, the myocardial sustained systolic, early diastolic (E) and late diastolic (A) velocities from the basal septal and basal lateral segments were calculated. Significant systolic and diastolic dyssynchrony was defined as a Max-ASE Sys and Max-ASE Dias of more than 100 ms, and Max-ASE to Mean Sys of more than 60 ms, as previously defined by Yu et al (4).
Figure 2).
Demonstration of the time from the onset of QRS to peak myocardial systolic velocity (Ts) calculation
Statistical analysis
Data were analyzed using a statistical software program (SPSS version 13.0; SPSS Inc, USA). Data are presented as mean ± SD, controlled for normal distribution by the Kolmogorov-Smirnov test and compared using the paired Student’s t test. Finally, nonparametric tests, such as the Mann-Whitney U test, were used when the distribution was not normal. Categorical data between two or more groups were compared by Pearson’s χ2 test. Sensitivity was defined by the number of true positives for the presence of fQRS complexes and a corresponding intraventricular dyssynchrony for suitable myocardial segments. Specificity was defined as the number of true negatives with no fQRS complexes and normally defined synchronicity. P<0.05 was considered to be significant.
RESULTS
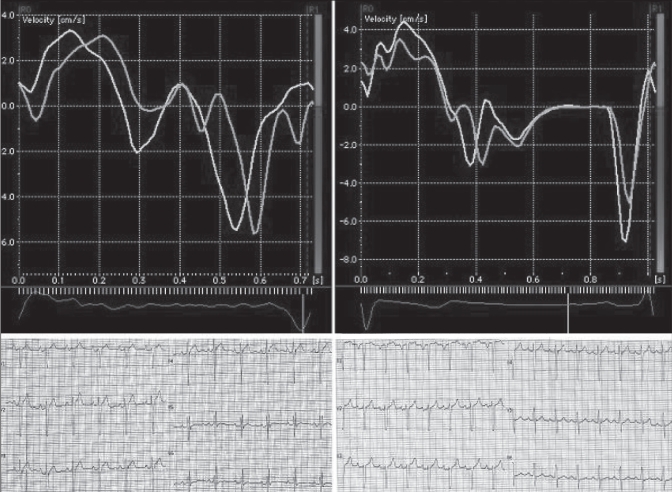
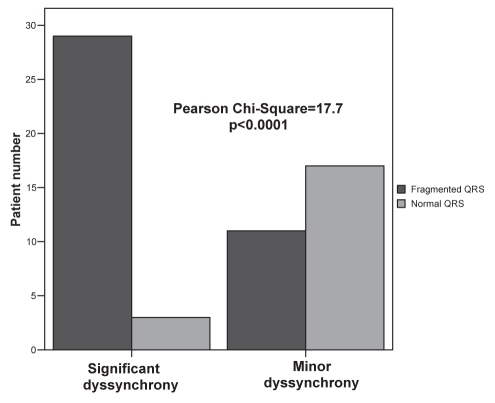
The study group included 19 women (31.7%) and 41 men (68.3%) with a mean (± SD) age of 38±15 years. Patients were categorized into two subgroups according to having (n=40; 66.7%) and not having (n=20; 33.3%) fQRS complexes in their basal ECGs. Demographic, clinical and echocardiographic characteristics of the study population and the differences between the two groups are shown in Tables 1 and 2. Max-ASE Sys and Max-ASE to Mean Sys were significantly higher in patients who had fQRS complexes in their resting ECGs (P=0.001 and P=0.003, respectively). Peak myocardial systolic velocities of two patients, one with fQRS and one with normal QRS, are demonstrated in Figure 3. There was no statistically significant difference between the two groups in other parameters. When the patients were split into two groups (Max-ASE Sys values of 100 ms or lower, or higher than 100 ms), 72.5% (29 of 40) of the patients with fQRS complexes had significant LV dyssynchrony, and 15% (3 of 20) of the patients without fQRS complexes had significant LV dyssynchrony (Pearson’s χ2=17.7, P<0.0001) (Figure 4). When the patients were characterized by a Max-ASE to Mean Sys of more than 60 ms, 62.5% (25 of 40) of patients with fQRS complexes had significant LV dyssynchrony, and 20% (4 of 20) of patients without fQRS complexes had significant LV dyssynchrony (P=0.002).
TABLE 1.
The characteristics of patients with fragmented and normal QRS
| Characteristic | Study population (n=60) | Fragmented QRS (n=40) | Normal QRS (n=20) | P |
|---|---|---|---|---|
| Sex, female:male | 19:41 | 12:28 | 7:13 | 0.695 |
| Age, years | 38±15 | 39±15 | 35±15 | 0.262 |
| NYHA, I to II:III to IV | 44:16 | 27:13 | 17:3 | 0.148 |
| Duration of HF, years | 1.6±0.2 | 1.7±0.3 | 1.5±0.4 | 0.467 |
| Beta-blockers, yes:no | 52:8 | 35:5 | 17:3 | 0.644 |
| ACE inhibitors, yes:no | 59:1 | 39:1 | 20:0 | 0.854 |
| Digitalis, yes:no | 8:52 | 5:35 | 3:17 | 0.556 |
| ASA, yes:no | 56:4 | 37:3 | 19:1 | 0.644 |
| LA, cm | 4.7±0.8 | 4.7±0.8 | 4.7±0.9 | 0.838 |
| LVEDD, cm | 6.9±0.9 | 7±0.9 | 6.8±0.8 | 0.327 |
| LVESD, cm | 6.1±0.8 | 6.1±0.8 | 5.9±0.9 | 0.461 |
| IVS, cm | 1±0.26 | 1±0.2 | 1±0.3 | 0.490 |
| PW, cm | 1±0.28 | 1±0.2 | 1±0.3 | 0.754 |
| LVEF, % | 26±7 | 26.6±8 | 26.2±7 | 0.765 |
| EPSS, cm | 2.4±0.5 | 2.4±0.5 | 2.4±0.6 | 0.691 |
| dP/dt, mmHg/ms | 508±157 | 487±156 | 541±158 | 0.178 |
| MR volume, mL | 22±14 | 24±15 | 18±10 | 0.180 |
| Mitral E velocity, m/s | 0.85±0.27 | 0.89±0.29 | 0.78±0.22 | 0.226 |
| Mitral A velocity, m/s | 0.46±0.18 | 0.49±0.20 | 0.39±0.13 | 0.106 |
| E/A | 2.2±1.1 | 2.1±1.1 | 2.3±1 | 0.441 |
| EDT, ms | 132±65 | 132±68 | 112±56 | 0.096 |
| IVRT, ms | 95±35 | 100±37 | 85±29 | 0.122 |
| PAP, mmHg | 47±12 | 47±11 | 50±16 | 0.935 |
| Max-ASE Sys, ms | 111±46 | 126±46 | 82±29 | 0.001 |
| Max-ASE to Mean Sys, ms | 62±25 | 70±25 | 48±18 | 0.003 |
Data presented as mean ± SD unless otherwise indicated. A Late diastolic; ACE Angiotensin-converting enzyme; ASA Acetylsalicylic acid; dP/dt Peak rate of rise in left ventricular pressure; E Early diastolic; EDT Early filling deceleration time; EPSS E point septal separation; HF Heart failure; IVRT Isovolumetric relaxation time; IVS Interventricular septum; LA Left atrium; LVEDD Left ventricular end diastolic diameter; LVEF Left ventricular ejection fraction; LVESD Left ventricular end systolic diameter; Max-ASE Sys Maximal systolic dyssynchrony; Mean Sys Mean time to the peak myocardial systolic velocity of all segments; MR Mitral regurgitation; NYHA New York Heart Association functional classification; PAP Pulmonary artery pressure; PW Posterior wall
TABLE 2.
Characteristics of patients with (group 1) and without (group 2) dyssynchrony
| Characteristic | Group 1 (n=29) | Group 2 (n=11) | P |
|---|---|---|---|
| Sex, female:male | 8:21 | 4:7 | 0.589 |
| Age, years | 42±14 | 30±15 | 0.015 |
| NYHA, I to II:III to IV | 21:8 | 6:5 | 0.281 |
| LA, cm | 4.8±0.7 | 4.7±1 | 0.881 |
| LVEDD, cm | 7.2±0.9 | 6.7±1 | 0.231 |
| LVESD, cm | 6.2±0.8 | 5.9±0.8 | 0.422 |
| IVS, cm | 1±0.2 | 0.95±0.2 | 0.163 |
| PW, cm | 1±0.2 | 0.94±0.4 | 0.122 |
| LVEF, % | 27.7±7 | 24±10 | 0.355 |
| EPSS, cm | 2.4±0.5 | 2.3±0.6 | 0.440 |
| dP/dt, mmHg/ms | 516±178 | 435±90 | 0.289 |
| MR volume, mL | 26±17 | 19±9 | 0.457 |
| Mitral E velocity, m/s | 1±0.3 | 0.8±0.3 | 0.155 |
| Mitral A velocity, m/s | 0.4±0.2 | 0.5±0.2 | 0.022 |
| E/A | 2.9±1.1 | 1.7±1 | 0.009 |
| EDT, ms | 103±33 | 158±73 | 0.034 |
| IVRT, ms | 78±22 | 109±38 | 0.01 |
| PAP, mmHg | 52±10 | 44±11 | 0.160 |
| Max-ASE Sys, ms | 150±29 | 63±15 | <0.0001 |
| Max-ASE to Mean Sys, ms | 81±19 | 39±9 | <0.0001 |
| RV TDI S velocity, cm/s | 7.4±2.3 | 6.6±2.5 | 0.563 |
| Septal TDI s velocity, cm/s | 3±1.1 | 2.5±0.8 | 0.154 |
| Septal TDI e velocity, cm/s | 3.2±1.8 | 3.9±2 | 0.170 |
| Septal TDI a velocity, cm/s | 4.1±1.8 | 3.6±1.9 | 0.361 |
| Lateral TDI s velocity, cm/s | 2.8±1.3 | 2.4±1.3 | 0.173 |
| Lateral TDI e velocity, cm/s | 4.6±3.2 | 5.1±3 | 0.496 |
| Lateral TDI a velocity, cm/s | 4.3±2.5 | 3.2±1.5 | 0.074 |
| E/e | 37±27 | 38±43 | 0.961 |
Data presented as mean ± SD unless otherwise indicated. A Late diastolic; dP/dt peak rate of rise in left ventricular pressure; E Early diastolic; EDT Early filling deceleration time; EPSS E point septal separation; IVRT Isovolumetric relaxation time; IVS Interventricular septum; LA Left atrium; LVEDD Left ventricular end diastolic diameter; LVEF Left ventricular ejection fraction; LVESD Left ventricular end systolic diameter; Max-ASE Sys Maximal systolic dyssynchrony; Mean Sys Mean time to the peak myocardial systolic velocity of all segments; MR Mitral regurgitation; NYHA New York Heart Association functional classification; PAP Pulmonary artery pressure; PW Posterior wall; RV TDI S Right ventricular tissue Doppler imaging systolic; TDI a Tissue Doppler imaging late diastolic; TDI e Tissue Doppler imaging early diastolic; TDI s Tissue Doppler imaging systolic
Figure 3).
Intraventricular dyssynchrony in a patient with fragmented QRS (left) and in a patient with a normal QRS complex (right)
Figure 4).
Number of dyssynchronic and nondyssynchronic patients within the normal or fragmented QRS group
The 40 patients with fQRS complexes were subdivided into two groups with either significant dyssynchrony (group 1, n=29; 72.5%) or without dyssynchrony (group 2, n=11; 27.5%) according to a Max-ASE Sys of more than 100 ms. Table 2 shows the clinical, demographic and echocardiographic characteristics of patients in groups 1 and 2. Group 1 was significantly older (P=0.015) and had higher E/A ratios (P=0.009) than group 2. Group 1 also had significantly lower mitral A-wave velocity (P=0.022), E-wave deceleration time (P=0.034) and isovolumetric relaxation time (P=0.01). The other echocardiographic and clinical parameters between the two groups were not statistically different. Among 40 patients with fQRS complexes and a Max-ASE Dias of more than 100 ms, significant diastolic dyssynchrony was present in nine (22.5%) patients; however, the other 31 (77.5%) patients did not have significant diastolic dyssynchrony. The presence of fQRS complexes in the basal ECG was found to detect intraventricular dyssynchrony with 90.6% sensitivity, 60.7% specificity, a positive predictive value of 72.5% and a negative predictive value of 85% (Table 3).
TABLE 3.
Relationship between fragmented QRS and intraventricular dyssynchrony
| Significant systolic dyssynchrony, n | No significant systolic dyssynchrony, n | Total, n | |
|---|---|---|---|
| Fragmented QRS | 29 (true positive) | 11 (false positive) | 40 |
| Normal QRS | 3 (false negative) | 17 (true negative) | 20 |
| Total | 32 | 28 | 60 |
Forty patients with fQRS complexes were classified by segmental involvement as follows: anterior (n=8); inferior (n=6); lateral (n=5); posterior (n=3); inferior and lateral (n=12); anterior and lateral (n=1); posterior and lateral (n=1); anterior and inferior (n=1); inferior and posterior (n=1); inferior, anterior and lateral (n=1); and inferior, posterior and lateral (n=1). Among 29 patients with significant dyssynchrony on echocardiography, 16 patients had ECG fragmentation in the maximal dyssynchronic segment and six patients had ECG fragmentation in one of the dyssynchronic segments. In seven patients, fragmented segments and dyssynchronic segments were not correlated. Among dyssynchronic patients, the sensitivity and specificity of a fragmented segment in an ECG to detect the maximal dyssynchronic segment or one of the dyssynchronic segments were 75.8% and 76%, respectively.
Discussion
Although most CRT studies have been performed in patients with wide QRS complexes, intraventricular dyssynchrony also occurs in 27% to 56% of patients with narrow QRS complexes (4,5,9–12,25,26). The screening for intraventricular dyssynchrony in patients with narrow QRS complexes requires simple and inexpensive methods because the benefit of CRT is not yet clear in this group. Echocardiographic evaluation of dyssynchrony is recommended in borderline and challenging patients when making a decision for CRT (27). Determination of longitudinal LV velocities using tissue Doppler imaging is the principal clinical method to assess dyssynchrony, although it has several limitations. In our study, we found that fQRS complexes present in the resting ECGs of patients with nonischemic cardiomyopathy were associated with intraventricular dyssynchrony. Our study also demonstrates the need for more investigation in patients with narrow QRS complexes to determine the best method for detection of dyssynchrony, the outcome and the benefit, if any, of CRT.
The presence of fQRS in 12-lead ECG is associated with increased adverse cardiac events and mortality in patients with coronary artery disease. Das et al (13) reported that the presence of an fQRS has a better sensitivity and negative predictive value than Q waves in an ECG for the detection of a myocardial scar in patients with narrow QRS complexes. Myocardial scars and/or ischemia cause nonhomogeneous ventricular activation, which results in fragmentation in ECG (28–31). fQRS complexes are also associated with LV aneurysms on left ventriculography (18). Intramyocardial inflammation, myocyte necrosis and fibrosis are common in nonischemic dilated cardiomyopathy (32–35). Dyssynchronic contraction pattern might be secondary to nonhomogeneous intraventricular activation and uncoordinated depolarization of viable myocyte groups, which are surrounded by fibrotic tissue.
There are no reports of nonischemic dilated cardiomyopathy patients that analyzed the association between the presence of fQRS complexes and intraventricular dyssynchrony. We found that fQRS complexes predicted intraventricular dyssynchrony with high sensitivity (90.6%) and negative predictive value (85%) in patients with nonischemic dilated cardiomyopathy and a narrow QRS. These findings agree with the study by Yu et al (4), which showed the association between intraventricular dyssynchrony and fQRS complexes in patients with a wide QRS interval. Our study suggests that resting ECGs can help identify nonischemic dilated cardiomyopathy patients with narrow QRS complexes who might benefit from CRT. Our study also showed that among dyssynchronic patients, the fragmented ECG segment has a high sensitivity (75.8%) and specificity (76%) to locate the maximal dyssynchronic segment or one of the dyssynchronic segments. These findings will facilitate the echocardiographic assessment of intraventricular dyssynchrony among nonischemic cardiomyopathy patients with a narrow QRS. Additional longitudinal studies will be needed to assess the clinical outcomes in patients with narrow QRS complexes who have CRT.
Study limitations
While there is active investigative interest in identifying patients with dyssynchrony and normal QRS duration, the clinical implications of these studies are uncertain at the present time. The RethinQ Trial did not demonstrate that patients with normal QRS duration and evidence of dyssynchrony benefit from biventricular pacing (36). Thus, while the physiological implications of the fQRS with respect to dyssynchrony may be accurate in our study, the clinical implications need more investigation. We recruited patients consecutively to our study from the heart failure clinic. This might create a potential selection bias because our population might not represent the spectrum of dilated cardiomyopathy cases. Finally, we did not use other basic electrocardiographic information, such as heart rate, PR intervals and QRS duration, in our analysis. This might explain the mismatch between depolarization time and mechanical activation time observed in some patients. Also, it is uncertain whether tissue Doppler indexes are the best measures of dyssynchrony.
CONCLUSION
Fragmentation in basal ECG is associated with intraventricular dyssynchrony in nonischemic cardiomyopathy patients with a narrow QRS interval and sinus rhythm. The presence of fragmentation in an ECG might help identify patients who may benefit from CRT.
REFERENCES
- 1.Guidelines for the evaluation and management of heart failure. Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 1995;92:2764–84. doi: 10.1161/01.cir.92.9.2764. [DOI] [PubMed] [Google Scholar]
- 2.Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–17. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 3.Zannad F, Briancon S, Juilliere Y, et al. Incidence, clinical and etiologic features, and outcomes of advanced chronic heart failure: The EPICAL study. J Am Coll Cardiol. 1999;33:734–42. doi: 10.1016/s0735-1097(98)00634-2. [DOI] [PubMed] [Google Scholar]
- 4.Yu CM, Lin H, Zhang Q, et al. High prevalence of left ventricular systolic and diastolic asynchrony in patients with congestive heart failure and normal QRS duration. Heart. 2003;89:54–60. doi: 10.1136/heart.89.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bleeker G, Schalij M, Molhoek S, et al. Frequency of left ventricular dyssynchrony in patients with heart failure and a narrow QRS complex. Am J Cardiol. 2005;95:140–2. doi: 10.1016/j.amjcard.2004.08.082. [DOI] [PubMed] [Google Scholar]
- 6.Auricchio A, Yu CM. Beyond the measurement of QRS complex toward mechanical dyssynchrony: Cardiac resynchronization therapy in heart failure patients with a normal QRS duration. Heart. 2004;90:479–81. doi: 10.1136/hrt.2003.024273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghio S, Constantin C, Klersy C, et al. Interventricular and intraventricular dyssynchrony are common in heart failure patients, regardless of QRS duration. Eur Heart J. 2004;25:571–8. doi: 10.1016/j.ehj.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 8.Dohi K, Suffoletto M, Murali S, et al. Benefit of cardiac resynchronization therapy to a patient with a narrow QRS complex and ventricular dyssynchrony identified by tissue synchronization imaging. Eur J Echocardiogr. 2005;6:455–60. doi: 10.1016/j.euje.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Cazeau S, Leclercq C, Lavergne T, et al. Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N Engl J Med. 2001;344:873–80. doi: 10.1056/NEJM200103223441202. [DOI] [PubMed] [Google Scholar]
- 10.Abraham WT, Fisher WG, Smith AL, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346:1845–53. doi: 10.1056/NEJMoa013168. [DOI] [PubMed] [Google Scholar]
- 11.Bristow MR, Saxon LA, Boehmer J, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–50. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 12.Nelson GS, Curry CW, Wyman BT, et al. Predictors of systolic augmentation from left ventricular preexcitation in patients with dilated cardiomyopathy and intraventricular conduction delay. Circulation. 2000;101:2703–9. doi: 10.1161/01.cir.101.23.2703. [DOI] [PubMed] [Google Scholar]
- 13.Das M, Khan B, Jacob S, et al. Significance of a fragmented QRS complex versus a Q wave in patients with coronary artery disease. Circulation. 2006;113:2495–501. doi: 10.1161/CIRCULATIONAHA.105.595892. [DOI] [PubMed] [Google Scholar]
- 14.Das M, Saha C, El Masry H, et al. Fragmented QRS on a 12-lead ECG: A predictor of mortality and cardiac events in patients with coronary artery disease. Heart Rhythm. 2007;4:1385–92. doi: 10.1016/j.hrthm.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 15.Pietrasik G, Goldenberg I, Zdzienicka J, et al. Prognostic significance of fragmented QRS complex for predicting the risk of recurrent cardiac events in patients with Q-wave myocardial infarction. Am J Cardiol. 2007;100:583–6. doi: 10.1016/j.amjcard.2007.03.063. [DOI] [PubMed] [Google Scholar]
- 16.Michael M, El Masry H, Khan B, et al. Electrocardiographic signs of remote myocardial infarction. Prog Cardiovasc Dis. 2007;50:198–208. doi: 10.1016/j.pcad.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 17.El-Sherif N. The rsR′ pattern in left surface leads in ventricular aneurysm. Br Heart J. 1970;32:440–8. doi: 10.1136/hrt.32.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reddy CV, Cheriparambill K, Saul B, et al. Fragmented left sided QRS in absence of bundle branch block: Sign of left ventricular aneurysm. Ann Noninvasive Electrocardiol. 2006;11:132–8. doi: 10.1111/j.1542-474X.2006.00094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sahn DJ, DeMaria A, Kisslo J, et al. Recommendations regarding quantitation in M-mode echocardiography: Results of a survey of echocardiographic measurements. Circulation. 1978;58:1072–83. doi: 10.1161/01.cir.58.6.1072. [DOI] [PubMed] [Google Scholar]
- 20.Bargiggia GS, Bertucci C, Recusani F, et al. A new method for estimating left ventricular dP/dt by continuous wave Doppler-echocardiography: Validation studies at cardiac catheterization. Circulation. 1989;80:1287–92. doi: 10.1161/01.cir.80.5.1287. [DOI] [PubMed] [Google Scholar]
- 21.Shiota T, Jones M, Teien DE, et al. Evaluation of mitral regurgitation using a digitally determined colour Doppler flow convergence ‘centerline’ acceleration method: Studies in an animal model with quantified mitral regurgitation. Circulation. 1994;89:2879–86. doi: 10.1161/01.cir.89.6.2879. [DOI] [PubMed] [Google Scholar]
- 22.Miyatake K, Yamagishi M, Tanaka N, et al. New method for evaluating left ventricular wall motion by color-coded tissue Doppler imaging: In vitro and in vivo studies. J Am Coll Cardiol. 1995;25:717–24. doi: 10.1016/0735-1097(94)00421-L. [DOI] [PubMed] [Google Scholar]
- 23.Gorcsan J, Strum DP, Mandarino WA, et al. Quantitative assessment of alterations in regional left ventricular contractility with color-coded tissue Doppler echocardiography. Comparison with sonomicrometry and pressure-volume relations. Circulation. 1997;95:2423–33. doi: 10.1161/01.cir.95.10.2423. [DOI] [PubMed] [Google Scholar]
- 24.Yu CM, Chau E, Sanderson JE, et al. Tissue Doppler echocardiographic evidence of reverse remodeling and improved synchronicity by simultaneously delaying regional contraction after biventricular pacing therapy in heart failure. Circulation. 2002;105:438–45. doi: 10.1161/hc0402.102623. [DOI] [PubMed] [Google Scholar]
- 25.Bleeker GB, Schalij MJ, Molhoek SG, et al. Relationship between QRS duration and left ventricular dyssynchrony in patients with end-stage heart failure. J Cardiovasc Electrophysiol. 2004;15:544–9. doi: 10.1046/j.1540-8167.2004.03604.x. [DOI] [PubMed] [Google Scholar]
- 26.Bader H, Garrigue S, Lafitte S, et al. Intra-left ventricular electromechanical asynchrony. J Am Coll Cardiol. 2004;43:248–56. doi: 10.1016/j.jacc.2003.08.038. [DOI] [PubMed] [Google Scholar]
- 27.Gorcsan J, Abraham T, Agler DA, et al. Echocardiography for cardiac resynchronization therapy: Recommendations for Performance and Reporting – A Report from the American Society of Echocardiography Dyssynchrony Writing Group Endorsed by the Heart Rhythm Society. J Am Soc Echocardiogr. 2008;21:191–213. doi: 10.1016/j.echo.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Flowers NC, Horan LG, Thomas JR, et al. The anatomic basis for high-frequency components in the electrocardiogram. Circulation. 1969;39:531–9. doi: 10.1161/01.cir.39.4.531. [DOI] [PubMed] [Google Scholar]
- 29.Lesh MD, Spear JF, Simson MB. A computer model of the electrogram: What causes fractionation? J Electrocardiol. 1988;21(Suppl):S69–73. doi: 10.1016/0022-0736(88)90061-1. [DOI] [PubMed] [Google Scholar]
- 30.Gardner PI, Ursell PC, Fenoglio JJ, Jr, et al. Electrophysiologic and anatomic basis for fractionated electrograms recorded from healed myocardial infarcts. Circulation. 1985;72:596–611. doi: 10.1161/01.cir.72.3.596. [DOI] [PubMed] [Google Scholar]
- 31.Friedman PL, Fenoglio JJ, Wit AL. Time course for reversal of electrophysiological and ultrastructural abnormalities in subendocardial Purkinje fibers surviving extensive myocardial infarction in dogs. Circ Res. 1975;36:127–44. doi: 10.1161/01.res.36.1.127. [DOI] [PubMed] [Google Scholar]
- 32.Timonen P, Magga J, Risteli J, Punnonen K, et al. Cytokines, interstitial collagen and ventricular remodelling in dilated cardiomyopathy. Int J Cardiol. 2008;124:293–300. doi: 10.1016/j.ijcard.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 33.Kawano H, Tsuneto A, Koide Y, et al. Magnetic resonance imaging in a patient with peripartum cardiomyopathy. Intern Med. 2008;47:97–102. doi: 10.2169/internalmedicine.47.0316. [DOI] [PubMed] [Google Scholar]
- 34.Marijianowski MM, Teeling P, Mann J, et al. Dilated cardiomyopathy is associated with an increase in the type I/type III collagen ratio: A quantitative assessment. J Am Coll Cardiol. 1995;25:1263–72. doi: 10.1016/0735-1097(94)00557-7. [DOI] [PubMed] [Google Scholar]
- 35.Gunja-Smith Z, Morales AR, Romanelli R, et al. Remodeling of human myocardial collagen in idiopathic dilated cardiomyopathy. Role of metalloproteinases and pyridinoline crosslinks. Am J Pathol. 1996;1448:1639–48. [PMC free article] [PubMed] [Google Scholar]
- 36.Beshai JF, Grimm RA, Nagueh SF, et al. Cardiac-resynchronization therapy in heart failure with narrow QRS complexes. N Engl J Med. 2007;357:2461–71. doi: 10.1056/NEJMoa0706695. [DOI] [PubMed] [Google Scholar]