Abstract
BACKGROUND:
Heart failure (HF) clinics are known to improve outcomes of patients with HF. Studies have been limited to single, usually tertiary centres whose experience may not apply to the general HF population.
OBJECTIVES:
To determine the effectiveness of HF clinics in reducing death or all-cause rehospitalization in a real-world population.
METHODS:
A retrospective analysis of the Improving Cardiovascular Outcomes in Nova Scotia (ICONS) disease registry was performed. All 8731 patients with a diagnosis of HF (844 managed in HF clinics) who were discharged from the hospital between October 15, 1997, and July 1, 2000, were identified. Patients enrolled in any one of four HF clinics (two community-based and two academic-based) were compared with those who were not. The primary outcome was the one-year combined hospitalization and mortality.
RESULTS:
Patients followed in HF clinics were younger (68 versus 75 years), more likely to be men (63% versus 48%), and had a lower ejection fraction (35% versus 44%), lower systolic blood pressure (137 mmHg verus 146 mmHg) and lower serum creatinine (121 μmol/L versus 130 μmol/L). There was no difference in the prevalence of hypertension (56%), diabetes (35%) or stroke/transient ischemic attack (16%). The one-year mortality rate was 23%, while 31% of patients were rehospitalized; the combined end point was 51%. Enrollment in an HF clinic was independently associated with reduced risk of total mortality (hazard ratio [HR] 0.69 [95% CI 0.51 to 0.90], P=0.008; number needed to treat for one year to prevent the occurrence of one event [NNT]=16), all-cause hospital readmission (HR 0.27 [95% CI 0.21 to 0.36], P<0.0001; NNT=4), and combined mortality or hospital readmission (HR 0.73 [95% CI 0.60 to 0.89], P<0.0015; NNT=5).
DISCUSSION:
HF clinics are associated with reductions in rehospitalization and mortality in an unselected HF population, independent of whether they are academic- or community-based. Such clinics should be made widely available to the HF population.
Keywords: Disease management, Heart failure, Morbidity, Mortality, Outcomes
Abstract
HISTORIQUE :
On sait que les cliniques d’insuffisance cardiaque (IC) améliorent l’issue des patients ayant une IC. Les études se limitent à des centres uniques, généralement de soins tertiaires, dont l’expérience ne s’applique peut-être pas à la population générale ayant une IC.
OBJECTIFS :
Déterminer l’efficacité des cliniques d’IC à réduire les décès ou les réhospitalisations toutes causes confondues dans une population réelle.
MÉTHODOLOGIE :
Les auteurs ont effectué une analyse rétrospective du registre ICONS sur l’amélioration des issues cardiovasculaires en Nouvelle-Écosse. On a repéré les 8 731 patients ayant un diagnostic d’IC (844 pris en charge dans une clinique d’IC) qui avaient obtenu leur congé de l’hôpital entre le 15 octobre 1997 et le 1er juillet 2000. Ils ont comparé les patients inscrits dans l’une des quatre cliniques d’IC (deux en milieu communautaire et deux en milieu universitaire) à ceux qui ne l’étaient pas. L’issue primaire était l’hospitalisation et la mortalité combinées au bout d’un an.
RÉSULTATS :
Les patients suivis dans une clinique d’IC étaient plus jeunes (68 ans par rapport à 75), plus susceptibles d’être des hommes (63 % par rapport à 48 %), ils avaient une fraction d’éjection plus faible (35 % par rapport à 44 %) une tension artérielle systolique plus faible (137 mmHg par rapport à 146 mmHg) et une créatinine sérique plus faible (121 μmol/L par rapport à 130 μmol/L). On n’observait aucune différence dans la prévalence d’hypertension (56 %), de diabète (35 %) ou d’accident vasculaire cérébral ou d’attaque ischémique transitoire (16 %). Le taux de mortalité au bout d’un an était de 23 %, tandis que 31 % des patients étaient réhospitalisés : le paramètre ultime combiné était de 51 %. La participation à une clinique d’IC s’associait de manière indépendante à une diminution du risque de mortalité totale (ratio de risque [RR] 0,69 [95 % IC 0,51 à 0,90], P=0,008; nombre nécessaire à traiter pendant un an pour éviter l’occurrence d’un événement [NNT]=16), réadmissions hospitalières toutes causes confondues (IC 0,27 [95 % IC 0,21 à 0,36], P<0,0001; NNT=4) et mortalité ou réhospitalisation combinées (IC 0,73 [95 % IC 0,60 à 0,89], P<0,0015; NNT=5).
EXPOSÉ :
Les cliniques d’IC s’associent à des réductions des réhospitalisations et de la mortalité dans une population ayant une IC non sélectionnée, qu’elles soient communautaires ou universitaires. Il faudrait rendre ces cliniques largement accessibles à la population ayant une IC.
Heart failure (HF) accounts for more hospital admissions of North American patients older than 65 years of age than any other condition (1). This common, lethal disorder confers a large burden on the health care system and may account for as much as $22 billion in direct costs per year in North America (2,3). The prevalence and disease burden of HF are expected to continue at this rate, at least until the year 2025 (4).
While many therapeutic advances in HF treatment, such as angiotensin-converting enzyme (ACE) inhibition, have translated into improved population outcomes (5,6), others – such as the use of spironolactone – have not (7). This is due, in part, to suboptimal use of effective therapies (8), an aging population, complex patient profiles and inadequate follow-up and support of patients with HF (9,10). One powerful intervention shown to improve outcomes is the HF clinic. Randomized trials (11–15) have reported the effectiveness of HF clinics, with reductions in morbidity and mortality, and cost-effectiveness in the management of HF. However, many of these studies have suffered from systematic bias because only individuals able or willing to participate were enrolled (16,17). Because recent reports demonstrated significant and pervasive differences in characteristics and outcomes between individuals who enroll in randomized studies and those who do not, one could hypothesize that widespread implementation of HF clinics may not confer the same benefits to a population of HF patients, as shown in reported studies (16,18). One method to help clarify the real-world impact of an intervention is to measure patient outcomes in a defined health care system, while correcting for baseline differences between those who received the intervention and those who did not. Although not randomized, such a study would help to define the potential global impact of the intervention – in this case, the HF clinic – on the HF population at large.
The Improving Cardiovascular Outcomes in Nova Scotia (ICONS) study (19) began in October 1997 and disease surveillance continues. All consecutive patients admitted to a Nova Scotia adult care hospital with an acute cardiac condition are automatically identified and followed. Nova Scotia has a closed health care system with universal hospital and specialized clinic access, thereby limiting an ability-to-pay bias. In the present study, detailed clinical, demographic and process of care data were collected. This, in combination with outcomes measurement, gives this project the ability to develop a detailed impact analysis of a defined intervention, such as an HF clinic. We hypothesized that the introduction of HF clinics in Nova Scotia would result in fewer hospital admissions for patients with HF, and would possibly have an impact on mortality rates.
METHODS
Study design and patient recruitment
As described in detail previously (10,19), ICONS was conceived as a large prospective cohort study exploring the effectiveness of a disease management approach in patients with HF, acute coronary syndrome or atrial fibrillation. The study protocol was approved by the Ethics Review Board of the Queen Elizabeth II Health Sciences Centre (Halifax, Nova Scotia) and other participating institutions across the province. In April 2002, the study concluded but the responsibility for ongoing data collection was assumed by the Nova Scotia Department of Health and continues for patients hospitalized with HF or acute coronary syndrome.
Cases contained in the ICONS registry are identified using daily patient lists obtained at all provincial institutions that provide adult medical care. Only the index HF admissions for patients eligible for the study were identified. No washout period was enforced. Detailed clinical information on all patients admitted to Nova Scotia hospitals with a clinical diagnosis of HF (ie, International Statistical Classification of Diseases and Related Health Problems, Ninth Revision code 428x) was collected by trained study abstractors and entered into the ICONS registry. Repeat hospitalizations were identified through ongoing surveillance of hospitalization, while deaths were ascertained through linkage to the provincial vital statistics registry. To determine the one-year repeat hospitalization and/or mortality rate, each unique patient was followed for at least 365 days following their index hospital discharge. This ensured comparable follow-up for the entire HF population. Patient accrual for the present study began on October 15, 1997 and concluded on December 31, 2002 to allow for 365 days of follow-up. The primary end point – combined all-cause mortality and hospitalization – was censored at the one-year follow-up. Secondary outcomes included the one-year total mortality and all-cause hospital readmission rate.
From July 1998 to January 2001, HF clinics (HF clinic group) were established in four geographically distinct locations within the province. Two of the four clinics were located within a large teaching hospital. The other two were within hospitals 96.6 km and 563.3 km away from any tertiary care institution. Patients were accepted by referral if there was a previous hospitalization for HF within the past three months. The structure of these clinics was very similar and consisted of a nurse specialist working in collaboration with three to six physician specialists with experience in the management of HF. In addition to assessment and provision of evidence-based therapies, patients and their caregivers underwent detailed and repeated educational sessions in which information relating to the diagnosis and causes of HF, an explanation of their treatment regimen, causes of decompensation, dietary advice and suggestions for actions should evidence of early decompensation occur were provided. Patients tended to be seen frequently (every one to two weeks) until clinical stabilization and successful negotiation of educational sessions, and then every one to three months. Patients were given a telephone number to call and clinics were conducted five times weekly; there was no weekend or after-hours coverage. Follow-up was determined on an individual basis; when evidence of clinical stability existed for longer than six months, patients could be discharged from the clinic and referred back to their family physician and/or referring specialist. Adverse outcomes that occurred in patients removed from active follow-up were attributed to the HF clinic group by an analogy to the intention-to-treat principle. No patient was counted twice within or between clinics. Individuals were included in the present study if they were a resident in the province and were admitted to a Nova Scotia hospital with a primary diagnosis of HF during the study period (HF population group). They were further included in the HF clinic group if they attended an HF clinic at least once during the study period.
Statistical analysis
The primary outcome was a composite of total mortality and hospitalization for any cause within 365 days following enrollment. Thus, any rehospitalization event that occurred before the first clinic visit was not included in the analysis of either the HF clinic or nonclinic groups. Secondary outcomes included the separate outcomes of mortality and hospitalization.
Descriptive statistics were calculated for each group (HF clinic versus the overall HF population) and raw outcomes reported. Medication usage at hospital discharge was reported but data regarding subsequent changes to medication were not available. A univariate regression analysis was performed using all 55 variables for the primary outcome (Appendix). No variable was forced into the analysis. Any variable that was predictive at a significance level of P<0.10 was included in a subsequent multiple stepwise logistic regression analysis in which the model of best fit was reported. Cox proportional hazard ratios (HRs) for total mortality, total repeat hospitalization and the composite of mortality or hospitalization (with 95% CIs) were calculated. This process was repeated while excluding patients who were not taking ACE inhibitors, and was repeated with other variables. The number needed to treat for one year to prevent the occurrence of one event (NNT) was based on the adjusted HR for outcomes compared with the overall population adverse event rates. An α<0.05 was used to determine statistical significance. A test for heterogeneity was performed to determine whether the results were influenced by any of the clinics. A cohort of 100 HF clinic patients older than 65 years of age was also extracted and compared (for occurrence of the primary end point) with 400 nonclinic patients matched for age, sex, renal function, hemoglobin and ACE inhibitor use at baseline.
RESULTS
During the study, 8731 patients were identified; 7741 in the nonclinic group and 990 in the HF clinic group (844 followed for one year). The four HF clinics each followed between 37 and 504 patients. The average number of patients followed in the HF clinic group increased from 44 in 1998 to 938 in 2002 (Table 1). The total number of patient visits was recorded in only one clinic, which showed a mean (± SD) of 17±8 clinic visits per patient during a one-year follow-up period. The number of non-clinic visits was not available for either group.
TABLE 1.
Yearly patient enrollment in the heart failure (HF) clinic group
|
Year |
|||||
|---|---|---|---|---|---|
| 1998 | 1999 | 2000 | 2001 | 2002 | |
| New patients* | 44 | 96 | 240 | 272 | 286 |
| Active patients† | 42 | 127 | 323 | 553 | 715 |
| Total patients‡ | 44 | 139 | 364 | 613 | 938 |
Patients seen in an HF clinic for ≥1 visit;
Patients alive and followed as of December 31 of each year;
All patients with ≥1 visit to an HF clinic
The baseline characteristics of both groups are provided in Table 2 and indicate several differences. The patients in the HF clinic group were younger, more likely to be men and had higher body mass, plasma hemoglobin and rates of previous myocardial infarction. They also demonstrated a lower ejection fraction (EF), serum creatinine and blood pressure at study entry, and had a decreased likelihood of receiving ACE inhibitor therapy at hospital discharge. There was no difference in baseline previous New York Heart Association functional class (median 3). Data regarding patient no-show or request for discontinuation of HF clinic care were available from the largest HF clinic. Of 504 patients, three (0.5%) did not show up for the first or subsequent visit and three (0.5%) requested discontinuation of HF clinic care.
TABLE 2.
Selected baseline characteristics of the heart failure clinic care versus usual care groups
| Characteristic | Clinic (n=984) | Usual care (n=7741) | P |
|---|---|---|---|
| Median age, years | 68±13 | 75±12 | <0.0001 |
| Female sex, n (%) | 364 (37) | 5121 (52) | <0.0001 |
| Income below poverty line ($20,000 annual income), n (%) | 344 (35) | (39) | 0.001 |
| History of MI, n (%) | 364 (37) | 2400 (31) | <0.0001 |
| History of diabetes, n (%) | 335 (34) | 2709 (35) | 0.51 |
| History of stroke/TIA, n (%) | 148 (15) | 1238 (16) | 0.10 |
| History of hypertension, n (%) | 561 (57) | 4335 (56) | 0.83 |
| Weight, kg | 81±21 | 78±21 | <0.0001 |
| Median heart rate, beats/min | 90±19 | 92±21 | 0.006 |
| Median BP, mmHg | 137/79 | 146/82 | <0.0001 |
| Median creatinine, μmol/L | 116±55 | 130±78 | <0.0001 |
| Median hemoglobin, g/L | 130±20 | 127±20 | <0.0001 |
| Median LDL, mmol/L | 2.7±1.0 | 2.7±1.0 | 0.19 |
| Median EF*, % | 35±17 | 44±16 | <0.0001 |
| Discharge ACE inhibitor, n (%) | 600 (61) | 5032 (65) | <0.0001 |
| Discharge beta-blocker, n (%) | 528 (53.7) | 4211 (54.4) | <0.0001 |
| Discharge ARB, n (%) | 53 (5.3) | 464 (6.0) | 0.07 |
| Discharge spironolactone, n (%) | 108 (11) | 619 (8) | <0.001 |
| Discharge calcium blocker, n (%) | 157 (16) | 2090 (27) | <0.0001 |
| EF measured†, %, n (%) | 817 (83) | 4180 (54) | <0.0001 |
| Cardiac catheterization any time within 180 days‡, n (%) | 502 (51) | 1626 (21) | <0.0001 |
Data presented as mean ± SD unless otherwise indicated. Variables documented at index hospital admission unless otherwise indicated.
Ejection fraction (EF) measured by radionuclide angiography, echocardiography or cardiac catheterization. Last measurement included if more than one test performed;
EF documented within 60 days of index hospitalization;
Cardiac catheterization within 6 months of index hospitalization. ACE Angiotensin-converting enzyme; ARB Angiotensin receptor blocker; BP Blood pressure; LDL Low-density lipoprotein; MI Myocardial infarction; TIA Transient ischemic attack
An analysis of unadjusted outcomes showed that the HF clinic group had a significant reduction in one-year mortality (11% versus 24%, P<0.0001), all-cause hospital readmission (24% versus 33%, P<0.0001) or the combination of total mortality and all-cause hospital readmission (31% versus 55%, P<0.00015). This included 4% of HF clinic patients who experienced hospitalization and 1% who died while waiting to be seen in one of the HF clinics. After correction for all covariates, except performance of cardiac catheterization or measurement of EF (it was believed that these variables would have been affected by clinic care), there was still a significant reduction in rehospitalization (Table 3). The test for heterogeneity for occurrence of the primary end point between clinics was nonsignificant (P=0.09), and was driven by an extremely low event rate in the smallest clinic (of 37 patients, there was only one hospitalization). With this clinic excluded, the P-value for heterogeneity was P=0.55, although the overall HR was not affected. The adjusted NNT for treatment for one year in an HF clinic to avoid one hospitalization or death (the primary outcome) was 5. The corresponding NNT for hospitalization was 4 and for total mortality, the NNT was 16. Survival curves for freedom from all-cause death and death/hospitalization between the HF clinic and nonclinic groups are shown in Figures 1 and 2. Other important multivariate predictors of mortality and hospitalization are listed in Table 4. The logistic regression was repeated for the primary end point including only patients receiving ACE inhibitors, yielding a similar hazard reduction of outcomes in association with HF clinic care. This analysis was also performed for several other variables for which there was a difference between the HF clinic and non-clinic groups (such as EF, blood pressure, age, etc), all with similar results. This analysis was also performed to exclude EF because there were many missing values for this variable; results similar to those described above were obtained. Finally, 100 HF clinic patients older than 65 years of age were case-matched with 400 nonclinic patients of the same age, sex and renal function (there was no significant difference in hemoglobin). There was a significant difference in the combined primary end point of death or hospitalization at one year (35% versus 58%, P<0.001).
TABLE 3.
Adjusted one-year outcomes for heart failure clinic care versus usual care patients
| 1-year outcome | Hazard ratio | 95% CI | NNT | P |
|---|---|---|---|---|
| Mortality | 0.69 | 0.51–0.90 | 16 | 0.008 |
| Readmission | 0.27 | 0.21–0.36 | 4 | <0.0001 |
| Mortality/readmission | 0.73 | 0.60–0.89 | 5 | 0.0015 |
NNT Number needed to treat for one year to prevent one event in the target population
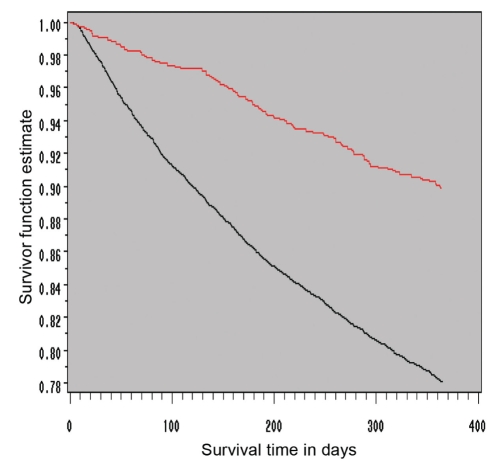
Figure 1).
Kaplan-Meier survival function estimate of one-year total mortality in heart failure clinic care (red line) versus usual care (black line) groups. Adjusted survival was significantly better for those who underwent care in a heart failure clinic than for those followed by usual care
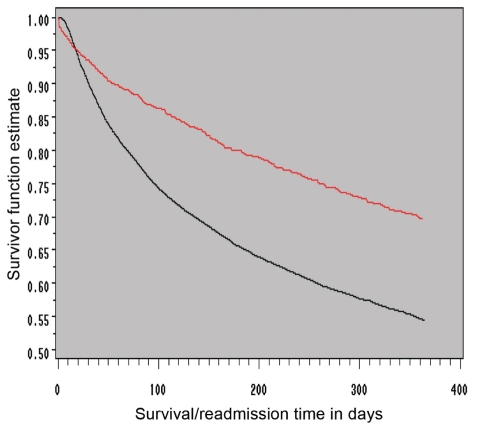
Figure 2).
Kaplan-Meier survival function estimate of one-year total mortality or all-cause hospitalization in heart failure clinic care (red line) versus usual care (black line) groups. Adjusted freedom from mortality or all-cause rehospitalization was significantly better for those who underwent care in a heart failure clinic than for those followed by usual care
TABLE 4.
Independent predictors of one-year mortality and hospitalization
| Variable | Hazard ratio | 95% CI | P |
|---|---|---|---|
| HF clinic group (versus usual care) | 0.73 | 0.60–0.89 | 0.0017 |
| Age (per 10-year increase) | 1.14 | 1.09–1.20 | <0.0001 |
| Sex (male versus female) | 1.21 | 1.06–1.37 | <0.0001 |
| History of diabetes | 1.41 | 1025–1.59 | <0.0001 |
| Previous myocardial infarction | 1.34 | 1.18–1.51 | <0.0001 |
| Weight (per 10 kg increase) | 0.994 | 0.991–0.997 | 0.0001 |
| Hemoglobin (per 100 g/L increase) | 0.95 | 0.92–0.98 | 0.0009 |
| Serum creatinine (per 10 μmol increase) | 1.01 | 1.00–1.02 | 0.002 |
| Systolic pressure (per 10 mmHg increase) | 0.91 | 0.85–0.95 | 0.002 |
| Ejection fraction (per 1% increase) | 0.96 | 0.93–0.98 | 0.02 |
HF Heart failure
Follow-up data on medication usage were unavailable in three of the HF clinics and in the nonclinic group. However, in the largest clinic, the one-year ACE inhibitor usage rate (by direct questioning) was 85%, and was similarly high for other medications (angiotensin receptor blockers 12%; ACE/angiotensin receptor blockers 95%; beta-blockers 85%; loop diuretic 96%; digoxin 60%; statin 45%; acetylsalicylic acid 65%; warfarin 32%; and calcium channel blockers 19%). These data attest to the high level of compliance to a complicated medical regimen that can be achieved in a disease management program such as an HF clinic.
DISCUSSION
Hospitalization for HF represents the most costly direct health care expense in North America and accounts for more than 80% of overall HF care expenditures (1,3,20,21). Our data show that implementation of a multidisciplinary HF clinic is associated with a significant reduction of hospitalization and mortality in a real-world setting of HF care. We calculated that for every five patients followed over the course of one year in an HF clinic, one less death or rehospitalization occurred. Given the very high number of adverse outcomes in this population, tremendous potential exists for a reduction of adverse health outcomes and system costs with more widespread implementation from the present 22% penetrance of HF clinic care in Nova Scotia.
Several meta-analyses of randomized trials of HF clinics have now been published that report reductions of mortality between 20% and 30%, and of repeat hospitalization between 25% and 70% (11,12,15,22). Follow-up in these studies has ranged from six months to more than four years. It is important to note that there does not seem to be an attenuation of the effects of HF clinic care over time (15). Thus, at least in the setting of randomized clinical trials, HF clinics are efficacious.
Despite the strengths of randomized trials, limitations do exist. Patients who participate in such studies are different from those in the general population. These differences exist across most variables, including age, demographics and clinical characteristics, and tend to limit the applicability of the results of randomized clinical trials to everyday practice. Evidence of efficacy does not necessarily translate into widespread effectiveness. This is best illustrated by the example of spironolactone, which was associated with a reduction in mortality in the Randomized Aldactone Evaluation Study (RALES) (7,23,24); however, no benefit was observed (there was even possible harm) when viewed from a global health care system perspective. Many reasons exist for such discordant observations between the clinical trial and ‘real-world’ populations, such as inappropriate application of the intervention in question (23,25).
Therefore, it is helpful to evaluate the overall impact of an intervention in a self-contained health care population. The ICONS registry is unique because it captures all consecutive HF hospitalizations and subsequent mortality data across an entire health care system. It offers the advantages of both a clinical study in terms of having detailed data capture (nearly 250 variables collected), and population studies through its capture of a complete and relatively unbiased real-world population.
Limitations
The data analysis was retrospective, even though the data itself was captured prospectively. Thus, other unmeasured variables may have impacted the results. Patient allocation to HF clinic care was not randomized; the possibility of referral bias may have affected the results. Additionally, it is possible that only patients who were able to visit HF clinics or those believed to benefit from increased testing would have been referred. Alternatively, patients in the nonclinic group may have been less amenable to such testing for a variety of reasons, such as patient refusal or inaccessibility to the HF clinic or testing facility. The population undergoing invasive assessment was also very unlikely to subsequently undergo an invasive procedure (6% of those catheterized). It should be noted that in a subgroup of patients followed at the largest clinic, the attendance of those referred was 98% of total visits, suggesting patients were unlikely to self-select away from this intervention. Moreover, it is clear that the two groups were different, as evidenced in several baseline characteristics, although many of the differences were not large in magnitude. The HF clinic group was younger, had a greater body weight, higher hemoglobin levels, and a slightly lower serum creatinine or likelihood of cerebrovascular disease, predisposing toward a better outcome. The nonclinic group had a higher mean EF and systolic blood pressure, factors that are associated with a better outcome. Both groups contained a large (but slightly different) percentage of patients living below the poverty line (35% for the HF clinic group versus 39% for the nonclinic group) or living more than 48.3 km from the clinic (41% versus 45%, respectively). The use of propensity analysis was advocated by some to better attenuate the effects of unmeasured bias. However, this remains largely theoretical, with one systematic review (26) suggesting only minor differences between the two methods for statistical control of confounders. Finally, one of the four clinics treated a population nearly identical to the nonclinic population in terms of age, baseline medication usage, number of comorbid conditions, blood pressure and renal function, with significant reduction in the primary end point (HR 0.69; 95% CI 0.44 to 0.86). While the effect of unmeasured variables such as referral bias cannot be fully controlled, when the above-mentioned additional analyses are combined, a true association is suggested.
We did not measure quality of care following hospital discharge other than cardiac catheterization and noninvasive measurement of EF. We did not account for specialist versus primary care physician involvement in our model. This is known to affect the quality of care afforded to patients with HF (8,27–29). For instance, a patient living in an isolated community or who did not have access to an HF clinic may have been at a general disadvantage to receive any type of care. Proximity to a single tertiary care hospital has been reported to be directly related to access to cardiac catheterization and coronary revascularization following myocardial infarction (30). The same may be true of access to advanced cardiac testing in patients with HF. In our study, neither income nor proximity to the HF clinic was a significant predictor of outcome. Hopefully, studies of alternative or additional disease management will shed light on this geographically disadvantaged population. Because all four clinics adhered to a similar practice philosophy and reached similar outcomes, we cannot determine which characteristics of our clinics were associated with the most benefit, although we were able to demonstate extremely high medication adherence in patients who attended the largest clinic. This issue will require further study. Similarly, longer-term follow-up of these cohorts, with cost data included, is ongoing and will provide a more complete understanding of the benefits of HF clinic care in a population.
CONCLUSION
Treatment of HF in HF clinics was associated with reduction in one-year morbidity and mortality. Strategies to widely implement this treatment model for HF should be instituted. Future research should focus on comparison of the relative benefits of different types of disease management programs in this population. In particular, alternative methods to deliver the HF clinic model of care to those without access to care should be researched.
Acknowledgments
Dr Cox receives salary support from a Canadian Institutes of Health Research/Regional Partnership Program Investigator Award and through a Clinical Research Scholarship from the Faculty of Medicine, Dalhousie University (Halifax, Nova Scotia). The Queen Elizabeth II Heart Function Clinic is supported by an unrestricted grant from AstraZeneca Canada Inc. The ICONS study was supported by a nondirected educational grant from Merck Frosst Canada Inc, and through in-kind support from the Queen Elizabeth II Health Sciences Centre and the Nova Scotia Department of Health. The authors acknowledge the work of the ICONS staff and Steering Committee, and thank Drs Simon Jackson, Catherine Kells, Miroslaw Rajda, Christiansen Koilpillai, Paul MacDonald and Bruce McClelland, as well as Ms Marian Malloy and Ms Heather Merry for their invaluable assistance in conducting the present study.
APPENDIX
Variables (n=55) used in analysis
|
Demographic and outcome variables (12 variables) |
| Length of index hospital stay |
| Subsequent hospitalization (and date)? |
| Number of days in hospital |
| Death (and date) |
| Age in years |
| Sex |
| Distance from tertiary care facility >30 min drive |
| Household annual income <$20,000.00 |
| Lives alone |
| Heart failure clinic enrollment? |
| Documentation of ejection fraction within 60 days (before or after) of index admission? (yes/no/measured but no ejection fraction available) |
| Ejection fraction numerical value (if available) |
|
Medical history (16 variables) |
| Hypertension |
| Dyslipidemia |
| Congestive heart failure |
| Myocardial infarction |
| Previous percutaneous transluminal coronary angioplasty |
| Previous coronary artery bypass grafting |
| Diabetes |
| Peripheral vascular disease |
| Stroke or transient ischemic attack |
| Renal insufficiency |
| Chronic obstructive pulmonary disease |
| Malignancy |
| Dementia |
| History of alcohol abuse (ever versus none) |
| History of smoking (never, previous, current) |
| Number of comorbid illnesses |
|
Index admission (11 variables) |
| Heart rate |
| Systolic blood pressure |
| Diastolic blood pressure |
| Mean arterial pressure |
| Blood glucose |
| Serum creatinine |
| Serum troponin |
| Serum sodium |
| Serum potassium |
| Plasma hemoglobin |
| Total white blood cell count |
|
Treatment at index hospital admission (1 variable) |
| Cardiac catheterization performed? |
|
Treatment at index hospital discharge (15 variables) |
| Calcium channel blocker |
| Beta-blocker |
| Angiotensin-converting enzyme inhibitor |
| Angiotensin II receptor blocker |
| Loop diuretic |
| Spironolactone |
| Warfarin |
| Acetylsalicylic acid |
| Clopidogrel |
| Statin |
| Digoxin |
| Insulin |
| Metformin |
| Nonsteroidal anti-inflammatory drug excluding cyclo-oxygenase-2 inhibitors |
| Cyclo-oxygenase-2 inhibitors |
REFERENCES
- 1.<http://circ.ahajournals.org/cgi/content/full/119/2/e21> (Version current at July 16, 2009).
- 2.Hunt SA, Baker DW, Chin MH, et al. ACC/AHA Guidelines for the Evaluation and Management of Chronic Heart Failure in the Adult: Executive Summary A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1995 Guidelines for the Evaluation and Management of Heart Failure): Developed in Collaboration With the International Society for Heart and Lung Transplantation; Endorsed by the Heart Failure Society of America. Circulation. 2001;104:2996–3007. doi: 10.1161/hc4901.102568. [DOI] [PubMed] [Google Scholar]
- 3.Funk M, Krumholz HM. Epidemiologic and economic impact of advanced heart failure. J Cardiovasc Nurs. 1996;10:1–10. doi: 10.1097/00005082-199601000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Johansen H, Strauss B, Arnold JM, Moe G, Liu P. On the rise: The current and projected future burden of congestive heart failure hospitalization in Canada. Can J Cardiol. 2003;19:430–5. [PubMed] [Google Scholar]
- 5.Schaufelberger M, Swedberg K, Koster M, Rosen M, Rosengren A. Decreasing one-year mortality and hospitalization rates for heart failure in Sweden; data from the Swedish Hospital Discharge Registry 1988 to 2000. Eur Heart J. 2004;25:300–7. doi: 10.1016/j.ehj.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 6.Lee DS, Mamdani MM, Austin PC, et al. Trends in heart failure outcomes and pharmacotherapy: 1992 to 2000. Am J Med. 2004;116:581–9. doi: 10.1016/j.amjmed.2003.11.025. [DOI] [PubMed] [Google Scholar]
- 7.Juurlink DN, Mamdani MM, Lee DS, et al. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N Engl J Med. 2004;351:543–51. doi: 10.1056/NEJMoa040135. [DOI] [PubMed] [Google Scholar]
- 8.Edep ME, Shah NB, Tateo IM, Massie BM. Differences between primary care physicians and cardiologists in management of congestive heart failure: Relation to practice guidelines. J Am Coll Cardiol. 1997;30:518–26. doi: 10.1016/s0735-1097(97)00176-9. [DOI] [PubMed] [Google Scholar]
- 9.Krumholz HM, Chen YT, Wang Y, Vaccarino V, Radford MJ, Horwitz RI. Predictors of readmission among elderly survivors of admission with heart failure. Am Heart J. 2000;139:72–7. doi: 10.1016/s0002-8703(00)90311-9. [DOI] [PubMed] [Google Scholar]
- 10.Ghali JK, Kadakia S, Cooper R, Ferlinz J. Precipitating factors leading to decompensation of heart failure. Traits among urban blacks. Arch Intern Med. 1988;148:2013–6. [PubMed] [Google Scholar]
- 11.Gustafsson F, Arnold JM. Heart failure clinics and outpatient management: Review of the evidence and call for quality assurance. Eur Heart J. 2004;25:1596–604. doi: 10.1016/j.ehj.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 12.Heidenreich PA, Ruggerio CM, Massie BM. Effect of a home monitoring system on hospitalization and resource use for patients with heart failure. Am Heart J. 1999;138:633–40. doi: 10.1016/s0002-8703(99)70176-6. [DOI] [PubMed] [Google Scholar]
- 13.Krumholz HM, Amatruda J, Smith GL, et al. Randomized trial of an education and support intervention to prevent readmission of patients with heart failure. J Am Coll Cardiol. 2002;39:83–9. doi: 10.1016/s0735-1097(01)01699-0. [DOI] [PubMed] [Google Scholar]
- 14.Rich MW, Beckham V, Wittenberg C, Leven CL, Freedland KE, Carney RM. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. N Engl J Med. 1995;333:1190–5. doi: 10.1056/NEJM199511023331806. [DOI] [PubMed] [Google Scholar]
- 15.Stewart S, Horowitz JD. Home-based intervention in congestive heart failure: Long-term implications on readmission and survival. Circulation. 2002;105:2861–6. doi: 10.1161/01.cir.0000019067.99013.67. [DOI] [PubMed] [Google Scholar]
- 16.Cox JL, Zitner D, Courtney KD, et al. Undocumented patient information: An impediment to quality of care. Am J Med. 2003;114:211–6. doi: 10.1016/s0002-9343(02)01481-x. [DOI] [PubMed] [Google Scholar]
- 17.Gross CP, Mallory R, Heiat A, Krumholz HM. Reporting the recruitment process in clinical trials: Who are these patients and how did they get there? Ann Intern Med. 2002;137:10–6. doi: 10.7326/0003-4819-137-1-200207020-00007. [DOI] [PubMed] [Google Scholar]
- 18.Howlett JG, Johnstone DE, Sketris I, O’Reilly M, Horne GS, Cox JL. Identifying opportunities to address the congestive heart failure burden: The Improving Cardiovascular Outcomes in Nova Scotia (ICONS) study. Can J Cardiol. 2003;19:439–44. [PubMed] [Google Scholar]
- 19.Cox JL. Optimizing disease management at a health care system level: The rationale and methods of the improving cardiovascular outcomes in Nova Scotia (ICONS) study. Can J Cardiol. 1999;15:787–96. [PubMed] [Google Scholar]
- 20.Ghali JK, Cooper R, Ford E. Trends in hospitalization rates for heart failure in the United States, 1973–1986. Evidence for increasing population prevalence. Arch Intern Med. 1990;150:769–73. [PubMed] [Google Scholar]
- 21.Joshi AV, D’Souza AO, Madhavan SS. Differences in hospital length-of-stay, charges, and mortality in congestive heart failure patients. Congest Heart Fail. 2004;10:76–84. doi: 10.1111/j.1527-5299.2004.02008.x. [DOI] [PubMed] [Google Scholar]
- 22.Phillips CO, Wright SM, Kern DE, Singa RM, Shepperd S, Rubin HR. Comprehensive discharge planning with postdischarge support for older patients with congestive heart failure: A meta-analysis. JAMA. 2004;291:1358–67. doi: 10.1001/jama.291.11.1358. [DOI] [PubMed] [Google Scholar]
- 23.Bozkurt B, Agoston I, Knowlton AA. Complications of inappropriate use of spironolactone in heart failure: When an old medicine spirals out of new guidelines. J Am Coll Cardiol. 2003;41:211–4. doi: 10.1016/s0735-1097(02)02694-3. [DOI] [PubMed] [Google Scholar]
- 24.Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–17. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 25.Svensson M, Gustafsson F, Galatius S, Hildebrandt PR, Atar D. How prevalent is hyperkalemia and renal dysfunction during treatment with spironolactone in patients with congestive heart failure? J Card Fail. 2004;10:297–303. doi: 10.1016/j.cardfail.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 26.Shah BR, Laupacis A, Hux JE, Austin PC. Propensity score methods gave similar results to traditional regression modeling in observational studies: A systematic review. J Clin Epidemiol. 2005;58:550–9. doi: 10.1016/j.jclinepi.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 27.Ashton CM, Kuykendall DH, Johnson ML, Wray NP, Wu L. The association between the quality of inpatient care and early readmission. Ann Intern Med. 1995;122:415–21. doi: 10.7326/0003-4819-122-6-199503150-00003. [DOI] [PubMed] [Google Scholar]
- 28.Foody JM, Rathore SS, Wang Y, et al. Predictors of cardiologist care for older patients hospitalized for heart failure. Am Heart J. 2004;147:66–73. doi: 10.1016/j.ahj.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 29.Howlett JG, Cox JL, Haddad H, Stanley J, McDonald M, Johnstone DE. Physician specialty and quality of care for CHF: Different patients or different patterns of practice? Can J Cardiol. 2003;19:371–7. [PubMed] [Google Scholar]
- 30.Gregory PM, Malka ES, Kostis JB, Wilson AC, Arora JK, Rhoads GG. Impact of geographic proximity to cardiac revascularization services on service utilization. Med Care. 2000;38:45–57. doi: 10.1097/00005650-200001000-00006. [DOI] [PubMed] [Google Scholar]