Abstract
BACKGROUND:
Multidetector computed tomography (MDCT) has demonstrated promise in the noninvasive evaluation of coronary artery disease.
OBJECTIVE:
To systematically review the literature regarding the improved diagnostic accuracy of 64-slice MDCT.
METHODS:
An EMBASE, OVID, PubMed and Cochrane Library database search was performed using the key words ‘computed tomography’ matched with the terms ‘coronary artery’ or ‘coronary angiography’ to identify English-language articles examining MDCT cardiac imaging. Studies that compared 16-slice or 64-slice MDCT with catheter-based coronary angiography for the detection of coronary artery disease in non-revascularized, poststent and post-coronary artery bypass graft patients were included. Data were pooled to obtain a weighted sensitivity, specificity and diagnostic accuracy for MDCT. Negative and positive predictive values, and likelihood ratios were calculated based on sensitivity and specificity.
RESULTS:
Currently, 15 studies involving 1008 patients have examined the efficacy of 64-slice MDCT in the assessment of coronary artery stenosis (more than 50% luminal narrowing). In these studies, 64-slice MDCT has demonstrated a sensitivity (89%), specificity (96%) and diagnostic accuracy (95%) similar to that of 16-slice MDCT. However, 64-slice MDCT was able to assess 5% more coronary artery segments than 16-slice MDCT. In revascularized patients, MDCT can accurately assess both bypass graft occlusion and stenosis. The 64-slice MDCT is also capable of adequately detecting in-stent restenosis. Improvements in spatial and temporal resolution with 64-slice technology have decreased the occurrence of high attenuation and motion artefacts that plagued the previous generation of MDCT scanners.
CONCLUSION:
MDCT offers an accurate assessment of the coronary arteries, stented arteries and bypass grafts. The improved accuracy and safety of MDCT may reduce the need for catheter-based coronary angiography.
Keywords: Angiography, Bypass, Computed tomography, Coronary disease, Restenosis
Abstract
HISTORIQUE :
La tomodensitométrie à détecteurs multiples (TDDM) s’est révélée prometteuse dans l’évaluation non effractive des coronaropathies.
OBJECTIF :
Procéder à une analyse bibliographique systématique au sujet de la plus grande exactitude de la TDDM à 64 coupes.
MÉTHODOLOGIE :
Les auteurs ont mené une recherche dans les bases de données EMBASE, OVID, PubMed et Cochrane au moyen du mot-clé computed tomography apparié aux termes coronary artery ou coronary angiography pour colliger les articles de langue anglaise portant sur l’imagerie cardiaque par TDDM. Ils ont inclus les études qui comparaient la TDDM à 16 coupes à celle à 64 coupes au moyen d’une coronarographie par sonde afin de déceler les coronaropathies chez des patients non revascularisés, s’étant fait implanter une endoprothèse ou ayant subi un pontage aortocoronarien. Ils ont groupé les données pour obtenir une sensibilité, une spécificité et une précision diagnostique pondérées de la TDDM. Ils ont calculé les valeurs prédictives positives et négatives et les ratios de probabilité d’après la sensibilité et la spécificité.
RÉSULTATS :
Quinze études portant sur 1 008 patients on traité de l’efficacité de la TDDM à 64 coupes pour évaluer la sténose de l’artère coronaire (plus de 50 % de rétrécissements de la lumière). Dans ces études, la TDDM à 64 coupes a démontré une sensibilité (89 %), une spécificité (96 %) et une précision diagnostique (95 %) similaires à celles de la TDDM à 16 coupes. Cependant, la TDDM à 64 coupes permettait d’évaluer 5 % de plus de segments de l’artère coronaire que la TDDM à 16 coupes. Chez les patients revascularisés, la TDDM permet d’évaluer avec précision tant l’occlusion d’un pontage que la sténose. La TDDM à 64 coupes permet également de bien déceler une nouvelle sténose dans une endoprothèse. L’amélioration de la résolution spatiale et temporelle obtenue grâce à la technologie à 64 coupes réduit l’occurrence d’une forte atténuation et de l’artéfact de mouvement qui affligeait la génération précédente de TDDM.
CONCLUSION :
La TDDM offre une évaluation exacte des artères coronaires, des artères munies d’une endoprothèse et des pontages. L’exactitude et la sécurité accrues de la TDDM peuvent limiter la nécessité de procéder à une coronarographie par sonde.
Multidetector computed tomography (MDCT) is increasingly used for the noninvasive assessment of coronary arteries. The gold standard for the diagnosis of coronary artery disease (CAD) is catheter-based coronary angiography (CCA). However, this technique is invasive, expensive and not without risks; it is associated with a major complication rate of 1.7% (1). Up to 20% of diagnostic CCAs fail to show obstructive lesions, while only one-third are associated with a concurrent intervention (2).
An accurate noninvasive method to assess the coronary arteries would be a major improvement from angiography. In addition to assessing suspected CAD, MDCT has shown promise in analyzing restenosis following percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG). The present article systematically reviews the literature regarding improvements in the diagnostic capacity of 64-slice MDCT compared with the previous generation of MDCT scanners.
METHODS
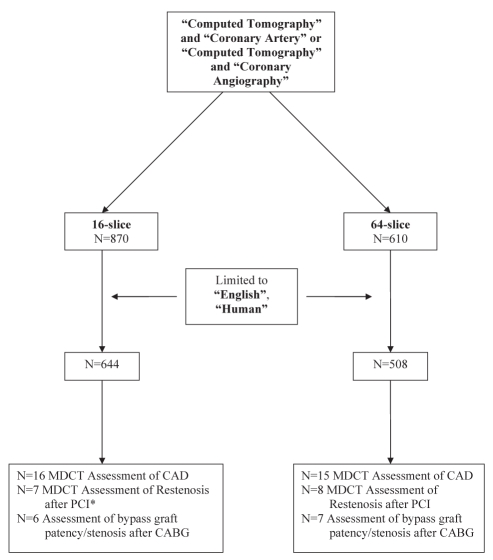
A PubMed, OVID, EMBASE and Cochrane Library database search was performed using key words describing MDCT assessment of CAD. Search terms included ‘computed tomography’ matched with ‘coronary artery’ or ‘coronary angiography’. Further data were obtained from the American Heart Association (AHA) and Canadian Cardiovascular Society (CCS) Web sites and task force recommendations. Studies that directly compared 16-slice or 64-slice MDCT with CCA for the detection of coronary artery stenosis in nonrevascularized, post-PCI and post-CABG patients were included (Figure 1). References from included studies were manually searched to supplement electronic searches.
Figure 1).
Quorum diagram. *One study of multidetector computed tomography (MDCT) assessment of resten osis after percutaneous coronary intervention (PCI) used 40-slice MDCT rather than 16-slice MDCT. CABG Coronary artery bypass grafting; CAD Coronary artery disease
In all studies included for analysis, the sensitivity and specificity of MDCT had to be noted or calculable from the data provided. Sensitivity was calculated as the percentage of patients with significant CAD who had a positive MDCT. Specificity was calculated as the percentage of patients without significant CAD who had a negative MDCT. Negative predictive value (NPV) was assessed by the percentage of patients with a negative MDCT who did not have significant CAD. Positive predictive value (PPV) was calculated by the percentage of patients with a positive MDCT who had significant CAD. The likelihood ratio for a positive test was calculated as sensitivity/(1 – specificity). The likelihood ratio for a negative test was calculated as (1 – sensitivity)/specificity. Data from all studies were pooled to obtain a weighted sensitivity, specificity, NPV, PPV and diagnostic accuracy for MDCT.
Original and review articles from the electronic database and manual search were reviewed, while all other forms of publication were excluded (eg, case reports, letters, editorials, animal and in vitro studies, abstracts only and case series with fewer than 15 patients). Additionally, all foreign-language articles were excluded. Articles that failed to provide a head-to-head comparison between MDCT and CCA were also excluded.
The quality of each study cited was reviewed using the quality information questionnaire from the University of Alberta Evidence-Based Working Group. All authors reviewed each citation.
Assessment of significant CAD using MDCT
The danger, cost and time burden associated with CCA suggests a need to develop noninvasive assessments for patients with suspected CAD. Initially, MDCT lacked the capacity to evaluate coronary artery stenosis, with diagnostic accuracies for four-slice MDCT ranging from 78% to 93% (3,4). Additionally, MDCT was unable to assess almost one-quarter of all coronary artery segments due to poor image quality. However, during the 10 years since its initial clinical use, progressive improvements in the spatial resolution of MDCT now allow accurate identification of stenotic coronary lesions, thereby offering a legitimate alternative to CCA.
Position statements by the AHA (5) and the CCS (6) noted the increasing accuracy of MDCT. A class IIa recommendation was provided for the use of 16-slice and 64-slice MDCT in the diagnosis of significant obstructive CAD. The use of MDCT was deemed harmful or of no benefit (class III) in patients with severe coronary calcification and irregular heart rhythms. Unfortunately, the CCS and AHA statements analyzed only three and six 64-slice MDCT studies, respectively.
Currently, 15 studies have examined 64-slice technology for the evaluation of suspected CAD (7–21) (Figure 2). In these studies, 64-slice MDCT was able to assess 97% of all coronary artery segments visualized by both CCA and MDCT (Table 1), which is approximately 5% more than previous 16-slice technology. This improvement was noted despite the inclusion of 64-slice MDCT studies that analyzed all vessel segments, compared with 16-slice MDCT studies, which generally excluded the analysis of segments smaller than 1.5 mm in diameter (Table 2) (22–37). When compared with CCA, 64-slice MDCT had a sensitivity of 89% and a specificity of 96% for the assessment of coronary artery stenosis (more than 50% luminal narrowing), similar to results obtained with 16-slice MDCT. The NPV, PPV, likelihood ratios and diagnostic accuracy of 64-slice MDCT were almost identical to those documented for 16-slice MDCT. Therefore, the advent of 64-slice scanners has allowed for substantial improvements in the number of vessel segments that can be assessed by MDCT while maintaining the efficacy of previous technologies.
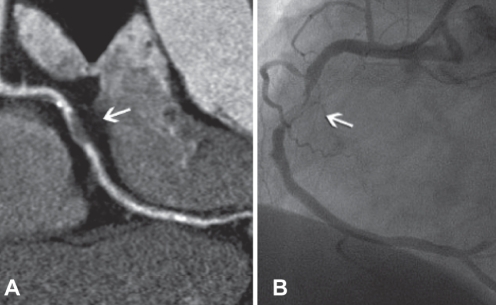
Figure 2).
Coronary artery stenosis (arrows). A Curved multiplanar reconstructed image of coronary artery stenosis. B Coronary artery stenosis confirmed by catheter-based coronary angiography. Reproduced with permission from reference 8
TABLE 1.
Accuracy of 64-slice multidetector computed tomography for the assessment of coronary artery stenosis compared with catheter-based coronary angiography (CCA)
| Reference | Patients, n | Patient population | Mean age, years | Men, % | Prevalence of CAD, % | Vessel diameter analyzed | Assessability, % | Sensitivity, % | Specificity, % | NPV, % | PPV, % | Diagnostic accuracy, % | Positive likelihood ratio | Negative likelihood ratio |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ong et al (7) | 134 | Scheduled angiography | 55 | 73 | 73 | ≥1.5 mm | 90 | 82 | 96 | 96 | 79 | 93 | 20.5 | 0.2 |
| Meijboom et al (8) | 104 | Suspected CAD | 59 | 72 | 85 | N/A | 100 | 92 | 91 | 99 | 60 | 91 | 10.2 | 0.1 |
| Ropers et al (9) | 84 | Scheduled angiography | 58 | 62 | 32 | ≥1.5 mm | 96 | 93 | 97 | 100 | 56 | 97 | 31.0 | 0.1 |
| Oncel et al (10) | 80 | Suspected CAD | 56 | 76 | 77 | ≥2 mm | 100 | 96 | 98 | 99 | 91 | 98 | 48 | 0 |
| Nikolaou et al (11) | 72 | Suspected CAD | 64 | 82 | 54 | ≥1.5 or2 mm | 90 | 82 | 95 | 97 | 72 | 93 | 27.3 | 0.2 |
| Raff et al (12)* | 70 | Suspected CAD | 59 | 73 | 54 | All vessels | 100 | 86 | 95 | 98 | 66 | 94 | 17.2 | 0.1 |
| Ehara et al (13)† | 69 | Suspected CAD | 69 | 75 | 88 | All vessels | 92 | 90 | 94 | 95 | 89 | 93 | 15.0 | 0.1 |
| Leschka et al (14)‡ | 67 | Suspected CAD | 62 | 74 | 70 | ≥1.5 mm | 100 | 94 | 97 | 99 | 87 | 97 | 31.3 | 0.1 |
| Schuijf et al (15) | 61 | Scheduled angiography | 60 | 77 | 52 | N/A | 99 | 85 | 98 | 99 | 82 | 93 | 42.5 | 0.3 |
| Leber et al (16) | 55 | Scheduled angiography | N/A | N/A | 56 | All vessels | 100 | 77 | 97 | 95 | 85 | 94 | 25.7 | 0.2 |
| Mollet et al (17) | 52 | Scheduled angiography | 59 | 65 | 90 | ≥1.5 or | 100 | 99 | 95 | 99 | 76 | 96 | 19.8 | 0.0 |
| Mühlenbruch et al (18)§ | 51 | Suspected CAD | 59 | 76 | 88 | N/A | 91 | 87 | 95 | 98 | 75 | 94 | 17.4 | 0.1 |
| Plass et al (19) | 50 | Scheduled angiography | 66 | 78 | 80 | ≥1.5 mm | 92 | 93 | 97 | 98 | 91 | 96 | 31 | 0.1 |
| Scheffel et al (20) | 38 | Scheduled angiography | 50 | 76 | 76 | ≥1.0 mm | 98 | 93 | 99 | 99 | 91 | 99 | 93 | 0.1 |
| Pugliese et al (21)* | 21 | Stable angina | 61 | 100 | 71 | All vessels | 100 | 99 | 96 | 99 | 78 | 96 | 24.8 | 0.0 |
| Total | 1008 | 97 | 89 | 96 | 98 | 79 | 95 | 22.3 | 0.1 |
All studies were blinded.
Quantitative CCA was not performed. Evaluation of coronary artery stenosis was made by visual analysis of CCA;
57% of patients had at least 1 stent;
Beta-blockers were not administered for heart rate reduction;
Defined coronary artery stenosis as >70% luminal narrowing of vessel. Coronary artery stenosis was defined as >50% luminal narrowing of vessel in all other studies. CAD Coronary artery disease; N/A Not available; NPV Negative predictive value; PPV Positive predictive value
TABLE 2.
Accuracy of 16-slice multidetector computed tomography for the assessment of coronary artery stenosis compared with catheter-based coronary angiography (CCA)
| Reference | Patients, n | Patient population | Mean age, years | Men, % | Prevalence of CAD, % | Vessel diameter analyzed | Assessability, % | Sensitivity, % | Specificity, % | NPV, % | PPV, % | Diagnostic accuracy, % | Positive likelihood ratio | Negative likelihood ratio |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Garcia et al (22) | 238 | Scheduled angiography | 60 | 68 | 100 | ≥1.5 mm | 71 | 85 | 91 | 99 | 36 | 91 | 67 | 0.3 |
| Mollet et al (23) | 128 | Stable angina | 59 | 88 | 83 | ≥2.0 mm | 100 | 92 | 95 | 98 | 79 | 95 | 18.4 | 0.1 |
| Hoffmann et al (24) | 103 | Scheduled angiography | 61.5 | 69 | 56 | ≥1.5 mm | 93 | 95 | 99 | 99 | 87 | 98 | 95.0 | 0.1 |
| Ropers et al (25) | 77 | Scheduled angiography | 58 | 65 | 53 | ≥1.5 mm | 88 | 91 | 93 | 98 | 78 | 93 | 13.0 | 0.1 |
| Kuettner et al (26) | 72 | Scheduled angiography | 64 | 58 | 50 | N/A | 100 | 82 | 98 | 97 | 87 | 96 | 41.0 | 0.2 |
| Ghersin et al (27)*† | 66 | Acute angina | 57 | 79 | N/A | ≥2.0 mm | 97 | 80 | 89 | 97 | 52 | 87 | 7.3 | 0.2 |
| Martuscelli et al (28) | 64 | Scheduled angiography | 58 | 92 | 67 | ≥1.5 mm | 84 | 89 | 98 | 98 | 90 | 97 | 44.5 | 0.1 |
| Nikolaou et al (29) | 64 | Suspected CAD | 60 | 53 | 83 | N/A | 92 | 80 | 99 | 99 | 57 | 99 | 80 | 0.2 |
| Kuettner et al (30) | 60 | Scheduled angiography | 58 | 73 | 60 | N/A | 100 | 70 | 97 | 97 | 70 | 95 | 23.3 | 0.3 |
| Nieman et al (31) | 59 | Scheduled angiography | 58 | 90 | 86 | ≥2.0 mm | 100 | 95 | 86 | 97 | 80 | 90 | 6.8 | 0.1 |
| Kefer et al (32) | 52 | Scheduled angiography | 50 | 64 | 80 | ≥1.5 mm | 75 | 67 | 99 | 98 | 88 | 97 | 67 | 0.3 |
| Mollet et al (33) | 51 | Typical/atypical angina | 59 | 73 | 63 | ≥2.0 mm | 100 | 95 | 98 | 99 | 87 | 98 | 47.5 | 0.1 |
| Achenbach et al (34) | 50 | Scheduled angiography | 62 | 50 | 54 | ≥1.5 mm | 96 | 94 | 96 | 99 | 68 | 96 | 23.5 | 0.1 |
| Cademartiri et al (35) | 40 | Suspected CAD | 59 | 90 | N/A | ≥2.0 mm | 100 | 96 | 96 | 99 | 86 | 96 | 24.0 | 0.0 |
| Manghat et al (36) | 36 | Scheduled angiography | 71 | 68 | 42 | N/A | 92 | 82 | 95 | 98 | 58 | 94 | 16.4 | 0.2 |
| Hoffmann et al (37) | 33 | Suspected CAD | 57 | 82 | 67 | N/A | 82 | 70 | 94 | 97 | 58 | 92 | 11.7 | 0.3 |
| Total | 1193 | 92 | 88 | 96 | 98 | 73 | 95 | 22.0 | 0.1 |
All studies were blinded. Coronary artery stenosis was defined as >50% luminal narrowing of vessel.
Quantitative CCA was not performed. Evaluation of coronary artery stenosis was made based on visual analysis of CCA;
Beta-blockers were not administered for heart rate reduction. CAD Coronary artery disease; N/A Not available; NPV Negative predictive value; PPV Positive predictive value
Like 16-slice MDCT, 64-slice technology is associated with a very high sensitivity for the diagnosis of coronary artery stenosis, suggesting that it may be effective in ruling out CAD, particularly in the emergency room. Furthermore, when using patient-based analysis, defined as the presence of one or more significant coronary artery lesions in a study subject, sensitivity for 64-slice MDCT ranged between 88% to 100% in the 15 studies reviewed (7–21). Additionally, in study populations with a low prevalence of CAD (32%), per-patient sensitivity remained very high (96%) (9). These results suggest angiography could be avoided in approximately nine of 10 patients with suspected CAD through the use of MDCT.
The assessment of heavily calcified atherosclerotic plaques remains a major limitation to the diagnostic accuracy of MDCT. Calcification, like metallic stents (discussed below), causes artefact due to the partial volume-averaging effect and beam hardening. The partial volume effect refers to the loss of contrast between neighbouring tissues of different densities present in the same voxel. When attenuation of materials is averaged within a voxel, high-density calcium in atherosclerotic plaques outweighs low-density tissue and blood, leading to image artefact (21). The beam-hardening artefact arises due to an increased proportion of lower-energy photons in an x-ray beam being absorbed when passing through high-density tissue (eg, calcified plaque, metallic stents), leaving a beam of increased intensity to be detected by MDCT. The latter effect usually results in an erroneously increased estimate of calcified plaque size (21). With 16-slice MDCT, plaque calcification prevented the assessment of vessel narrowing in 5% of arteries and was responsible for up to 94% of the false-positive findings (37,38). Similarly, with 64-slice MDCT, 40% to 65% of all nonassessable coronary segments were attributed to severe calcification (9,15,19). Sensitivity for significant stenosis in severely calcified coronary segments remained high (93% to 100%) for 64-slice MDCT (8,12,17). Unfortunately, specificity for significant stenosis was variable (67% to 92%) in the setting of severe calcification, suggesting that calcification-associated artefact with 64-slice MDCT leads predominantly to overestimation of lesion obstruction (8,12,17).
Although previous MDCT technology has been able to identify the presence or absence of coronary lesions, quantification of coronary artery stenosis could not be performed accurately. Using 64-slice technology, Leber et al (16) found a weak correlation between MDCT and CCA in quantifying coronary artery stenosis (r=0.54). In contrast, Raff et al (12) found that MDCT and CCA correlated well (r=0.76) with regard to quantification of lesional obstruction. In the latter study, the average difference between measurements of percentage luminal stenosis in arteries with significant obstruction was only 1.3%, but the SD was greater than 14%. The limited number of studies using MDCT to quantify coronary artery stenosis, along with the variability of results within these studies, suggests that MDCT cannot be used as a tool for accurate quantification of lesional obstruction. This has important clinical implications because the therapeutic sequelae of 50% and 100% obstructive lesions are very different.
Sixty-four-slice MDCT can accurately assess for significant coronary artery stenosis in patients with suspected CAD. Enhanced spatial and temporal resolution allow for excellent diagnostic accuracy despite the inclusion of vessels with small diameters and severe calcification in studies. Unfortunately, 64-slice MDCT is still not capable of adequately quantifying coronary artery stenosis.
Assessment of in-stent restenosis using MDCT
The six-month rate of restenosis after PCI remains at approximately 9%, despite the advent of drug-eluting stents (39). Evaluation of restenosis is usually performed with CCA. However, a number of studies have examined the accuracy of MDCT for the noninvasive assessment of in-stent restenosis. Thus far, the delineation of stent lumens by MDCT has been noted to be feasible in larger vessels such as the iliac and renal arteries (40,41). However, initial in vitro observations using four-slice MDCT for coronary artery assessment after PCI found that only 20% to 40% of the vessel lumen was visible at nondiseased stented sites due to high attenuation artefact (42).
The AHA position statement (5) notes that there is no benefit or potential harm (class III indication) in using MDCT to evaluate in-stent restenosis. Analysis for this statement included only one study using 64-slice MDCT. No recommendations on MDCT in the diagnosis of in-stent restenosis have been made by the CCS.
The development of 16-slice MDCT has allowed for better assessment of restenosis of coronary arteries after PCI. In six studies (38,43–47) assessing in-stent restenosis (more than 50% luminal narrowing), 16-slice MDCT had a sensitivity of 73%, a specificity of 95%, an NPV of 94%, a PPV of 94%, a positive likelihood ratio of 14.6, a negative likelihood ratio of 0.3 and a diagnostic accuracy of 94% compared with CCA (Table 3). Unfortunately, only 72% of all stented vessels were assessable in study populations. Recently, Gaspar et al (48) attempted to assess the efficacy of 40-slice MDCT in similar evaluations of post-PCI restenosis. This study found that 96% of stented vessels could be assessed by 40-slice MDCT. However, the study noted that 40-slice MDCT had a sensitivity of 78%, a specificity of 83%, an NPV of 92%, a PPV of 58%, a positive likelihood ratio of 4.6, a negative likelihood ratio of 0.3 and a diagnostic accuracy of 81% for the assessment of coronary artery in-stent restenosis.
TABLE 3.
Accuracy of 16-slice, 40-slice and 64-slice multidetector computed tomography (MDCT) for the assessment of in-stent restenosis compared with catheter-based coronary angiography (CCA)
| Reference | Patients, n | Mean age, years | Men, % | Assessability, % | Sensitivity (per stent), % | Specificity (per stent), % | NPV, % | PPV, % | Diagnostic accuracy, % | Positive likelihood ratio | Negative likelihood ratio |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 16-slice MDCT | |||||||||||
| Gilard et al (43)* | 143 | 68 | 71 | 54 | 65 | 100 | 94 | 100 | 94 | Undefined | 0.4 |
| Kefer et al (38) | 50 | 64 | 80 | 100 | 67 | 98 | 89 | 92 | 90 | 33.5 | 0.3 |
| Soon et al (44) | 37 | 57 | 89 | 96 | 71 | 97 | 95 | 83 | 93 | 23.7 | 0.3 |
| Schuijf et al (45)* | 22 | 62 | 91 | 78 | 78 | 100 | 95 | 100 | 94 | Undefined | 0.2 |
| Kitagawa et al (46)† | 16 | N/A | N/A | N/A | 100 | 100 | 100 | 100 | 100 | Undefined | 0.0 |
| Ohnuki et al (47) | 16 | 65 | 69 | 95 | 100 | 87 | 100 | 66 | 90 | 7.7 | 0.0 |
| Total | 284 | 72 | 73 | 95 | 94 | 94 | 94 | 14.6 | 0.3 | ||
| 40-slice MDCT | |||||||||||
| Gaspar et al (48)‡ | 65 | 64 | 74 | 96 | 78 | 83 | 92 | 58 | 81 | 4.6 | 0.3 |
| 64-slice MDCT | |||||||||||
| Cademartiri et al (49) | 182 | 58 | 84 | 93 | 95 | 93 | 99 | 63 | 93 | 13.6 | 0.1 |
| Pugliese et al (50) | 100 | 62 | 78 | 95 | 94 | 92 | 98 | 77 | 93 | 11.8 | 0.1 |
| Cademartiri et al (51) | 95 | 76 | 58 | N/A | 93 | 89 | 99 | 57 | 89 | 8.5 | 0.1 |
| Rixe et al (52) | 64 | 58 | 64 | 58 | 86 | 98 | 98 | 86 | 97 | 44.5 | 0.1 |
| Das et al (53) | 53 | 54 | 85 | 97 | 97 | 88 | 99 | 78 | 91 | 8.1 | 0.0 |
| Schuijf et al (54) | 50 | 60 | 80 | 86 | 100 | 100 | 100 | 100 | 100 | Undefined | 0.0 |
| Carbone et al (55) | 41 | 62 | 83 | 72 | 75 | 86 | 89 | 71 | 83 | 5.4 | 0.3 |
| Oncel et al (56) | 30 | 58 | 90 | 100 | 89 | 95 | 90 | 94 | 92 | 17.8 | 0.1 |
| Total | 615 | 88 | 92 | 92 | 97 | 75 | 92 | 11.5 | 0.1 | ||
All studies were blinded. Coronary artery stenosis was defined as >50% luminal narrowing of vessel. All studies assessed coronary artery stenosis with quantitative CCA.
Beta-blockers were not administered for heart rate reduction;
Only a subset of the study population was used for comparing the efficacy of MDCT with CCA for the examination of in-stent restenosis;
5 stents were not visible by MDCT, but were included in analysis and categorized as stenotic (stenosis could not be excluded). N/A Not available; NPV Negative predictive value; PPV Positive predictive value
More recently, the efficacy of 64-slice MDCT in assessing in-stent restenosis was evaluated (Figure 3). The 64-slice MDCT was capable of assessing 88% of all stented arteries (49–56). In the eight clinical studies examined, 64-slice MDCT had a sensitivity of 92%, a specificity of 92%, an NPV of 97%, a PPV of 75%, a positive likelihood ratio of 11.5, a negative likelihood ratio of 0.1 and a diagnostic accuracy of 92% for the assessment of coronary in-stent restenosis.
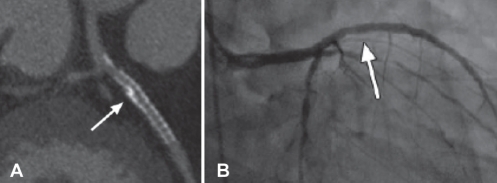
Figure 3).
In-stent restenosis (arrows). A Curved multiplanar reconstruction demonstrating in-stent restenosis. B Confirmation of middle and distal in-stent restenosis with catheter-based coronary angiography. Reproduced with permission from reference 53
In patients with coronary artery stents, high-attenuation artefact is attributable to beam-hardening and partial volume-averaging effects. Increased spatial resolution and concordant reductions in voxel size lessen these effects, and partially account for the greater accuracy of 64-slice MDCT. Despite improved MDCT technology, inherent properties of metallic stents, such as stent diameter, altered beam-hardening and partial volume-averaging-related artefact. The sensitivity, specificity and diagnostic accuracy of 64-slice MDCT were reduced by more than 10% when analyzing restenosis in stents smaller than 2.75 mm (50). Both four- and 16-slice MDCT were incapable of evaluating restenosis in stents smaller than 2.5 mm (46).
Stent strut thickness and material were noted to increase high- attenuation artefact in 16-slice MDCT. Stents made of tantalum and gold (BeStent2; Medtronic Inc, USA) were associated with the greatest amount of artefact, making lumen visibility difficult or even completely unassessable (46). With regard to stent strut thickness, 16-slice MDCT was capable of analyzing only 59% of stents with struts greater than 140 μm in thickness (45). In contrast, 89% of stents with a strut thickness smaller than 140 μm could be interpreted. The effects of stent material or strut thickness have not been fully evaluated in 64-slice MDCT.
The increased ability to assess stented arteries and greater accuracy of 64-slice MDCT indicates marked improvement from its 16- and 40-slice predecessors. However, even with 64-slice technology, MDCT is not capable of adequately evaluating restenosis in patients with stents smaller than 2.75 mm, representing a major limitation compared with angiography. Additionally, the effects of artefact related to stent material and strut thickness need to be further researched using 64-slice technology.
Assessment of bypass graft occlusion and restenosis using MDCT
The presence of significant atherosclerotic disease can be seen in 75% of bypass grafts at 10 years (57). Currently, CCA remains the gold standard for the assessment of suspected occlusion and restenosis after CABG. Unfortunately, the use of CCA, particularly in saphenous vein grafts, has been associated with arrhythmia, stroke, myocardial infarction and damage to grafts (58). MDCT has been proposed as a noninvasive substitute for the evaluation of obstructive bypass graft lesions.
The AHA states that there is a class IIb indication for the use of MDCT in assessing bypass graft stenosis (5). No specific recommendations were made regarding the efficacy of MDCT in evaluating bypass graft occlusion. Again, this statement did not include any studies involving 64-slice MDCT. The CCS has no current position statement guidelines for the use of MDCT in assessing bypass grafts.
The assessment of bypass graft patency would be of considerable use in the acute postoperative setting (less than one month), where graft occlusion can be seen in 10% to 15% of patients following CABG (59). In four studies analyzed, 64-slice MDCT was capable of assessing 98% of all bypass grafts (60–63). The sensitivity, specificity and diagnostic accuracy of 64-slice MDCT for the detection of bypass graft occlusion were 100%, 100% and 99%, respectively. The 64-slice MDCT had an NPV of 100%, a PPV of 98%, an undefined positive likelihood ratio and a negative likelihood ratio of 0.0 (Table 4). Consistent with the results for nonrevascularized coronary arteries, 64-slice MDCT was able to assess more bypass grafts and was more accurate than 16-slice MDCT (64–69).
TABLE 4.
Accuracy of 16-slice and 64-slice multidetector computed tomography (MDCT) for the assessment of bypass graft occlusion following coronary artery bypass grafting compared with catheter-based coronary angiography (CCA)
| Reference | Patients, n | Mean age, years | Men, % | Assessability, % | Sensitivity, % | Specificity, % | NPV, % | PPV, % | Diagnostic accuracy, % | Positive likelihood ratio | Negative likelihood ratio |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 16-slice MDCT | |||||||||||
| Martuscelli et al (64) | 96 | 62 | 83 | 88 | 100 | 100 | 100 | 100 | 100 | Undefined | 0.0 |
| Chiurlia et al (65) | 52 | 63 | 87 | 99 | 100 | 100 | 100 | 100 | 100 | Undefined | 0.0 |
| Houslay et al (66) | 50 | 66 | 86 | 100 | 100 | 93 | 100 | 85 | 95 | 14.3 | 0.0 |
| Yamamoto et al (67)* | 42 | 64 | 86 | 99 | 100 | 92 | 100 | 33 | 94 | 12.5 | 0.0 |
| Anders et al (68) | 32 | 67 | 91 | 100 | 100 | 98 | 100 | 96 | 99 | 50 | 0.0 |
| Salm et al (69)*† | 25 | 67 | 96 | 92 | 100 | 95 | 99 | 100 | 99 | 20 | 0.0 |
| Total | 297 | 94 | 100 | 98 | 99 | 92 | 98 | 49 | 0.0 | ||
| 64-slice MDCT | |||||||||||
| Onuma et al (60) | 54 | 65 | 81 | 95 | 100 | 98 | 100 | 94 | 99 | 50 | 0.0 |
| Jabara et al (61) | 50 | 64 | 90 | 99 | 100 | 100 | 100 | 100 | 100 | Undefined | 0.0 |
| Ropers et al (62) | 50 | 67 | 76 | 100 | 100 | 100 | 100 | 100 | 100 | Undefined | 0.0 |
| Feuchtner et al (63) | 40 | 62 | 80 | 100 | 100 | 100 | 100 | 100 | 100 | Undefined | 0.0 |
| Total | 194 | 98 | 100 | 100 | 100 | 98 | 99 | Undefined | 0.0 | ||
Bypass graft occlusion was defined as 100% luminal narrowing of vessel. All studies assessed bypass graft occlusion with quantitative CCA.
Blinding was not specified;
Beta-blockers were not administered for heart rate reduction. NPV Negative predictive value; PPV Positive predictive value
Chronic bypass graft vasculopathy is generally attributable to vessel stenosis and occlusion secondary to intimal hyperplasia. The efficacy of 16- and 64-slice MDCT in examining vein and arterial graft stenosis (more than 50% luminal narrowing) has been evaluated (70–73) (Figure 4). As with graft patency, 64-slice MDCT was capable of assessing stenosis in 98% of all vein and arterial grafts. In the seven clinical studies using 64-slice MDCT to evaluate graft stenosis, the sensitivity and specificity were 98% and 97%, respectively, while an NPV of 99%, a PPV of 92%, a positive likelihood ratio of 32.3, a negative likelihood ratio of 0.0 and a diagnostic accuracy of 97% were attained compared with CCA (Table 5). Similar diagnostic efficacy was noted for bypass graft stenosis with 16-slice and 64-slice MDCT. This is despite the fact that two of seven studies using 64-slice technology included patients with irregular or elevated heart rates (71,72). The increased accuracy and ‘real-world’ applicability of 64-slice MDCT suggests that it may be used clinically in the assessment of bypass graft patency and stenosis immediately following surgery and consistently afterwards.
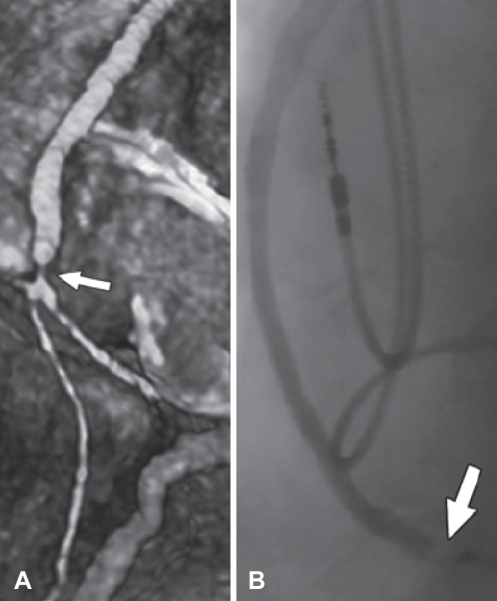
Figure 4).
Bypass graft stenosis (arrows). A Three-dimensional reconstruction of 64-slice multidetector computed tomography image showing bypass graft stenosis at the distal anastamotic site. B Bypass graft stenosis verified by catheter-based coronary angiography. Reproduced with permission from reference 63
TABLE 5.
Accuracy of 16-slice and 64-slice multidetector computed tomography (MDCT) for the assessment of bypass graft stenosis following coronary artery bypass grafting compared with catheter-based coronary angiography (CCA)
| Reference | Patients, n | Mean age, years | Men, % | Assessability, % | Sensitivity, % | Specificity, % | NPV, % | PPV, % | Diagnostic accuracy, % | Positive likelihood ratio | Negative likelihood ratio |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 16-slice MDCT | |||||||||||
| Martuscelli et al (64) | 96 | 62 | 83 | 88 | 97 | 100 | 99 | 100 | 99 | Undefined | 0.0 |
| Chiurlia et al (65) | 52 | 63 | 87 | 99 | 99 | 100 | 95 | 100 | 99 | Undefined | 0.0 |
| Moore et al (70)*† | 50 | 63 | 78 | 97 | 100 | 99 | 100 | 98 | 99 | 100 | 0.0 |
| Anders et al (68) | 32 | 67 | 91 | 100 | 95 | 89 | 96 | 86 | 91 | 8.6 | 0.1 |
| Total | 230 | 94 | 98 | 98 | 99 | 98 | 98 | 25.0 | 0.0 | ||
| 64-slice MDCT | |||||||||||
| Meyer et al (71) | 138 | 68 | 88 | 98 | 97 | 97 | 99 | 93 | 97 | 32.3 | 0.0 |
| Onuma et al (60) | 54 | 65 | 81 | 95 | 100 | 98 | 100 | 94 | 99 | 50 | 0.0 |
| Malagutti et al (73) | 52 | 65 | 87 | 100 | 100 | 98 | 100 | 98 | 99 | 50 | 0.0 |
| Jabara et al (61) | 50 | 64 | 90 | 99 | 100 | 100 | 100 | 100 | 100 | Undefined | 0.0 |
| Ropers et al (62) | 50 | 67 | 76 | 100 | 100 | 94 | 100 | 76 | 95 | 16.7 | 0.0 |
| Feuchtner et al (63) | 40 | 62 | 80 | 100 | 85 | 95 | 97 | 80 | 93 | 17.0 | 0.2 |
| Pache et al (72) | 31 | 68 | 84 | 97 | 98 | 89 | 98 | 90 | 94 | 8.9 | 0.0 |
| Total | 415 | 98 | 98 | 97 | 99 | 92 | 97 | 32.3 | 0.0 | ||
Bypass graft stenosis was defined as >50% luminal narrowing of vessel. All studies assessed bypass graft stenosis with quantitative CCA.
Blinding was not specified;
Beta-blockers were not administered for heart rate reduction. NPV Negative predictive value; PPV Positive predictive value
Previous generations of MDCT scanners were unable to consistently evaluate obstructive lesions at anastamotic sites between bypass grafts and native coronary arteries. Analysis of distal anastamotic sites was particularly difficult due to small vessel size and the presence of metallic clip-related artefacts. The 64-slice MDCT was capable of assessing between 94% and 98% of distal anastamotic sites (60,72). Metallic clip-related artefacts prevented the analysis of only 2% to 3% of bypass grafts with 64-slice MDCT (63,72). Unfortunately, only two studies have explicitly examined the ability of 64-slice MDCT in assessing distal anastamosis sites.
The 64-slice MDCT has excellent diagnostic accuracy in assessing bypass graft stenosis and occlusion. Compared with the previous generation of MDCT scanners, preliminary studies suggest that 64-slice MDCT may allow for the assessment of obstructive lesions at distal anastamotic sites, while also maintaining very high diagnostic accuracy in patients with elevated or irregular heart rates.
RECOMMENDATIONS
64-slice MDCT accurately assesses coronary artery stenosis in patients with suspected CAD (Table 6). The capacity to assess a greater proportion of coronary artery segments and the improved accuracy of 64-slice MDCT make it an increasingly feasible alternative to CCA. In studies using 64-slice MDCT, patient-based analysis consistently revealed high sensitivities for the assessment of coronary artery stenosis, suggesting that MDCT may now be a particularly effective tool for ruling out CAD.
TABLE 6.
Summary of weighted analysis for 16-slice, 40-slice and 64-slice multidetector computed tomography (MDCT)
| Assessment | Patients, n | Assessability, % | Sensitivity, % | Specificity, % | NPV, % | PPV, % | Diagnostic accuracy, % | Positive likelihood ratio | Negative likelihood ratio |
|---|---|---|---|---|---|---|---|---|---|
| 16-slice MDCT | |||||||||
| Stenosis in suspected CAD | 1193 | 92 | 88 | 96 | 98 | 73 | 95 | 22.0 | 0.1 |
| In-stent restenosis | 284 | 72 | 73 | 95 | 94 | 94 | 94 | 14.6 | 0.3 |
| Post-CABG occlusion | 297 | 94 | 100 | 98 | 99 | 92 | 98 | 49 | 0.0 |
| Post-CABG stenosis | 230 | 94 | 100 | 96 | 100 | 94 | 98 | 25.0 | 0.0 |
| 40-slice MDCT | |||||||||
| In-stent restenosis | 65 | 64 | 74 | 96 | 78 | 83 | 92 | 58 | 81 |
| 64-slice MDCT | |||||||||
| Stenosis in suspected CAD | 957 | 97 | 89 | 96 | 98 | 79 | 95 | 22.3 | 0.1 |
| In-stent restenosis | 615 | 88 | 92 | 92 | 97 | 75 | 92 | 11.5 | 0.1 |
| Post-CABG occlusion | 194 | 98 | 100 | 100 | 100 | 98 | 99 | Undefined | 0.0 |
| Post-CABG stenosis | 415 | 98 | 98 | 97 | 99 | 92 | 97 | 32.3 | 0.0 |
Coronary artery or bypass graft stenosis was defined as >50% luminal narrowing of vessel. All studies assessed vessel stenosis or occlusion with quantitative or visually based catheter-based coronary angiography. CABG Coronary artery bypass graft; CAD Coronary artery disease; NPV Negative predictive value; PPV Positive predictive value
Previous 16-slice and 40-slice MDCT were not able to evaluate in-stent restenosis. However, the improved spatial and temporal resolution associated with 64-slice technology has allowed for improved image quality and greater accuracy for evaluating in-stent restenosis. Additionally, the accuracy of 64-slice technology in assessing bypass graft occlusion and restenosis after CABG is excellent. The efficacy of 64-slice MDCT in assessing for obstructive coronary lesions in both revascularized and nonrevascularized study populations indicates that it may be used in broad patient groups. However, due to the considerable risks associated with radiation and contrast administration (described below), MDCT should predominantly be used in patients who are candidates for subsequent CCA.
LIMITATIONS
High attenuation artefact attributable to beam-hardening and partial volume-averaging effects remains a significant obstacle for MDCT. Calcified atherosclerotic plaques, metallic surgical clips and stents are all associated with artefact that limits the ability of MDCT to accurately assess coronary artery stenosis. Improvements in spatial resolution have progressively reduced the effects of high attenuation artefact.
Motion artefact is a major cause of diagnostic error in MDCT assessment of coronary artery stenosis (74). Elevated heart rates, seen more prominently with arrhythmias, are a significant cause of motion artefact. The presence of atrial fibrillation usually serves as an exclusion criteria in most MDCT analyses. More recently, studies using MDCT (71,72) have included patients with atrial fibrillation and have maintained excellent diagnostic accuracy, likely attributable to the increased temporal resolution associated with 64-slice technology.
The radiation dose associated with MDCT is higher than that in CCA. Thus far, most studies have yielded effective radiation dose values of 6 mSv to 16 mSv for 16-slice scanners and 11 mSv to 21 mSv for 64-slice scanners, compared with approximately 6 mSv for CCA (75–77). In practical terms, the National Council on Radiation Protection and Measurements identifies a risk factor for lifetime cancer mortality of 5×10−2 per 1 Sv exposure, which, given the above data, translates into a risk of inducing a fatal cancer of 0.05% to 0.11% for each 64-slice MDCT and 0.03% for each CCA (1). This risk increases significantly among younger patients who may require more than one MDCT scan in their lifetime. In fact, if every North American between the ages of 55 and 64 years (estimated to be approximately 35,000,000 individuals by Statistics Canada and the United States Census Bureau) were screened with 64-slice MDCT for assessment of significant CAD, approximately 17,500 to 35,000 new cancers would potentially arise based on risk estimates (78,79). If screening was performed approximately every five years, patients aged 55 to 64 years would likely require at least two scans in their lifetime, resulting in a doubling (35,000 to 70,000) of expected cancers. More recently, newer measures, including lower scan voltage and algorithms involving electrocardiogram-gated image acquisition, have been initiated to reduce the effective radiation dose associated with MDCT (76).
Along with radiation, the administration of contrast required for MDCT can also lead to adverse events, most notably contrast-associated allergic reactions and contrast-induced nephropathy (CIN). The use of noniodinated contrast for radiological examinations is associated with severe allergic reactions in 0.2% to 0.7% of patients (1). The incidence of renal dysfunction after administration of contrast is approximately 3% in the general patient population, but substantially higher in patients with multiple risk factors for CIN (eg, diabetic nephropathy, low-volume states, etc) (80). In patients undergoing PCI, the incidence of CIN has been estimated to be as high as 19% (81). Additionally, patients who develop CIN after undergoing cardiovascular intervention have worse clinical outcomes, as well as increased in-hospital and long-term mortality rates compared with patients without CIN (82). Therefore, MDCT studies should be largely avoided in patients with previous severe contrast-associated allergic reactions and those deemed to be at high risk for the development of CIN.
The volume of scans performed at hospital centres likely plays a significant role in determining the accuracy of MDCT in the assessment of patients with suspected CAD. Numerous variables, including heart rate, the presence of arrhythmia and patient motion, must be assessed to ensure the efficacy of MDCT scans, underscoring the importance of experience in conducting and interpreting studies. Although interobserver agreement for MDCT scan interpretation has been noted to be between good and excellent (kappa coefficient 0.68 to 0.95), the majority of published studies have been performed at experienced or high-volume centres (18,23,28,33,37,43). The importance of experience will be increasingly relevant as the number of hospital centres that have access to MDCT technology continues to rise. In a recent nationwide survey of hospitals in the United States that use 64-slice MDCT, 44% of sites had ‘low’ (fewer than 100 scans to date) levels of experience with scans (83). An analysis examining the accuracy of MDCT at high- and low-volume centres will have to be completed to determine where scans should be performed and interpreted.
CONCLUSION
MDCT technology is constantly improving and becoming increasingly viable for the assessment of coronary artery stenosis in both revascularized and nonrevascularized patients. Further research in MDCT must be aimed at improving diagnostic accuracy in diverse patient populations, particularly those with arrhythmias, and reducing the considerable radiation and contrast exposure faced by patients. If successful, MDCT will be able to diminish the significant cost and health risk associated with diagnostic evaluation of CAD by reducing the need for CCA.
REFERENCES
- 1.Zanzonico P, Rothenberg LN, Strauss HW. Radiation exposure of computed tomography and direct intracoronary angiography: Risk has its reward. J Am Coll Cardiol. 2006;47:1846–9. doi: 10.1016/j.jacc.2005.10.075. [DOI] [PubMed] [Google Scholar]
- 2.American Heart Association . 2002 Heart and Stroke Statistical Update. Dallas: American Heart Association; 2001. [Google Scholar]
- 3.Achenbach S, Giesler T, Ropers D, et al. Detection of coronary artery stenoses by contrast-enhanced, retrospectively electrocardiographically-gated, multislice spiral computed tomography. Circulation. 2001;103:2535–8. doi: 10.1161/01.cir.103.21.2535. [DOI] [PubMed] [Google Scholar]
- 4.Leber AW, Knez A, Becker C, et al. Non-invasive intravenous coronary angiography using electron beam tomography and multislice computed tomography. Heart. 2003;89:633–9. doi: 10.1136/heart.89.6.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Budoff MJ, Achenbach S, Blumenthal RS, et al. American Heart Association Committee on Cardiovascular Imaging and Intervention; American Heart Association Council on Cardiovascular Radiology and Intervention; American Heart Association Committee on Cardiac Imaging, Council on Clinical Cardiology Assessment of coronary artery disease by cardiac computed tomography: A scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention, Council on Cardiovascular Radiology and Intervention, and Committee on Cardiac Imaging, Council on Clinical Cardiology. Circulation. 2006;114:1761–91. doi: 10.1161/CIRCULATIONAHA.106.178458. [DOI] [PubMed] [Google Scholar]
- 6.Beanlands RS, Chow BJ, Dick A, et al. Canadian Cardiovascular Society; Canadian Association of Radiologists; Canadian Association of Nuclear Medicine; Canadian Nuclear Cardiology Society; Canadian Society of Cardiac Magnetic Resonance CCS/CAR/CANM/CNCS/CanSCMR joint position statement on advanced noninvasive cardiac imaging using positron emission tomography, magnetic resonance imaging and multidetector computed tomographic angiography in the diagnosis and evaluation of ischemic heart disease – executive summary. Can J Cardiol. 2007;23:107–19. doi: 10.1016/s0828-282x(07)70730-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ong TK, Chin SP, Liew CK, et al. Accuracy of 64-row multidetector computed tomography in detecting coronary artery disease in 134 symptomatic patients: Influence of calcification. Am Heart J. 2006;151:1323.e1–6. doi: 10.1016/j.ahj.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 8.Meijboom WB, Mollet NR, Van Mieghem CA, et al. 64-slice CT coronary angiography in patients with non-ST elevation acute coronary syndrome. Heart. 2007;93:1386–92. doi: 10.1136/hrt.2006.112771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ropers D, Rixe J, Anders K, et al. Usefulness of multidetector row spiral computed tomography with 64- × 0.6-mm collimation and 330-ms rotation for the noninvasive detection of significant coronary artery stenoses. Am J Cardiol. 2006;97:343–8. doi: 10.1016/j.amjcard.2005.08.050. [DOI] [PubMed] [Google Scholar]
- 10.Oncel D, Oncel G, Tastan A, Tamci B. Detection of significant coronary artery stenosis with 64-section MDCT angiography. Eur J Radiol. 2007;62:394–405. doi: 10.1016/j.ejrad.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 11.Nikolaou K, Knez A, Rist C, et al. Accuracy of 64-MDCT in the diagnosis of ischemic heart disease. AJR Am J Roentgenol. 2006;187:111–7. doi: 10.2214/AJR.05.1697. [DOI] [PubMed] [Google Scholar]
- 12.Raff GL, Gallagher MJ, O’Neill WW, Goldstein JA. Diagnostic accuracy of noninvasive coronary angiography using 64-slice spiral computed tomography. J Am Coll Cardiol. 2005;46:552–7. doi: 10.1016/j.jacc.2005.05.056. [DOI] [PubMed] [Google Scholar]
- 13.Ehara M, Surmely JF, Kawai M, et al. Diagnostic accuracy of 64-slice computed tomography for detecting angiographically significant coronary artery stenosis in an unselected consecutive patient population: Comparison with conventional invasive angiography. Circ J. 2006;70:564–71. doi: 10.1253/circj.70.564. [DOI] [PubMed] [Google Scholar]
- 14.Leschka S, Alkadhi H, Plass A, et al. Accuracy of MSCT coronary angiography with 64-slice technology: First experience. Eur Heart J. 2005;26:1482–7. doi: 10.1093/eurheartj/ehi261. [DOI] [PubMed] [Google Scholar]
- 15.Schuijf JD, Pundziute G, Jukema JW, et al. Diagnostic accuracy of 64-slice multislice computed tomography in the noninvasive evaluation of significant coronary artery disease. Am J Cardiol. 2006;98:145–8. doi: 10.1016/j.amjcard.2006.01.092. [DOI] [PubMed] [Google Scholar]
- 16.Leber AW, Becker A, Knez A, et al. Accuracy of 64-slice computed tomography to classify and quantify plaque volumes in the proximal coronary system: A comparative study using intravascular ultrasound. J Am Coll Cardiol. 2006;47:672–7. doi: 10.1016/j.jacc.2005.10.058. [DOI] [PubMed] [Google Scholar]
- 17.Mollet NR, Cademartiri F, van Mieghem CA, et al. High-resolution spiral computed tomography coronary angiography in patients referred for diagnostic conventional coronary angiography. Circulation. 2005;112:2318–23. doi: 10.1161/CIRCULATIONAHA.105.533471. [DOI] [PubMed] [Google Scholar]
- 18.Mühlenbruch G, Seyfarth T, Soo CS, Pregalathan N, Mahnken AH. Diagnostic value of 64-slice multi-detector row cardiac CTA in symptomatic patients. Eur Radiol. 2007;17:603–9. doi: 10.1007/s00330-006-0429-5. [DOI] [PubMed] [Google Scholar]
- 19.Plass A, Grünenfelder J, Leschka S, et al. Coronary artery imaging with 64-slice computed tomography from cardiac surgical perspective. Eur J Cardiothorac Surg. 2006;30:109–16. doi: 10.1016/j.ejcts.2006.03.048. [DOI] [PubMed] [Google Scholar]
- 20.Scheffel H, Leschka S, Plass A, et al. Accuracy of 64-slice computed tomography for the preoperative detection of coronary artery disease in patients with chronic aortic regurgitation. Am J Cardiol. 2007;100:701–6. doi: 10.1016/j.amjcard.2007.03.087. [DOI] [PubMed] [Google Scholar]
- 21.Pugliese F, Mollet NR, Runza G, et al. Diagnostic accuracy of non-invasive 64-slice CT coronary angiography in patients with stable angina pectoris. Eur Radiol. 2006;16:575–82. doi: 10.1007/s00330-005-0041-0. [DOI] [PubMed] [Google Scholar]
- 22.Garcia MJ, Lessick J, Hoffmann MH, CATSCAN Study Investigators Accuracy of 16-row multidetector computed tomography for the assessment of coronary artery stenosis. JAMA. 2006;296:403–11. doi: 10.1001/jama.296.4.403. [DOI] [PubMed] [Google Scholar]
- 23.Mollet NR, Cademartiri F, Nieman K, et al. Multislice spiral computed tomography coronary angiography in patients with stable angina pectoris. J Am Coll Cardiol. 2004;43:2265–70. doi: 10.1016/j.jacc.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 24.Hoffmann MH, Shi H, Schmitz BL, et al. Noninvasive coronary angiography with multislice computed tomography. JAMA. 2005;293:2471–8. doi: 10.1001/jama.293.20.2471. [DOI] [PubMed] [Google Scholar]
- 25.Ropers D, Baum U, Pohle K, et al. Detection of coronary artery stenoses with thin-slice multi-detector row spiral computed tomography and multiplanar reconstruction. Circulation. 2003;107:664–6. doi: 10.1161/01.cir.0000055738.31551.a9. [DOI] [PubMed] [Google Scholar]
- 26.Kuettner A, Beck T, Drosch T, et al. Diagnostic accuracy of noninvasive coronary imaging using 16-detector slice spiral computed tomography with 188 ms temporal resolution. J Am Coll Cardiol. 2005;45:123–7. doi: 10.1016/j.jacc.2004.10.050. [DOI] [PubMed] [Google Scholar]
- 27.Ghersin E, Litmanovich D, Dragu R, et al. 16-MDCT coronary angiography versus invasive coronary angiography in acute chest pain syndrome: A blinded prospective study. AJR Am J Roentgenol. 2006;186:177–84. doi: 10.2214/AJR.04.1232. [DOI] [PubMed] [Google Scholar]
- 28.Martuscelli E, Romagnoli A, D’Eliseo A, et al. Accuracy of thin-slice computed tomography in the detection of coronary stenoses. Eur Heart J. 2004;25:1043–8. doi: 10.1016/j.ehj.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 29.Nikolaou K, Rist C, Wintersperger BJ, et al. Clinical value of MDCT in the diagnosis of coronary artery disease in patients with a low pretest likelihood of significant disease. AJR Am J Roentgenol. 2006;186:1659–68. doi: 10.2214/AJR.05.0726. [DOI] [PubMed] [Google Scholar]
- 30.Kuettner A, Trabold T, Schroeder S, et al. Noninvasive detection of coronary lesions using 16-detector multislice spiral computed tomography technology: Initial clinical results. J Am Coll Cardiol. 2004;44:1230–7. doi: 10.1016/j.jacc.2004.05.079. [DOI] [PubMed] [Google Scholar]
- 31.Nieman K, Cademartiri F, Lemos PA, Raaijmakers R, Pattynama PM, de Feyter PJ. Reliable noninvasive coronary angiography with fast submillimeter multislice spiral computed tomography. Circulation. 2002;106:2051–4. doi: 10.1161/01.cir.0000037222.58317.3d. [DOI] [PubMed] [Google Scholar]
- 32.Kefer J, Coche E, Legros G, et al. Head-to-head comparison of three-dimensional navigator-gated magnetic resonance imaging and 16-slice computed tomography to detect coronary artery stenosis in patients. J Am Coll Cardiol. 2005;46:92–100. doi: 10.1016/j.jacc.2005.03.057. [DOI] [PubMed] [Google Scholar]
- 33.Mollet NR, Cademartiri F, Krestin GP, et al. Improved diagnostic accuracy with 16-row multi-slice computed tomography coronary angiography. J Am Coll Cardiol. 2005;45:128–32. doi: 10.1016/j.jacc.2004.09.074. [DOI] [PubMed] [Google Scholar]
- 34.Achenbach S, Ropers D, Pohle FK, et al. Detection of coronary artery stenoses using multi-detector CT with 16 × 0.75 collimation and 375 ms rotation. Eur Heart J. 2005;26:1978–86. doi: 10.1093/eurheartj/ehi326. [DOI] [PubMed] [Google Scholar]
- 35.Cademartiri F, Runza G, Marano R, et al. Diagnostic accuracy of 16-row multislice CT angiography in the evaluation of coronary segments. Radiol Med (Torino) 2005;109:91–7. [PubMed] [Google Scholar]
- 36.Manghat NE, Morgan-Hughes GJ, Broadley AJ, et al. 16-detector row computed tomographic coronary angiography in patients undergoing evaluation for aortic valve replacement: Comparison with catheter angiography. Clin Radiol. 2006;61:749–57. doi: 10.1016/j.crad.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 37.Hoffmann U, Moselewski F, Cury RC, et al. Predictive value of 16-slice multidetector spiral computed tomography to detect significant obstructive coronary artery disease in patients at high risk for coronary artery disease: Patient-versus segment-based analysis. Circulation. 2004;110:2638–43. doi: 10.1161/01.CIR.0000145614.07427.9F. [DOI] [PubMed] [Google Scholar]
- 38.Kefer JM, Coche E, Vanoverschelde JL, Gerber BL. Diagnostic accuracy of 16-slice multidetector-row CT for detection of in-stent restenosis vs detection of stenosis in nonstented coronary arteries. Eur Radiol. 2007;17:87–96. doi: 10.1007/s00330-006-0291-5. [DOI] [PubMed] [Google Scholar]
- 39.Babapulle MN, Joseph L, Belisle P, Brophy JM, Eisenberg MJ. A hierarchical Bayesian meta-analysis of randomised clinical trials of drug-eluting stents. Lancet. 2004;364:583–91. doi: 10.1016/S0140-6736(04)16850-5. [DOI] [PubMed] [Google Scholar]
- 40.Maintz D, Fischbach R, Juergens K, Allkemper T, Wessling J, Heindel W. Multislice CT-angiography of the iliac arteries in the presence of various stents: In vitro evaluation of artefacts and lumen visibility. Invest Radiol. 2001;36:699–704. doi: 10.1097/00004424-200112000-00004. [DOI] [PubMed] [Google Scholar]
- 41.Raza SA, Chughtai AR, Wahba M, Cowling MG, Taube D, Wright AR. Multislice CT angiography in renal artery stent evaluation: Prospective comparison with intra-arterial digital subtraction angiography. Cardiovasc Intervent Radiol. 2004;27:9–15. doi: 10.1007/s00270-003-2685-y. [DOI] [PubMed] [Google Scholar]
- 42.Maintz D, Juergens KU, Wichter T, Grude M, Heindel W, Fischbach R. Imaging of coronary artery stents using multislice computed tomography: In vitro evaluation. Eur Radiol. 2003;13:830–5. doi: 10.1007/s00330-002-1651-4. [DOI] [PubMed] [Google Scholar]
- 43.Gilard M, Cornily JC, Pennec PY, et al. Assessment of coronary artery stents by 16 slice computed tomography. Heart. 2006;92:58–61. doi: 10.1136/hrt.2004.056010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soon KH, Cox N, Chaitowitz I, et al. Non-invasive computed tomography angiography in the assessment of coronary stent patency: An Australian experience. Intern Med J. 2007;37:360–4. doi: 10.1111/j.1445-5994.2007.01363.x. [DOI] [PubMed] [Google Scholar]
- 45.Schuijf JD, Bax JJ, Jukema JW, et al. Feasibility of assessment of coronary stent patency using 16-slice computed tomography. Am J Cardiol. 2004;94:427–30. doi: 10.1016/j.amjcard.2004.04.057. [DOI] [PubMed] [Google Scholar]
- 46.Kitagawa T, Fujii T, Tomohiro Y, et al. Noninvasive assessment of coronary stents in patients by 16-slice computed tomography. Int J Cardiol. 2006;109:188–94. doi: 10.1016/j.ijcard.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 47.Ohnuki K, Yoshida S, Ohta M, et al. New diagnostic technique in multi-slice computed tomography for in-stent restenosis: Pixel count method. Int J Cardiol. 2006;108:251–8. doi: 10.1016/j.ijcard.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 48.Gaspar T, Halon DA, Lewis BS, et al. Diagnosis of coronary in-stent restenosis with multidetector row spiral computed tomography. J Am Coll Cardiol. 2005;46:1573–9. doi: 10.1016/j.jacc.2005.07.049. [DOI] [PubMed] [Google Scholar]
- 49.Cademartiri F, Schuijf JD, Pugliese F, et al. Usefulness of 64-slice multislice computed tomography coronary angiography to assess in-stent restenosis. J Am Coll Cardiol. 2007;49:2204–10. doi: 10.1016/j.jacc.2007.02.045. [DOI] [PubMed] [Google Scholar]
- 50.Pugliese F, Weustink AC, Van Mieghem C, et al. Dual-source coronary computed tomography angiography for detecting in-stent restenosis. Heart. 2008;94:848–54. doi: 10.1136/hrt.2007.126474. [DOI] [PubMed] [Google Scholar]
- 51.Cademartiri F, Palumbo A, Maffei E, et al. Diagnostic accuracy of 64-slice CT in the assessment of coronary stents. 2007;112:526–37. doi: 10.1007/s11547-007-0159-z. [DOI] [PubMed] [Google Scholar]
- 52.Rixe J, Achenbach S, Ropers D, et al. Assessment of coronary artery stent restenosis by 64-slice multi-detector computed tomography. Eur Heart J. 2006;27:2567–72. doi: 10.1093/eurheartj/ehl303. [DOI] [PubMed] [Google Scholar]
- 53.Das KM, El-Menyar AA, Salam AM, et al. Contrast-enhanced 64-section coronary multidetector CT angiography versus conventional coronary angiography for stent assessment. Radiology. 2007;245:424–32. doi: 10.1148/radiol.2452061389. [DOI] [PubMed] [Google Scholar]
- 54.Schuijf JD, Pundziute G, Jukema JW, et al. Evaluation of patients with previous coronary stent implantation with 64-section CT. Radiology. 2007;245:416–23. doi: 10.1148/radiol.2452061199. [DOI] [PubMed] [Google Scholar]
- 55.Carbone I, Francone M, Algeri E, et al. Non-invasive evaluation of coronary artery stent patency with retrospectively ECG-gated 64-slice CT angiography. Eur Radiol. 2008;18:234–4. 3. doi: 10.1007/s00330-007-0756-1. [DOI] [PubMed] [Google Scholar]
- 56.Oncel D, Oncel G, Karaca M. Coronary stent patency and in-stent restenosis: Determination with 64-section multidetector CT coronary angiography – initial experience. Radiology. 2007;242:403–9. doi: 10.1148/radiol.2422060065. [DOI] [PubMed] [Google Scholar]
- 57.FitzGibbon GM, Leach AJ, Kafka HP, Keon WJ. Coronary bypass graft fate: Long-term angiographic study. J Am Coll Cardiol. 1991;17:1075–80. doi: 10.1016/0735-1097(91)90834-v. [DOI] [PubMed] [Google Scholar]
- 58.Wyman RM, Safian RD, Portway V, Skillman JJ, McKay RG, Baim DS. Current complications of diagnostic and therapeutic cardiac catheterization. J Am Coll Cardiol. 1988;12:1400–6. doi: 10.1016/s0735-1097(88)80002-0. [DOI] [PubMed] [Google Scholar]
- 59.Fitzgibbon GM, Kafka HP, Leach AJ, Keon WJ, Hooper GD, Burton JR. Coronary bypass graft fate and patient outcome: Angiographic follow-up of 5,065 grafts related to survival and reoperation in 1,388 patients during 25 years. J Am Coll Cardiol. 1996;28:616–26. doi: 10.1016/0735-1097(96)00206-9. [DOI] [PubMed] [Google Scholar]
- 60.Onuma Y, Tanabe K, Chihara R, et al. Evaluation of coronary artery bypass grafts and native coronary arteries using 64-slice multidetector computed tomography. Am Heart J. 2007;154:519–26. doi: 10.1016/j.ahj.2007.04.054. [DOI] [PubMed] [Google Scholar]
- 61.Jabara R, Chronos N, Klein L, et al. Comparison of multidetector 64-slice computed tomographic angiography to coronary angiography to assess the patency of coronary artery bypass grafts. Am J Cardiol. 2007;99:1529–34. doi: 10.1016/j.amjcard.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 62.Ropers D, Pohle FK, Kuettner A, et al. Diagnostic accuracy of noninvasive coronary angiography in patients after bypass surgery using 64-slice spiral computed tomography with 330-ms gantry rotation. Circulation. 2006;114:2334–41. doi: 10.1161/CIRCULATIONAHA.106.631051. [DOI] [PubMed] [Google Scholar]
- 63.Feuchtner GM, Schachner T, Bonatti J, et al. Diagnostic performance of 64-slice computed tomography in evaluation of coronary artery bypass grafts. AJR Am J Roentgenol. 2007;189:574–80. doi: 10.2214/AJR.07.2174. [DOI] [PubMed] [Google Scholar]
- 64.Martuscelli E, Romagnoli A, D’Eliseo A, et al. Evaluation of venous and arterial conduit patency by 16-slice spiral computed tomography. Circulation. 2004;110:3234–8. doi: 10.1161/01.CIR.0000147277.52036.07. [DOI] [PubMed] [Google Scholar]
- 65.Chiurlia E, Menozzi M, Ratti C, Romagnoli R, Modena MG. Follow-up of coronary artery bypass graft patency by multislice computed tomography. Am J Cardiol. 2005;95:1094–7. doi: 10.1016/j.amjcard.2004.12.067. [DOI] [PubMed] [Google Scholar]
- 66.Houslay ES, Lawton T, Sengupta A, Uren NG, McKillop G, Newby DE. Non-invasive assessment of coronary artery bypass graft patency using 16-slice computed tomography angiography. J Cardiothorac Surg. 2007;2:27. doi: 10.1186/1749-8090-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yamamoto M, Kimura F, Niinami H, Suda Y, Ueno E, Takeuchi Y. Noninvasive assessment of off-pump coronary artery bypass surgery by 16-channel multidetector-row computed tomography. Ann Thorac Surg. 2006;81:820–7. doi: 10.1016/j.athoracsur.2005.08.069. [DOI] [PubMed] [Google Scholar]
- 68.Anders K, Baum U, Schmid M, et al. Coronary artery bypass graft (CABG) patency: Assessment with high-resolution submillimeter 16-slice multidetector-row computed tomography (MDCT) versus coronary angiography. Eur J Radiol. 2006;57:336–44. doi: 10.1016/j.ejrad.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 69.Salm LP, Bax JJ, Jukema JW, et al. Comprehensive assessment of patients after coronary artery bypass grafting by 16-detector-row computed tomography. Am Heart J. 2005;150:775–81. doi: 10.1016/j.ahj.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 70.Moore RK, Sampson C, MacDonald S, Moynahan C, Groves D, Chester MR. Coronary artery bypass graft imaging using ECG-gated multislice computed tomography: Comparison with catheter angiography. Clin Radiol. 2005;60:990–8. doi: 10.1016/j.crad.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 71.Meyer TS, Martinoff S, Hadamitzky M, et al. Improved noninvasive assessment of coronary artery bypass grafts with 64-slice computed tomographic angiography in an unselected patient population. J Am Coll Cardiol. 2007;49:946–50. doi: 10.1016/j.jacc.2006.10.066. [DOI] [PubMed] [Google Scholar]
- 72.Pache G, Saueressig U, Frydrychowicz A, et al. Initial experience with 64-slice cardiac CT: Non-invasive visualization of coronary artery bypass grafts. Eur Heart J. 2006;27:976–80. doi: 10.1093/eurheartj/ehi824. [DOI] [PubMed] [Google Scholar]
- 73.Malagutti P, Nieman K, Meijboom WB, et al. Use of 64-slice CT in symptomatic patients after coronary bypass surgery: Evaluation of grafts and coronary arteries. Eur Heart J. 2007;28:1879–85. doi: 10.1093/eurheartj/ehl155. [DOI] [PubMed] [Google Scholar]
- 74.Brodoefel H, Reimann A, Heuschmid M, et al. Non-invasive coronary angiography with 16-slice spiral computed tomography: Image quality in patients with high heart rates. Eur Radiol. 2006;16:1434–41. doi: 10.1007/s00330-006-0155-z. [DOI] [PubMed] [Google Scholar]
- 75.Coles DR, Smail MA, Negus IS, et al. Comparison of radiation doses from multislice computed tomography coronary angiography and conventional diagnostic angiography. J Am Coll Cardiol. 2006;47:1840–5. doi: 10.1016/j.jacc.2005.11.078. [DOI] [PubMed] [Google Scholar]
- 76.Hausleiter J, Meyer T, Hadamitzky M, et al. Radiation dose estimates from cardiac multislice computed tomography in daily practice: Impact of different scanning protocols on effective dose estimates. Circulation. 2006;113:1305–10. doi: 10.1161/CIRCULATIONAHA.105.602490. [DOI] [PubMed] [Google Scholar]
- 77.Peebles C. Computed tomographic coronary angiography: How many slices do you need? Heart. 2006;92:582–4. doi: 10.1136/hrt.2005.082198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Statistics Canada Population and demographics (updated November 29, 2007). <http://www40.statcan.ca/l01/cst01/demo10a.htm> (Version current at January 2008).
- 79.United States Census Bureau Population estimate by sex and age (updated July 1, 2006). <http://www.census.gov/popest/national/asrh/NC-EST2006-sa.html> (Version current at January 2008).
- 80.Toprak O. Risk markers for contrast-induced nephropathy. Am J Med Sci. 2007;334:283–90. doi: 10.1097/MAJ.0b013e318068ddf9. [DOI] [PubMed] [Google Scholar]
- 81.Marenzi G, Lauri G, Assanelli E, et al. Contrast-induced nephropathy in patients undergoing primary angioplasty for acute myocardial infarction. J Am Coll Cardiol. 2004;44:1780–5. doi: 10.1016/j.jacc.2004.07.043. [DOI] [PubMed] [Google Scholar]
- 82.McCullough P. Outcomes of contrast-induced nephropathy: Experience in patients undergoing cardiovascular intervention. Catheter Cardiovasc Interv. 2006;67:335–43. doi: 10.1002/ccd.20658. [DOI] [PubMed] [Google Scholar]
- 83.Johnson PT, Eng J, Pannu HK, Fishman EK. 64-MDCT angiography of the coronary arteries: Nationwide survey of patient preparation practice. AJR Am J Roentgenol. 2008;190:743–7. doi: 10.2214/AJR.07.2620. [DOI] [PubMed] [Google Scholar]