Abstract
CD4+FoxP3+ Tregs are essential mediators of the peripheral immune response to self-antigens. Accordingly, the homeostatic regulation of Treg activity and number would impact on the immune response to both self- and non-self antigens. Because the sympathetic nervous system (SNS) interacts chemically and physically with the central and peripheral immune system and exerts a direct influence on antigen-presenting cells and effector lymphocytes, we have investigated the effect of chemical ablation of the SNS on the number and function of peripheral Treg. Removal of murine peripheral sympathetic innervation by 6-hydroxydopamine induced an increase in splenic and lymph node CD4+FoxP3+ Tregs by a TGF-β-dependent mechanism. Further, this increase in Tregs coincides with an inhibition of the induction of experimental autoimmune encephalomyelitis. Our results demonstrate that the SNS is an important contributor to the maintenance of peripheral Treg and TGF-β acts as a bridge between the immune system and the nervous system. Neurological events mediated by the SNS, such as a stress response, may affect the number of T cells that regulate an immune response. Additionally, targeting Tregs via the SNS may be a novel approach to the prevention or treatment of autoimmune diseases.
Keywords: EAE, neuroimmunology, sympathectomy
Introduction
Immune tolerance to self-antigens is maintained by mechanisms that control potentially pathogenic autoreactive lymphocytes by deletion, clonal anergy, and suppression by CD4+FoxP3+ Tregs [1, 2]. Therefore, the manipulation of Foxp3+ Tregs has proved to be a promising way to restore self-tolerance; and prevent autoimmune or inflammatory conditions [3,4,5,6,7,8,9]. Classically, Tregs that occur naturally or are induced have been defined as CD4+ T lymphocytes, expressing the interleukin-2 receptor α-chain (CD25) on the cell surface and the transcription factor FoxP3 [4, 10]. Although CD25 has been the most used marker to enumerate Treg cells [4, 8], the CD4+CD25+ phenotype is not Treg cell specific because some CD25− T cells also display regulatory activity [5, 11, 12]. The mechanism(s) by which the number of Treg cells is controlled in the periphery remains poorly understood but holds potential toward treating autoimmune diseases.
The SNS interacts with the immune system chemically and physically, thereby exerting a significant influence on an immune response (reviewed in [13,14,15,16,17]). The influence of the SNS on the immune response has been demonstrated by the ablation of the SNS innervations (sympathectomy) of mice with the neurotoxin 6-OHDA that when administered peripherally depletes NE levels in the lymphoid organs by more than 90% [18]. Because 6-OHDA does not cross the blood-brain barrier (BBB), peripheral administration does not cause any reduction in the levels of CNS catecholamines [19]. Administration of 6-OHDA directly into the CNS of rats induces cells that transfer the suppression of humoral immunity, or an increase in OX8+ cells concomitant with the suppression of EAE [20,21,22]. Peripheral administration of 6-OHDA to mice 1) depletes peripheral catecholamine levels [23, 24], 2) prevents the expression of, cell-mediated immunity but not necessarily humoral immunity [24, 25]; 3) prevents the generation of antigen-specific CD8+ splenic regulatory T cells induced by an injection of antigen into the anterior chamber of an eye [25]. This inability to generate CD8+ regulatory T cells in 6-OHDA-treated mice may be due to a loss of NE-dependent regulatory NK T cells [26], necessary to induce the CD8+ regulatory T cells [27]. Taken together, these observations suggest that the SNS has a profound influence on immune homeostasis by participating in the maintenance of T cells that regulate both humoral and cell-mediated immune responses. But despite a plethora of data published on the SNS and immune regulation [13,14,15, 20,21,22,23,24,25], a causative association between the SNS and cell-mediated immune regulation requires further investigation.
Accordingly, the current study was undertaken to evaluate the influence of the SNS on immune regulation and tolerance by exploring the possibility that SNS innervations in the periphery may be an important modulator of Tregs. For this purpose, we investigated the affect of 6-OHDA-induced peripheral sympathectomy on the number and function of peripheral CD4+FoxP3+ Tregs and its affect on EAE. We show that the ablation of sympathetic innervations in the periphery causes a robust increase in the number of CD4+FoxP3+ Tregs in the spleens and lymph nodes of mice treated with 6-OHDA. Further, we found that this increase in Treg number was due to an induction of Tregs from naïve T cells mediated by TGF-β. Consistent with a role of Tregs in maintaining immune tolerance, 6-OHDA-treated animals with an increased Treg number did not develop EAE. Thus, these results demonstrate that the SNS plays an important role in immune homeostasis by maintaining the number of Tregs in the periphery.
MATERIALS AND METHODS
Animals
Female C57BL/6 mice 6–8 weeks old were purchased from Charles River Laboratories (Wilmington, MA, USA). TGFbRII mice obtained from Dr. Richard Flavell, Yale University, New Haven, USA, are maintained at the University of Connecticut Health Center. Cbl-b−/− mice are maintained at the University of Connecticut Health Center. All animals were maintained by the Center for Laboratory Animal Care at the University of Connecticut Health Center. The use of animals adhered to the Association for Research in Vision and Ophthalmology resolution on the use of animals in ophthalmic and vision research. All work with animals has been reviewed and approved by the University of Connecticut Health Center Animal Care Committee (ACC 2004-380).
Reagents
6-Hydroxydopamine hydrobromide (6-OHDA), desipramine, and propranolol were purchased from Sigma (St. Louis, MO, USA). MOG35–55 (MEVGWYRSPFSRVVHLYRNGK) peptide was prepared by the W. M. Keck facility at Yale University, New Haven, CT. IFA and heat-killed Mycobacterium tuberculosis H37Ra were purchased from Difco Laboratory (Detroit, MI, USA). Pertussis toxin was purchased from List Biological Labs (Campbell, CA, USA). For flow cytometry, anti-CD3, anti-CD4, anti-FoxP3, anti-CD8, and anti-CD122 antibodies with appropriate isotype controls were purchased from eBiosciences (San Diego, CA, USA). FoxP3 staining kit was purchased from eBiosciences. For cell proliferation and stimulation assays, anti-CD3 (145-2C11) was purchased from BD PharMingen (San Jose, CA, USA). CFSE dye was purchased from Molecular Probes (Carlsbad, CA, USA).
Sympathetic denervation
Two days before the removal of spleens and lymph nodes, mice received 6-OHDA intraperitoneally (200 mg/kg body weight). A solution of 6-OHDA in PBS (pH 7.2) was prepared fresh for each experiment and used immediately. To determine the specificity of denervation by 6-OHDA, some mice received concurrently intraperitoneal 6-OHDA and 10 mg/kg body weight of the antagonist desipramine (Sigma) or desipramine 2 h before the injection of 6-OHDA. Some groups received 5 mg/kg propranolol 1 h after 6-OHDA injection.
Flow cytometry
Cell samples were washed in PBS (PBS, pH 7.2) containing 0.2% BSA and 0.1% NaN3 (FACS buffer). Aliquots containing ∼106 cells were incubated with 100 μl of appropriately diluted Abs for 30 min at 4°C. For the identification of Foxp3+ Treg cells, cells stained with anti-CD3ε, anti-CD4, were permeabilized using fixation/permeabilization buffer (eBiosciences) following the manufacturer’s protocol, and stained using anti-Foxp3-allophycocyanin (FJK-16 s) with corresponding isotype controls, IgG2a-allophycocyanin (eBiosciences). Flow cytometry analysis was done using FACSCalibur (BD Biosciences, San Jose, CA, USA) and FLOWJO (Tree Star, Ashland, OR, USA) software.
In vitro T-cell proliferation assay
The proliferative responses of CD4+ T cells to CD4+ Fox P3+ Tregs obtained from PBS and 6-OHDA-treated animals were compared. Tregs and CD4+ T effectors were separated from spleen cells using the MACS CD4+CD25+ Regulatory T cell isolation Kit, as per the manufacturer’s instructions. Cell preparations were depleted of dead and dying cells with a Dead Cell Removal kit (Miltenyi Biotec, Bergisch Gladbach, Germany). Populations of CD4+ CD25– T cells were incubated with 1 μM carboxyfluoroscein succinimidyl ester (CFSE) for 10 min at room temperature. These T-cell subsets (5 × 104 cells /well) were stimulated for 3 days in triplicate wells with 5 × 104 irradiated (3,000 rad) autologous PBMCs as antigen-presenting cells and 0.5 μg/ml of anti-CD3 antibody in Corning 96-well round-bottomed plates, in the presence or absence of isolated purified Tregs, at different ratios. On the fourth day, proliferation was measured by CFSE dilution using a FACS Calibur apparatus with FlowJo software (Becton Dickinson, San Jose, CA, USA).
Real-time qRT-PCR analysis
Total RNA was isolated from spleen cells using the RNeasy mini kit (Qiagen, Valencia, CA, USA) and treated with Turbo DNA-free DNase (Ambion, Austin, TX, USA). cDNA was synthesized using Superscript III reverse transcriptase (Invitrogen, Carlsbad, CA, USA). Ten micrograms of cDNA was amplified by qRT-PCR in a 25-μl reaction using SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA). Primers were designed using Primer Express software v3.0 and primer efficiency verified by slope analysis to be 100% ± 2.5%. qRT-PCR was performed using an ABI 7500 fast system and data analyzed using the Δct method (SDS software v3.0). Primer sequences (Invitrogen and IDT, Coralville, IA, USA) are listed in Table 1. Amplicon sizes were ∼100 bp.
TABLE 1.
Primers Used in qRT-PCR Analysis of TGF-β
| Gene | Forward primer | Reverse primer |
|---|---|---|
| TGF-β1 | TCCCCCGAGAGGCAGATC | ATCGAGATGAGCGCTCTCTGA |
| RPL-19 | CCAAGAAGATTGACCGCCAT | CAGCTTGTGGATGTGCTCCAT |
Immunizations
Experimental autoimmune encephalomyelitis
For the induction of active experimental autoimmune encephalomyelitis (EAE), 2 days after the injection of 6-OHDA or PBS, C57BL/6 mice were injected intradermally with 200 μg of emulsion containing 200 μg MOG35–55 in IFA supplemented with 500 μg M. tuberculosis. Mice were injected i.p. with 200 ng of pertussis toxin (PTX) in 100 μl of PBS shortly after and 48 h after the first immunization. Following immunization, animals were kept under observation to score the disease. The study was done in a blinded fashion. The clinical scale measured was as follows: 0 = normal, 1 = limp tail, 2 = paraparesis with a clumsy gait, 3 = hind limb paralysis, 4 = quadriplegia, and 5 = death.
Induction of Delayed-Type Hypersensitivity
Mice were immunized with an injection of 200 μg MOG peptide in CFA into a flank. The control group did not receive any immunization. Seven days after immunization the mice were anesthetized with ketamine (75 mg/kg) and xylazine (15 mg/kg) and footpad thickness of both hind footpads measured with a digital micrometer (Mitatoyo, Tokyo, Japan). One footpad was challenged with an intradermal (ID) injection of 50 μg MOG peptide in PBS and the other footpad was challenged with vehicle only. Approximately 24 h later, the mice were anesthetized with ketamine/xylazine and footpad thickness measured. Swelling was computed as the difference in thickness (in μm) of the challenged footpad at 24 h minus the difference in thickness (in μm) of the vehicle-challenged footpad at 24 h. After we assessed the swelling, the mice were killed, and antigen or vehicle-challenge feet were removed and fixed with 10% buffered formalin. The feet were processed by the Department of pathology Research histology Laboratory and stained with hematoxylin and eosin.
Isolation of CD4+ T cells and adoptive transfer
CD4+ T cells were isolated using the CD4+ T cell isolation kit (Miltenyi Biotec) as per the manufacturer’s instructions. Cell preparations were depleted of dead and dying cells with a Dead Cell Removal kit (Miltenyi Biotec). The isolated population was between 90 and 95% pure for CD4+ T cells. For adoptive transfer, a total of 1 × 106 cells were injected i.v. via the tail vein. Injections were done on the day of immunization with MOG peptide.
Statistics
Foot pad swelling, TGF-β mRNA levels, and frequency of Tregs were compared between PBS- treated and 6-OHDA-treated groups by paired t tests. When comparing more than two groups, repeated-measures ANOVA was used. In all comparisons, P < 0.05 was used to determine statistical significance.
RESULTS
Delayed-type hypersensitivity swelling response to MOG peptide is reduced in mice receiving 6-OHDA
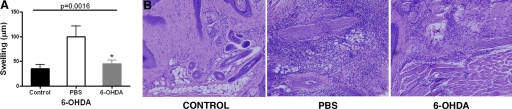
To document further the reduction in delayed-type hypersensitivity (DTH) in mice receiving 6-OHDA, mice were immunized with MOG and CFA 2 days after the injection of 6-OHDA. The dose of 6-OHDA used to ablate the SNS did not cause any abnormality or apparent neurological symptoms in the animals. The swelling of a DTH reaction induced by antigenic (MOG) challenge of mice immunized with MOG after receiving 6-OHDA was reduced or did not occur (Fig. 1A). There was a significant increase in monocytic cells at the site challenged by antigen (in 6-OHDA-treated mice) relative to the unchallenged footpad, although the number of infiltrated cells was somewhat less than that of mice receiving PBS (Fig. 1B).
Figure 1.
Abrogation of DTH reaction in 6-OHDA-treated, immunized mice. Mice were immunized with MOG/CFA 2 days after receiving i.p. dose of 6-OHDA (200 mg/kg). Control groups did not receive any immunization. Seven days after immunizing, one footpad was challenged with the MOG and the other footpad with vehicle only. (A) The thickness of footpads measured 24 h after challenge. Bar graph represents the mean ± SE footpad swelling of 6 mice/group, 2 experiments. (B) Histology of the footpad 24 h after challenge with MOG from immunized mice without challenge (Control), immunized mice that received an i.p. injection of PBS or 6-OHDA 2 days before immunization and then challenged. Mice showed perivascular infiltration of cells in the PBS or 6-OHDA-treated groups. Magnification ×20.
Peripheral chemical sympathectomy by 6-OHDA induces an increase in the number of peripheral Tregs
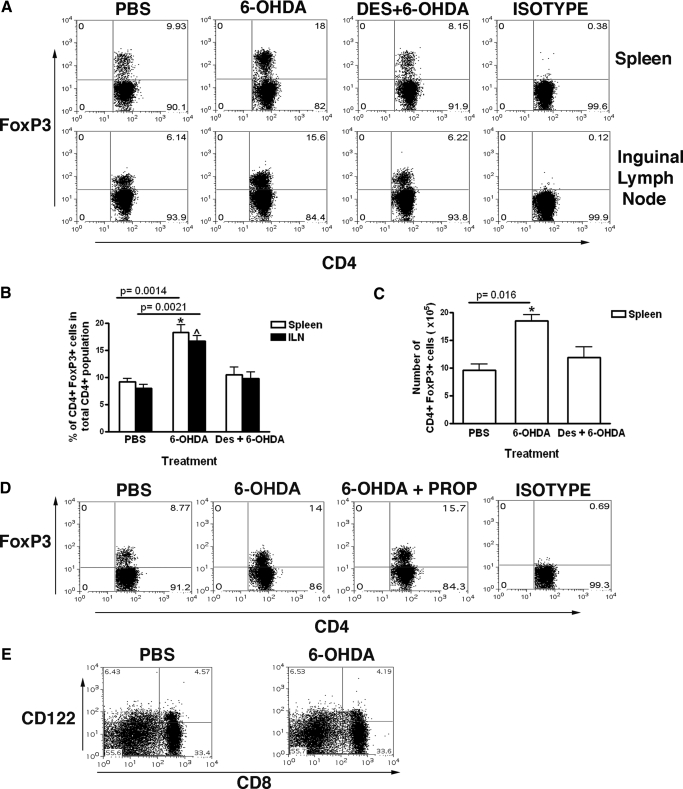
To investigate the effect of 6-OHDA on Treg, 48 h following 6-OHDA administration, spleen and inguinal lymph node cell suspensions were stained with anti-CD4, CD25, and FoxP3. There was a robust increase in the frequency of CD4+ FoxP3 + T cells, from ∼9.8% to 18% of the total CD4+ T cells, both in the spleen and the inguinal lymph nodes (ILN) (Fig. 2A, B). This increase in the number of T regs was found to be consistent when the absolute number of cells with the same phenotype was computed (Fig. 2C). To ensure that the increase in T cells with a Treg phenotype was due to 6-OHDA and to determine the specificity of 6-OHDA-induced sympathetic denervation, we used desipramine (DES) to specifically prevent the uptake of 6-OHDA by sympathetic neurons. Treatment with DES 2 h before 6-OHDA administration completely prevented the 6-OHDA-mediated increase in the number of CD4+ FoxP3+ T cells (Fig. 2A–C). Moreover, treatment with propranolol (a β-blocker that prevents the uptake of NE) after 6-OHDA injection did not affect the 6-OHDA-induced increase in the number of splenic and lymph node CD4+FoxP3+ T cells, suggesting that the affect of 6-OHDA was not due to a bolus release of norepinephrine after sympathectomy (Fig. 2D). Although there are no well-defined markers for CD8+ regulatory T cells [28, 29], recently, it has been shown that CD8+ CD122+ regulatory T cells play an essential role in protecting mice from EAE [30]. Therefore, we determined the number of CD8+CD122+ T regs before and after 6-OHDA treatment. There was no change in the frequency of CD8+ T cells in the spleen after treatment with 6-OHDA (Fig. 2E).
Figure 2.
Peripheral chemical sympathectomy by 6-OHDA induces an increase in the number of Tregs. (A) Representative dot plots show flow cytometric analysis of mouse (C57BL/6) spleen and inguinal lymph node cells at 48-h post injection of PBS, 6-OHDA, or desipramine and 6-OHDA. Plots represent gated CD4 T cells. The values in the upper-right quadrant represent CD4+ FoxP3+ T cells as a percentage of total CD4 T cells. (B) Bar graph shows the mean results (n=12–15). (C) Bar graph showing the absolute number of CD4+ FoxP3+ Tregs from the above experiment. Numbers were calculated from flow cytometry data from the spleen. (D) Propranolol fails to prevent the increase in Tregs by chemical sympathectomy: representative dot plots show flow cytometric analysis of mouse (C57BL/6) spleen and inguinal lymph node cells at 48 h postinjection of PBS, 6-OHDA, or 6-OHDA followed by propranolol. Plots represent gated CD4 T cells. The values in the upper-right quadrant represent CD4+ FoxP3+ T cells as a percentage of total CD4 T cells. (E) 6-OHDA does not effect the number of total CD8+ cells or CD8+ CD122+ cells. Representative dot plots show flow cytometric analysis of mouse (C57BL/6) spleen cells at 48 h postinjection of PBS or 6-OHDA. Cells are gated for CD3+. The values in the upper right quadrant represent CD8+ CD122+ T cells.
Tregs from 6-OHDA-treated mice are functionally similar to Tregs from PBS-treated mice
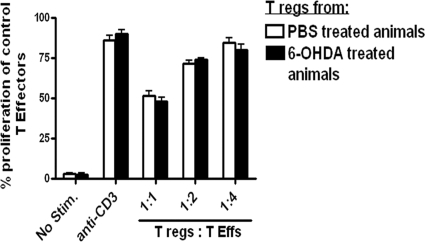
The effect of chemical sympathectomy on the functional ability of the Tregs was investigated with an in vitro suppression assay of anti-CD3-induced proliferation of effector T cells. Splenic Treg cells isolated from sympathectomized mice or PBS- treated mice were co-cultured with CFSE-labeled CD4+ T effectors (CD4 T effs) from naïve mice, in the presence of anti-CD3 and antigen presenting cells. After 3 days of culture, anti- CD3- induced proliferation of the T effs in the presence or absence of Tregs was measured on the basis of CFSE dilution. We found that there was no change in the ability of the Tregs isolated from sympathectomized animals to suppress anti-CD3-induced proliferation of T effs, as compared with PBS-treated controls (Fig. 3). These results suggest that the SNS may be involved in Treg homeostasis, but without affecting their function.
Figure 3.
Tregs from 6-OHDA-treated mice are functionally similar to Tregs from PBS-treated mice. In vitro Treg functional assay: CD4+CD25– T cells (T effs) from the spleen of naïve C57BL/6 were labeled with CFSE, in each group. CFSE-labeled responder T effs did not proliferate in unstimulated conditions (No Stim.) but did proliferate in the presence of soluble anti-CD3 as demonstrated by the dilution of CFSE in the stimulated group. Tregs cocultured with T effs and antigen-presenting cells (live splenic DCs) in the presence of anti-CD3, as described, suppress the proliferation of responder T eff cells as quantified by CFSE dilution. Tregs were isolated from control or 6-OHDA-treated animals and cocultured with naïve T effs at a 1:1, 1:2, 1:4 Treg-to-T eff ratio.
6-OHDA-induced sympathectomy does not change the frequency of total CD4+ T cells
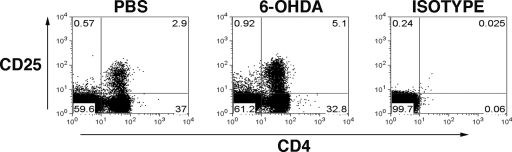
To determine mechanisms responsible for the 6-OHDA-induced increase in peripheral CD4+FoxP3+ T cells, we considered that the increase in Treg could be due to a direct effect of 6-OHDA on T effector cells by “converting” them to a Treg phenotype. Alternatively, 6-OHDA could affect other factors that induce Tregs or there might be an enhanced proliferation of existing Tregs after 6-OHDA treatment. 6-OHDA did not cause a change in the frequency of total CD4+ T cells in the spleen; although the frequency of CD4+ FoxP3+ Tregs and CD4+ CD25+ cells increased (Figs. 1 and 4), suggesting that 6-OHDA induced a conversion of T effectors to a Treg phenotype. To determine a direct affect of 6-OHDA on T cells, T effectors and Tregs were recovered from naïve mice and incubated with 6-OHDA in vitro. We observed no effect on the proliferation and/or induction of a Treg phenotype by culture with 6-OHDA (data not shown).
Figure 4.
6-OHDA induced sympathectomy does not change the frequency of total CD4+ T cells. C57BL/6 mice were treated with 6-OHDA, while control mice received PBS. Forty-eight hours after injection, total spleen cells were stained. Representative FACS dot plots demonstrate the percentage of CD4+ CD25+ T cells in the spleen, 48 h after treatment. Plots represent a total lymphocyte gate. The values represent different cell types, as a percentage of total lymphocytes.
6-OHDA-induced increase in Tregs is mediated by TGF-β
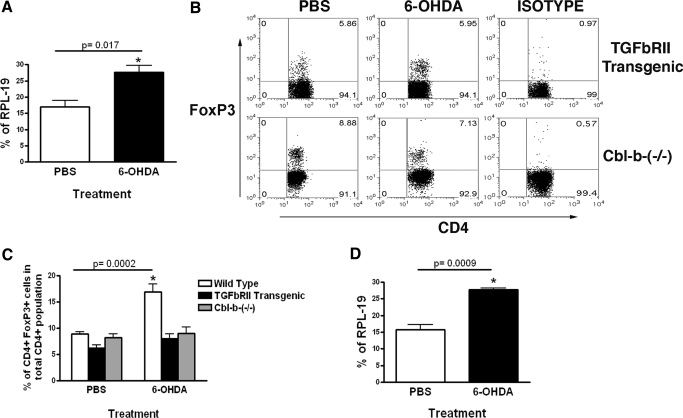
TGF-β has been defined as a major factor involved in the induction and survival of FoxP3+ Tregs [3, 7, 10, 31, 32]. Therefore, we investigated the levels of TGF-β in the spleen following chemical sympathectomy. Injection of 6-OHDA in the periphery resulted in a significant increase in the expression of TGF-β mRNA in spleens as assessed by qRT-PCR (Fig. 5A). To determine whether this increase in TGF-β mRNA is relevant to the 6-OHDA-induced increase in splenic Treg, TGFbRII transgenic and the Cbl-b−/− mice were injected with 6-OHDA. T cells of TGFbRII transgenic mice express a dominant-negative TGF-β receptor II [33], rendering CD4 T cells resistant to TGF-β. Cbl-b−/− T effs also show a reduced responsiveness to TGF-β, despite expressing normal levels of type II TGF-β receptors [34]. Forty-eight hours after 6-OHDA injection, there was no significant change in the Treg frequency in the spleens of TGFbRII transgenic or Cbl-b−/− mice (Fig. 5B, C). However qRT-PCR from the spleen showed that after 6-OHDA injection, there was an increase in the expression of TGF-β mRNA in the TGFbRII transgenic mouse (Fig. 5D). This lack of increase in CD4+ FoxP3+ Tregs following 6-OHDA administration in mice whose T cells are resistant to TGF-β suggests that TGF-β signaling is involved in the 6-OHDA-induced increase of splenic and lymph node Tregs.
Figure 5.
6-OHDA-induced increase in Tregs is mediated by TGF-β. (A) TGF-β mRNA expression in PBS or 6-OHDA-treated C57BL/6 mice, assessed by qRT-PCR from total spleen cells taken 48 h after injection. Bar graph shows TGF-β mRNA expression as a percentage of RPL-19 (a housekeeping gene) expression. (B) Representative FACS dot plots demonstrating the percentage of CD4+ FoxP3+ Tregs in the spleens of PBS-treated or 6-OHDA treated TGFbRII transgenic and Cbl-b-(−/−) mouse, 48 h after treatment. Plots represent gated CD4 T cells. The values in the upper right quadrant represent CD4+ FoxP3+ T cells as a percentage of total CD4 T cells. (C) Bar graph shows the mean result (n=10). (D) TGF-β mRNA expression before and after 6-OHDA induced peripheral sympathectomy in TGFbRII mice qRT-PCR from total spleen cells taken 48 h after injection. Bar graph shows TGF-β mRNA expression as percentage of RPL-19 expression.
6-OHDA-induced peripheral chemical sympathectomy prevents EAE
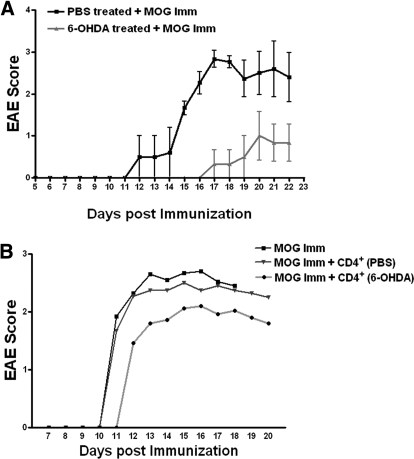
Because 6-OHDA injection caused an increase in the number of CD4+ FoxP3+ Tregs and these cells are known to suppress EAE [4, 8, 9, 35, 36], we explored the possibility of using 6-OHDA as a preventive measure against EAE. The onset of EAE was delayed, and the expression of EAE was reduced significantly in mice that received 6-OHDA before immunization with myelin oligodendrocyte glycoprotein (MOG) (Fig. 6A). To investigate whether the 6-OHDA-induced increase in CD4+ T regs affected the induction of EAE, 1 × 106 splenic CD4+ T cells from PBS or 6-OHDA-treated mice were injected into recipient mice immediately after they were immunized with MOG. CD4+ T cells from the PBS-treated mice did not affect the induction of EAE (Fig. 6B). However 1 × 106 CD4+ T from a 6-OHDA-treated mice did have an ameliorating effect on EAE in the recipient (Fig. 6B), although the suppression was not significant.
Figure 6.
6-OHDA-induced peripheral chemical sympathectomy prevents EAE. (A) Female C57BL/6 mice, 7 to 8 wk old were injected with 6-OHDA, and then, 48 h later were immunized with MOG, as described previously. Clinical scores of vehicle-treated mice (light shade) and mice treated with 6-OHDA (dark shade) (0, normal; 1, limp tail; 2, paraparesis with a clumsy gait; 3, hind limb paralysis; 4, quadriplegia; 5, death) were assessed each day. Data represent mean scores (n=10, vehicle and; n=12, 6-OHDA) ± SE. (B) Mice were immunized with MOG peptide and received 1 × 106 CD4+ T cells (i.v.) from PBS or 6-OHDA-treated mice immediately after immunization. Clinical scores of uninjected (black), PBS-CD4+ T cells-injected (—▾—) and 6-OHDA- CD4+ T cells-injected (light shade) mice, assessed each day. Data represent mean scores (n=10, uninjected; n=10, PBS-CD4+ T cells injected; and n=12, 6-OHDA-CD4+ T cells injected).
DISCUSSION
The administration of 6-OHDA into the CNS (central administration) suppressed EAE in rats [22, 37] and impaired a humoral immune response in mice, possibly by enhancing T-suppressor cell activity [20, 21]. The central administration of 6-OHDA prior to immunization 1) did not affect the number of mononuclear cells infiltrating the spinal cord but increased the number of cells bearing the T-suppressor cell phenotype OX8 in the perivascular lesions in rats [22] and 2) enhanced splenic T suppressor cell activity in mice [20]. However, because 6-OHDA cannot cross the BBB, the effects of a peripheral administration of 6-OHDA on regulatory T cells remain inconclusive. In contrast to the central administration of 6-OHDA, it has been reported that chemical ablation of the SNS by peripheral administration of 6-OHDA enhanced EAE in Lewis rats [38, 39] and mice [40]. Newborn Lewis rats were used to induce peripheral sympathectomy [38, 39]. Because in newborn rodents, the BBB is imperfectly developed, 6-OHDA may enter the brain and damage central dopaminergic and noradrenergic neurons in addition to its peripheral action. Taken together, these studies suggest that different routes of neurotoxin administration produce different patterns of sympathetic denervation that may be involved in disease development and progression, as seen in the case of adjuvant-induced arthritis [41]. Moreover, although earlier investigations suggested that chemical sympathectomy enhanced regulatory/suppressor T cells [20, 22], definitive demonstration on phenotypically identified Tregs is still lacking.
Therefore, we investigated the affects of peripheral sympathectomy of mice by 6-OHDA on the expression and function of peripheral Tregs. The dose of 6-OHDA used to sympathectomize the mice (200 mg/kg) did not induce obvious neurologic damage. In fact, antibody production is not affected or is sometimes enhanced in mice receiving this dose of 6-OHDA [25]. DTH-induced swelling was reduced significantly in 6-OHDA-treated, MOG-immunized mice. Although somewhat less monocytic cells accumulated at the site challenged by MOG in mice receiving 6-OHDA, the number of infiltrated cells was considerably higher than that of controls. Similar results were obtained when DTH to trinitrophenol (TNP) was measured in TNP-immunized mice, treated with 6-OHDA (S. Bhowmick, unpublished data). Moreover 6-OHDA treatment does not prevent the induction of IFN-γ-producing cells, although DTH swelling is suppressed [25]. The local suppression of a DTH reaction by CD8+ regulatory T cells prevents the recruitment of monocytic cells to the site challenged by antigen [42]. Hence, the suppression in swelling was not mediated by the CD8+ regulatory T cells, whose number did not change following 6-OHDA injection. The majority of monocytic cells recruited to a DTH site after antigenic challenge is not specific for the challenged antigen [43]. These observations suggest that 6-OHDA treatment did not inhibit the induction of a cell-mediated response but may have prevented a vascular response at the site challenged with antigen. These observations are consistent with reports that synovial plasma extravasation induced by bradykinin is dependent on sympathetic neurons [44, 45].
Peripheral administration of 6-OHDA increased the frequency and number of CD4+FoxP3+ T regs in the spleen and lymph nodes but did not increase the frequency of all CD4+ or CD8+ T cells, suggesting a selective increase in CD4+T cells with a Treg phenotype. Although there was an increase in the number of CD4+FoxP3+ T regs, we did not observe any change in the functional ability of the Tregs isolated from sympathectomized mice. Our findings indicate that the targeted ablation of sympathetic innervations in the peripheral tissues may be a novel approach to induce active tolerance by inducing Tregs, which may serve as therapy for autoimmune diseases.
In vivo, the majority of Tregs seems to be generated in the thymus [4]; however, these cells may also be generated in the periphery [10, 46]. Indeed, increasing evidence suggests that peripheral Tregs can be generated not only by peripheral expansion of thymic Tregs [32, 47] but also by the de novo conversion from conventional CD4+Foxp3– T cells [48,49,50]. The possibility of an expansion of existing Tregs is ruled out by the observation that 6-OHDA injection did not cause any change in the frequency of total CD4+ T cells. Also, we did not see any direct effect of 6-OHDA on the phenotype or proliferation of T effectors and Tregs by exposing naïve T effectors or Tregs to 6-OHDA in vitro (data not shown). Accordingly, the conversion of CD4+ Tregs is likely due to secondary effects of 6-OHDA on sympathetic neurons.
TGF-β has been defined as a major factor involved in the induction and survival of FoxP3+ Tregs [4, 6, 31, 51, 52]. Peripheral ablation of the sympathetic innervations caused a significant increase in the level of TGF-β mRNA in the spleen. This increase in TGF-β is directly involved in causing an increase in the frequency of Tregs, because 6-OHDA administration did not induce an increase in CD4+ FoxP3+ Treg numbers in TGFbRII or the Cbl-b −/− mice in which T cells are resistant to TGF-β. Nevertheless, 6-OHDA did cause an increase in the TGF-β mRNA levels in the spleens of the TGFbRII mice. NE might have a suppressive effect on TGF-β production, as suggested by an in vitro study, which showed that NE can decrease or increase TGF-β produced in vitro in hepatocytes in a dose-dependent manner [53]. In addition, intracellular levels of TGF-β are raised by an increase in intracellular oxidation conditions [54]. Therefore, an increase in neuronal oxidation conditions by 6-OHDA might increase neuronal TGF-β. The mechanism of increase in TGF-β induced by peripheral sympathectomy and the source of TGF-β is under investigation. Neurons from the murine cerebellum have been shown to produce TGF-β, which can result into the generation of regulatory T cells from encephalitogenic T cells [55]. Accordingly, neurons could be a major source of TGF-β in our system also. Moreover, our results suggest that sympathetic neurons regulate Treg generation. Also, our study suggests a peripheral mechanism, which can suppress EAE at the induction phase of EAE, as compared with Liu et al. [55], who studied the effect of cerebellar neurons on activated encephalitogenic T cells. Because systemically administered 6-OHDA does not penetrate the BBB, it is unlikely that cerebellar neurons are the source of TGF-β in our study.
Many investigations have established the protective role of Tregs in autoimmune disorders like EAE [3, 4, 8, 9]. Here, we show that 6-OHDA injection 48 h prior to immunization with MOG prevented the development of MOG induced EAE in mice. This result further confirms a previous finding that augmentation of CD4+FoxP3+ T regs by 50% inhibits the induction of EAE [35]. 6-OHDA treatment increased the number of CD4+FoxP3+ T regs by ∼50% in vivo. Adoptive transfer of 1×106 CD4+ T cells from 6-OHDA-treated animals reduced the induction of EAE, but this reduction was not statistically significant. However, that the total CD4+ T-cell population from 6-OHDA-treated animals had more CD4+FoxP3+ T regs compared with the PBS-treated group, and the CD4+ T cells from the 6-OHDA-treated animals were more suppressive than those from PBS-treated animals. Accordingly, although we could suppress EAE efficiently by the direct injection of 6-OHDA (which increases the CD4+ T reg numbers by ∼50%), suppression by the adoptive transfer of total CD4+ T cells was not efficient due to the low CD4+ T Fox P3+ T reg numbers in the transferred population. Although this does not convincingly show that the suppression of the induction of EAE following chemical sympathectomy is due to the increased Treg number, it is likely that these cells are involved in this suppression because 1) adoptive transfer of Tregs suppress EAE in the recipient 2) Tregs prevent the activity of autoreactive MOG specific T cells [8, 9]. Here, it is important to note that although sympathectomy before immunization may induce Tregs that prevent the induction of EAE, sympathectomy may sometimes enhance EAE [40], possibly by the inhibition of the induction of CD8+ Tregs [25] that inhibit activated T cells [56]. Accordingly, the timing of sympathectomy relative to immunization and also the route of administration could determine whether EAE is suppressed or enhanced.
Taken together, our results suggest that the number of Tregs in the periphery is tuned by SNS signals and that TGF-β act as a bridge between the SNS and the immune system. Additionally, our observations define a close relationship between the production of TGF-β in the peripheral immune system and the SNS. These observations indicate that the neurologic events mediated by the SNS, such as a stress response could affect the number of T cells that regulate an immune response. An influence of the SNS on Treg would, therefore, impact many SNS-immune system interactions, including stress and autoimmune diseases. Targeting of sympathetic innervations in the lymphoid organs may indeed be an attractive approach to inducing active tolerance and protection from autoimmune diseases. The method of Treg expansion described here may be promising because the technique of sympathectomy has been in use to treat primary palmar and axillary hyperhidrosis, Raynaud’s phenomenon and occlusive vascular disease [57,58,59,60].
ACKNOWLEDGMENTS
This work was funded in part by National Institutes of Health grants EY017537 and EY017289 (to R. E. C.), the Connecticut Lions Eye Research Foundation (to J. O. R.), and the University of Connecticut Health Center Research Advisory Committee. The authors sincerely thank Amanda Vang (Department of Immunology, University of Connecticut Health Center) for her help with the designing of primers and PCR assays.
DISCLOSURE
The authors have no conflicting financial interests.
Footnotes
Abbreviations: 6-OHDA=6-hyrdoxydopamine, BBB=blood brain barrier, DTH=delayed-type hypersensitivity, EAE=experimental autoimmune encephalomyelitis, FoxP3+=forkhead box P3+, MOG=myelin oligodendrodyte glycoprotein, NE=norepinephrine, qRT-PCR=quantitative RT-PCR, RPL-19=ribosomal protein L-19, SNS=sympathetic nervous system, Treg=T regulatory cell
References
- Hogquist K A, Baldwin T A, Jameson S C. Central tolerance: learning self-control in the thymus. Nat Rev Immunol. 2005;5:772–782. doi: 10.1038/nri1707. [DOI] [PubMed] [Google Scholar]
- Goodnow C C, Sprent J, Fazekas de St Groth B, Vinuesa C G. Cellular and genetic mechanisms of self tolerance and autoimmunity. Nature. 2005;435:590–597. doi: 10.1038/nature03724. [DOI] [PubMed] [Google Scholar]
- Fontenot J D, Gavin M A, Rudensky A Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- Annacker O, Pimenta-Araujo R, Burlen-Defranoux O, Barbosa T C, Cumano A, Bandeira A. CD25+ CD4+ T cells regulate the expansion of peripheral CD4 T cells through the production of IL-10. J Immunol. 2001;166:3008–3018. doi: 10.4049/jimmunol.166.5.3008. [DOI] [PubMed] [Google Scholar]
- Chen W, Jin W, Hardegen N, Lei K J, Li L, Marinos N, McGrady G, Wahl S M. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot J D, Rasmussen J P, Gavin M A, Rudensky A Y. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- Mills K H. Regulatory T cells: friend or foe in immunity to infection? Nat Rev Immunol. 2004;4:841–855. doi: 10.1038/nri1485. [DOI] [PubMed] [Google Scholar]
- O'Connor R A, Anderton S M. Foxp3+ regulatory T cells in the control of experimental CNS autoimmune disease. J Neuroimmunol. 2008;193:1–11. doi: 10.1016/j.jneuroim.2007.11.016. [DOI] [PubMed] [Google Scholar]
- Huber S, Schramm C. TGF-β and CD4+CD25+ regulatory T cells. Front Biosci. 2006;11:1014–1023. doi: 10.2741/1859. [DOI] [PubMed] [Google Scholar]
- Annacker O, Pimenta-Araujo R, Burlen-Defranoux O, Bandeira A. On the ontogeny and physiology of regulatory T cells. Immunol Rev. 2001;182:5–17. doi: 10.1034/j.1600-065x.2001.1820101.x. [DOI] [PubMed] [Google Scholar]
- Liu W, Putnam A L, Xu-Yu Z, Szot G L, Lee M R, Zhu S, Gottlieb P A, Kapranov P, Gingeras T R, Fazekas de St Groth B, Clayberger C, Soper D M, Ziegler S F, Bluestone J A. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger D L, Millar B A, Perez S, Carter J, Wood C, ThyagaRajan S, Molinaro C, Lubahn C, Lorton D. Sympathetic modulation of immunity: relevance to disease. Cell Immunol. 2008;252:27–56. doi: 10.1016/j.cellimm.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elenkov I J, Wilder R L, Chrousos G P, Vizi E S. The sympathetic nerve—an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev. 2000;52:595–638. [PubMed] [Google Scholar]
- Wrona D. Neural-immune interactions: an integrative view of the bidirectional relationship between the brain and immune systems. J Neuroimmunol. 2006;172:38–58. doi: 10.1016/j.jneuroim.2005.10.017. [DOI] [PubMed] [Google Scholar]
- Weihe E, Nohr D, Michel S, Muller S, Zentel H J, Fink T, Krekel J. Molecular anatomy of the neuro-immune connection. Int J Neurosci. 1991;59:1–23. doi: 10.3109/00207459108985446. [DOI] [PubMed] [Google Scholar]
- Madden K S, Sanders V M, Felten D L. Catecholamine influences and sympathetic neural modulation of immune responsiveness. Annu Rev Pharmacol Toxicol. 1995;35:417–448. doi: 10.1146/annurev.pa.35.040195.002221. [DOI] [PubMed] [Google Scholar]
- Madden K S, Stevens S Y, Felten D L, Bellinger D L. Alterations in T lymphocyte activity following chemical sympathectomy in young and old Fischer 344 rats. J Neuroimmunol. 2000;103:131–145. doi: 10.1016/s0165-5728(99)00243-x. [DOI] [PubMed] [Google Scholar]
- Kostrzewa R M, Jacobowitz D M. Pharmacological actions of 6-hydroxydopamine. Pharmacol Rev. 1974;26:199–288. [PubMed] [Google Scholar]
- Cross R J, Brooks W H, Roszman T L. Modulation of T-suppressor cell activity by central nervous system catecholamine depletion. J Neurosci Res. 1987;18:75–81. doi: 10.1002/jnr.490180113. [DOI] [PubMed] [Google Scholar]
- Cross R J, Jackson J C, Brooks W H, Sparks D L, Markesbery W R, Roszman T L. Neuroimmunomodulation: impairment of humoral immune responsiveness by 6-hydroxydopamine treatment. Immunology. 1986;57:145–152. [PMC free article] [PubMed] [Google Scholar]
- Karpus W J, Konkol R J, Killen J A. Central catecholamine neurotoxin administration. 1. Immunological changes associated with the suppression of experimental autoimmune encephalomyelitis. J Neuroimmunol. 1988;18:61–73. doi: 10.1016/0165-5728(88)90135-x. [DOI] [PubMed] [Google Scholar]
- Felten D L, Livnat S, Felten S Y, Carlson S L, Bellinger D L, Yeh P. Sympathetic innervation of lymph nodes in mice. Brain Res Bull. 1984;13:693–699. doi: 10.1016/0361-9230(84)90230-2. [DOI] [PubMed] [Google Scholar]
- Leo N A, Bonneau R H. Mechanisms underlying chemical sympathectomy-induced suppression of herpes simplex virus-specific cytotoxic T lymphocyte activation and function. J Neuroimmunol. 2000;110:45–56. doi: 10.1016/s0165-5728(00)00336-2. [DOI] [PubMed] [Google Scholar]
- Li X, Taylor S, Zegarelli B, Shen S, O'Rourke J, Cone R E. The induction of splenic suppressor T cells through an immune-privileged site requires an intact sympathetic nervous system. J Neuroimmunol. 2004;153:40–49. doi: 10.1016/j.jneuroim.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Minagawa M, Oya H, Yamamoto S, Shimizu T, Bannai M, Kawamura H, Hatakeyama K, Abo T. Intensive expansion of natural killer T cells in the early phase of hepatocyte regeneration after partial hepatectomy in mice and its association with sympathetic nerve activation. Hepatology. 2000;31:907–915. doi: 10.1053/he.2000.5850. [DOI] [PubMed] [Google Scholar]
- Sonoda K H, Exley M, Snapper S, Balk S P, Stein-Streilein J. CD1-reactive natural killer T cells are required for development of systemic tolerance through an immune-privileged site. J Exp Med. 1999;190:1215–1226. doi: 10.1084/jem.190.9.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Cantor H. Generation and regulation of CD8(+) regulatory T cells. Cell Mol Immunol. 2008;5:401–406. doi: 10.1038/cmi.2008.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T R, Kumar V. Revival of CD8+ Treg-mediated suppression. Trends Immunol. 2008;29:337–342. doi: 10.1016/j.it.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Lee Y H, Ishida Y, Rifa'i M, Shi Z, Isobe K, Suzuki H. Essential role of CD8+CD122+ regulatory T cells in the recovery from experimental autoimmune encephalomyelitis. J Immunol. 2008;180:825–832. doi: 10.4049/jimmunol.180.2.825. [DOI] [PubMed] [Google Scholar]
- Fontenot J D, Rasmussen J P, Williams L M, Dooley J L, Farr A G, Rudensky A Y. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Gavin M A, Clarke S R, Negrou E, Gallegos A, Rudensky A. Homeostasis and anergy of CD4(+)CD25(+) suppressor T cells in vivo. Nat Immunol. 2002;3:33–41. doi: 10.1038/ni743. [DOI] [PubMed] [Google Scholar]
- Gorelik L, Flavell R A. Abrogation of TGFβ signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 2000;12:171–181. doi: 10.1016/s1074-7613(00)80170-3. [DOI] [PubMed] [Google Scholar]
- Wohlfert E A, Callahan M K, Clark R B. Resistance to CD4+CD25+ regulatory T cells and TGF-β in Cbl-b−/− mice. J Immunol. 2004;173:1059–1065. doi: 10.4049/jimmunol.173.2.1059. [DOI] [PubMed] [Google Scholar]
- Kohm A P, Carpentier P A, Anger H A, Miller S D. Cutting edge: CD4+CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J Immunol. 2002;169:4712–4716. doi: 10.4049/jimmunol.169.9.4712. [DOI] [PubMed] [Google Scholar]
- Korn T, Reddy J, Gao W, Bettelli E, Awasthi A, Petersen T R, Backstrom B T, Sobel R A, Wucherpfennig K W, Strom T B, Oukka M, Kuchroo V K. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med. 2007;13:423–431. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konkol R J, Wesselmann U, Karpus W J, Leo G L, Killen J A, Roerig D L. Suppression of clinical weakness in experimental autoimmune encephalomyelitis associated with weight changes, and post-decapitation convulsions after intracisternal-ventricular administration of 6-hydroxydopamine. J Neuroimmunol. 1990;26:25–34. doi: 10.1016/0165-5728(90)90116-5. [DOI] [PubMed] [Google Scholar]
- Chelmicka-Schorr E, Checinski M, Arnason B G. Chemical sympathectomy augments the severity of experimental allergic encephalomyelitis. J Neuroimmunol. 1988;17:347–350. doi: 10.1016/0165-5728(88)90125-7. [DOI] [PubMed] [Google Scholar]
- Chelmicka-Schorr E, Kwasniewski M N, Wollmann R L. Sympathectomy augments adoptively transferred experimental allergic encephalomyelitis. J Neuroimmunol. 1992;37:99–103. doi: 10.1016/0165-5728(92)90160-m. [DOI] [PubMed] [Google Scholar]
- Pal E, Yamamura T, Tabira T. Autonomic regulation of experimental autoimmune encephalomyelitis in IL-4 knockout mice. J Neuroimmunol. 1999;100:149–155. doi: 10.1016/s0165-5728(99)00209-x. [DOI] [PubMed] [Google Scholar]
- Lorton D, Lubahn C, Klein N, Schaller J, Bellinger D L. Dual role for noradrenergic innervation of lymphoid tissue and arthritic joints in adjuvant-induced arthritis. Brain Behav Immun. 1999;13:315–334. doi: 10.1006/brbi.1999.0564. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay S, O'Rourke J, Cone R E. Implication for the CD94/NKG2A-Qa-1 system in the generation and function of ocular-induced splenic CD8+ regulatory T cells. Int Immunol. 2008;20:509–516. doi: 10.1093/intimm/dxn008. [DOI] [PubMed] [Google Scholar]
- McCluskey R T, Benacerraf B, McCluskey J W. Studies on the specificity of the cellular infiltrate in delayed hypersensitivity reactions. J Immunol. 1963;90:466–477. [PubMed] [Google Scholar]
- Khalil Z, Helme R D. Sympathetic neurons modulate plasma extravasation in the rat through a non-adrenergic mechanism. Clin Exp Neurol. 1989;26:45–50. [PubMed] [Google Scholar]
- Miao F J, Janig W, Levine J. Role of sympathetic postganglionic neurons in synovial plasma extravasation induced by bradykinin. J Neurophysiol. 1996;75:715–724. doi: 10.1152/jn.1996.75.2.715. [DOI] [PubMed] [Google Scholar]
- Wan Y Y, Flavell R A. The roles for cytokines in the generation and maintenance of regulatory T cells. Immunol Rev. 2006;212:114–130. doi: 10.1111/j.0105-2896.2006.00407.x. [DOI] [PubMed] [Google Scholar]
- Walker L S, Chodos A, Eggena M, Dooms H, Abbas A K. Antigen-dependent proliferation of CD4+ CD25+ regulatory T cells in vivo. J Exp Med. 2003;198:249–258. doi: 10.1084/jem.20030315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang S, Alard P, Zhao Y, Parnell S, Clark S L, Kosiewicz M M. Conversion of CD4+ CD25- cells into CD4+ CD25+ regulatory T cells in vivo requires B7 costimulation, but not the thymus. J Exp Med. 2005;201:127–137. doi: 10.1084/jem.20041201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig M C, von Boehmer H. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol. 2005;6:1219–1227. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- Knoechel B, Lohr J, Kahn E, Bluestone J A, Abbas A K. Sequential development of interleukin 2-dependent effector and regulatory T cells in response to endogenous systemic antigen. J Exp Med. 2005;202:1375–1386. doi: 10.1084/jem.20050855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- Marie J C, Letterio J J, Gavin M, Rudensky A Y. TGF-β1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J Exp Med. 2005;201:1061–1067. doi: 10.1084/jem.20042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher S A, Absher M. Norepinephrine and ANG II stimulate secretion of TGF-β by neonatal rat cardiac fibroblasts in vitro. Am J Physiol. 1995;268:C910–C917. doi: 10.1152/ajpcell.1995.268.4.C910. [DOI] [PubMed] [Google Scholar]
- Ayache N, Boumediene K, Mathy-Hartert M, Reginster J Y, Henrotin Y, Pujol J P. Expression of TGF-βs and their receptors is differentially modulated by reactive oxygen species and nitric oxide in human articular chondrocytes. Osteoarthritis Cartilage. 2002;10:344–352. doi: 10.1053/joca.2001.0499. [DOI] [PubMed] [Google Scholar]
- Liu Y, Teige I, Birnir B, Issazadeh-Navikas S. Neuron-mediated generation of regulatory T cells from encephalitogenic T cells suppresses EAE. Nat Med. 2006;12:518–525. doi: 10.1038/nm1402. [DOI] [PubMed] [Google Scholar]
- Yang J, Brook M O, Carvalho-Gaspar M, Zhang J, Ramon H E, Sayegh M H, Wood K J, Turka L A, Jones N D. Allograft rejection mediated by memory T cells is resistant to regulation. Proc Natl Acad Sci USA. 2007;104:19954–19959. doi: 10.1073/pnas.0704397104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey D M, Brookes V S, Cooke W T. Sympathectomy in the treatment of hypertension; review of 122 cases. Lancet. 1953;1:403–408. doi: 10.1016/s0140-6736(53)91589-x. [DOI] [PubMed] [Google Scholar]
- Pope J E. Treatment of systemic sclerosis. Rheum Dis Clin North Am. 1996;22:893–907. doi: 10.1016/s0889-857x(05)70307-0. [DOI] [PubMed] [Google Scholar]
- Moak J P, Eldadah B, Holmes C, Pechnik S, Goldstein D S. Partial cardiac sympathetic denervation after bilateral thoracic sympathectomy in humans. Heart Rhythm. 2005;2:602–609. doi: 10.1016/j.hrthm.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Gordon A, Zechmeister K, Collin J. The role of sympathectomy in current surgical practice. Eur J Vasc Surg. 1994;8:129–137. doi: 10.1016/s0950-821x(05)80447-5. [DOI] [PubMed] [Google Scholar]