Abstract
We reported that PARP-1 exhibits differential roles in expression of inflammatory factors. Here, we show that PARP-1 deletion was associated with a significant reduction in inflammatory cell recruitment to mouse airways upon intratracheal administration of LPS. However, PARP-1 deletion exerted little effect in response to TNF exposure. LPS induced massive neutrophilia and moderate recruitment of macrophages, and TNF induced recruitment of primarily macrophages with smaller numbers of neutrophils in the lungs. Following either exposure, macrophage recruitment was blocked severely in PARP-1−/− mice, and this was associated with a marked reduction in MCP-1 and MIP-1α. This association was corroborated partly by macrophage recruitment in response to intratracheal administration of MCP-1 in PARP-1−/− mice. Surprisingly, although neutrophil recruitment was reduced significantly in LPS-treated PARP-1−/− mice, neutrophil numbers increased in TNF-treated mice, suggesting that PARP-1 deletion may promote a macrophagic-to-neutrophilic shift in the inflammatory response upon TNF exposure. Neutrophil-specific chemokines mKC and MIP-2 were reduced significantly in lungs of LPS-treated but only partially reduced in TNF-treated PARP-1−/− mice. Furthermore, the MIP-2 antagonist abrogated the shift to a neutrophilic response in TNF-exposed PARP-1−/− mice. Although CXCR2 expression increased in response to either stimulus in PARP-1+/+ mice, the DARC increased only in lungs of TNF-treated PARP-1+/+ mice; both receptors were reduced to basal levels in treated PARP-1−/− mice. Our results show that the balance of pro-neutrophilic or pro-macrophagic stimulatory factors and the differential influence of PARP-1 on these factors are critical determinants for the nature of the airway inflammatory response.
Keywords: inflammation, neutrophils, monocytes/macrophages, cytokines, lipopolysaccharide, transgenic/knockout mice, lung
Introduction
Airway inflammation is a multifactorial and complex process that is elicited by numerous stimuli, from microbial infection to allergen exposure. The types of inflammatory cells recruited to the airways are often determined by the nature of the stimulus and the chemokines induced by the first line of defense, such as macrophages, or somatic cells, such as epithelial cells. A great deal of effort has been made to understand the details of this process. Interfering with the expression of adhesion and chemotactic molecules in response to inflammatory factors such as LPS or TNF prevents inflammatory cell adhesion and transendothelial migration of leukocytes. In many situations, blocking inflammatory processes may have therapeutic potential by impairing the establishment or progression of inflammatory diseases. For example, the inflammatory response triggered by LPS is associated with a massive recruitment of neutrophils and to a lesser extent, macrophages [1]. Production of numerous cytokines such as TNF and the CC and CXC chemokines MCP-1, IL-8, MIP-1α, MIP-2, mKC, as well as many others is induced upon exposure to inflammatory factors [2]. On the other hand, many factors and cell or tissue processes promote modulation or termination of the inflammatory process. For instance, apoptosis of neutrophils is an important process for resolution of inflammation [3]. There is also an increasing body of evidence supporting the existence of factors that modulate inflammation, such as the class of silent chemokine receptors, which include the DARC, D6, and CCX-CKR [4,5,6]. These receptors do not harbor a G protein-associated function as other heptahelical receptors but may function as decoy receptors and serve as a sink for chemokines by taking up ligands without eliciting a response. It is noteworthy that DARC functioning as a modulator of inflammatory response and leukocyte recruitment has been challenged by several studies [5, 7,8,9], further demonstrating the complexity of the inflammatory process.
Our laboratory has investigated the involvement of PARP-1 in tissue injury and its implication in several conditions associated with oxidative stress and inflammation, including allergic airway inflammation and atherosclerosis [10,11,12,13,14,15]. PARP-1 is emerging as a viable therapeutic target for the treatment of inflammatory diseases. In addition to its effects on cell and tissue homeostasis through NAD+ metabolism, PARP-1 is thought to participate in inflammation by regulating the expression of a number of inflammatory factors directly or indirectly, including adhesion molecules, MCP-1, MIP-1α, and others (reviewed in refs. [16,17,18]). This activity is connected with the ability of PARP-1 to regulate signal transduction events that result in the activation of NF-κB, ERK, and AP-1 [19,20,21,22]. NF-κB is a pleiotropic transcription factor that plays a decisive role in regulating expression of various inflammatory response genes, including adhesion molecules, MCP-1, and MIP-1α [23]. We and others [24, 25] have reported that PARP-1 expression is required for the efficient translocation of NF-κB to the nucleus in response to LPS. However, we reported recently that this requirement does not apply when the stimulus is TNF [26]. Interestingly, although NF-κB nuclear translocation in TNF-treated smooth muscle cells was sufficient for the expression of the adhesion molecule VCAM-1, ICAM-1 expression showed a critical requirement for PARP-1. Such differential effects were independent of NF-κB signal transduction [26]. These findings suggest that NF-κB nuclear translocation may be sufficient for certain genes and may be insufficient for others, providing a novel insight into the role of NF-κB in driving target gene expression.
In the present study, we investigated the consequences of the differential requirement for PARP-1 in recruitment of inflammatory cells and expression of inflammatory genes using a model of acute airway inflammation mediated by LPS or TNF exposure.
MATERIALS AND METHODS
Animals and intratracheal administration of TNF or LPS
Mice were bred in a specific pathogen-free facility at LSUHSC (New Orleans, LA, USA) and allowed unlimited access to sterilized chow and water. The LSUHSC Animal Care and Use Committee approved the maintenance, experimental protocols, and procedures. C57BL/6 WT mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA). The generation of C57BL/6 PARP-1−/− mice was described previously [27]. All animals were genotyped by PCR.
Mice were anesthetized with ketamine/xylazine (60 and 3 mg/kg, respectively) and administered, intratracheally, TNF (100 ng/mouse) or LPS (50 μg/mouse), diluted in 50 μl saline. Some WT mice received an intratracheal administration of rMCP-1 (100 ng/mouse). Some PARP-1−/− mice received the MIP-2 antagonist antileukinate Ac-RRWWCR-NH2 (Anaspec, San Jose, CA, USA; 0.2 mg/kg), intratracheally, in combination with TNF-α. Mice were killed 6 or 24 h later for lung collection or BALF, respectively.
Organ recovery and tissue staining
Animals were killed, and lungs were isolated as described [13, 25, 27]. Lungs were immersed in RNA Later solution (Qiagen Inc., Valencia, CA, USA) for RNA extraction or subjected to BAL, and BALF were subjected to cytospin and stained with H&E for the assessment of inflammatory cells.
Conventional and real-time PCR
RNA was extracted from lungs or SMCs using standard methods, and cDNA was generated using RT III (Invitrogen, Carlsbad, CA, USA). Oligonucleotide primers were all purchased from Integrated DNA Technologies (Coralville, IA, USA) to amplify a fragment of the tested genes specifically. The specific primers were as follows: MCP-1, forward primer, ACTGAAGCCAGCTCTCTCTTCCTC, reverse primer, TTCCTTCTTGGGGTCAGCACAGAC; MIP-1α, forward primer, ACCACTGCCCTTGCTGTTC, reverse primer, TCTGCCGGTTTCTCTTAGTCAG; CCR2, forward primer, CCACACCCTGTTTCGCTG, reverse primer, ACCTTCGGAACTTCTCTCCAAC; MIP-2, forward primer, CACTCTCAAGGGCGGTCAAA, reverse primer TACGATCCAGGCTTCCCGGGT; mKC, forward primer, CGCTGCTGCTGCTGGCCACCA, reverse primer, GGCTATGACTTCGGTTTGGGTGCAG; CXCR2, forward primer, GGCGGGGTAAGACAAGAATC, reverse primer, GGCAAGGTCAGGGCAAAGAA; DARC, forward primer, GGCACTTATCTTGGAGCCAC, reverse primer, GTCACTCGAGAGTTCATAGG; and β-actin, forward primer, ACCGTGAAAAGATGACCCAGATC, reverse primer, TAGTTTCATGGATGCCACAGG. The annealing temperatures and cycle numbers were optimized for each primer pair. The PCR products were then incubated for 15 min at 72°C. The resulting PCR products were subjected to electrophoresis in a 2% agarose gel and stained with ethidium bromide. For real-time PCR, the same specific primer sets were used. Quantitative determination of gene expression levels using a two-step cycling protocol was performed on a MyIQ Cycler (Bio-Rad, Hercules, CA, USA). Relative expression levels were calculated using the 2(–Δ Δ comparative threshold) method [28]. Quantities of all targets from the test samples were normalized to the mouse β-actin gene.
Data analysis
All data are expressed as means ± sd of values from at least four mice/group. Prism software (GraphPad, San Diego, CA, USA) was used to analyze the differences between experimental groups by one-way ANOVA, followed by Dunnett’s multiple comparison test.
RESULTS
Effects of PARP-1 gene deletion on airway inflammatory cell recruitment in response to intratracheal administration of LPS or TNF in mice
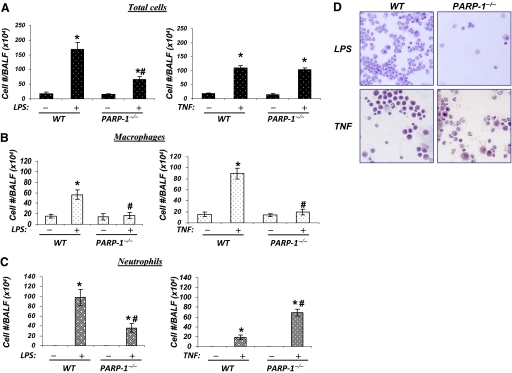
Figure 1, A and B, shows that intratracheal administration of LPS induced a massive recruitment of inflammatory cells to the airways of WT mice. Within 24 h, neutrophils are the most common type of recruited cells, as assessed by cell staining in the BALF. As reported by others, PARP-1 gene deletion significantly reduced the overall total number of inflammatory cells to the airways with a drastic effect on neutrophil recruitment (reviewed in refs. [16,17,18]). Intratracheal TNF administration promoted recruitment of macrophages and neutrophils to the airways, and macrophages were the predominant cell type. Unlike the response to LPS, PARP-1 gene deletion did not promote a significant reduction in the overall number of inflammatory cells but had a drastic effect on macrophage recruitment. Interestingly, neutrophils accounted for most of the recruited inflammatory cells in the airways of TNF-exposed PARP-1−/− mice, suggesting that PARP-1 gene deletion may promote a shift in the nature of the inflammatory response from macrophages to neutrophils following exposure TNF. These results suggest a critical difference in the role of PARP-1 in the process of airway inflammation mediated by exposure to LPS or TNF.
Figure 1.
Effects of PARP-1 gene deletion on airway inflammatory cell recruitment in response to intratracheal administration of LPS or TNF in mice. C57BL/6 WT or PARP-1−/− mice were subjected to a single intratracheal administration of LPS (50 μg/mouse) or TNF (100 ng/mouse) in 50 μl saline. Twenty-four hours after treatment, BALF were collected and centrifuged; cells were then differentially stained and visualized by light microscopy, followed by a count of total cells (A), macrophages (B), and neutrophils (C). Data are given as means ± sd of values obtained from at least four mice/group. *, Difference from respective untreated control mice, P < 0.01; #, difference from LPS- or TNF-treated WT mice, P < 0.01. (D) A sample of H&E-stained cells collected from BALF of LPS- or TNF-treated WT or PARP-1−/− mice.
The effect of PARP-1 gene deletion on TNF-induced macrophage recruitment to the airways is associated with severe effects on expression of MCP-1 and MIP-1α
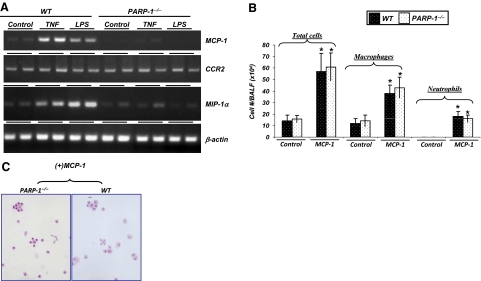
To investigate the mechanism(s) behind the differential effect of PARP-1−/− gene deletion on the type of inflammatory response to LPS or TNF-α exposure, we examined initially whether the complete reduction in macrophage recruitment to either stimulus was associated with critical factors involved in the recruitment, including MCP-1, CCR2, and MIP-1α [2]. Figure 2A shows that PARP-1 gene deletion drastically reduced expression of MCP-1 and MIP-1α with little effect on CCR2, as assessed by RT-PCR of RNA isolated from lungs of control and treated mice 6 h after intratracheal administration of TNF or LPS.
Figure 2.
Association between the effect of PARP-1 gene deletion on TNF- or LPS-induced inflammatory cell airway recruitment to effects on expression of MCP-1 and MIP-1α. (A) C57BL/6 WT or PARP-1−/− mice were subjected to a single intratracheal administration of LPS or TNF in 50 μl saline. Six hours after treatment, mice were killed, and whole lungs were collected and subjected to RNA extraction followed by cDNA generation. Prepared cDNA was subjected to RT-PCR for MCP-1, CCR2, MIP-1α, or β-actin. (B and C) C57BL/6 WT or PARP-1−/− mice (as in A) were subjected to a single intratracheal administration of MCP-1 (100 ng/mouse) in 50 μl saline or saline alone. Twenty-four hours after treatment, BALF were collected and centrifuged; cells were then differentially stained with H&E and visualized by light microscopy, followed by a count of total cells, neutrophils, and macrophages. Data are given as means ± sd of values obtained from at least four mice/group. *, Difference from untreated control mice, P < 0.01.
We examined the effect of MCP-1 re-establishment in promoting inflammatory cell recruitment to airways of PARP-1−/− mice to determine whether the effect of PARP-1 gene deletion on macrophage recruitment was a result of the expression of chemokines, with or without an additive effect on cell migration to the airways. Figure 2B shows that intratracheal administration of MCP-1 promoted significant macrophage recruitment to the airways of PARP-1−/− mice, similar to levels observed upon TNF or LPS exposure. Neutrophil recruitment upon MCP-1 exposure was less pronounced. Similar types and numbers of inflammatory cells were observed in WT mice treated intratracheally with MCP-1. Figure 2C depicts the different cell types recruited to the airways of PARP-1 or WT mice upon MCP-1 exposure. These results indicate that the defect in macrophage and neutrophil recruitment in response to TNF or LPS was primarily a result of the modulatory effect of PARP-1 gene deletion on expression of MCP-1 rather than a defect in the ability of PARP-1−/− cells to migrate toward chemotactic factors. Although reversal of MIP-1α was not tested in this study, the effect PARP-1 gene deletion on the chemokine remains a potentially critical factor in the effect of PARP-1 gene deletion on macrophage recruitment.
The blockade or promotion of neutrophil recruitment to airway of PARP-1−/− mice in response to LPS and TNF-α treatment correlates with levels of mKC and MIP-2 expression
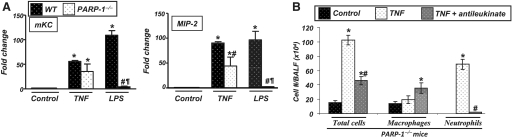
The role of mKC and MIP-2α in the specific recruitment of neutrophils is well-established [2]. We assessed the expression levels of these two chemokines to examine the reason behind neutrophil recruitment to the airways of PARP-1−/− mice upon TNF treatment. Figure 3A shows quantitative data as assessed by real-time PCR. Similar to LPS treatment, TNF exposure markedly increased expression of mKC and MIP-2α in the lungs of WT animals. Interestingly, PARP-1 gene deletion exerted only a minor-to-moderate effect on the expression of these chemokines after TNF treatment. These results suggest that mKC and MIP-2 expression may contribute to neutrophil recruitment in the airways of PARP-1−/− mice in response to TNF.
Figure 3.
Correlation between the levels of neutrophil recruitment to airway of PARP-1−/− mice in response to LPS or TNF with levels of mKC and MIP-2 expression. C57BL/6 WT or PARP-1−/− mice were subjected to a single intratracheal administration of LPS or TNF. Six hours after treatment, mice were killed, and whole lungs were collected and subjected to RNA extraction, followed by cDNA generation. Prepared cDNA were subjected to real-time PCR with primers specific to mKC and MIP-2; β-actin was used as an internal control for normalization of expression values. *, Difference from respective untreated control mice; #, difference from TNF- or LPS-treated WT mice; ¶, difference from TNF-treated PARP-1–/– mice, P < 0.01. (B) PARP-1−/− mice were subjected to a single intratracheal administration of TNF, with or without the MIP-2 antagonist antileukinate (0.2 mg/kg). Twenty-four hours later, BALF were collected and centrifuged; cells were then differentially stained and visualized by light microscopy, followed by a count of total cells, neutrophils, and macrophages. Data are given as means ± sd of values obtained from at least four mice/group. *, Difference from untreated control mice, P < 0.01; #, difference from PARP-1−/− mice treated with TNF alone, P < 0.01.
To provide additional support for the latter hypothesis, we examined whether the MIP-2 antagonist antileukinate could block neutrophil recruitment to airways of PARP-1−/− mice treated with TNF. Figure 3B shows that antileukinate abrogated TNF-induced recruitment of neutrophils completely in PARP-1−/− mice, strengthening the hypothesis that expression of MIP-2 in PARP-1−/− mice was a critical factor in the observed inflammatory response shift. Interestingly, we observed a moderate increase in macrophage recruitment, although the reasons for this increase are not clear. Overall, these results show that the differential requirement for PARP-1 in the expression of inflammatory factors is a determining factor for the type of inflammatory response induced by TNF.
Critical differences in CXCR2 and DARC expression after TNF or LPS treatment may be associated with differential promotion of inflammatory cell recruitment to the airways of WT mice and the promotion of neutrophilia in airways of TNF-treated PARP-1−/− mice
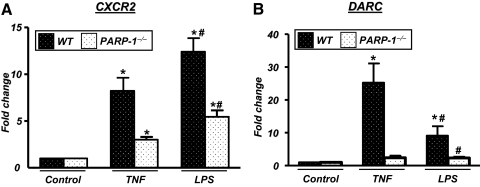
We next examined whether the differential expression of chemokines in the presence or absence of PARP-1 in TNF- or LPS-treated animals was associated with a differential expression of CXCR2 and DARC. Figure 4A shows that CXCR2 expression displayed a moderate increase from basal levels after TNF treatment, which is consistent with a recent report using endothelial cells [29]. However, the expression of CXCR2 was markedly elevated in lungs of LPS-treated WT mice.
Figure 4.
Effect of PARP-1 gene deletion on expression of CXCR2 and DARC in lungs of TNF- or LPS-treated mice. cDNA from samples from TNF- or LPS-treated WT or PARP-1−/− mice, described in Figure 2, was subjected to real-time PCR with primers specific to CXCR2 or DARC; β-actin was used as an internal control for normalization of expression values. *, Difference from respective untreated control mice; #, difference from TNF- or LPS-treated WT mice; P < 0.01.
As CXCR2 is involved in neutrophil recruitment, the differences in expression in airways of LPS- or TNF-treated WT mice may be associated with the observed massive or modest neutrophilia, respectively. Figure 4A also shows that PARP-1 gene deletion reduced the receptor expression severely in response to LPS, which is highly consistent with a severe reduction in neutrophilia observed in treated PARP-1−/− mice. PARP-1 gene deletion also caused a reduction in CXCR2 expression levels in airways of TNF-treated mice (Fig. 4A).
It is noteworthy that the moderate expression levels of CXCR2 in the airways of TNF-treated WT mice were accompanied with elevated levels of mKC and MIP-2 (see Fig. 3A). This does not provide a sufficient explanation for the mostly phagocytic inflammatory response following exposure to TNF compared with the mostly neutrophilic response to LPS. The persistence of these two chemokines in TNF-treated PARP-1−/− mice does not fully explain the switch in the inflammatory response from phagocytic to neutrophilic. Several reports suggest that DARC may play a role in the modulation of neutrophil recruitment in response to inflammatory agents including LPS [7, 30]. We assessed DARC expression in the different experimental groups to determine whether DARC expression patterns correlate with the inflammatory responses elicited by TNF and by LPS and the shift from mainly macrophagic to mainly neutrophilic in treated PARP-1−/− mice. Figure 4B shows that TNF treatment induced significantly high amounts of DARC compared with LPS treatment. Interestingly, PARP-1 gene deletion abrogated DARC expression completely in response to TNF or LPS.
Altogether, these results (Fig. 5) suggest that the combination of increased CXCR2 and its ligands mKC and MIP-2 with low levels in DARC may be responsible for the mostly neutrophilic response observed in LPS-treated WT mice. In parallel, moderate levels of CXCR2 and the high levels of DARC in combination with robust MCP-1 and MIP-1α expression may account for the mostly phagocytic response in TNF-treated WT mice. Accordingly, the nearly complete inhibition of DARC, moderate levels of CXCR2, the persistence of mKC and MIP-2, and the complete absence of MCP-1 and MIP-1α may account for the shift in the inflammatory response from phagocytic to neutrophilic in airways of PARP-1−/− mice in response to TNF treatment.
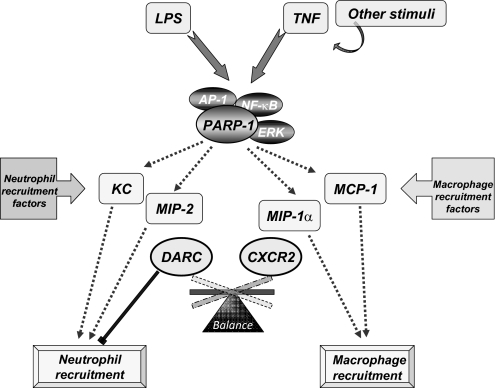
Figure 5.
Model for the potential involvement of PARP-1 in the balance of pro-neutrophilic or pro-macrophagic stimulatory factors and the differential influence of PARP-1 on these factors as critical determinants for the nature of airway inflammatory response. Upon exposure to LPS, TNF, or TNF-producing stimuli that involve a number of intricate processes, PARP-1 participates in the process of chemokine expression potentially through NF-κB-, AP-1-, and/or ERK-mediated signal transduction pathways and processes that have yet to be fully determined. The balance of pro-neutrophilic chemokines (e.g., mKC and MIP-2) or pro-macrophagic chemokines (e.g., MCP-1 and MIP-1α) and the differential effects of PARP-1 on these factors may play important and determining roles for the nature of airway inflammatory response. This balance is influenced further by the levels of stimulatory (e.g., CXCR2 and CCR2) or modulatory (e.g., DARC) receptors (see Discussion).
DISCUSSION
Our recent reports show that PARP-1 gene deletion may have differential effects on the expression of inflammatory genes and could exert completely different effects on transcription factors such as NF-κB nuclear translocation and activation of ERK [10, 14, 15, 25, 31]. For instance, we have shown that NF-κB nuclear translocation is defective in PARP-1−/− cells upon exposure to LPS or H2O2, and NF-κB translocation is unaffected in TNF-treated cells [26]. Despite the lack of an inhibitory effect on NF-κB nuclear translocation by PARP-1 gene deletion in response to TNF, we observed selective activation of the NF-κB-dependent gene VCAM-1, but not ICAM-1. The current study was designed to address the intricacies of PARP-1 in an animal model of acute airway inflammation mediated by TNF or LPS. It is important to note that TNF and LPS are inflammatory factors that have similar mechanisms of action despite their mediation of inflammatory responses through distinct receptors. Our results show that intratracheally delivered TNF and LPS mediate very distinct responses. Although both factors induced recruitment of neutrophils and macrophages to the airways, the nature of the recruited inflammatory cells was strikingly different. LPS treatment induced a massive neutrophilia and a moderate recruitment of phagocytes, and TNF induced massive macrophage recruitment with somewhat subdued neutrophil recruitment, which is consistent with reports by Couillin’s group [32]. PARP-1 gene deletion modulated recruitment of phagocytes in response to either stimulus. PARP-1 gene deletion caused a severe reduction in neutrophil recruitment in response to LPS, which is consistent with published reports [33, 34], but recruitment of neutrophils actually increased in response to TNF. The overall number of recruited inflammatory cells did not change in TNF-treated PARP-1−/− mice compared with WT animals. We examined the expression patterns of different inflammatory genes that are specific to macrophage or neutrophil recruitment and performed reversal experiments. PARP-1 gene deletion affects expression of selected genes in response to TNF, and it exerts a more general inhibitory effect in response to LPS. This differential effect on gene expression may explain the different inflammatory responses elicited by LPS or TNF in WT and PARP-1−/− mice. Whether PARP-1 exerts its regulatory function on inflammatory genes equally in cell types is not clear and remains to be examined. For instance, the role of PARP-1 in expression of several inflammatory genes in isolated pulmonary SMCs was consistent for some genes but inconsistent for others (see Supplemental Fig. 1 and Supplemental Material). PARP-1 gene deletion reduced MCP-1 and mKC expression in LPS-treated SMCs; however, such an effect was absent completely in response to TNF. Additionally, expression of MIP-2 was not affected by gene deletion in LPS- or TNF-treated SMCs. These results exemplify the complexity of the role of PARP-1 in the expression of inflammatory genes and certainly invite new experimentations to reach a better understanding of such involvement.
When total inflammatory cell numbers are considered, PARP-1 gene deletion exhibits an excellent inhibitory effect on inflammation mediated by LPS, but little to no effect on TNF-mediated inflammation. The consequences of tissue injury and repair after exposure to TNF are not clear and remain to be determined. LPS-mediated inflammation in the airways may involve TNF [2]. The stage at which LPS differs from TNF in mediating inflammation in our experimental model is unknown, but it is clear that the two stimuli can induce different sets of genes, which may constitute the basis for the difference. It is interesting that PARP-1 gene deletion caused a severe reduction in macrophage recruitment in response to LPS or TNF and is associated with almost a complete inhibition of MCP-1 and MIP-1α in response to either stimulus. It is important to note that in addition to macrophages, MIP-1α can play a role in the recruitment process of neutrophils in mice [35, 36]. However, it is not clear to what extent this chemokine exerts its chemoattracting properties on neutrophils in the presence of other predominant factors such as mKC, MIP-2, and MCP-1, as is the case in our animal model. We and others [15, 25, 37] have reported that PARP-1 is required for MCP-1 expression. The role of MCP-1 as a potential key factor in mediating macrophage recruitment in response to LPS or TNF was confirmed by examining the consequences of intratracheal administration of rMCP-1 to PARP-1−/− mice. We observed that inflammatory cells migrated to the lungs of PARP-1−/− mice, indicating that PARP-1 does not play an important role in the actual migratory function of inflammatory cells toward chemotactic factors. This observation was confirmed in vitro using a variety of chemokines (data not shown). These results confirm that the defect in macrophage recruitment in response to TNF or LPS was primarily a result of the effect of PARP-1 gene deletion on chemokine expression but was not a result of a defect in the ability of PARP-1−/− cells to migrate toward chemotactic factors. These results are consistent with our previous results showing that administration of IL-5 in PARP-1−/− mice re-establishes recruitment of eosinophils to the airways in an animal model of allergen-induced lung inflammation [27].
PARP-1 deficiency was also associated with a modulation of neutrophilia, only in response to LPS but not in response to TNF. Pharmacological PARP inhibition blocks LPS-induced neutrophilia in rodents [33, 34]. The effects of PARP-1 gene knockout on expression of mKC, MIP-2, and CXCR2 in response to LPS result in severe modulatory effects on neutrophil recruitment. A variety of studies have reported that antagonists to mKC and MIP-2 block neutrophil recruitment [2]. In response to TNF, PARP-1 gene knockout was associated with a drastic increase rather than a decrease in neutrophil recruitment to the airways. This result was surprising, as PARP-1 gene deletion exerted modest inhibitory effects on mKC and MIP-2. A modest reduction in mKC and MIP-2 may result in an equally modest reduction in the small number of recruited neutrophils in the airways of TNF-treated PARP-1−/− mice. An important difference was that LPS induced robust expression of CXCR2 coinciding with high levels of mKC and MIP-2, which may create conditions conducive for neutrophil recruitment. On the other hand, exposure to TNF caused a modest increase in receptor expression, suggesting that the presence of the chemokines without threshold levels of the CXCR2 receptor may not lead to the efficient recruitment of neutrophils. These results are consistent with a number of studies reporting that neutralization of CXCR2 by antagonists or by gene deletion prevents neutrophilia [38, 39]. Increased CXCR2 expression in the lungs of LPS-treated WT mice may also be related to an actual increase in CXCR2-positive neutrophils. A recent study by Kesteman et al. [40] reported that i.v. LPS injection reduces CXCR2 immunoreactivity in the spleen, resulting in a decrease in splenic neutrophil emigration.
More importantly, DARC may explain further the observed differences between the effects mediated by LPS and those by TNF as well as the role of PARP-1. Many studies have suggested that DARC functions as a decoy receptor and a sink for chemokines such as mKC and MIP-2 to modulate inflammation [41, 42]. Such a trait is related directly to its inability to mediate signal transduction upon binding of chemokines as a result of a structural difference from general G-protein-coupled receptors [42]. DARC lacks the peptide motif in its second intracellular loop, which is required for signaling through G-proteins. However, the receptor has also been shown to be associated with an active recruitment of neutrophils upon stimulation by LPS or other inflammatory factors [42]. Such discrepancies relate to the complexity of the potential “function” of DARC and may be associated with the type of cells that express this receptor and/or this type of inflammatory factor, as well as the specifics of the experimental system used to conduct the study. In this study, we show that DARC expression was activated by TNF in the lungs of WT mice but to a much lower extent by LPS and that PARP-1 gene deletion abrogated the increased expression completely. The increase in DARC expression in response to TNF and the effect of PARP-1 gene deletion on such increase were confirmed at the protein level by immunohistochemical analysis of lung sections with antibodies to murine DARC (Supplemental Fig. 2 and Supplemental Material). DARC appeared to be expressed predominantly by macrophages in lungs of treated WT mice. DARC expression in lungs of LPS-treated WT mice appeared to be lower than that observed in TNF-treated animals, which is consistent with the patterns of mRNA expression assessed by RT-PCR.
LPS and TNF exert different effects on CXCR2 and DARC expression. It is tempting to speculate that these effects may determine the nature of the recruited cells (neutrophils vs. macrophages) upon treatment with LPS or TNF. When DARC is expressed, chemokines important for neutrophil recruitment such as mKC and/or MIP-2 may be diminished, as DARC functions as a decoy or sink receptor, allowing chemokines such as MCP-1 and MIP-1α to dominate and recruit mostly macrophages. When DARC expression is low, mKC and MIP-2 dominate and promote mostly a neutrophilic response (see Fig. 5). Obviously, an extensive additional experimentation is required to decipher the molecular mechanism(s) by which PARP-1 affects the specific expression of each affected cytokine and/or receptor and the signaling pathways associated with the expression of these critical inflammatory factors.
Overall, a number of questions remain about PARP-1 involvement in airway inflammation and the mechanisms by which it contributes to these processes. Our laboratory is continuing its efforts to decipher the role(s) of PARP-1 in inflammatory gene expression with the ultimate goal of providing further evidence that PARP-1 is a viable target for therapies to treat inflammatory diseases. However, PARP-1 inhibition may not be a general strategy for all inflammatory diseases. More experimentation is required to define which diseases can benefit from therapeutic PARP-1 targeting.
ACKNOWLEDGMENTS
This work was supported in part by grant HL072889 from the National Institutes of Health and funds from the Louisiana Cancer Research Consortium to A. H. B. We thank Dr. Stephania Cormier for critically reading the manuscript.
Supplementary Material
Footnotes
Current address: Department of Cardiology, Tulane University Medical Center, New Orleans, LA 70112, USA.
Current address: Department of Animal Science and Biotechnology, Chonbuk National University, Jeonju, Korea.
Abbreviations: BALF=brochoalveolar lavage fluid(s), DARC=Duffy antigen receptor for chemokines, LSUHSC=Louisiana State University Health Sciences Center, mKC=mouse keratinocyte-derived chemokine, PARP-1=poly(ADP-ribose) polymerase-1, SMC=smooth muscle cell, WT=wild-type
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
References
- Gonzalez P K, Zhuang J, Doctrow S R, Malfroy B, Benson P F, Menconi M J, Fink M P. Role of oxidant stress in the adult respiratory distress syndrome: evaluation of a novel antioxidant strategy in a porcine model of endotoxin-induced acute lung injury. Shock. 1996;6:S23–S26. [PubMed] [Google Scholar]
- Kobayashi Y. The role of chemokines in neutrophil biology. Front Biosci. 2008;13:2400–2407. doi: 10.2741/2853. [DOI] [PubMed] [Google Scholar]
- Hallett J M, Leitch A E, Riley N A, Duffin R, Haslett C, Rossi A G. Novel pharmacological strategies for driving inflammatory cell apoptosis and enhancing the resolution of inflammation. Trends Pharmacol Sci. 2008;29:250–257. doi: 10.1016/j.tips.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Pruenster M, Rot A. Throwing light on DARC. Biochem Soc Trans. 2006;34:1005–1008. doi: 10.1042/BST0341005. [DOI] [PubMed] [Google Scholar]
- Comerford I, Litchfield W, Harata-Lee Y, Nibbs R J, McColl S R. Regulation of chemotactic networks by “atypical” receptors. Bioessays. 2007;29:237–247. doi: 10.1002/bies.20537. [DOI] [PubMed] [Google Scholar]
- Borroni E M, Bonecchi R, Buracchi C, Savino B, Mantovani A, Locati M. Chemokine decoy receptors: new players in reproductive immunology. Immunol Invest. 2008;37:483–497. doi: 10.1080/08820130802191318. [DOI] [PubMed] [Google Scholar]
- Luo H, Chaudhuri A, Zbrzezna V, He Y, Pogo A O. Deletion of the murine Duffy gene (Dfy) reveals that the Duffy receptor is functionally redundant. Mol Cell Biol. 2000;20:3097–3101. doi: 10.1128/mcb.20.9.3097-3101.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarbock A, Schmolke M, Bockhorn S G, Scharte M, Buschmann K, Ley K, Singbartl K. The Duffy antigen receptor for chemokines in acute renal failure: a facilitator of renal chemokine presentation. Crit Care Med. 2007;35:2156–2163. doi: 10.1097/01.ccm.0000280570.82885.32. [DOI] [PubMed] [Google Scholar]
- Horton L W, Yu Y, Zaja-Milatovic S, Strieter R M, Richmond A. Opposing roles of murine Duffy antigen receptor for chemokine and murine CXC chemokine receptor-2 receptors in murine melanoma tumor growth. Cancer Res. 2007;67:9791–9799. doi: 10.1158/0008-5472.CAN-07-0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulares A H, Zoltoski A J, Sherif Z A, Jolly P, Massaro D, Smulson M E. Gene knockout or pharmacological inhibition of poly(ADP-ribose) polymerase-1 prevents lung inflammation in a murine model of asthma. Am J Respir Cell Mol Biol. 2003;28:322–329. doi: 10.1165/rcmb.2001-0015OC. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Masini E, Mazzocca C, Cuzzocrea S, Ciampa A, Suzuki H, Bani D. Inhibition of poly(ADP-ribose) polymerase prevents allergen-induced asthma-like reaction in sensitized Guinea pigs. J Pharmacol Exp Ther. 2004;311:1241–1248. doi: 10.1124/jpet.104.072546. [DOI] [PubMed] [Google Scholar]
- Boulares H, Zoltoski A J, Kandan S, Akbulut T, Yakovlev A, Oumouna M. Correlation between decreased sensitivity of the Daudi lymphoma cells to VP-16-induced apoptosis and deficiency in DNAS1L3 expression. Biochem Biophys Res Commun. 2006;341:653–662. doi: 10.1016/j.bbrc.2006.01.014. [DOI] [PubMed] [Google Scholar]
- Naura A S, Hans C P, Zerfaoui M, You D, Cormier S A, Oumouna M, Boulares A H. Post-allergen challenge inhibition of poly(ADP-ribose) polymerase harbors therapeutic potential for treatment of allergic airway inflammation. Clin Exp Allergy. 2008;38:839–846. doi: 10.1111/j.1365-2222.2008.02943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naura A S, Datta R, Hans C P, Zerfaoui M, Rezk B M, Errami Y, Oumouna M, Matrougui K, Boulares A H. Reciprocal regulation of iNOS and PARP-1 during allergen-induced eosinophilia. Eur Respir J. 2008;33:252–262. doi: 10.1183/09031936.00089008. [DOI] [PubMed] [Google Scholar]
- Hans C P, Zerfaoui M, Naura A, Troxclair D, Strong J P, Matrougui K, Boulares H. Thieno[2,3-c]isoquinolin-5-one, a potent poly(ADP-ribose) polymerase inhibitor, promotes atherosclerotic plaque regression in high fat diet-fed ApoE-deficient mice: effects on inflammatory markers and lipid content. J Pharmacol Exp Ther. 2009;329:150–158. doi: 10.1124/jpet.108.145938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieper A A, Verma A, Zhang J, Snyder S H. Poly (ADP-ribose) polymerase, nitric oxide and cell death. Trends Pharmacol Sci. 1999;20:171–181. doi: 10.1016/s0165-6147(99)01292-4. [DOI] [PubMed] [Google Scholar]
- Tentori L, Portarena I, Graziani G. Potential clinical applications of poly(ADP-ribose) polymerase (PARP) inhibitors. Pharmacol Res. 2002;45:73–85. doi: 10.1006/phrs.2001.0935. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S. Shock, inflammation and PARP. Pharmacol Res. 2005;52:72–82. doi: 10.1016/j.phrs.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Hassa P O, Hottiger M O. The functional role of poly(ADP-ribose)polymerase 1 as novel coactivator of NF-κB in inflammatory disorders. Cell Mol Life Sci. 2002;59:1534–1553. doi: 10.1007/s00018-002-8527-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassa P O, Hottiger M O. A role of poly (ADP-ribose) polymerase in NF-κB transcriptional activation. Biol Chem. 1999;380:953–959. doi: 10.1515/BC.1999.118. [DOI] [PubMed] [Google Scholar]
- Cohen-Armon M, Visochek L, Rozensal D, Kalal A, Geistrikh I, Klein R, Bendetz-Nezer S, Yao Z, Seger R. DNA-independent PARP-1 activation by phosphorylated ERK2 increases Elk1 activity: a link to histone acetylation. Mol Cell. 2007;25:297–308. doi: 10.1016/j.molcel.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Kraus W L, Lis J T. PARP goes transcription. Cell. 2003;113:677–683. doi: 10.1016/s0092-8674(03)00433-1. [DOI] [PubMed] [Google Scholar]
- Karin M, Cao Y, Greten F R, Li Z W. NF-κB in cancer: from innocent bystander to major culprit. Nat Rev Cancer. 2002;2:301–310. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- Oliver F J, Menissier-de Murcia J, Nacci C, Decker P, Andriantsitohaina R, Muller S, de la Rubia G, Stoclet J C, de Murcia G. Resistance to endotoxic shock as a consequence of defective NF-κB activation in poly (ADP-ribose) polymerase-1 deficient mice. EMBO J. 1999;18:4446–4454. doi: 10.1093/emboj/18.16.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oumouna-Benachour K, Hans C P, Suzuki Y, Naura A, Datta R, Belmadani S, Fallon K, Woods C, Boulares A H. Poly(ADP-ribose) polymerase inhibition reduces atherosclerotic plaque size and promotes factors of plaque stability in apolipoprotein E-deficient mice: effects on macrophage recruitment, nuclear factor-κB nuclear translocation, and foam cell death. Circulation. 2007;115:2442–2450. doi: 10.1161/CIRCULATIONAHA.106.668756. [DOI] [PubMed] [Google Scholar]
- Zerfaoui M, Suzuki Y, Naura A S, Hans C P, Nichols C, Boulares A H. Nuclear translocation of p65 NF-κB is sufficient for VCAM-1, but not ICAM-1, expression in TNF-stimulated smooth muscle cells: differential requirement for PARP-1 expression and interaction. Cell Signal. 2008;20:186–194. doi: 10.1016/j.cellsig.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oumouna M, Datta R, Oumouna-Benachour K, Suzuki Y, Hans C, Matthews K, Fallon K, Boulares A H. PARP-1 inhibition prevents eosinophil recruitment by modulating Th2 cytokines in a murine model of allergic airway inflammation: a potential specific effect on IL-5. J Immunol. 2006;177:6489–6496. doi: 10.4049/jimmunol.177.9.6489. [DOI] [PubMed] [Google Scholar]
- Livak K J, Schmittgen T D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(–Δ Δ C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Matsuda A, Fukuda S, Matsumoto K, Saito H. Th1/Th2 cytokines reciprocally regulate in vitro pulmonary angiogenesis via CXC chemokine synthesis. Am J Respir Cell Mol Biol. 2008;38:168–175. doi: 10.1165/rcmb.2007-0162OC. [DOI] [PubMed] [Google Scholar]
- Lee J S, Frevert C W, Wurfel M M, Peiper S C, Wong V A, Ballman K K, Ruzinski J T, Rhim J S, Martin T R, Goodman R B. Duffy antigen facilitates movement of chemokine across the endothelium in vitro and promotes neutrophil transmigration in vitro and in vivo. J Immunol. 2003;170:5244–5251. doi: 10.4049/jimmunol.170.10.5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hans C P, Zerfaoui M, Naura A S, Catling A, Boulares A H. Differential effects of PARP inhibition on vascular cell survival and ACAT-1 expression favoring atherosclerotic plaque stability. Cardiovasc Res. 2008;78:429–439. doi: 10.1093/cvr/cvn018. [DOI] [PubMed] [Google Scholar]
- Togbe D, Schnyder-Candrian S, Schnyder B, Doz E, Noulin N, Janot L, Secher T, Gasse P, Lima C, Coelho F R, Vasseur V, Erard F, Ryffel B, Couillin I, Moser R. Toll-like receptor and tumor necrosis factor dependent endotoxin-induced acute lung injury. Int J Exp Pathol. 2007;88:387–391. doi: 10.1111/j.1365-2613.2007.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingarelli B, Szabo C, Salzman A L. Blockade of Poly(ADP-ribose) synthetase inhibits neutrophil recruitment, oxidant generation, and mucosal injury in murine colitis. Gastroenterology. 1999;116:335–345. doi: 10.1016/s0016-5085(99)70130-7. [DOI] [PubMed] [Google Scholar]
- Szabo C, Lim L H, Cuzzocrea S, Getting S J, Zingarelli B, Flower R J, Salzman A L, Perretti M. Inhibition of poly (ADP-ribose) synthetase attenuates neutrophil recruitment and exerts antiinflammatory effects. J Exp Med. 1997;186:1041–1049. doi: 10.1084/jem.186.7.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos C D, Canetti C, Souto J T, Silva J S, Hogaboam C M, Ferreira S H, Cunha F Q. MIP-1α[CCL3] acting on the CCR1 receptor mediates neutrophil migration in immune inflammation via sequential release of TNF-α and LTB4. J Leukoc Biol. 2005;78:167–177. doi: 10.1189/jlb.0404237. [DOI] [PubMed] [Google Scholar]
- Ekman A K, Fransson M, Rydberg C, Adner M, Cardell L O. Nasal challenge with LPS stimulates the release of macrophage inflammatory protein 1α. Int Arch Allergy Immunol. 2009;149:154–160. doi: 10.1159/000189199. [DOI] [PubMed] [Google Scholar]
- Farivar A S, Woolley S M, Fraga C H, Thomas R, Salzman A L, Szabo C, Mulligan M S. Intratracheal poly (ADP) ribose synthetase inhibition ameliorates lung ischemia reperfusion injury. Ann Thorac Surg. 2004;77:1938–1943. doi: 10.1016/j.athoracsur.2003.10.120. [DOI] [PubMed] [Google Scholar]
- McColl S R, Clark-Lewis I. Inhibition of murine neutrophil recruitment in vivo by CXC chemokine receptor antagonists. J Immunol. 1999;163:2829–2835. [PubMed] [Google Scholar]
- Reutershan J, Morris M A, Burcin T L, Smith D F, Chang D, Saprito M S, Ley K. Critical role of endothelial CXCR2 in LPS-induced neutrophil migration into the lung. J Clin Invest. 2006;116:695–702. doi: 10.1172/JCI27009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesteman N, Vansanten G, Pajak B, Goyert S M, Moser M. Injection of lipopolysaccharide induces the migration of splenic neutrophils to the T cell area of the white pulp: role of CD14 and CXC chemokines. J Leukoc Biol. 2008;83:640–647. doi: 10.1189/jlb.0807578. [DOI] [PubMed] [Google Scholar]
- Dawson T C, Lentsch A B, Wang Z, Cowhig J E, Rot A, Maeda N, Peiper S C. Exaggerated response to endotoxin in mice lacking the Duffy antigen/receptor for chemokines (DARC) Blood. 2000;96:1681–1684. [PubMed] [Google Scholar]
- Bonecchi R, Borroni E M, Savino B, Buracchi C, Mantovani A, Locati M. Non-signaling chemokine receptors: mechanism of action and role in vivo. J Neuroimmunol. 2008;198:14–19. doi: 10.1016/j.jneuroim.2008.04.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.