Abstract
IL-17+ T cells make up the majority of the infiltrating cells in the inflamed eye during the development of EAU. However, the role of IL-17 in ocular inflammation is poorly defined. Given that the primary target cells for IL-17 are parenchymal cells of the tissue, we investigated the in vitro effect of IL-17 on mouse RACs and RPE cells. Our results showed that although RACs and RPE cells expressed the IL-17R, RACs responded to IL-17 by producing increased amounts of proinflammatory cytokines and chemokines, leading to increased migration of granulocytes, whereas RPE cells responded to the same concentration of IL-17 by expressing increased levels of SOCS proteins, resulting in only limited production of proinflammatory cytokines and chemokines and an increased amount of suppressive cytokines, such as LIF. The combination of IL-17 and IFN-γ had a synergistic effect on cell migration with RACs but an antagonistic effect with RPE. In addition, specific inhibitors of the PI3K/Akt signaling pathway completely blocked inflammatory cell migration induced by chemokines released by IL-17-stimulated RACs. Our results demonstrate that IL-17 can induce a pro- or anti-inflammatory effect in the eye, depending on the parenchymal cells stimulated.
Keywords: autoimmunity, inflammation, cytokines, tissue cells
Introduction
Uveitis is a common inflammatory disease that affects the uvea and retina. Although its etiology is not fully understood, Th1 cells and the cytokines that they produce are thought to play a role in its development. Recently, IL-17-producing Th17 cells were reported to be involved in the pathogenesis of uveitis [1] and the corresponding animal model, EAU [2,3,4,5]. Th17 cells are present in human PBMCs, and their numbers increase during active uveitis and scleritis and decrease following treatment [1]. IL-17 levels in the eye are increased in EAU, suggesting a mechanism by which Th17 cells contribute to ocular pathology.
Previous studies have shown that the biological actions of IL-17 are proinflammatory. IL-17 induces epithelial, endothelial, and fibroblastic cells to produce other inflammatory cytokines and chemokines, including IL-6, MIP-2, G-CSF, and MCP-1, also known as CCL-2 [6, 7]. IL-17 also acts synergistically with other cytokines, such as TNF-α or IL-1β, to induce further chemokine expression [8, 9]. Therapeutic agents that target IL-17 or inhibit the Th17 cell population directly have shown promise in animal models of autoimmunity [10,11,12,13]. However, as many cytokines, such as IFN-γ, TNF-α, and IL-6, have pro- and anti-inflammatory activity [14,15,16,17,18], we have recently reported a similar dual function of IL-17 in uveitis [19].
Although the complete IL-17R signaling cascade is far from defined completely, it seems that signaling transduction pathways involving PI3K/Akt, NF-κB, MAPK, and JAK/STAT may be activated after IL-17 binds to the IL-17R.
We have shown previously that RACs and RPE cells are two major types of ocular resident cells that participate in immune responses in the eye. For example, RACs from uveitis-susceptible B10RIII mice are able to express MHC class II molecules and costimulatory molecules after exposure to proinflammatory cytokines [2]. They also present antigen to uveitogenic T cells, inducing them to proliferate and produce inflammatory cytokines [2]. On the other hand, RPE cells, preactivated by IFN-γ alone or IFN-γ plus TNF-α, induce the same T cells to produce IL-6 and IL-10 but fail to induce IL-2 production or T cell proliferation [2, 20]. In this study, we explored the proinflammatory effects of IL-17 on RACs but not on RPE cells. We found that although both cell types express the IL-17RA, exposure to IL-17 results in different effects, and RACs produce large amounts of inflammatory cytokines and chemokines, and RPE cells produce lower levels of cytokines and chemokines and increased levels of LIF. The weak inflammatory effect of RPE cells in response to IL-17 seems to be regulated by the expression of SOCS proteins.
MATERIALS AND METHODS
Animals, reagents, and cell culture
Pathogen-free female C57BL/6 (B6) mice (6–10 weeks old) were purchased from Jackson Laboratory (Bar Harbor, ME, USA) and were housed and maintained in the animal facilities of the University of Louisville (KY, USA). All animal studies conformed to the Association for Research in Vision and Ophthalmology statement on the use of animals in ophthalmic and vision research. Institutional approval was obtained and institutional guidelines regarding animal experimentation followed.
Murine rIL-17 and rIFN-γ were purchased from R&D Systems (Minneapolis, MN, USA). LY294002, MG-132, and PD98059 were obtained from Calbiochem (San Diego, CA, USA) and SB216763 from Sigma-Aldrich (St. Louis, MO, USA).
IRBP-specific CD4 T cell lines were prepared from IRBP1–20-immunized B6 mice using the methods of generating antigen-specific T cells as described previously [21]. Bone marrow-derived macrophages were collected and differentiated in vitro as described [22].
Isolation and culture of primary RACs and RPE
The methods for the isolation of RACs and RPE cells have been described previously [2]. The purity of the RACs or RPE cells was >95%, as assessed by staining with, respectively, primary antibodies against glial fibrillary acidic protein (Sigma-Aldrich) and S-100 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) or anti-pan keratin antibody (clone PCK-26, Sigma-Aldrich) and anti-RPE65 antibody (Novus, Littleton, CO, USA). RACs and RPE cells were used in experiments at three to five passages.
Detection of genetic expression by RT-PCR or real-time qRT-PCR
Total RNA from cells was extracted using an RNA isolation kit (Invitrogen, Carlsbad, CA, USA), treated with DNase I (GE Healthcare, Piscataway, NJ, USA), and reverse-transcribed into cDNA using a Moloney murine leukemia virus-RT kit (Invitrogen). Except IL-17R, which was determined by RT-PCR (forward primer: 5′-AATAGTACTTGTCTGGATGACAGC; reverse primer: 5′-ACAGCCGCTCATTGGTGTTC), all of the other genes were detected by qRT-PCR. Each cDNA sample was amplified for the gene of interest and β-actin (TaqMan assays; Mx3000P system; Stratagene, La Jolla, CA, USA). The concentration of the gene of interest was determined using the comparative threshold cycle number and normalized to that of the internal β-actin control. The primers used for qRT-PCR are listed in Table 1.
TABLE 1.
Primers Used for RT-PCR
| Name | Forward primer | Reverse primer |
|---|---|---|
| β-actin | 5′-ATCTACGAGGGCTATGCTCTCC | 5′-ACGCTCGGTCAGGATCTTCAT |
| TNF-α | 5′-AAATGGCCTCCCTCTCATCAG | 5′-GCTTGTCACTCGAATTTTGAGAAG |
| IL-6 | 5′-CCTTCTTGGGACTGATGCTG | 5′-TCTGTTGGGAGTGGTATCCTC |
| CCL2 | 5′-AGTAGGCTGGAGAGCTACAAG | 5′-GTAGGTTCTGATCTCATTTGGTTC |
| CCL7 | 5′-GAGGATCTCTGCCACGCTTC | 5′-TTCCCAGGGACACCGACTAC |
| SOCS-1 | 5′-CACTCACTTCCGCACCTTCC | 5′-GACTGTCGCGCACCAAGAAG |
| SOCS-3 | 5′-GCAGGAGAGCGGATTCTACTG | 5′-CCTCACACTGGATGCGTAGG |
ELISA
Levels of IL-6, TNF-α, CCL2, and LIF were measured using commercially available ELISA kits (R&D Systems).
Chemotaxis assay
Splenocytes (3×105 cells/well) from B6 mice immunized s.c. with 100 μl emulsion containing 300 μg IRBP peptide 1–20 (Sigma-Aldrich) and 500 μg Mycobacterium tuberculosis H37Ra in IFA [23] were added to the upper wells of a microchemotaxis device (5 μm pore size; 24-well; Transwell; Corning-Costar, Corning, NY, USA). The supernatants from RACs or RPE cultured for 24 h in fresh culture medium after incubation with or without 100 ng/ml IL-17 were added to the lower wells. Cells that migrated to the lower wells after 2 h were collected, counted, stained with antibodies against CD4, CD8, CD11b, Gr-1, TCR, NK1.1, CD19, or CD11c, and analyzed by flow cytometry. All assays were performed three times, each in triplicate.
Flow cytometry analysis
Aliquots (1×106 cells) were double-stained with combinations of FITC- or PE-conjugated mAb against mouse TCR, IL-17R, IL-17, IFN-γ, CD11b, or Gr-1 (eBioscience, San Diego, CA, USA). For intracellular cytokine staining, splenic T cells from immunized mice or infiltrated cells from the eye were cultured for 5 h with 1 μg/mL Brefeldin A, 1 μg/mL ionomycin, and 50 ng/mL PMA (Sigma-Aldrich) and then permeabilized using a kit (Cytofix/Cytoperm Plus, BD PharMingen, San Diego, CA, USA), according to the manufacturer’s protocol before reaction with antibody. Data collection and analysis were performed using a flow cytometer (FACSCalibur, BD PharMingen) and appropriate software (CellQuest, BD PharMingen).
Statistics
Experiments were repeated at least twice and usually three or more times. An unpaired Student’s t-test or one-way ANOVA with Bonferroni’s correction was used for statistical analysis. A P value <0.05 was considered as significant. Values determined to be significantly different from controls are marked with an asterisk in the figures.
RESULTS
Expression of IL-17R (IL-17RA) on RACs and RPE cells
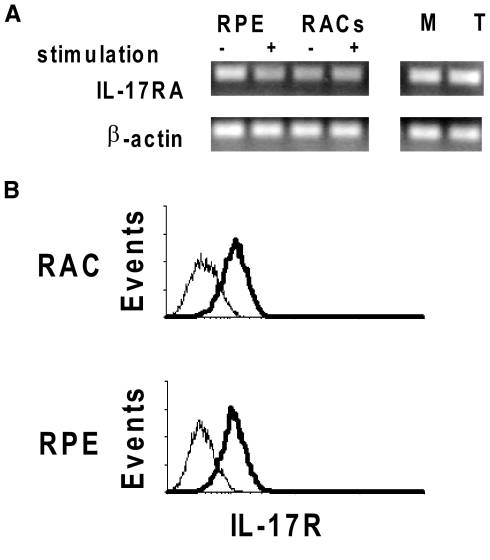
To determine whether RACs and RPE cells expressed the IL-17RA, we assessed IL-17RA mRNA levels in these cells using RT-PCR. As shown in Figure 1A, RACs and RPE cells expressed IL-17RA mRNA, as did macrophages and splenic T cells. IL-17RA mRNA was constitutively expressed in RACs and RPE cells, and stimulation with IFN-γ and TNF-α did not result in a significant change in expression. Analyzing their protein expression by flow cytometry revealed that RACs and RPE expressed a similar level of IL-17R on their cell surface (Fig. 1B).
Figure 1.
Expression of IL-17R in RACs and RPE cells. (A) Total RNA was extracted from RACs and RPE cells incubated with or without culture medium containing IFN-γ and TNF-α and also from macrophages (M) or a CD4 T cell line (T). Levels of IL-17RA mRNA were determined by RT-PCR. (B) The receptor expression on RACs and RPE was also tested by flow cytometry at protein level. IL-17R expression by cells is shown by the shift in fluorescence intensity of the specific antibody (thick lines) over the isotype control (thin lines).
Responses of RACs and RPE cells to IL-17 stimulation
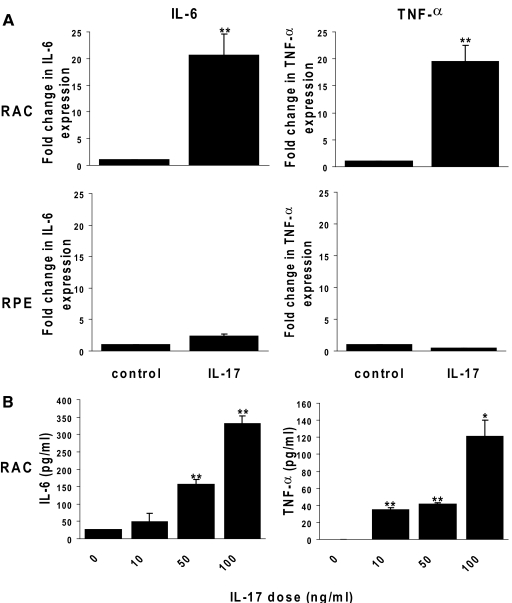
We then determined whether exposure to IL-17 induced the IL-17-mediated production of proinflammatory cytokines by RACs and RPE cells. Production of IL-6 and TNF-α by IL-17-stimulated RACs and RPE cells was assessed using real-time PCR and ELISA. As shown in Figure 2A, IL-6 and TNF-α mRNA levels increased in cultured RACs by up to 20-fold after exposure to 100 ng/ml IL-17 for 24 h. A dose-dependent effect of IL-17 on IL-6 and TNF-α protein production by RACs was shown by ELISA (Fig. 2B). Interestingly, IL-17 did not induce significant expression of IL-6 or TNF-α mRNA in RPE cells (Fig. 2A). In contrast, RPE cells produced IL-6 protein in the absence of exgogeneous stimulation, and exposure to IL-17 enhanced IL-6 production by two- or 2.5-fold at the concentration of 10 or 100 ng/ml, respectively (data not shown). RPE cells produced low amounts of TNF-α protein, and levels were barely detectable after IL-17 stimulation (data not shown).
Figure 2.
Effects of IL-17 on IL-6 and TNF-α production by RACs and RPE cells. RPCs and RPE cells were incubated for 24 h in medium with or without 100 ng/ml rIL-17 (A) or different concentrations of rIL-17 (B). Then, IL-6 and TNF-α mRNAs were measured by qRT-PCR analysis (A) and cytokines released into the supernatants measured by ELISA (B). The data are the mean ± sd for triplicate wells and are representative of those obtained in three independent experiments. *, P < 0.05; **, P < 0.01.
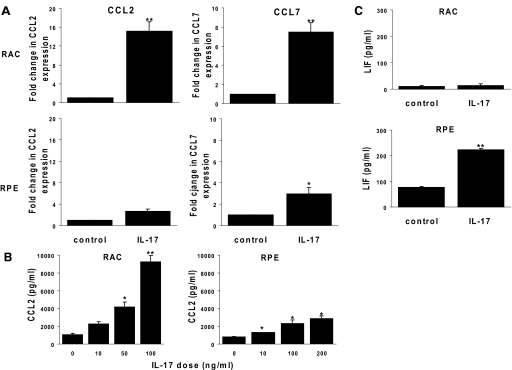
RT-PCR studies showed that RACs and RPE cells expressed increased levels of chemokines after IL-17 treatment. At the concentration of 100 ng/ml, IL-17 induced higher levels of CCL2 and CCL7 mRNAs in RACs than in RPE cells (Fig. 3A). Production of CCL2 protein by RACs, but not by RPE cells, was increased in a dose-dependent manner following IL-17 stimulation (Fig. 3B). In contrast, IL-17 induced a marked increase in LIF production by RPE cells but not by RACs (Fig. 3C).
Figure 3.
Effects of IL-17 on chemokine production by RACs and RPE cells. RACs and RPE cells were incubated with or without 100 ng/ml rIL-17 (A and C) or with different concentrations of IL-17 (B). Then, CCL2 and CCL7 mRNAs were measured by qRT-PCR (A) and CCL2 (B), or LIF (C) protein in the supernatants was measured by ELISA. The data represent typical samples performed in triplicate in three independent experiments. *, P < 0.05; **, P < 0.01.
Chemoattractive activity in the supernatants of IL-17-stimulated RACs and RPE cells
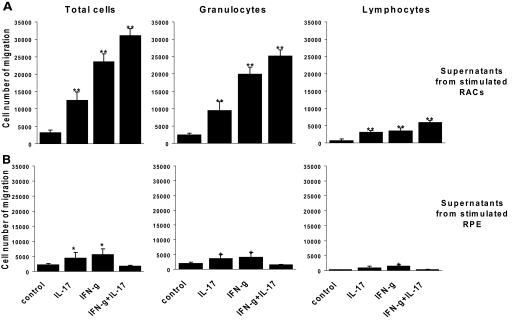
We compared chemoattractive activity in the culture supernatants of IL-17-stimulated and nonstimulated RACs and RPE cells by measuring the effect on the migration of T cells and polymorphonuclear leukocytes, the two dominant, inflammatory cells in ocular inflammation [24, 24, 25]. Activated splenocytes from B6 mice immunized with IRBP1–20 were placed in the top chambers of culture wells separated from the bottom chamber by culture inserts, and the migration of cells to the lower chamber was estimated and their phenotype determined by FACS analysis. As shown in Figure 4, significant numbers of leukocytes and T cells were migrated to the cytokines and chemokines in the supernatants of IL-17-stimulated RACs in comparison with the same IL-17-treated RPE. Cell migration was increased markedly to the supernatants of IFN-γ or IL-17- and IFN-γ-treated RACs. In contrast, migration was reduced to the combination of IL-17- and IFN-γ-treated RPE.
Figure 4.
Cytokines and chemokines released into the supernatants of IL-17-stimulated RACs attract more inflammatory cells than those produced by IL-17-stimulated RPE cells. Spleen cells from IRBP1–20-immunized B6 mice that migrated to the lower chamber containing supernatants collected from RACs (A) or RPE cells (B) pretreated for 24 h with medium, IL-17 (100 ng/ml), IFN-γ (500 U/ml), or IL-17 and IFN-γ were counted after 2 h, stained with rat mAb against mouse TCR or Gr-1, and analyzed by flow cytometry. The results are representative of those obtained in three experiments. The values shown are the means ± sd. *, P < 0.05; **, P < 0.01.
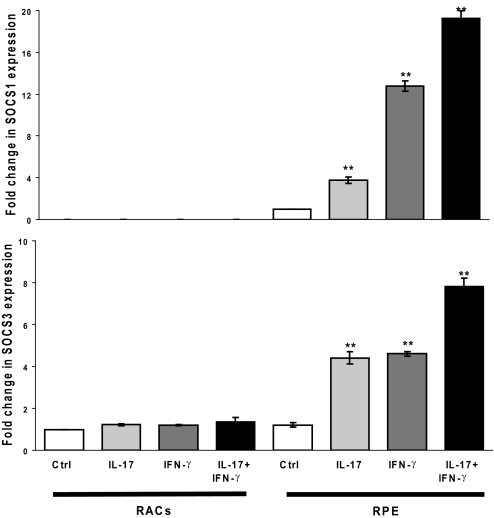
IL-17 induces expression of SOCS-1 and SOCS-3 in RPE cells but not in RACs
To determine how IL-17 causes the different inflammatory potential of RACs and RPE cells, we measured levels of mRNAs coding for SOCS-1 and SOCS-3 in RAC and RPE cells before and after exposure to IL-17 using RT-PCR. As shown in Figure 5, RACs did not express SOCS-1 mRNA with or without IL-17 treatment but constitutively expressed SOCS-3 mRNA, and the level of expression was not altered significantly after exposure to IL-17. RPE cells, on the other hand, constitutively expressed SOCS-1 and SOCS-3 mRNAs, and the levels of both were increased significantly when the RPE cells were pretreated with IL-17 or IFN-γ. In addition, the combination of IL-17 and IFN-γ had an additive effect, resulting in even higher levels of SOCS-1 and SOCS-3 mRNAs.
Figure 5.
SOCS-1 and SOCS-3 expression following IL-17 treatment of RACs and RPE cells. RACs and RPE cells were cultured to confluency and then treated with medium alone or 100 ng/ml IL-17 and/or 500 U/ml IFN-γ for 6 h or 24 h. Then, SOCS-1 and SOCS-3 mRNAs were measured by RT-PCR. The data shown are after treatment for 6 h; similar results were obtained after treatment for 24 h. **, P < 0.01.
Signaling pathways involved in the IL-17/IL-17R interaction on RACs
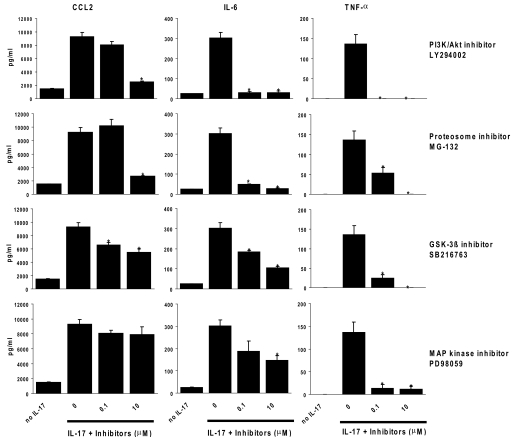
To determine which signaling pathway was involved in the induction of IL-6, TNF-α, and CCL2 expression by IL-17 in RACs, we assessed whether LY294002, PD98059, or SB216763, which, respectively, inhibits PI3K/Akt 1, MAPK, and GSK-3, or MG-132, a proteosome inhibitor that inhibits the activation of NF-κB, had an effect on the production of IL-6, TNF-α, and CCL2 by IL-17-treated RACs. RACs were preincubated with 0–10 μM each inhibitor for 2 h and then incubated with 100 ng/ml IL-17 for 24 h, and the supernatants were collected for detection of IL-6, TNF-α, and CCL2 by ELISA. As shown in Figure 6, each of the four inhibitors caused a marked dose-dependent reduction in TNF-α production, and the IL-17-mediated production of IL-6 and CCL2 was inhibited significantly by the PI3K/Akt 1, NF-κB, and GSK-3 inhibitors but inhibited less efficiently by the MAPK inhibitor.
Figure 6.
Signaling pathways involved in IL-17-mediated induction of expression of IL-6, TNF-α, and CCL2 by RACs. RACs were preincubated with medium or an inhibitor of PI3K/Akt1 (LY294002), NF-κB (MG-132), MAPK (PD98059), or GSK-3 (SB216763) at the indicated concentrations for 2 h and then incubated with 100 ng/ml IL-17 for 24 h and the supernatant collected for measurement of IL-6, TNF-α, and CCL2 protein by ELISA. The data presented are the mean values (±sd) obtained from triplicate samples for each concentration. *, P < 0.05, compared with untreated cells.
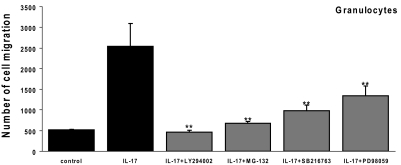
Blockade of IL-17-mediated granulocyte migration by signaling pathway inhibitors
We then examined whether these inhibitors were able to block the migration of granulocytes induced by supernatants from IL-17-treated RACs. As shown in Figure 7, each of the four signaling inhibitors suppressed granulocyte migration significantly. The PI3K/Akt1 inhibitor LY294002 had the strongest effect, followed by the NF-κB inhibitor MG-132 and the GSK-3 inhibitor SB216763, and the MAPK inhibitor PD98059 was the least effective.
Figure 7.
Reduced migration caused by signaling pathway inhibitors. A similar experiment to that described in Figure 4 was performed, except that spleen cells from IRBP1–20-immunized B6 mice were attracted to the lower chamber containing supernatants collected from RACs pretreated with medium or IL-17 (100 ng/ml) in the absence or presence of the indicated signaling pathway inhibitors for 24 h. The results are representative of those obtained in three experiments. **, P < 0.01.
DISCUSSION
In the inflammatory environment of the eye, multiple cytokines and chemokines will accumulate and affect parenchymal cells. To dissect the impact of Th17 cells, we focused on the effect of IL-17, which is released primarily by Th17 cells on two types of parenchymal cells, RACs and RPE cells. We found that although RACs and RPE cells in the eye expressed IL-17RA, the responses of the two cell types to IL-17 were different. In response to IL-17 treatment, RACs expressed increased levels of both cytokines, such as IL-6 and TNF-α, and chemokines, such as CCL2, a neutrophil-directed chemokine. In contrast, RPE cells produced low or undetectable levels of IL-6, TNF-α, and CCL2 but high levels of LIF. As a result, the high levels of cytokines and chemokines in the supernatant of IL-17-stimulated RACs resulted in marked attraction of inflammatory cells, mainly granulocytes, whereas the supernatants from IL-17-stimulated RPE had only a weak ability to attract inflammatory cells. Similar to the effect of IL-17, the cytokines and chemokines released in the supernatant of IFN-γ-treated RACs enhanced the migration of granulocytes and mononuclear cells, whereas the cytokines and chemokines released in the supernatant of IFN-γ-treated RPE attract a low amount of infiltrated cells. Interestingly, IL-17 and IFN-γ had a synergistic effect on cell migration with RACs but an antagonistic effect with RPE. Thus, these results reveal cell-specific effects of IL-17 in the eye.
RPE cells can be induced to produce many pro- or anti-inflammatory cytokines and chemokines, depending on the mediators involved. For example, primary cultures of human fetal RPE stimulated with an inflammatory cytokine mixture containing IL-1β, TNF-α, and IFN-γ secrete exceptionally high amounts of CCL2 (MCP-1) onto the apical and basal sides of the cells [26]. The difference in our results from those of Shi et al. [26] may be related to a difference in species (mouse RPE vs. human fetal RPE) and/or culture conditions (IL-17 vs. multiple cytokines including IL-1β, TNF-α, and IFN-γ). A low level of CCL2 may be protective [26].
The opposing responses of mouse RACs and RPE cells to IL-17 exposure seem to be regulated by SOCS proteins, which have a suppressive effect on proinflammatory cytokine-mediated immune reactions. SOCS proteins have been reported to regulate cellular responses to cytokines. In immune cells, numerous cytokines induce expression of SOCS proteins, which provide negative feedback by inhibiting the JAK/STAT pathway [27]. SOCS-1 is involved in regulating tissue IFN-γ sensitivity [28], whereas SOCS-3 modulates IL-6 signaling [29,30,31]. SOCS protein levels are increased in the blood of uveitis patients [32] and in the retina in EAU [33]. In addition to immune cells, other tissue cells, such as astrocytes in the CNS, have been reported to express SOCS proteins in response to inflammatory stimuli including IFN-γ as a protective mechanism within the tissues [34]. In neurons and Schwann cells, SOCS-1 suppresses IFN-γ-induced MHC I expression [35]. The expression of SOCS-1 or SOCS-3 seems to be different in different cells responding to different stimuli. SOCS-1 was identified initially in a murine monocytic cell line treated with IL-6 [36, 37]. Exposure to CpG oligonucleotides or LPS induces SOCS-1 and SOCS-3 expression in murine macrophages and dendritic cells [38, 39]. In mouse astrocytes isolated from the CNS [34], exposure to IL-6 increases the expression of SOCS-3 but not of SOCS-1. In the present study, we showed, for the first time, that IL-17 or IFN-γ had little effect, alone or in combination, in inducing SOCS expression by RACs, whereas IL-17 or IFN-γ induced expression of SOCS-1 and SOCS-3 in RPE cells, and the combination of the two enhanced SOCS protein expression greatly. Our observation supports the possibility that ocular inflammation may cause increased production of IFN-γ and IL-17 in the eye and that these act on RPE cells, damping down ocular inflammation. It is likely that such a regulatory mechanism is involved in remission in relapsing-remitting uveitis or EAU. This result also suggests that modulation of SOCS protein expression in the eye or peripheral cells by genetic manipulation or drug administration may be a therapeutic strategy for treating inflammatory eye disease.
The signaling pathways by which IL-17 altered cytokine and chemokine production by RACs were explored further. Our results showed that inhibitors of PI3K, GSK, MAPK, and NF-κB markedly inhibited IL-17-mediated IL-6 and TNF-α production. The PI3K, NF-κB, and GSK inhibitors but not the MAPK inhibitor also reduced CCL2 production, indicating that the IL-17-induced production of inflammatory cytokines involves a number of kinases and resultant NF-κB activation.
In conclusion, our study demonstrates that IL-17 induces expression of IL-6, TNF, and CCL2 by RACs, suggesting that exposure of RACs to IL-17 may enhance proinflammatory responses in the eye. In contrast, on exposure to IL-17, RPE cells produce only limited amounts of proinflammatory cytokines as a result of expressing increased levels of SOCS proteins. Our observations suggest that IL-17 may have a dual effect in ocular inflammation, being able to promote or ameliorate inflammatory responses in the eye, depending on which parenchymal cells are stimulated. The severity of ocular inflammation might be regulated by the net effect of these responses.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants EY12974 and EY14599, Vision Research Infrastructure Development grant R24 EY015636, a Research to Prevent Blindness Career Development Award (H. S.), and the Commonwealth of Kentucky Research Challenge Trust Fund (H. J. K.). The authors thank Tom Barkas for editorial assistance.
Footnotes
Abbreviations: EAU=experimental autoimmune uveitis, GSK=glycogen synthase kinase, IRBP=interphotoreceptor binding protein, LIF=leukocyte inhibitory factor, qRT-PCR=quantitative RT-PCR, RAC=retinal astrocyte, RPE=retinal pigment epithelial, SOCS=suppressor of cytokine signaling
References
- Amadi-Obi A, Yu C R, Liu X, Mahdi R M, Clarke G L, Nussenblatt R B, Gery I, Lee Y S, Egwuagu C E. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med. 2007;13:711–718. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- Jiang G, Ke Y, Sun D, Han G, Kaplan H J, Shao H. Reactivation of uveitogenic T cells by retinal astrocytes derived from experimental autoimmune uveitis-prone B10RIII mice. Invest Ophthalmol Vis Sci. 2008;49:282–289. doi: 10.1167/iovs.07-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y, Han G, Shao H, Wang Y, Kaplan H J, Sun D. Characterization of IL-17+ interphotoreceptor retinoid-binding protein-specific T cells in experimental autoimmune uveitis. Invest Ophthalmol Vis Sci. 2007;48:4153–4161. doi: 10.1167/iovs.07-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Zhu W, Silver P B, Su S B, Chan C C, Caspi R R. Autoimmune uveitis elicited with antigen-pulsed dendritic cells has a distinct clinical signature and is driven by unique effector mechanisms: initial encounter with autoantigen defines disease phenotype. J Immunol. 2007;178:5578–5587. doi: 10.4049/jimmunol.178.9.5578. [DOI] [PubMed] [Google Scholar]
- Yoshimura T, Sonoda K H, Miyazaki Y, Iwakura Y, Ishibashi T, Yoshimura A, Yoshida H. Differential roles for IFN-γ and IL-17 in experimental autoimmune uveoretinitis. Int Immunol. 2008;20:209–214. doi: 10.1093/intimm/dxm135. [DOI] [PubMed] [Google Scholar]
- Aggarwal S, Gurney A L. IL-17: prototype member of an emerging cytokine family. J Leukoc Biol. 2002;71:1–8. [PubMed] [Google Scholar]
- Gaffen S L. An overview of IL-17 function and signaling. Cytokine. 2008;43:402–407. doi: 10.1016/j.cyto.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic D V, Di Battista J A, Martel-Pelletier J, Jolicoeur F C, He Y, Zhang M, Mineau F, Pelletier J P. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-{β} and TNF-{α}, by human macrophages. J Immunol. 1998;160:3513–3521. [PubMed] [Google Scholar]
- Chabaud M, Fossiez F, Taupin J L, Miossec P. Enhancing effect of IL-17 on IL-1-induced IL-6 and leukemia inhibitory factor production by rheumatoid arthritis synoviocytes and its regulation by Th2 cytokines. J Immunol. 1998;161:409–414. [PubMed] [Google Scholar]
- Hofstetter H H, Ibrahim S M, Koczan D, Kruse N, Weishaupt A, Toyka K V, Gold R. Therapeutic efficacy of IL-17 neutralization in murine experimental autoimmune encephalomyelitis. Cell Immunol. 2005;237:123–130. doi: 10.1016/j.cellimm.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Koenders M I, Joosten L A, van den Berg W B. Potential new targets in arthritis therapy: interleukin (IL)-17 and its relation to tumor necrosis factor and IL-1 in experimental arthritis. Ann Rheum Dis. 2006;65:iii29–iii33. doi: 10.1136/ard.2006.058529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang W, Kolls J K, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y, Han G, Shao H, Wang Y, Kaplan H J, Sun D. Characterization of IL-17+ interphotoreceptor retinoid-binding protein-specific T cells in experimental autoimmune uveitis. Invest Ophthalmol Vis Sci. 2007;48:4153–4161. doi: 10.1167/iovs.07-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewal I S, Grewal K D, Wong F S, Picarella D E, Janeway C A, Jr, Flavell R A. Local expression of transgene encoded TNF α in islets prevents autoimmune diabetes in nonobese diabetic (NOD) mice by preventing the development of auto-reactive islet-specific T cells. J Exp Med. 1996;184:1963–1974. doi: 10.1084/jem.184.5.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christen U, Wolfe T, Mohrle U, Hughes A C, Rodrigo E, Green E A, Flavell R A, von Herrath M G. A dual role for TNF-{{α}} in type 1 diabetes: islet-specific expression abrogates the ongoing autoimmune process when induced late but not early during pathogenesis. J Immunol. 2001;166:7023–7032. doi: 10.4049/jimmunol.166.12.7023. [DOI] [PubMed] [Google Scholar]
- Flaishon L, Topilski I, Shoseyov D, Hershkoviz R, Fireman E, Levo Y, Marmor S, Shachar I. Cutting edge: anti-inflammatory properties of low levels of IFN-{γ} J Immunol. 2002;168:3707–3711. doi: 10.4049/jimmunol.168.8.3707. [DOI] [PubMed] [Google Scholar]
- Billiau A, Heremans H, Vandekerckhove F, Dijkmans R, Sobis H, Meulepas E, Carton H. Enhancement of experimental allergic encephalomyelitis in mice by antibodies against IFN-γ. J Immunol. 1988;140:1506–1510. [PubMed] [Google Scholar]
- Xing Z, Gauldie J, Cox G, Baumann H, Jordana M, Lei X F, Achong M K. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J Clin Invest. 1998;101:311–320. doi: 10.1172/JCI1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke Y, Liu K, Huang G Q, Cui Y, Kaplan H J, Shao H, Sun D. Anti-inflammatory role of IL-17 in experimental autoimmune uveitis. J Immunol. 2009;182:3183–3190. doi: 10.4049/jimmunol.0802487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Enzmann V, Lei S, Sun S L, Kaplan H J, Shao H. Retinal pigment epithelial cells activate uveitogenic T cells when they express high levels of MHC class II molecules, but inhibit T cell activation when they express restricted levels. J Neuroimmunol. 2003;144:1–8. doi: 10.1016/s0165-5728(03)00248-0. [DOI] [PubMed] [Google Scholar]
- Shao H, Lei S, Sun S L, Kaplan H J, Sun D. Conversion of monophasic to recurrent autoimmune disease by autoreactive T cell subsets. J Immunol. 2003;171:5624–5630. doi: 10.4049/jimmunol.171.10.5624. [DOI] [PubMed] [Google Scholar]
- Sun D, Lohmann-Matthes M L. Functionally different subpopulations of mouse macrophages recognized by monoclonal antibodies. Eur J Immunol. 1982;12:134–140. doi: 10.1002/eji.1830120207. [DOI] [PubMed] [Google Scholar]
- Shao H, Liao T, Ke Y, Shi H, Kaplan H J, Sun D. Severe chronic experimental autoimmune uveitis (EAU) of the C57BL/6 mouse induced by adoptive transfer of IRBP1–20-specific T cells. Exp Eye Res. 2006;82:323–331. doi: 10.1016/j.exer.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Kerr E C, Copland D A, Dick A D, Nicholson L B. The dynamics of leukocyte infiltration in experimental autoimmune uveoretinitis. Prog Retin Eye Res. 2008;27:527–535. doi: 10.1016/j.preteyeres.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Liao T, Ke Y, Shao W H, Haribabu B, Kaplan H J, Sun D, Shao H. Blockade of the interaction of leukotriene B4 with its receptor prevents development of autoimmune uveitis. Invest Ophthalmol Vis Sci. 2006;47:1543–1549. doi: 10.1167/iovs.05-1238. [DOI] [PubMed] [Google Scholar]
- Shi G, Maminishkis A, Banzon T, Jalickee S, Li R, Hammer J, Miller S S. Control of chemokine gradients by the retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2008;49:4620–4630. doi: 10.1167/iovs.08-1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander W S. Suppressors of cytokine signaling (SOCS) in the immune system. Nat Rev Immunol. 2002;2:410–416. doi: 10.1038/nri818. [DOI] [PubMed] [Google Scholar]
- Alexander W S, Starr R, Fenner J E, Scott C L, Handman E, Sprigg N S, Corbin J E, Cornish A L, Darwiche R, Owczarek C M, Kay T W, Nicola N A, Hertzog P J, Metcalf D, Hilton D J. SOCS1 is a critical inhibitor of interferon γ signaling and prevents the potentially fatal neonatal actions of this cytokine. Cell. 1999;98:597–608. doi: 10.1016/s0092-8674(00)80047-1. [DOI] [PubMed] [Google Scholar]
- Croker B A, Krebs D L, Zhang J G, Wormald S, Willson T A, Stanley E G, Robb L, Greenhalgh C J, Forster I, Clausen B E, Nicola N A, Metcalf D, Hilton D J, Roberts A W, Alexander W S. SOCS3 negatively regulates IL-6 signaling in vivo. Nat Immunol. 2003;4:540–545. doi: 10.1038/ni931. [DOI] [PubMed] [Google Scholar]
- Lang R, Pauleau A L, Parganas E, Takahashi Y, Mages J, Ihle J N, Rutschman R, Murray P J. SOCS3 regulates the plasticity of gp130 signaling. Nat Immunol. 2003;4:546–550. doi: 10.1038/ni932. [DOI] [PubMed] [Google Scholar]
- Yasukawa H, Yajima T, Duplain H, Iwatate M, Kido M, Hoshijima M, Weitzman M D, Nakamura T, Woodard S, Xiong D, Yoshimura A, Chien K R, Knowlton K U. The suppressor of cytokine signaling-1 (SOCS1) is a novel therapeutic target for enterovirus-induced cardiac injury. J Clin Invest. 2003;111:469–478. doi: 10.1172/JCI16491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egwuagu C E, Yu C R, Li Z, Nussenblatt R B. SOCS5 mRNA levels in peripheral blood mononuclear cells (PBMC): a potential bio-marker for monitoring response of uveitis patients to Daclizumab therapy. J Autoimmun. 2005;24:39–46. doi: 10.1016/j.jaut.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Takase H, Yu C R, Liu X, Fujimoto C, Gery I, Egwuagu C E. Induction of suppressors of cytokine signaling (SOCS) in the retina during experimental autoimmune uveitis (EAU): potential neuroprotective role of SOCS proteins. J Neuroimmunol. 2005;168:118–127. doi: 10.1016/j.jneuroim.2005.07.021. [DOI] [PubMed] [Google Scholar]
- Stark J L, Lyons J A, Cross A H. Interferon-γ produced by encephalitogenic cells induces suppressors of cytokine signaling in primary murine astrocytes. J Neuroimmunol. 2004;151:195–200. doi: 10.1016/j.jneuroim.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Turnley A M, Starr R, Bartlett P F. Failure of sensory neurons to express class I MHC is due to differential SOCS1 expression. J Neuroimmunol. 2002;123:35–40. doi: 10.1016/s0165-5728(01)00480-5. [DOI] [PubMed] [Google Scholar]
- Starr R, Willson T A, Viney E M, Murray L J, Rayner J R, Jenkins B J, Gonda T J, Alexander W S, Metcalf D, Nicola N A, Hilton D J. A family of cytokine-inducible inhibitors of signaling. Nature. 1997;387:917–921. doi: 10.1038/43206. [DOI] [PubMed] [Google Scholar]
- Fujimoto M, Naka T. Regulation of cytokine signaling by SOCS family molecules. Trends Immunol. 2003;24:659–666. doi: 10.1016/j.it.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Dalpke A H, Opper S, Zimmermann S, Heeg K. Suppressors of cytokine signaling (SOCS)-1 and SOCS-3 are induced by CpG-DNA and modulate cytokine responses in APCs. J Immunol. 2001;166:7082–7089. doi: 10.4049/jimmunol.166.12.7082. [DOI] [PubMed] [Google Scholar]
- Crespo A, Filla M B, Murphy W J. Low responsiveness to IFN-γ, after pretreatment of mouse macrophages with lipopolysaccharides, develops via diverse regulatory pathways. Eur J Immunol. 2002;32:710–719. doi: 10.1002/1521-4141(200203)32:3<710::AID-IMMU710>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]