Abstract
Background: The experience of undergoing surgery is known to induce a short-term, fight-or-flight physiological stress response. As an optimum immune response at the site of surgery would enhance tissue repair, we examined surgical stress-induced immune cell redistribution profiles as predictors, and potential mediators, of short and long-term postoperative recovery. We tested the a priori hypothesis that predefined adaptive immune cell redistribution profiles observed during surgery will predict enhanced postoperative recovery.
Methods: This prospective longitudinal study involved fifty-seven patients undergoing meniscectomy. Knee function was assessed preoperatively and at one, three, eight, sixteen, twenty-four, and forty-eight weeks postoperatively with use of the clinically validated Lysholm scale, which assesses mechanical function, pain, mobility, and the ability to perform daily activities. Surgery-induced immune cell redistribution was measured in the blood at baseline, before surgery, and after surgery.
Results: Mixed-model repeated-measures analyses revealed a main effect of immune cell redistribution: patients who showed the predefined “adaptive” lymphocyte and monocyte redistribution profiles during surgery showed enhanced recovery. Interesting differences were also observed between the sexes: women as a group showed less adaptive redistribution and correspondingly showed significantly delayed maximum recovery, requiring forty-eight weeks, compared with men, who required only sixteen weeks. Inter-individual differences in leukocyte redistribution predicted the rate of recovery across both sexes.
Conclusions: Immune cell redistribution that is induced by the stress of undergoing surgery can predict (and may partially mediate) postoperative healing and recovery. These findings may provide the basis for identifying patients (either prospectively or during surgery) who are likely to show good as opposed to poor recovery following surgery and for designing interventions that would maximize protective immune responses and enhance the rate and extent of recovery.
Level of Evidence: Prognostic Level I. See Instructions to Authors for a complete description of levels of evidence.
Nearly 1.3 million Americans undergo knee surgery annually1. The rate of recovery is known to be affected by damage-related factors such as the severity, chronicity, and location of injury; the specific tissues and structures involved; preexisting conditions (e.g., degenerative joint changes); and age2-6. Importantly, a robust immune response mediates effective wound-healing, protects against infection, and is likely to be a key determinant of recovery following surgery. However, much remains to be known about the influence of immune factors on postoperative outcomes.
In the present study, we tested the hypothesis that specific blood immune cell redistribution profiles, which can be identified with relative ease during surgery, would predict inter-individual differences in recovery following surgery. This a priori hypothesis was based on findings of preclinical studies showing that, in contrast with chronic stress, which mediates well-known health-aversive effects7-11, an acute, fight-or-flight stress response, lasting for minutes to hours, induces specific changes in blood leukocyte distribution and other immune parameters and enhances innate and adaptive immune responses in organs (e.g., skin, subcutaneous tissue, and sentinel lymph nodes) to which leukocytes traffic during stress12-17. Early during an acute stress response (within ten to thirty minutes), lymphocyte and monocyte numbers increase in the blood stream as these cell types are mobilized from organs like the spleen and the marginated pool18-22. Later (within 0.5 to four hours), blood lymphocyte and monocyte numbers decrease as these cell types traffic to (and marginate within) blood vessels of tissues like the skin, subcutaneous tissues, and sentinel lymph nodes15,19-23. Importantly, such leukocyte redistribution is related to a robust enhancement of innate and adaptive immune responses within target organs to which leukocytes traffic during acute stress12,14,16,17,19,20,24,25.
We reasoned that stress that is experienced during surgery is likely to elicit a clinically relevant fight-or-flight stress response during which immunoenhancement is likely to be beneficial for wound-healing and recovery. On the basis of preclinical findings, we predefined “adaptive” surgery-induced lymphocyte and monocyte redistribution profiles as those involving an increase in leukocyte numbers early during surgery, followed by a decrease later, and an “adaptive” neutrophil redistribution profile as one involving an overall increase in neutrophil numbers. To our knowledge, this model of leukocyte redistribution has never before been tested in human subjects, and no studies have tested the predictive power of leukocyte redistribution in terms of clinical recovery. Here we proposed that changes in blood leukocyte numbers would provide a clinically relevant index of leukocyte redistribution in the body. We tested the hypothesis that adaptive blood leukocyte redistribution profiles observed during surgery will predict enhanced recovery. Moreover, because research has indicated that women undergoing major orthopaedic procedures such as total hip or knee arthroplasty and laminectomy tend to have lower preoperative and postoperative function than men do26, we examined differences between the sexes in terms of blood leukocyte distribution profiles and recovery.
Materials and Methods
Participants
Patients who had been referred to a university-affiliated sports medicine clinic for elective arthroscopic knee surgery were recruited to participate in a prospective, longitudinal study examining factors promoting surgical recovery. The inclusion criteria were an age of seventeen to eighty years, nonsmoking status, no previous surgery on the symptomatic knee, normal knee function of the contralateral knee, no history of bilateral knee injury, no chronic comorbid conditions (including collagen, heart, or lung-related diseases) that resulted in restricted physical activity, no pregnancy, and no requirement for emergency surgery. All participants had been physically active prior to the meniscal injury. Only three participants required cane or crutch support; the remaining participants were able to walk without support. All procedures were scheduled to be performed in the morning to control for circadian differences in the numbers of circulating leukocytes. Power analyses were conducted on the basis of the results of a pilot study that determined that a sample size of sixty would be larger than required to observe group differences in the variables of interest. Of the sixty-five patients who completed a preoperative interview and baseline blood drawings, three were excluded postoperatively because of the need for more extensive surgery, and five patients dropped out of the study at the time of surgery, leaving fifty-seven patients, including twenty women (35%) and thirty-seven men (65%). All fifty-seven patients completed a minimum of two postoperative interviews; fifty-one (89.5%) of these patients completed the final postoperative interview, with 7% of the outcome data missing across visits. Six patients were lost to follow-up through Week 48. Approval for all procedures was obtained from the Yale University Human Investigations Committee. Participation in the study was completely voluntary and did not affect the delivery of health care in any way.
Procedures
Patients scheduled for meniscectomy were screened and recruited by telephone three to sixty days prior to surgery. All procedures were performed by the same surgeon (P.J.). All surgical procedures were performed through a two-portal arthroscopic anterior approach. Procedures included meniscectomy and related repairs, including minor arthroscopic procedures inside the knee joint such as débridement, chondroplasty, and simple intra-articular meniscal repairs; no inside-out or outside-in repairs were included. Most patients underwent a partial meniscectomy, and some also had a local chondroplasty or related synovectomy. Patients undergoing other, more involved procedures were excluded. No patient required an arthrotomy. In addition to ensuring that all procedures were performed by the same surgeon, general gas anesthesia conditions were also kept as uniform as possible among patients. The severity of osteoarthritis was graded with use of the modified Outerbridge articular surface grading scale27. A score for each of the medial, lateral, and patellar surfaces was recorded by the surgeon at the time of surgery on a rating scale ranging from 0 to 4. The articular surface grading scale score of the most arthritic of the three joint surfaces was used as the final score.
Data Collected at Medical Appointments
Data were collected one to thirty days preoperatively and at one, three, eight, sixteen, twenty-four, and forty-eight weeks postoperatively. At each visit, the patient was examined by an orthopaedic surgeon who completed structured ratings of effusion and knee function. Effusion was rated on a 4-point scale as 0 (none), 1 (mild), 2 (moderate), or 3 (tense). These ratings were later collapsed for analyses to classify patients into two groups: (1) no or mild effusion, and (2) moderate or tense effusion. The Lysholm scale was used to measure knee function (as described below). Age, sex, and body mass index data were collected at the preoperative time point. Demographic information was also collected during this interview. Each postoperative interview lasted approximately fifteen minutes. Patients who were enrolled in the immunology sub-study agreed to repeated blood testing. A trained research assistant conducted morning “baseline” blood drawings three to ten days prior to surgery. Drawing the blood in the morning ensured control for circadian effects. On the day of surgery, an anesthesiologist obtained blood samples in the operating room immediately prior to the administration of anesthesia (the “pre-surgery” time point). A nurse obtained the postoperative blood sample immediately on the patient's arrival in the recovery room approximately 0.5 to one hour following surgery (the “post-surgery” time point). Blood samples were collected in heparinized Vacutainer tubes (Becton Dickinson, Franklin Lakes, New Jersey) at room temperature and were transported at room temperature in an insulated container to the immunology laboratory for analyses.
Primary Outcome Measure
The Lysholm rating scale, a gold-standard measure for knee function, assesses mechanical function of the knee, mobility, pain, and the ability to perform daily activities28. It is the most widely used patient-reported knee function outcome instrument in orthopaedic research29-32 and has demonstrated reliability and validity33. Items are differentially weighted on the basis of clinical criteria, and a summary score ranging from 0 to 100 points is obtained, with a higher score reflecting better knee functioning. Lysholm scores were assessed preoperatively and at all postoperative interviews. Trained research staff administered the Lysholm scale. Each Lysholm scale item was read aloud by the staff, and patients were given the responses for each item to review, were allowed to ask questions if necessary, and then were asked to provide a response. This method has the advantage of quantifying postoperative recovery in various dimensions that do not involve a subjective evaluation by the treating physician.
Leukocyte Mobilization, Trafficking, and Redistribution
White blood-cell counts were obtained with a hematology analyzer (Sysmex America, Mundelein, Illinois). Lymphocyte, monocyte, and neutrophil differentials were based on forward-versus-side-scatter parameters on a flow cytometer (FACSCalibur; Becton Dickinson, San Jose, California). The definitions of mobilization, trafficking, and redistribution (calculated as described below) were based on findings from preclinical studies12,13,15,18,19,21,22. Mobilization is defined as a short-term increase (relative to resting baseline) in the absolute numbers of immune cells in the blood19-22. This increase is mediated by immune cells leaving organs like the spleen and bone marrow and entering the bloodstream. Mobilization makes more leukocytes available for recruitment at sites of immune activation (e.g., a site of surgery). Mobilization was calculated by subtracting resting baseline numbers (obtained three to ten days before surgery) from preoperative numbers (obtained immediately before anesthesia) of blood lymphocytes, monocytes, and neutrophils. Trafficking is defined as a short-term decrease (relative to the resting baseline or peak mobilization numbers) in the absolute numbers of immune cells in the blood that reflects leukocytes leaving the lumen of the circulatory system and either extravasating through normal tissue-surveillance pathways or marginating within blood vessels of tissues like the skin and subcutaneous tissues that are likely to be compromised during stress (e.g., wounding by a predator or, in this case, the surgeon)19-22. Lymphocyte and monocyte trafficking scores were derived by subtracting postoperative cell numbers (obtained immediately after surgery) from preoperative cell numbers. In contrast to blood lymphocyte and monocyte numbers that initially increase (mobilization) and then decrease (trafficking to surveillance pathways) during stress, neutrophils tend to show a biphasic increase during stress13,34,35, which is in keeping with their function, which does not involve extravascular surveillance in the absence of inflammation. Therefore, neutrophil increase scores were calculated by subtracting baseline neutrophil numbers from postoperative neutrophil numbers. Redistribution is defined as the total movement of a specific immune cell subpopulation from the “barracks” (spleen, bone marrow) to the “boulevards” (bloodstream) and on to potential “battle-stations” (skin, subcutaneous tissue, sentinel lymph nodes)19-22. Redistribution scores were obtained for lymphocytes and monocytes by taking the mean of the mobilization and trafficking scores for each individual. Neutrophil redistribution was calculated by taking the mean of the neutrophil mobilization and increase scores. For each variable, higher scores represented a hypothesized more adaptive immune response to surgery. Median splits were calculated for each redistribution score, and patients were classified as “high redistributors” or “low redistributors” for each immune cell variable.
Statistical Methods
Mixed-model repeated-measures analyses controlling for age were used to test whether immunological redistribution profiles were significant predictors of recovery over time and whether differences between the sexes in terms of surgical stress-induced leukocyte distribution corresponded with differences between the sexes in terms of postoperative recovery. An advantage of mixed-model repeated-measures analytic strategies is their ability to estimate missing observations. In addition, mixed-model analyses offer solutions for other potential problems, including serial correlation, time-varying covariates, and irregular measurement periods36-38. Analyses involving Pearson correlations examined individual relationships between leukocyte redistribution scores and recovery scores at postoperative Weeks 1 and 24. T tests and chi-square analyses compared differences between the sexes in terms of demographic and immunological status prior to surgery. T tests examined differences between the sexes in terms of demographic and immunological status prior to surgery only in order to determine preexisting differences between the sexes with variables that could potentially qualify results. T tests were used on numerical data only, and the two groups were always male/female; no T tests were used to compare before/after values. Chi-square analyses were used with categorical data. Chi-square analyses were also conducted to examine the association between effusion and immunological status.
Source of Funding:
Funding for this research came from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) (RO1-AR-46299). NIAMS did not play any other role in this investigation. Funds were used to provide salary support and materials for the study.
Results
Sample Characteristics
The patients ranged in age from twenty-four to seventy-five years. Table I shows demographic characteristics and Lysholm scores according to sex. Participants were predominantly white and of moderate to high social class. Women were less likely than men to be married or living with a partner (p < 0.01), had a lower level of familial income (p < 0.05), and had lower preoperative knee function as indicated by a lower mean Lysholm score (41.7 compared with 63.6; p < 0.001), confirming previous reports26.
TABLE I.
Baseline Demographic and Clinical Characteristics of Study Participants
| Characteristic | Women (N = 20) | Men (N = 37) | Statistic |
|---|---|---|---|
| Demographic | |||
| Age*(yr) | 51.0 ± 9.0 | 48.8 ± 11.1 | t = 0.8 |
| White race (no. of patients) | 18 (90.0%) | 34 (91.9%) | χ2 = 0.06 |
| Married/partner (no. of patients) | 10 (50.0%) | 31 (83.8%) | χ2 = 7.3† |
| Education (no. of patients) | χ2 = 1.7 | ||
| High school or less | 4 (20.0%) | 3 (8.1%) | |
| College (1 to 4 years) | 10 (50.0%) | 22 (59.5%) | |
| Graduate degree | 6 (30.0%) | 12 (32.4%) | |
| Total family income (no. of patients) | χ2 = 7.1‡ | ||
| <$50,000 | 8 (40.0%) | 4 (10.8%) | |
| $50,000 to $100,000 | 2 (10.0%) | 9 (24.3%) | |
| >$100,000 | 10 (50.0%) | 24 (64.9%) | |
| Clinical | |||
| Preoperative Lysholm score* | 41.7 ± 16.2 | 63.6 ± 16.8 | t = 4.7§ |
| Body mass index*(kg/m2) | 31.3 ± 9.3 | 28.9 ± 3.8 | t = 1.1 |
| History of prior knee injury (no. of patients) |
7 (35.0%) |
12 (32.4%) |
χ2 = 0.04 |
The values are given as the mean and the standard deviation.
P < 0.01.
P < 0.05.
P < 0.001.
Immune Cell Redistribution Profiles as Predictors of Recovery
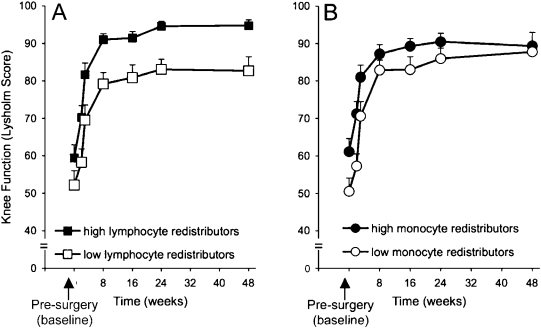
A mixed-model repeated-measures analysis involving time, sex, age, the extent of osteoarthritis, and lymphocyte, monocyte, and neutrophil redistribution profiles was conducted to determine the effects of redistribution on recovery (Table II). The analysis revealed that time and sex were significant predictors of recovery, with Lysholm scores increasing in a linear fashion over time as expected. Men had higher Lysholm scores over time. In addition, the analyses also revealed that lymphocyte and monocyte redistribution profiles were significant predictors of recovery. Figure 1 presents the estimated average Lysholm scores over time for high and low lymphocyte (Fig. 1, A) and monocyte (Fig. 1, B) redistributors with men and women combined. Subjects who showed a greater magnitude of lymphocyte and monocyte redistribution during surgery stress also showed better recovery.
TABLE II.
Surgical Stress-Induced Immune Cell Redistribution Profiles as Predictors of Recovery*
| Effect Source | Estimate | T Value | P Value |
|---|---|---|---|
| Intercept | 97.92 | 15.98 | 0.000 |
| Time | 0.22 | 6.66 | 0.000 |
| Sex | −7.87 | −3.06 | 0.004 |
| Age | −0.22 | −1.86 | 0.069 |
| Osteoarthritis | −1.12 | −0.71 | 0.480 |
| Lymphocyte redistribution | −6.47 | −2.68 | 0.010 |
| Monocyte redistribution | −4.85 | −2.02 | 0.049 |
| Neutrophil redistribution |
−0.41 |
−0.17 |
0.862 |
The “Effect Source” column lists the parameters that were analyzed. The “Estimate” column lists the value for the estimated effect of each parameter.
Fig. 1.
Line graphs showing recovery of knee function over time for patients who showed high as compared with low lymphocyte and monocyte redistribution during surgery. The figure depicts the average Lysholm scores of high and low lymphocyte (A) and monocyte (B) redistributor groups. Data are expressed as the mean and the standard error of the mean.
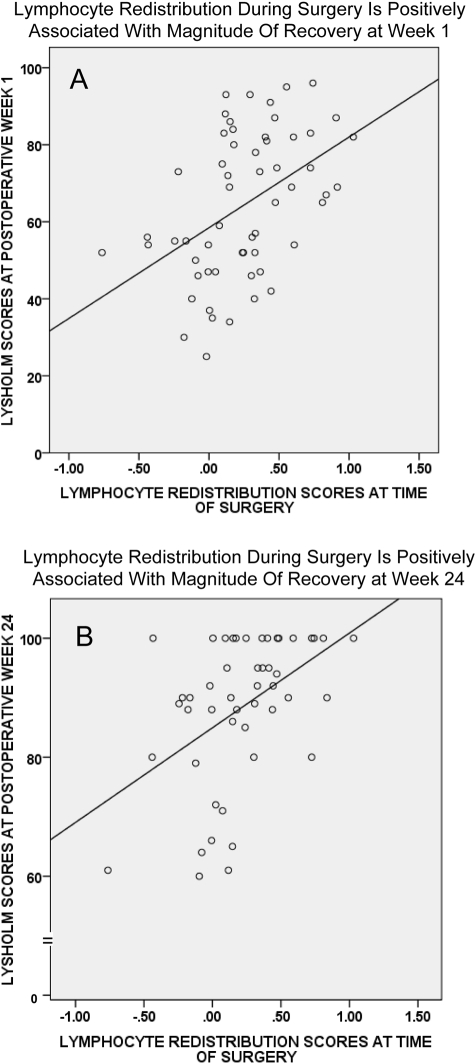
Moreover, in order to examine relationships between surgery-induced blood leukocyte redistribution profiles and postoperative recovery at specific weeks, we correlated lymphocyte and monocyte redistribution scores with Lysholm scores at Weeks 1 and 24. These time points were chosen because Week 1 represents the start of the recovery trajectory and Week 24 represents the time by which maximum recovery is attained. Lymphocyte redistribution during surgery was significantly and positively correlated with Lysholm scores at Week 1 (r = 0.45, p < 0.001) and Week 24 (r = 0.46, p < 0.001). Monocyte redistribution during surgery was significantly and positively correlated with Lysholm scores at Week 1 (r = 0.28, p < 0.05) but not at Week 24 (r = 0.17, p = 0.233). Figure 2 shows scatter plots for lymphocyte redistribution during surgery and recovery at Week 1 (Fig. 2, A) and Week 24 (Fig. 2, B).
Fig. 2.
Scatter plots showing the relationship between lymphocyte redistribution scores during surgery and postoperative recovery at Week 1 (A) and Week 24 (B). Lymphocyte redistribution during surgery was significantly and positively correlated with Lysholm scores at Week 1 (r = 0.45, p < 0.001) and Week 24 (r = 0.46, p < 0.001).
We also examined relationships between surgery-induced lymphocyte, monocyte, and neutrophil redistribution profiles and preoperative and postoperative effusion that served as an index of local inflammation of the affected joint. Chi-square analyses revealed that there were no associations between preoperative levels of effusion and surgery-induced immune cell redistribution (i.e., preoperative effusion scores were not associated with the classification of the patient as a high or low lymphocyte, monocyte, or neutrophil redistributor) (p > 0.05 for all). Interestingly, for the postoperative effusion measure, analyses revealed that high lymphocyte redistributors were more likely to have a significantly lower effusion at Week 1 following surgery (chi square = 4.94, p = 0.026). This was in agreement with the fact that patients who were high lymphocyte redistributors also showed higher Lysholm scores at Week 1, thus showing better recovery as had been hypothesized.
Differences Between Sexes in Immune Cell Redistribution Profiles
There were significant differences between the sexes in immune cell redistribution such that women showed less adaptive redistribution profiles than men did (Table III). For lymphocytes and monocytes, women showed lower mobilization, trafficking, and overall redistribution (p < 0.05), whereas no significant differences were observed between the sexes in terms of neutrophil redistribution.
TABLE III.
Sex Differences in Blood Immune Cell Distribution During Surgery
| Change in Blood Immune Cell Distribution* |
|||
|---|---|---|---|
| Women (N = 20) | Men (N = 37) | T Value | |
| Lymphocytes (1000/μL) | |||
| Mobilization | −0.04 ± 0.49 | 0.28 ± 0.31 | −2.55† |
| Trafficking | 0.15 ± 0.37 | 0.37 ± 0.43 | −2.05† |
| Redistribution | 0.09 ± 0.41 | 0.34 ± 0.31 | −2.55† |
| Monocytes (1000/μL) | |||
| Mobilization | −0.07 ± 0.15 | 0.05 ± 0.14 | −2.64† |
| Trafficking | 0.05 ± 0.13 | 0.13 ± 0.12 | −2.40† |
| Redistribution | −0.01 ± 0.11 | 0.09 ± 0.12 | −3.03‡ |
| Neutrophils (1000/μL) | |||
| Mobilization | −0.34 ± 1.60 | −0.12 ± 1.29 | −0.53 |
| Increase | 0.63 ± 1.47 | 0.16 ± 1.14 | 1.34 |
| Redistribution |
0.21 ± 1.23 |
0.06 ± 0.83 |
0.54 |
The values are given as the mean and the standard deviation.
P < 0.05.
P < 0.01.
Differences Between Sexes in Postoperative Recovery
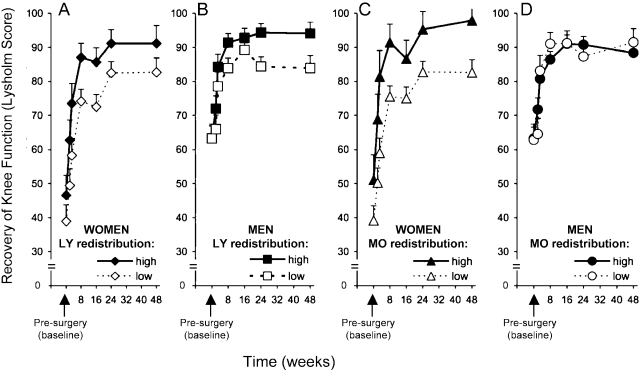
Differences between the sexes in terms of surgical stress-induced immune cell redistribution corresponded with differences between the sexes in terms of recovery following surgery. Women showed lower knee function at baseline as compared with men. We examined whether there were differences between the sexes in the extent of osteoarthritis, but the correlation between sex and articular surface grading scale scores was not significant. Additional mixed-model analyses examining recovery of knee function in the context of sex by leukocyte redistribution interaction effects revealed significant three-way interaction effects for two leukocyte subpopulations: lymphocyte redistributor group × sex × time (F = 3.29, p = 0.02) and monocyte redistributor group × sex × time (F = 1.94, p = 0.05). Follow-up post hoc tests were conducted to determine the nature of these interactions. Figure 3, A and B, presents the estimated average Lysholm scores over time for high and low lymphocyte redistributors stratified according to sex. Post hoc univariate analyses comparing the four groups in Figure 3, A and B, revealed differences between the sexes in the low lymphocyte redistributor group during the early phases of recovery: women who were low lymphocyte redistributors had significantly worse Lysholm scores through postoperative Week 16 than did men who were low lymphocyte redistributors (p < 0.05 across all time points). Both male and female high lymphocyte redistributors showed similar recovery patterns. Among patients of the same sex, low and high lymphocyte redistributors displayed different patterns of recovery. Women who were high lymphocyte redistributors had significantly higher Lysholm scores than did women who were low lymphocyte redistributors at Weeks 3, 8, and 16 (p < 0.05). Men who were high lymphocyte redistributors had significantly higher Lysholm scores than did men who were low lymphocyte redistributors during later recovery (at Weeks 24 and 48) (p < 0.05) but not at earlier time points. This finding indicates that women who were high lymphocyte redistributors showed enhanced early recovery and that men who were high lymphocyte redistributors showed higher maximum knee function. There were no differences in Lysholm scores between women and men who were high lymphocyte redistributors across all postoperative time points. However, women who were low lymphocyte redistributors had significantly lower Lysholm scores through Week 16 than did men who were low lymphocyte redistributors (p < 0.05 at all time points).
Fig. 3.
Line graphs showing recovery of knee function over time for women and men who showed high as compared with low lymphocyte and monocyte redistribution during surgery. The figure depicts average Lysholm scores of women (A and C) and men (B and D) divided into high and low lymphocyte (LY) (A and B) and monocyte (MO) (C and D) redistributor groups. Data are expressed as the mean and the standard error of the mean.
Figure 3, C and D, presents the estimated average Lysholm scores over time for high and low monocyte redistributors stratified according to sex. Men and women were divided into high and low monocyte redistributor groups. Post hoc univariate analyses comparing male and female high and low monocyte redistributors revealed that women who were high monocyte redistributors showed significantly enhanced recovery compared with women who were low monocyte redistributors. Men who were high monocyte redistributors showed a similar magnitude of recovery compared with men who were low monocyte redistributors. With the exception of Week 16, women who were high redistributors had significantly higher Lysholm scores than did women who were low redistributors (p < 0.05 for all), whereas no significant differences were observed in the Lysholm scores of men who showed high monocyte redistribution as compared with those who showed low monocyte redistribution. Moreover, women who were low monocyte redistributors had significantly lower Lysholm scores than did men who were low monocyte redistributors (p < 0.02 through postoperative Week 16). By Week 24, women and men who were low monocyte redistributors had comparable Lysholm scores. In contrast, women and men who were high monocyte redistributors had comparable Lysholm scores throughout the entire recovery period. Thus, when lymphocyte and monocyte redistribution is high across both sexes, the difference between the sexes in terms of recovery disappears.
Discussion
The present study represents the first clinical test of the ability of the stress-induced leukocyte redistribution model to predict the rate and magnitude of recovery following surgery. On the basis of the results of preclinical studies, we hypothesized that patients who show an increase in blood lymphocyte or monocyte numbers early during surgery, and/or a decrease later during surgery, will show enhanced recovery. Our results confirmed this hypothesis. The results of the present study showed that surgical stress-induced leukocyte redistribution can predict (and may partially mediate) inter-individual differences in recovery; specifically, individuals who showed the predefined “adaptive” leukocyte redistribution profile during surgical stress showed higher Lysholm scores following surgery (Figs. 1, 2, and 3). These results also suggest that leukocyte redistribution profiles can predict the observed differences between the sexes in terms of recovery: women showed less adaptive leukocyte redistribution during surgical stress and also showed lower Lysholm scores than men did (Fig. 3).
Immune Cell Redistribution and Recovery from Surgery: Biological Mechanisms
While the findings described here need to be validated and examined mechanistically, taken together with results from numerous preclinical studies12-17,20-23, they suggest that patients who show the predefined “adaptive” profiles of stress-induced immune cell redistribution during surgery also show enhanced wound-healing. Enhanced wound-healing and recovery is likely to be mediated by a cascade of biological events launched when a patient mounts an adaptive fight-or-flight stress response at the time of surgery. Initially, the short-term physiological stress response induces a redistribution of immune cells from their “barracks” (e.g., spleen, bone marrow, and marginated pool) into the “boulevards” (bloodstream). This results in an initial increase in blood leukocyte numbers (mediated largely by norepinephrine and epinephrine) that makes more leukocytes available for recruitment at potential sites of immune activation, including the site of surgery21,22,39. As the physiological stress response progresses, the number of immune cells in the blood decreases (mediated by epinephrine and cortisol) as cells begin to move out of the bloodstream and into potential “battle-stations” (e.g., skin, subcutaneous tissues, sentinel lymph nodes) and actual “battle-stations” (e.g., site of surgery)21,22,39. In addition to increasing leukocyte trafficking, it is also likely that acute stress enhances the functional capacity of leukocytes arriving at the site of surgery12,13,21,22. Thus, the immune response at the site of surgery is optimized and enhanced by the activation of acute stress physiology and mediates more efficient clearance of damaged tissue and/or pathogens during the days following surgery. An optimized immune response also facilitates and promotes enhanced tissue proliferation and remodeling that go on for weeks and months, respectively, following surgery. The net long-term result of an optimized immune and wound-healing response is likely to be reduced scar-tissue formation and improved knee function as reflected in the higher Lysholm scores that were observed in patients who showed “adaptive” immune cell redistribution during the stress of surgery.
It is also noteworthy that women undergoing major orthopaedic procedures (i.e., total hip or knee arthroplasty, laminectomy) tend to have lower preoperative and postoperative function as compared with men26. However, in spite of the prevalence of minimally invasive surgery, little is known about the influence of sex on the variability of outcome. Our results showed that as with major orthopaedic surgery, women as a group demonstrated slower postoperative recovery than men did, even in the case of arthroscopy. Men showed significantly greater surgery-induced mobilization and trafficking of lymphocytes and monocytes (Table III). Interestingly, men also showed significantly enhanced recovery (Fig. 3). Furthermore, while women on the average had worse recovery than men did, recovery in women who were high lymphocyte and monocyte redistributors was comparable with that in men (Fig. 3). Additional research is needed to determine why women in particular showed worse leukocyte redistribution during surgery and impaired recovery following surgery.
Factors mediating inter-individual and sex-related differences in leukocyte redistribution during short-term stressors such as surgery merit additional investigation. Trait differences in genes and in long-term exposure to stress, sex, and other hormones are likely to be important factors. State-dependent differences in relative concentrations of stress hormones (principally epinephrine, norepinephrine, and cortisol) released during surgery are also likely to be important as it has been shown that stress-induced changes in leukocyte distribution are mediated by glucocorticoid and catecholamine hormones13,19,35,40. Although neutrophils and monocytes/macrophages play a prominent role during early stages of wound-healing, lymphocytes are involved in later stages, regulate postoperative healing and angiogenesis, and are thought to be critical for effective and optimized wound-healing41-47. Our data suggest that lymphocyte redistribution profiles during surgery are significant predictors of recovery. These data also raise the possibility that lymphocyte and monocyte redistribution may be trait-like measures and that individuals who show adaptive leukocyte redistribution during stress may also experience the immunological benefits of more efficient mobilization and trafficking during immune surveillance (before and during surgical wounding) and during the proliferation and remodeling phases of wound-healing (during the weeks to months following surgery) and, as a result, show enhanced recovery. Clearly, additional studies are required to elucidate the mechanisms by which surgical stress-induced changes in leukocyte redistribution predict (and may mediate) recovery.
Immune Cell Redistribution and Preoperative and Postoperative Inflammation
Preoperative inflammation is an index of the extent of tissue damage, and, if chronic, may adversely affect postoperative healing independent of the amount of original tissue damage. However, it is important to note that inflammation is also essential for successful wound-healing. An optimum inflammatory response has three characteristics: (1) it is mounted rapidly and robustly (in terms of the numbers and concentrations of immune cells and factors) following wounding, (2) it quickly removes damaged cells and pathogens, and (3) it is resolved efficiently and effectively, with most immune cells leaving the site of inflammation within days after wounding to pave the way for the proliferative and remodeling phases of the wound-healing cascades. With use of a measure of joint effusion as an index of inflammation, our study revealed that preoperative effusion was not related to immune cell redistribution during surgery, suggesting that the magnitude of preexisting joint inflammation did not affect the magnitude or profile of leukocyte redistribution during surgery. Interestingly, we observed that patients who showed higher lymphocyte redistribution during surgery correspondingly showed lower levels of effusion one week following surgery, suggesting a faster resolution of postoperative inflammation, which may have contributed to the more efficient recovery that was also observed for high lymphocyte redistributors.
Stress and Surgery
At first glance, the results described here may appear to go against the widespread expectation that stress should inhibit rather than enhance postoperative recovery. This expectation is likely to arise because numerous studies have shown that distress suppresses or dysregulates various aspects of immune function8-11 and also impairs wound-healing48,49. It becomes clear that our results complement rather than contradict the well-known immunosuppressive/dysregulatory effects of stress as soon as one distinguishes the short-term, adaptive, fight-or-flight stress response from long-term, maladaptive, chronic stress or distress12. Studies showing stress-induced immunosuppression/dysregulation have examined largely chronic stressors, and studies involving acute stressors have not accounted for stress effects on leukocyte distribution. Acute stressors have been shown to induce large changes in immune cell trafficking and function that lead to robust enhancements in innate, adaptive, primary, and secondary immune responses12,14,16-21,24,25. It has been proposed that the acute stress response is one of nature's fundamental survival mechanisms, without which neither predator nor prey could stay alive12,18. It has been argued that just as this response prepares the musculoskeletal, cardiovascular, and neuroendocrine systems for fight-or-flight situations, it similarly prepares the immune system for responding to challenges such as wounding or infection that may arise due to the actions of a stressor (e.g., an attack by a lion, or a surgical procedure)13,18,20.
At first glance, these findings may call into question the usefulness of stress-reduction procedures administered prior to surgery. Although additional studies are required, we propose that a reduction in chronic stress levels is likely to have beneficial effects because preclinical studies have suggested that a reduction of chronic stress may increase the magnitude of adaptive, short-term stress responses and their salubrious or health-promoting effects20. In agreement with these findings, clinical studies have shown that, in comparison with controls, patients undergoing abdominal surgery who had been given preoperative relaxation instructions showed decreased anxiety before and after surgery but more robust cortisol and epinephrine responses (physiological indicators of a stronger acute stress response) during surgery50. Importantly, studies have shown that patients who scored higher for trait anxiety or Type-A personality (likely to result in higher levels of chronic stress) showed lower cortisol and epinephrine responses (indicating a weaker acute stress response) during abdominal surgery51. Therefore, interventions that reduce chronic stress and optimize acute stress at the time of surgery or immune activation are likely to be beneficial.
Strengths, Weaknesses, and Future Directions
The strengths of the present study include the widely used surgical model, the prospective longitudinal design, the specific patient population, and the objective measure of recovery. This study also addresses an important gap in research because there has been limited prospective research on musculoskeletal injury and surgical recovery that has taken a comprehensive approach to identifying preoperative determinants of postoperative outcomes. A major strength is the support that the present study provides for the novel idea that the acute stress response is one of nature's fundamental survival mechanisms that may be clinically harnessed (see below) to enhance protective immunity during surgery. A major contribution comes from the fact that the findings described here could lead to the use of patient profiles of leukocyte redistribution during surgery as prognostic indicators of recovery and to the development of biobehavioral and pharmacological interventions designed to manipulate leukocyte redistribution during surgery in a way that maximizes postoperative healing and recovery. A weakness of the present study is the absence of biological measurements at the site of surgery that would help to identify specific cellular and molecular mediators of enhanced recovery. Another weakness involves the correlational nature of our findings because correlation does not prove causation. However, given the substantial amount of preclinical data on which our leukocyte redistribution model was based, it is likely that surgical stress-induced changes in leukocyte distribution at least partially mediate recovery. There are also several issues pertinent to this study that merit future investigation. First, the present study suggests that age may be inversely related to recovery, although the association did not reach significance. Therefore, it would be useful for future studies to be designed to examine the effects of age on acute stress-induced leukocyte redistribution and its relationship to recovery following surgery. However, the important finding of the present study is that when age and immune cell redistribution were included in the model, immune cell redistribution variables were significantly associated with recovery but age was not. Second, although we attempted to keep anesthesia conditions as uniform as possible among patients, future studies should be powered to quantify the potential effects of the type of anesthetic agent and the depth of anesthesia on immune cell redistribution. Third, additional research is required to determine the psychological, physiological, and immunological mediators of acute stress-induced changes in leukocyte redistribution and the acute stress-induced enhancement of postoperative recovery.
Clinical Implications
Replicating and validating these findings is a necessary and important step toward clinical application. Once that is done, there are several ways (all of which require additional validation) in which these findings could enhance clinical practice. First, given how inexpensive and straightforward it is to quantify blood leukocyte numbers, surgeons could monitor changes in leukocyte distribution during surgery. This would provide information instantaneously during surgery about whether additional intraoperative or postoperative intervention is indicated to enhance recovery, especially for patients who show a maladaptive immune cell redistribution. Second, the principal stress hormones that are known to mediate stress-induced changes in leukocyte distribution (epinephrine, norepinephrine, and cortisol)13,18,19,21,22,35,52 could be administered at specific times during surgery to induce “adaptive” leukocyte redistribution in patients who fail to show an adaptive response. Third, the identification of mechanisms mediating adaptive as opposed to maladaptive leukocyte redistribution during stress would enable the development of pharmacological or biobehavioral interventions designed to maximize adaptive surgical stress-induced leukocyte redistribution and the related enhancement of postoperative recovery. Fourth, we hypothesize that the ability to mount adaptive immune cell redistribution responses during stress may be a characteristic that is expressed in a similar manner across different stressors. If this hypothesis is confirmed, it would open the possibility of prospectively identifying patients who are likely to show adaptive as opposed to maladaptive stress-induced changes in immune cell distribution through the administration of an appropriately designed stress test before surgery. Such prospective identification coupled with the interventions mentioned above could maximize the probability of a patient showing adaptive leukocyte redistribution during surgery, leading to enhanced recovery following surgery.
The present study identifies potential mediators of the well-known, but hard-to-explain, observation that patients with similar demographic, physical, and clinical characteristics often show large differences in the rate and extent of postoperative recovery. Our results may be applicable to a wide range of surgical procedures because the physiological stress response that is known to drive stress-induced changes in immune cell distribution13,14,16,19,21,22,35,40 is likely to be similar during different types of surgery. The present study lays the foundation for prospectively identifying patients who are likely to show low as opposed to high leukocyte redistribution during surgery and for designing psychological, biobehavioral, and pharmacological interventions to maximize the rate and extent of recovery. The important personal and economic benefits of enhanced recovery include reduced morbidity, fewer days lost from work or sports activities, more complete and long-lasting return to sports and life activities, reduced risk of reinjury, and reduced individual and societal health-care costs. 
Supplementary Material
Acknowledgments
Note: Firdaus S. Dhabhar designed the immunological aspects of the study, formulated and operationalized leukocyte redistribution concepts, guided and interpreted analyses, and wrote the manuscript. Patricia H. Rosenberger was Project Director, conducted and interpreted statistical analyses with guidance from Trace Kershaw, and wrote the initial draft of the manuscript. Jeannette R. Ickovics was Principal Investigator and oversaw study design and conduct. Jean M. Tillie and Firdaus S. Dhabhar made all immunological measurements. Elissa Epel played a substantial role in study design, operationalization of leukocyte redistribution concepts, data analyses, interpretation, and writing. Eric Nadler assisted with initial data analysis. Peter Jokl was the Medical Director of the study, performed operations, evaluated patients in the immunology sub-study as well as the parent study, and was centrally responsible for implementation and clinical oversight. John P. Fulkerson performed operations and evaluated patients for the parent study. All authors contributed to manuscript review and editing. Funding for this research came from NIAMS (RO1-AR-46299). The authors are grateful to Dr. Trace Kershaw for help and guidance with statistical analyses.
A commentary by Martin I. Boyer, MD, MSc, FRCS(C), is available at www.jbjs.org/commentary and as supplemental material to the online version of this article.
Disclosure: In support of their research for or preparation of this work, one or more of the authors received, in any one year, outside funding or grants in excess of $10,000 from NIH-NIAMS RO1-AR-46299. Neither they nor a member of their immediate families received payments or other benefits or a commitment or agreement to provide such benefits from a commercial entity. No commercial entity paid or directed, or agreed to pay or direct, any benefits to any research fund, foundation, division, center, clinical practice, or other charitable or nonprofit organization with which the authors, or a member of their immediate families, are affiliated or associated.
Investigation performed at the Department of Orthopaedics and Department of Epidemiology and Public Health, Yale University, New Haven, Connecticut; Department of Psychiatry, University of California San Francisco, San Francisco; and Department of Psychiatry and Behavioral Sciences, Stanford University School of Medicine, Stanford, California
References
- 1.Schappert SM. Ambulatory care visits of physician offices, hospital outpatient departments, and emergency departments: United States, 1995. Vital Health Stat 13. 1997;129:1-38. [PubMed] [Google Scholar]
- 2.Rosenberger PH, Jokl P, Ickovics JR. Psychosocial factors and surgical outcome: an evidence-based literature review. J Am Acad Orthop Surg. 2006;14:397-405. [DOI] [PubMed] [Google Scholar]
- 3.Schimmer RC, Brülhart KB, Duff C, Glinz W. Arthroscopic partial meniscectomy: a 12-year follow-up and two-step evaluation of the long-term course. Arthroscopy. 1998;14:136-42. [DOI] [PubMed] [Google Scholar]
- 4.Daniel DM. Selecting patients for ACL surgery. In: Jackson DW, Arnoczky SP, Woo SL-Y, Frank CB, Simon TM. The anterior cruciate ligament. Current and future concepts. New York: Raven Press; 1993. p 251-8.
- 5.McConville OR, Kipnis JM, Richmond JC, Rockett SE, Michaud MJ. The effect of meniscal status on knee stability and function after anterior cruciate ligament reconstruction. Arthroscopy. 1993;9:431-9. [DOI] [PubMed] [Google Scholar]
- 6.Swenson TM, Harner CD. Knee ligament and meniscal injuries. Current concepts. Orthop Clin North Am. 1995;26:529-46. [PubMed] [Google Scholar]
- 7.McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171-9. [DOI] [PubMed] [Google Scholar]
- 8.Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5:243-51. [DOI] [PubMed] [Google Scholar]
- 9.Coe CL, Laudenslager ML. Psychosocial influences on immunity, including effects on immune maturation and senescence. Brain Behav Immun. 2007;21:1000-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Irwin MR. Human psychoneuroimmunology: 20 years of discovery. Brain Behav Immun. 2008;22:129-39. [DOI] [PubMed] [Google Scholar]
- 11.Butts CL, Sternberg EM. Neuroendocrine factors alter host defense by modulating immune function. Cell Immunol. 2008;252:7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dhabhar FS. Enhancing versus suppressive effects of stress on immune function: implications for immunoprotection and immunopathology. Neuroimmunomodulation. 2009;16:300-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dhabhar FS, Miller AH, McEwen BS, Spencer RL. Effects of stress on immune cell distribution. Dynamics and hormonal mechanisms. J Immunol. 1995;154:5511-27. [PubMed] [Google Scholar]
- 14.Dhabhar FS, McEwen BS. Stress-induced enhancement of antigen-specific cell-mediated immunity. J Immunol. 1996;156:2608-15. [PubMed] [Google Scholar]
- 15.Viswanathan K, Dhabhar FS. Stress-induced enhancement of leukocyte trafficking into sites of surgery or immune activation. Proc Natl Acad Sci U S A. 2005;102:5808-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dhabhar FS, Viswanathan K. Short-term stress experienced at time of immunization induces a long-lasting increase in immunologic memory. Am J Physiol Regul Integr Comp Physiol. 2005;289:R738-44. [DOI] [PubMed] [Google Scholar]
- 17.Viswanathan K, Daugherty C, Dhabhar FS. Stress as an endogenous adjuvant: augmentation of the immunization phase of cell-mediated immunity. Int Immunol. 2005;17:1059-69. [DOI] [PubMed] [Google Scholar]
- 18.Dhabhar FS, McEwen BS. Bidirectional effects of stress on immune function: possible explanations for salubrious as well as harmful effects. In: Ader R, editor. Psychoneuroimmunology. 4th ed. Boston: Elsevier; 2007. p 723-60.
- 19.Dhabhar FS, Miller AH, McEwen BS, Spencer RL. Stress-induced changes in blood leukocyte distribution. Role of adrenal steroid hormones. J Immunol. 1996;157:1638-44. [PubMed] [Google Scholar]
- 20.Dhabhar FS, McEwen BS. Acute stress enhances while chronic stress suppresses immune function in vivo: a potential role for leukocyte trafficking. Brain Behav Immun. 1997;11:286-306. [DOI] [PubMed] [Google Scholar]
- 21.Dhabhar FS. Stress-induced enhancement of cell-mediated immunity. Ann N Y Acad Sci. 1998;840:359-72. [DOI] [PubMed] [Google Scholar]
- 22.Dhabhar FS, McEwen BS. Bidirectional effects of stress and glucocorticoid hormones on immune function: possible explanations for paradoxical observations. In: Ader R, Felten DL, Cohen N, editors. Psychoneuroimmunology. 3rd ed. San Diego: Academic Press; 2001. p 301-38.
- 23.Dhabhar FS, McEwen BS, Spencer RL. Adaptation to prolonged or repeated stress—comparison between rat strains showing intrinsic differences in reactivity to acute stress. Neuroendocrinology. 1997;65:360-8. [DOI] [PubMed] [Google Scholar]
- 24.Bilbo SD, Dhabhar FS, Viswanathan K, Saul A, Yellon SM, Nelson RJ. Short day lengths augment stress-induced leukocyte trafficking and stress-induced enhancement of skin immune function. Proc Natl Acad Sci U S A. 2002;99:4067-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saint-Mezard P, Chavagnac C, Bosset S, Ionescu M, Peyron E, Kaiserlian D, Nicolas JF, Bérard F. Psychological stress exerts an adjuvant effect on skin dendritic cell functions in vivo. J Immunol. 2003;171:4073-80. [DOI] [PubMed] [Google Scholar]
- 26.Katz JN, Wright EA, Guadagnoli E, Liang MH, Karlson EW, Cleary PD. Differences between men and women undergoing major orthopedic surgery for degenerative arthritis. Arthritis Rheum. 1994;37:687-94. [DOI] [PubMed] [Google Scholar]
- 27.Cameron ML, Briggs KK, Steadman JR. Reproducibility and reliability of the Outerbridge classification for grading chondral lesions of the knee arthroscopically. Am J Sports Med. 2003;31:83-6. [DOI] [PubMed] [Google Scholar]
- 28.Tegner Y, Lysholm J. Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res. 1985;198:43-9. [PubMed] [Google Scholar]
- 29.Weitzel PP, Richmond JC. Critical evaluation of different scoring systems of the knee. Sports Med Arthrosc. 2002;10:183-90. [Google Scholar]
- 30.Hoser C, Fink C, Brown C, Reichkendler M, Hackl W, Bartlett J. Long-term results of arthroscopic partial lateral meniscectomy in knees without associated damage. J Bone Joint Surg Br. 2001;83:513-6. [DOI] [PubMed] [Google Scholar]
- 31.Kartus JT, Russell VJ, Salmon LJ, Magnusson LC, Brandsson S, Pehrsson NG, Pinczewski LA. Concomitant partial meniscectomy worsens outcome after arthroscopic anterior cruciate ligament reconstruction. Acta Orthop Scand. 2002;73:179-85. [DOI] [PubMed] [Google Scholar]
- 32.Rosenberger PH, Ickovics JR, Epel ES, D'Entremont D, Jokl P. Physical recovery in arthroscopic knee surgery: unique contributions of coping behaviors to clinical outcomes and stress reactivity. Psychol Health. 2004;19:307-20. [Google Scholar]
- 33.Marx RG, Jones EC, Allen AA, Altchek DW, O'Brien SJ, Rodeo SA, Williams RJ, Warren RF, Wickiewicz TL. Reliability, validity, and responsiveness of four knee outcome scales for athletic patients. J Bone Joint Surg Am. 2001;83:1459-69. [DOI] [PubMed] [Google Scholar]
- 34.Ceddia MA, Price EA, Kohlmeier CK, Evans JK, Lu Q, McAuley E, Woods JA. Differential leukocytosis and lymphocyte mitogenic response to acute maximal exercise in the young and old. Med Sci Sports Exerc. 1999;31:829-36. [DOI] [PubMed] [Google Scholar]
- 35.Benschop RJ, Rodriguez-Feuerhahn M, Schedlowski M. Catecholamine-induced leukocytosis: early observations, current research, and future directions. Brain Behav Immun. 1996;10:77-91. [DOI] [PubMed] [Google Scholar]
- 36.Gibbons RD, Hedeker D, Elkin I, Waternaux C, Kraemer HC, Greenhouse JB, Shea MT, Imber SD, Sotsky SM, Watkins JT. Some conceptual and statistical issues in analysis of longitudinal psychiatric data. Application to the NIMH treatment of Depression Collaborative Research Program dataset. Arch Gen Psychiatry. 1993;50:739-50. [DOI] [PubMed] [Google Scholar]
- 37.Donner A, Klar N. Statistical considerations in the design and analysis of community intervention trials. J Clin Epidemiol. 1996;49:435-9. [DOI] [PubMed] [Google Scholar]
- 38.Murray DM. Design and analysis of group-randomized trials. New York: Oxford University Press; 1998.
- 39.Dhabhar FS. Acute stress enhances while chronic stress suppresses skin immunity. The role of stress hormones and leukocyte trafficking. Ann N Y Acad Sci. 2000;917:876-93. [DOI] [PubMed] [Google Scholar]
- 40.Miller AH, Spencer RL, Hassett J, Kim C, Rhee R, Ciurea D, Dhabhar F, McEwen B, Stein M. Effects of selective type I and II adrenal steroid agonists on immune cell distribution. Endocrinology. 1994;135:1934-44. [DOI] [PubMed] [Google Scholar]
- 41.Fishel RS, Barbul A, Beschorner WE, Wasserkrug HL, Efron G. Lymphocyte participation in wound healing. Morphologic assessment using monoclonal antibodies. Ann Surg. 1987;206:25-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schäffer M, Barbul A. Lymphocyte function in wound healing and following injury. Br J Surg. 1998;85:444-60. [DOI] [PubMed] [Google Scholar]
- 43.Shimaoka M, Hosotsubo K, Sugimoto M, Sakaue G, Taenaka N, Yoshiya I, Kiyono H. The influence of surgical stress on T cells: enhancement of early phase lymphocyte activation. Anesth Analg. 1998;87:1431-5. [DOI] [PubMed] [Google Scholar]
- 44.Stabile E, Kinnaird T, la Sala A, Hanson SK, Watkins C, Campia U, Shou M, Zbinden S, Fuchs S, Kornfeld H, Epstein SE, Burnett MS. CD8+ T lymphocytes regulate the arteriogenic response to ischemia by infiltrating the site of collateral vessel development and recruiting CD4+ mononuclear cells through the expression of interleukin-16. Circulation. 2006;113:118-24. Erratum in: Circulation. 2006;113:e711. [DOI] [PubMed] [Google Scholar]
- 45.Efron JE, Frankel HL, Lazarou SA, Wasserkrug HL, Barbul A. Wound healing and T-lymphocytes. J Surg Res. 1990;48:460-3. [DOI] [PubMed] [Google Scholar]
- 46.Kloth LC, McCulloch JM. Wound healing: alternatives in management. 3rd ed. Philadelphia: FA Davis; 2002. p 568.
- 47.Boyce DE, Jones WD, Ruge F, Harding KG, Moore K. The role of lymphocytes in human dermal wound healing. Br J Dermatol. 2000;143:59-65. [DOI] [PubMed] [Google Scholar]
- 48.Kiecolt-Glaser JK, Marucha PT, Malarkey WB, Mercado AM, Glaser R. Slowing of wound healing by psychological stress. Lancet. 1995;346:1194-6. [DOI] [PubMed] [Google Scholar]
- 49.Broadbent E, Petrie KJ, Alley PG, Booth RJ. Psychological stress impairs early wound repair following surgery. Psychosom Med. 2003;65:865-9. [DOI] [PubMed] [Google Scholar]
- 50.Manyande A, Chayen S, Priyakumar P, Smith CC, Hayes M, Higgins D, Kee S, Phillips S, Salmon P. Anxiety and endocrine responses to surgery: paradoxical effects of preoperative relaxation training. Psychosom Med. 1992;54:275-87. [DOI] [PubMed] [Google Scholar]
- 51.Salmon P, Pearce S, Smith CC, Manyande A, Heys A, Peters N, Rashid J. Anxiety, type A personality and endocrine responses to surgery. Br J Clin Psychol. 1989;28(Pt 3):279-80. [DOI] [PubMed] [Google Scholar]
- 52.Fauci AS, Dale DC. The effect of in vivo hydrocortisone on subpopulations of human lymphocytes. J Clin Invest. 1974;53:240-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.