Abstract
Aims
Some authors recommend avoiding fusion with left ventricular (LV) intrinsic depolarization during cardiac resynchronization therapy (CRT). If fusion is still present during optimized biventricular (Biv) pacing and its long-term effects on the response to CRT are currently unknown. The aim of the study was to analyse the endocardial LV activation pattern induced by echocardiographically optimized Biv pacing and its influence on LV reverse remodelling.
Methods and results
Contact electro-anatomical mapping was performed in 15 heart failure (HF) patients with left bundle branch block and echocardiographically optimized CRT (seven ischaemic aetiology, 64 ± 8 years, three women, New York Heart Association class 3 ± 0.4, LV ejection fraction 25 ± 5%). Left ventricular activation maps were performed in sinus rhythm (SR), during DDD right ventricular apical (RVA) and optimized Biv pacing. Fusion with intrinsic rhythm during pacing was considered when LV septal activation was produced at least partially by intrinsic depolarization, when compared with LV activation map during SR. Patients were considered responders to CRT if they had ≥10% reduction in LV end-systolic volume (LVESV) after 6 months of CRT. During SR, the LV breakthrough was mid-septal (n = 12), basal septum (n = 2), and apical (n = 1). During RVA pacing, LV breakthrough shifted apical in all patients. Right ventricular apical/Biv pacing proved fusion with intrinsic depolarization in 8 of 15 patients. The PR interval was shorter in patients with fusion RVA/Biv pacing (164 ± 24 vs. 234 ± 55 ms, P = 0.006). There was a trend for shorter LV activation time (LVat) in patients with fusion during RVA pacing (87 ± 33 vs. 113 ± 21 ms, P = 0.08) as well as during optimized Biv pacing (83 ± 18 vs. 104 ± 24 ms, P = 0.07), although LVat was similar in SR (100 ± 22 vs. 106 ± 20, P = NS). In patients with fusion, 6 months responder rate was significantly higher (100 vs. 28.5%, P < 0.007) as was the degree of LVESV reduction (39 ± 17 vs. 1.0 ± 14%, P < 0.001).
Conclusion
Biventricular pacing with fusion may substantially increase the structural responder rate probably by shortening LVat.
Keywords: Cardiac resynchronization therapy, RVA pacing, Fusion, Activation time
Introduction
In patients with wide QRS and New York Heart Association (NYHA) class 3 or 4 heart failure (HF) on optimal medical therapy, cardiac resynchronization therapy (CRT) improves functional status and quality of life and reduces HF-related hospitalizations and total mortality.1 Unfortunately, only 601 to 70%2 of patients have a clinical response. Moreover, long-term survival is improved with CRT only if associated with LV reverse remodelling, defined as ≥10% reduction in LV end-systolic volume (LVESV),3 a structural response that is present in only 56% of patients.2 Although identification of mechanical intraventricular dyssynchrony seemed appealing, with some data showing a superior effect of CRT in patients with a concordance between maximum delay area and LV lead position,4 a recent prospective trial did not showed a good correlation of any of the echocardiographic parameters used to assess baseline intraventricular dyssynchrony with clinical or structural response to CRT.2 Besides poor reproducibility of these parameters5 and complex torsion movement of the asynchronous, failing LV,6 another possible explanation could reside in the pacing configuration used. Acute data suggest that in patients with normal AV conduction allowing partial intrinsic depolarization of the LV (fusion) during CRT produce a superior haemodynamic response.7 However, due to presumed variability in AV conduction and lack of data on chronic effects of fusion, the majority of authors try to avoid it8 and prefer biventricular (Biv) pacing with the shortest possible AV delay, as determined by echocardiography. This introduces right ventricular (RV) pacing, which might change the timing and the pattern of LV depolarization.
The present investigation aimed to analyse endocardial LV activation pattern to test if fusion is still present after echocardiographically optimized Biv pacing and to determine its influence on LV reverse remodelling.
Methods
Patients
Between October 2006 and February 2007, 15 consecutive patients admitted to CRT were included in the study. Eligibility criteria for CRT were chronic moderate to severe HF (NYHA class III or IV), moderate to severe LV systolic dysfunction [LV ejection fraction (LVEF) ≤35%], and a QRS complex ≥120 ms. Ischaemic heart disease was considered the aetiology of LV systolic dysfunction in the presence of significant coronary artery stenosis (≥50% in one or more of the major epicardial coronary arteries) and/or a history of myocardial infarction and/or previous coronary revascularization. The study protocol was approved by the Institution's Ethics Committee and written informed consent was obtained in all cases.
Cardiac resynchronization therapy device implantation
The right atrial and ventricular leads were positioned conventionally [right atrial appendage and RV apical (RVA)]. After coronary sinus (CS) was cannulated with an 8F guiding catheter, an occlusive retrograde CS angiogram was obtained. The LV pacing lead (Easytrak 4512–80, Guidant Corporation, St Paul, MN, USA) was inserted in a lateral or postero-lateral vein. All leads were connected to a dual-chamber Biv implantable pacemaker or cardioverter-defibrillator (Contak Renewal IV or H195, Guidant Corporation).
Echocardiographic evaluation
All patients underwent standard transthoracic 2D and colour-Doppler echocardiography before implantation, 1 day after implantation (for optimization), and after 6 months of CRT. Images were obtained in the parasternal and apical views with a commercially available system (Vivid 7, General Electric, Milwaukee, WI, USA), using a 3.5 MHz transducer. Left ventricular volumes (LVEDV and LVESV) and LVEF were calculated using the biplane Simpson's technique. Echocardiographic optimization was performed step-wise: first AV interval (by the Ritter method, choosing the shortest AV interval that yielded the longest diastolic filling time without truncation of the A wave) followed by the VV interval (the optimum VV was considered as the one that yielded the best intraventricular synchrony as demonstrated by TDI, with a greater superposition of the displacement curves of two opposite LV walls as described previously).9 Patients were classified as responders to CRT if they had LV reverse remodelling (≥10% reduction in LVESV at 6 months follow-up).
Mapping procedure
Contact electro-anatomical activation mapping (CARTO, Biosense Webster) of the LV endocardium was performed from 1 to 3 days after the implantation of a CRT or CRT-D device. Activation maps were obtained during intrinsic rhythm, during RVA and during Biv pacing (both in DDD mode with AV interval programmed equal to echocardiographically optimized AV interval). Fusion with intrinsic rhythm during pacing was considered when LV septal activation was produced at least partially by intrinsic depolarization, when compared with LV activation map during sinus rhythm (SR), i.e. single-fused wave-front or double wave-front resulting from intrinsic depolarization and apical depolarization due to RVA pacing. Using multiple radioscopic projections, the LV lead electrode position was localized within the anatomical map. Rectilinear distance between the LV lead electrode and the area of maximum delay was determined in each map. Activation maps (intrinsic vs. RVA pacing) were superposed, and rectilinear distance between the areas of maximum delay was determined. The time interval from the beginning of QRS (from 12-lead electrocardiogram) to the earliest (breakthrough) point on the LV activation map during intrinsic rhythm as well as total LV activation time (LVat) were measured. The time interval from the beginning of QRS to RV-detected electrogram (RVEGM) was determined with the CRT device programmed in the ODO mode. We arbitrarily defined a significant shift in the location of the most delayed area as an increase of >3 cm in the distance from most delayed area to LV lead electrodes with RVA pacing (Δd).
Statistical analysis
The measured values are expressed as mean ± SD. Data showing Gaussian distribution were compared in the subgroups using paired and Student's t-tests. Dichotomous variables and non-parametric data were compared using χ2 and Wilcoxon testing, respectively. The level of significance was set at 0.05.
Results
Patients
Baseline characteristics of the 15 patients included in this study are summarized in Table 1. Mean age was 67 ± 8 years (three women); the aetiology of LV systolic dysfunction was ischaemic in seven patients. Mean baseline LVEF was 25 ± 5%. All patients were in SR and QRS morphology was left bundle branch block (LBBB) type in all 15 patients.
Table 1.
Baseline patient characteristics (n = 15)
| Sex (female/male) | 3/12 |
| Age (years) | 67 ± 8 |
| Aetiology (ischaemic/idiopathic) | 7/8 |
| NYHA functional class | 3 ± 0.4 |
| LV end-diastolic dimension (mm) | 73 ± 10 |
| LV end-systolic dimension (mm) | 59 ± 12 |
| LV ejection fraction (%) | 25 ± 5 |
| Sinus rhythm | 15 (100%) |
| PR interval (ms) | 197 ± 54 |
| QRS width (ms) | 181 ± 13 |
| Interval [bQRS–earliest point on the LV activation map] during intrinsic rhythm (ms) | 7 ± 7 |
| Interval [bQRS–RVEGM] during intrinsic rhythm (ms) | 50 ± 14 |
| Interval [bQRS–earliest point on the LV activation map] during RVA pacing (ms) | 30 ± 10 |
| Total LV endocardial activation time, intrinsic rhythm (ms) | 99 ± 30 |
| Total LV endocardial activation time, RVA pacing (ms) | 102 ± 21 |
| Total LV endocardial activation time, optimized (ms) | 93 ± 23 |
NYHA, New York Heart Association; LV, left ventricular; PR, QRS, and nQRS, narrow QRS complex; LBBB, left bundle branch block; bQRS, beginning of QRS; RVA, right ventricular apical; RVEGM, RV lead detected intracardiac electrogram.
The breakthrough
The breakthrough of LV endocardial activation during SR was mid-septal (n = 12, 80%), basal septum (n = 2, 13%), and apical (n = 1, 7%). Mean time interval from the beginning of QRS complex on 12-lead electrocardiogram to the earliest point on the LV activation map during intrinsic rhythm was 7 ± 7 ms; mean time interval from the beginning of QRS complex to RVEGM was 50 ± 14 ms. With RVA pacing, the LV endocardial breakthrough was always apical (Figure 1), 30 ± 10 ms from the RV pacing artefact on 12-lead electrocardiogram.
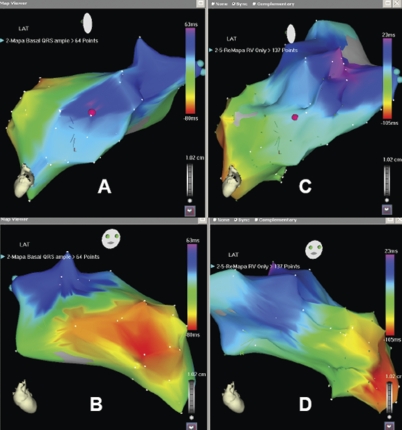
Figure 1.
Left-ventricular activation maps from the same patient during intrinsic rhythm (A and B) and RVA pacing (C and D) in left lateral (A and C) and RAO (B and D) projections. Baseline there was a perfect concordance between the area of maximum delay and LV lead position (pink floating spot). During RVA pacing, the area of maximum delay shifted 5 cm in a more basal position.
Maximum delay area
The most delayed area during SR was postero-lateral or lateral in 13 patients (87%) and antero-lateral in 2 patients (13%). During RVA pacing, the most delayed area was unchanged only in three patients (20%), in 80% of them (n = 12) shifting to more anterior positions (Figure 1). The mean shift was 33 ± 9 mm. However, the mean distance from most delayed area to LV lead electrode was not significantly changed in this population (39.5 ± 20.5 mm during intrinsic rhythm vs. 41.8 ± 10.8 mm with RVA pacing, P = NS). When we compared patients with significant shift during RVA pacing (n = 5) vs. patients with a non-significant shift (n = 10), there was no difference in the rate of structural responders: only 60% in the first group vs. 70% in the second group. Overall, there was also a slight increase in total LVat with RVA pacing (102 ± 21 vs. 99 ± 30 ms during intrinsic rhythm, P = NS).
Effects of fusion with left ventricular intrinsic depolarization
According to LV activation maps, with the optimized echocardiographic AV interval used for DDD RVA/Biv pacing, there was fusion with intrinsic rhythm in 8 of 15 patients (Figure 2). The mean shift in the most delayed area of activation during RVA pacing as well as the mean distance from most delayed area to LV lead electrodes in SR and during RVA pacing were similar with fusion and with pure RVA pacing (32 ± 11 vs. 30 ± 10 mm, 37 ± 23 vs. 43 ± 18 mm, respectively 40 ± 14 vs. 44 ± 7, P = NS for all comparisons). There was a trend towards shorter LVat in the fusion group during RVA pacing (87 ± 32 vs. 113 ± 21 ms, P = 0.08) as well as during optimum Biv pacing (83 ± 18 vs. 104 ± 24, P = 0.07). All the patients with fusion (100%) were structural responders at 6 months vs. only two of seven (28.5%) in the group with pure Biv pacing (P < 0.007), despite a similar baseline clinical profile (Table 2), except for a significantly shorter PR interval (164 ± 24 vs. 234 ± 55 ms, P = 0.006). In the group with fusion, the degree of reverse remodelling (evaluated by LVESV reduction) was also significantly higher than that in patients with pure RVA pacing: 39 ± 17 vs. 1.0 ± 14% (P < 0.001).
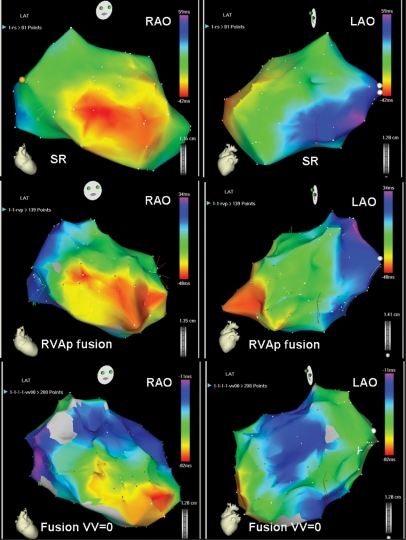
Figure 2.
Example in which echocardiographic AV optimization permitted CRT with fusion. On the top, activation maps during SR showing a mid-septal breakthrough, a postero-lateral most delayed activation area and LVat of 101 ms. On the middle, activation maps during RVA pacing with fusion with the intrinsic depolarization, showing the two septal breakthroughs, a change in the most delayed activation area (antero-lateral) and a shortening in LVat to 82 ms. On the bottom, activation maps with the addition of a third wave front from the CS lead, showing the maintenance of the same septal breakthroughs, a change of the most delayed area of activation towards intermediate positions and a further shortening of LVat to 71 ms.
Table 2.
Comparison between characteristics of patients with pure RVA pacing (n = 7) and with RVA/Biv pacing with fusion (n = 8)
| RVA (n = 7) | Fusion (n = 8) | P-value | |
|---|---|---|---|
| Sex (female/male) | 1/6 | 2/6 | NS |
| Age (years) | 73 ± 7 | 63 ± 6 | NS |
| Aetiology (ischaemic/idiopathic) | 4/3 | 3/5 | NS |
| NYHA functional class | 3.2 ± 0.4 | 2.9 ± 0.4 | NS |
| PR interval (ms) | 234 ± 55 | 164 ± 24 | 0.006 |
| AV interval (ms) | 136 ± 12 | 140 ± 0 | NS |
| VV interval (ms) | −9 ± 15 | −15 ± 16 | NS |
| QRS width (ms) | 164 ± 34 | 179 ± 14 | NS |
| LV lead | |||
| Maximum delay area intrinsic (mm) | 39.3 ± 17.8 | 36.5 ± 23 | NS |
| Maximum delay area RVA pacing (mm) | 42.6 ± 13.4 | 40.2 ± 13.6 | NS |
| Mean shift in activation pattern (mm) | 32.3 ± 9.3 | 32.0 ± 11 | NS |
| Significant shift (n'/n) | 2/7 | 3/8 | NS |
| Total LV activation time intrinsic rhythm (ms) | 106 ± 20 | 100 ± 22 | NS |
| Total LV activation time RVA pacing (ms) | 113 ± 21 | 87 ± 33 | 0.08 |
| Total LV activation time optimized pacing (ms) | 104 ± 24 | 83 ± 18 | 0.07 |
| Baseline LV ejection fraction (%) | 23 ± 5 | 25 ± 5 | NS |
| Baseline LVESV (mL) | 221 ± 56 | 176 ± 49 | NS |
| 6 months follow-up LVESV (mL) | 218 ± 50 | 107 ± 48 | <0.001 |
| Reduction of LVESV (%) | 1 ± 14 | 39 ± 17 | <0.001 |
| Structural responders (%; n'/n) | 28.5 (2/7) | 100 (8/8) | 0.001 |
NYHA, New York Heart Association; AV, atrioventricular; LV, left ventricular; PR, QRS, and nQRS, narrow QRS complex; RVA, right ventricular apical.
Discussion
Although it was previously assumed that RVA pacing is similar to LBBB in the presence of LV systolic dysfunction, this study confirms that in patients with chronic HF (CHF) subjected to CRT, RVA pacing changes the LV activation pattern, even in the presence of LBBB, producing overall an increase (although not statistically significant) in LVat and in the distance from maximum delay area to the LV lead. More importantly, the rate of structural responders to CRT was impressively higher (P < 0.007) in patients in whom RVA/Biv pacing allows partial intrinsic activation of the LV than in patients with pure capture during RVA/Biv pacing. A possible explanation is that CRT with fusion creates three activation wave fronts (instead of two during pure Biv pacing), therefore shortening LVat (Figure 3).
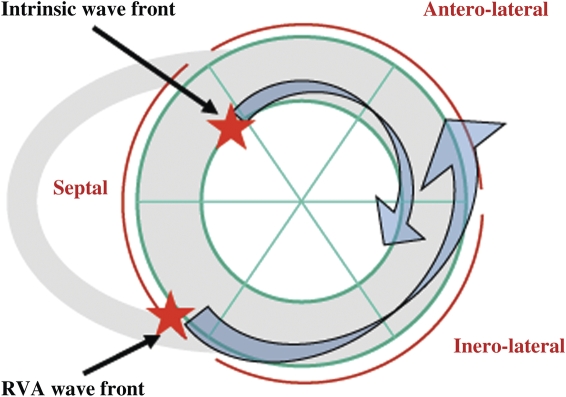
Figure 3.
Schematic of the mechanism by which the fusion between RVA pacing and partial intrinsic LV endocardial depolarization shortens LVat. The most delayed area during SR (intrinsic LV endocardial depolarization) was postero-lateral or lateral in >80% of patients. During RVA pacing, the most delayed area shifted to anterior or antero-lateral in ∼90% of patients. Thus, fusion between these two wave fronts takes less time to depolarize the LV. Adding a third wave front from the epicardial LV further diminishes the total duration of the LVat.
Breakthrough area
In this series of CHF patients with LBBB, the breakthrough of LV activation was most frequently mid-septal and only rarely apical (1 of 15 patients). This finding differs from a previous study of contact and non-contact mapping in 23 patients with CHF and LBBB,10 in which the breakthrough was most often apical (by trans-septal depolarization through RBBB). However, the majority of publications are consistent with our data: classical electrophysiological mapping,11 contact mapping,12 and non-contact mapping13 have shown that the location of breakthrough is mid- or basal septum14 in the majority of patients. Consistently, in our series of patients, the time interval from the beginning of QRS to the earliest point on the LV activation map is by far shorter than the time interval from the beginning of QRS to the detected RVEGM (7 ± 7 vs. 50 ± 14 ms, P < 0.05) despite the fact that RV lead was placed apical (i.e. in the proximity of RBB) (Table 1).
Area of left ventricular maximum delay
Right ventricular apical pacing has deleterious effects in patients with LV systolic dysfunction15 as well as in patients with normal baseline LV systolic function,16 mainly by induction of intraventricular dyssynchrony. In our study, RVA pacing increases the LVat and changes the location of the latest LV endocardial activation area (in 80% of cases to more anterior positions). The LVat increase with RVA pacing is smaller than that in other studies because they assessed it epicardially,17 but the presence of fusion in 8 of 15 patients probably contributed as well. Studies of classic LV endocardial mapping18 and contact electro-anatomical endocardial mapping19 also showed changes in the LV endocardial activation pattern during RVA pacing, with shifts in the location of maximum delay area in more postero-inferior18 or antero-lateral19 positions.
Currently, there is no direct evidence for a significant shift of activation on LV epicardium (where the LV lead is usually placed). However, indirect data on epicardial activation using body surface potential mapping consistently suggest that LV epicardial activation patterns change during RVA pacing, even in patients with LV systolic dysfunction and LBBB.20,21 Moreover, in studies of surgically delivered LV lead for CRT, placing the LV leads at sites of maximum electrical delay during RVA pacing significantly increased the percentage of responders.22
More important, recent mechanical data on 2D radial strain seems to confirm that RVA pacing changes the location of the area with the most delayed systolic peak.23
Effects of fusion during cardiac resynchronization therapy
It seems tempting to try to avoid LV activation shift induced by RVA pacing, at least in CRT patients with normal AV conduction and concordant LV lead position. One alternative would be to program the CRT device to allow partial or complete intrinsic depolarization of the interventricular septum (fusion pacing), creating three activation fronts instead of two during pure Biv pacing. Acute invasive haemodynamic data proved that in patients with normal AV conduction, CRT with fusion is superior to any optimized Biv configuration in improving LV7,24 as well as RV systolic performance.25 Our study suggests that the mechanism of this improved performance is a shorter LVat, which probably produces superior resynchronization. Despite the theoretical drawbacks of fusion (potential for loosing optimal fusion because of disease progression or spontaneous variation in AV conduction, lack of data on long-term effects of fusion CRT), in this study CRT with fusion produced an impressive rate of 100% structural responders at 6 months. Programming the AV delay to obtain CRT with optimal fusion can be challenging considering the variability of PR interval. However, a recent long-term prospective study in 40 patients used a non-invasive algorithm to obtain CRT with optimal fusion, observing the same high rate of structural response, with a similar level of reverse remodelling at 6 months.26 On the top of that, the same group recently demonstrated by serial ECG and ECG exercise test that CRT with optimal fusion can be maintained and that a Wenckebach point during atrial stimulation of less 500 ms can reliably predict fusion maintenance.27 The significantly shorter PR interval, a non-significant smaller LVESV at baseline in patients with fusion during CRT, may suggest also that they had a less advanced heart disease and a less dilated LV. However, the LVat during intrinsic rhythm that was not different in patients with fusion vs. patients with pure Biv pacing, which is an argument against this hypothesis. Concordantly, MIRACLE trial found similar baseline LV dimensions in non-responders when compared with responders, despite a significantly larger proportion of patients with long PR interval in the former group.28 It seems tempting to speculate that the optimization method which starts with AV interval (diastolic optimization) might also contribute, since the ‘optimum’ AVI was practically identical in these two patient populations despite very different baseline PR interval. In patients with long PR interval, this constantly introduces RVA pacing, possibly worsening LV function. This is suggested also by the trend to increase LVat in long PR interval patients during DDD RVA pacing as well as during Biv pacing at the optimized AVI. Invasive haemodynamical data showed that in one-third of patients with similar long PR, there is a worsening of LV systolic and diastolic function when paced with short AVI.29
An alternative to fusion would be to correct the effects of RVA pacing by triple site pacing, although this might be technically challenging. Recent data show that dual site RV pacing associated with LV pacing produced superior LV resynchronization and systolic performance when compared with conventional Biv pacing30 and that reverse remodelling with dual site LV pacing associated with RV pacing is superior to conventional Biv pacing.31 These data strengthen the idea of the superiority of three activation fronts during CRT (a situation reproduced during CRT with fusion).
Limitations
The results might be limited by the contact mapping technology. Rectilinear measurements underestimate distances on a curved surface. However, the precision in anatomical details is superior to non-contact mapping, although the latter can map the activation sequence using one beat. The protocol used for RVA pacing (DDD with echocardiographically optimized AVI), which permits fusion with intrinsic rhythm in a significant proportion of patients, may also obscure the changes in LV activation. Finally, this study is limited by our small sample size, which could explain the lack of statistical significance in the differences in LVat. However, the important difference observed with pure RVA pacing and with fusion warrants attention and further research.
Conclusions
In the majority of CHF patients eligible for CRT, LV activation breakthrough during intrinsic rhythm is located in mid- or basal septum. Pure RVA pacing changes LV endocardial activation sequence and prolongs LVat in the vast majority of cases. Partial intrinsic depolarization of the LV during CRT may substantially increase the rate of structural responders. This suggests that pure RVA/Biv pacing should be avoided whenever possible.
Conflict of interest: none declared.
Funding
Funding to pay the Open Access publication charges for this article was provided by Fundació Privada Clinic per a la Recerca Biomèdica.
References
- 1.McAlister FA, Ezekowitz J, Hooton N, Vandermeer B, Spooner C, Dryden DM, et al. Cardiac resynchronization therapy for patients with left ventricular systolic dysfunction: a systematic review. JAMA. 2007;297:2502–14. doi: 10.1001/jama.297.22.2502. [DOI] [PubMed] [Google Scholar]
- 2.Chung ES, Leon AR, Tavazzi L, Sun J-P, Nihoyannopoulos P, Merlino J, et al. Results of the Predictors of Response to CRT (PROSPECT) Trial. Circulation. 2008;117:2608–16. doi: 10.1161/CIRCULATIONAHA.107.743120. [DOI] [PubMed] [Google Scholar]
- 3.Yu CM, Bleeker GB, Fung JW, Schalij MJ, Zhang Q, van der Wall EE, et al. Left ventricular reverse remodeling but not clinical improvement predicts long-term survival after cardiac resynchronization therapy. Circulation. 2005;112:1580–6. doi: 10.1161/CIRCULATIONAHA.105.538272. [DOI] [PubMed] [Google Scholar]
- 4.Suffoletto MS, Dohi K, Cannesson M, Saba S, Gorcsan J. Novel speckle-tracking radial strain from routine black-and-white echocardiographic images to quantify dyssynchrony and predict response to cardiac resynchronization therapy. Circulation. 2006;113:960–8. doi: 10.1161/CIRCULATIONAHA.105.571455. [DOI] [PubMed] [Google Scholar]
- 5.Vesely MR, Li S, Kop WJ, Reese A, Marshall J, Shorofsky SR, et al. Test-retest reliability of assessment for intraventricular dyssynchrony by tissue Doppler imaging echocardiography. Am J Cardiol. 2008;101:645–50. doi: 10.1016/j.amjcard.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 6.Anderson LJ, Miyazaki C, Sutherland GR, Oh JK. Patient selection and echocardiographic assessment of dyssynchrony in cardiac resynchronization therapy. Circulation. 2008;117:2009–23. doi: 10.1161/CIRCULATIONAHA.107.721332. [DOI] [PubMed] [Google Scholar]
- 7.Van Gelder BM, Bracke FA, Meijer A, Pijls NHJ. The hemodynamic effect of intrinsic conduction during left ventricular pacing as compared to biventricular pacing. J Am Coll Cardiol. 2005;46:2305–10. doi: 10.1016/j.jacc.2005.02.098. [DOI] [PubMed] [Google Scholar]
- 8.Barold SS, Ilercil A, Herweg B. Echocardiographic optimization of the atrioventricular and interventricular intervals during cardiac resynchronization. Europace. 2008;10((Suppl 3):iii88–95. doi: 10.1093/europace/eun220. [DOI] [PubMed] [Google Scholar]
- 9.Vidal B, Sitges M, Marigliano A, Díaz-Infante E, Azqueta M, Tamborero D, et al. Relation of response to cardiac resynchronization therapy to left ventricular reverse remodeling. Am J Cardiol. 2006;97:876–81. doi: 10.1016/j.amjcard.2005.09.137. [DOI] [PubMed] [Google Scholar]
- 10.Auricchio A, Fantoni C, Regoli F, Carbucicchio C, Goette A, Geller C, et al. Characterization of left ventricular activation in patients with heart failure and left bundle-branch block. Circulation. 2004;109:1133–9. doi: 10.1161/01.CIR.0000118502.91105.F6. [DOI] [PubMed] [Google Scholar]
- 11.Vassallo JA, Cassidy DM, Marchlinski FE, Buxton AE, Waxman HL, Doherty JU, et al. Endocardial activation of left bundle branch block. Circulation. 1984;69:914–23. doi: 10.1161/01.cir.69.5.914. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez L-M, Timmermans C, Nabar A, Beatty G, Wellens HJJ. Variable patterns of septal activation in patients with left bundle branch block and heart failure. J Cardiovasc Electrophysiol. 2003;14:135–41. doi: 10.1046/j.1540-8167.2003.02421.x. [DOI] [PubMed] [Google Scholar]
- 13.Fung JW-H, Yu C-M, Yip G, Zhang Y, Chan H, Kum C-C, et al. Variable left ventricular activation pattern in patients with heart failure and left bundle branch block. Heart. 2004;90:17–9. doi: 10.1136/heart.90.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riedlbauchova L, Cihak R, Bytexnık J, Vanura V, Frıdl P, Hoxkova L, et al. Optimization of right ventricular lead position in cardiac resynchronisation therapy. Eur J Heart Fail. 2006;8:609–14. doi: 10.1016/j.ejheart.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 15.Wilkoff BL, Cook JR, Epstein AE, Greene HL, Hallstrom AP, Hsia H, et al. Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: the Dual Chamber and VVI Implantable Defibrillator (DAVID) Trial. JAMA. 2002;288:3115–23. doi: 10.1001/jama.288.24.3115. [DOI] [PubMed] [Google Scholar]
- 16.Vatasescu R, Shalganov T, Paprika D, Kornyei L, Prodan Z, Bodor G, et al. Evolution of left ventricular function in paediatric patients with permanent right ventricular pacing for isolated congenital heart block: a medium term follow-up. Europace. 2007;9:228–32. doi: 10.1093/europace/eum008. [DOI] [PubMed] [Google Scholar]
- 17.Varma N. Left ventricular conduction delays induced by right ventricular apical pacing: effect of left ventricular dysfunction and bundle branch block. J Cardiovasc Electrophysiol. 2008;19:114–22. doi: 10.1111/j.1540-8167.2007.00995.x. [DOI] [PubMed] [Google Scholar]
- 18.Vassallo JA, Cassidy DM, Miller JM, Buxton AE, Marchlinski FE, Josephson ME. Left ventricular endocardial activation during right ventricular pacing: effect of underlying heart disease. J Am Coll Cardiol. 1986;57:1228–33. doi: 10.1016/s0735-1097(86)80140-1. [DOI] [PubMed] [Google Scholar]
- 19.Peichl P, Kautzner J, Cihak R, Riedlbauchova L, Bytesnik J. Ventricular activation patterns during different pacing modes. An insight from electroanatomical mapping. Kardiol Pol. 2005;63:622–32. [PubMed] [Google Scholar]
- 20.Pastore CA, Tobias N, Samesima N, Filho MM, Pedrosa A, Nishioka S, et al. Body surface potential mapping investigating the ventricular activation patterns in the cardiac resynchronization of patients with left bundle-branch block and heart failure. J Electrocardiol. 2006;39:93–102. doi: 10.1016/j.jelectrocard.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Jia P, Ramanathan C, Ghanem RN, Ryu K, Varma N, Rudy Y. Electrocardiographic imaging of cardiac resynchronization therapy in heart failure: observation of variable electrophysiologic responses. Heart Rhythm. 2006;3:296–310. doi: 10.1016/j.hrthm.2005.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edgerton JR, Edgerton ZJ, Mack MJ, Hoffman S, Dewey TM, Herbert MA. Ventricular epicardial lead placement for resynchronization by determination of paced depolarization intervals: technique and rationale. Ann Thorac Surg. 2007;83:89–92. doi: 10.1016/j.athoracsur.2006.08.048. [DOI] [PubMed] [Google Scholar]
- 23.Vasile A, Vatasescu R, Iorgulescu C, Dumitrescu N, Constantinescu D, Dorobantu M. Right ventricular pacing changes the localization of left ventricular maximum delay area in patients with congestive heart failure and intraventricular mechanic dyssynchrony. Eur J Echocardiogr. 2008 S104. [Google Scholar]
- 24.Kurzidim K, Reinke H, Sperzel J, Schneider HJ, Danilovic D, Siemon G, et al. Invasive optimization of cardiac resynchronization therapy: role of sequential biventricular and left ventricular pacing. Pacing Clin Electrophysiol. 2005;28:754–61. doi: 10.1111/j.1540-8159.2005.00181.x. [DOI] [PubMed] [Google Scholar]
- 25.Lee KL, Burnes JE, Mullen TJ, Hettrick DA, Tse HF, Lau CP. Avoidance of right ventricular pacing in cardiac resynchronization therapy improves right ventricular hemodynamics in heart failure patients. J Cardiovasc Electrophysiol. 2007;18:497–504. doi: 10.1111/j.1540-8167.2007.00788.x. [DOI] [PubMed] [Google Scholar]
- 26.Vatasescu R, Berruezo A, Iorgulescu C, Vasile A, Constantinescu D, Dorobantu M. New algorithm for pacing with optimal fusion during cardiac resynchronization therapy induces extensive reverse-remodeling in heart failure patients with normal atrio-ventricular conduction and concordant left ventricular lead position. Circulation. 2009 Suppl (in press) [Google Scholar]
- 27.Vatasescu R, Berruezo A, Iorgulescu C, Fruntelata A, Dorobantu M. Cardiac resynchronization therapy with fusion can be maintained during long term follow-up in HF patients with normal AV conduction. Eur Heart J. 2009;30:1017. [Google Scholar]
- 28.Pires LA, Abraham WT, Young JB, Johnson KM MIRACLE MIRACLE-ICD Investigators. Clinical predictors and timing of New York Heart Association class improvement with cardiac resynchronization therapy in patients with advanced chronic heart failure: results from the Multicenter InSync Randomized Clinical Evaluation (MIRACLE) and Multicenter InSync ICD Randomized Clinical Evaluation (MIRACLE-ICD) Trials. Am Heart J. 2006;151:837–43. doi: 10.1016/j.ahj.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 29.Auricchio A, Ding J, Spinelli JC, Kramer JC, Salo RW, Hoersch W, et al. for the PATH-CHF Study. Cardiac resynchronization therapy restores optimal atrioventricular mechanical timing in heart failure patients with ventricular conduction delay. J Am Coll Cardiol. 2002;39:1163–9. doi: 10.1016/s0735-1097(02)01727-8. [DOI] [PubMed] [Google Scholar]
- 30.Yoshida K, Seo Y, Yamasaki H, Tanoue K, Murakoshi N, Ishizu T, et al. Effect of triangle ventricular pacing on haemodynamics and dyssynchrony in patients with advanced heart failure: a comparison study with conventional bi-ventricular pacing therapy. Eur Heart J. 2007;28:2610–9. doi: 10.1093/eurheartj/ehm441. [DOI] [PubMed] [Google Scholar]
- 31.Leclercq C, Gadler F, Kranig W, Ellery S, Gras D, Lazarus A, et al. for the TRIP-HF study. A randomized comparison of triple-site versus dual-site ventricular stimulation in patients with congestive heart failure. J Am Coll Cardiol. 2008;51:1455–62. doi: 10.1016/j.jacc.2007.11.074. [DOI] [PubMed] [Google Scholar]