Abstract
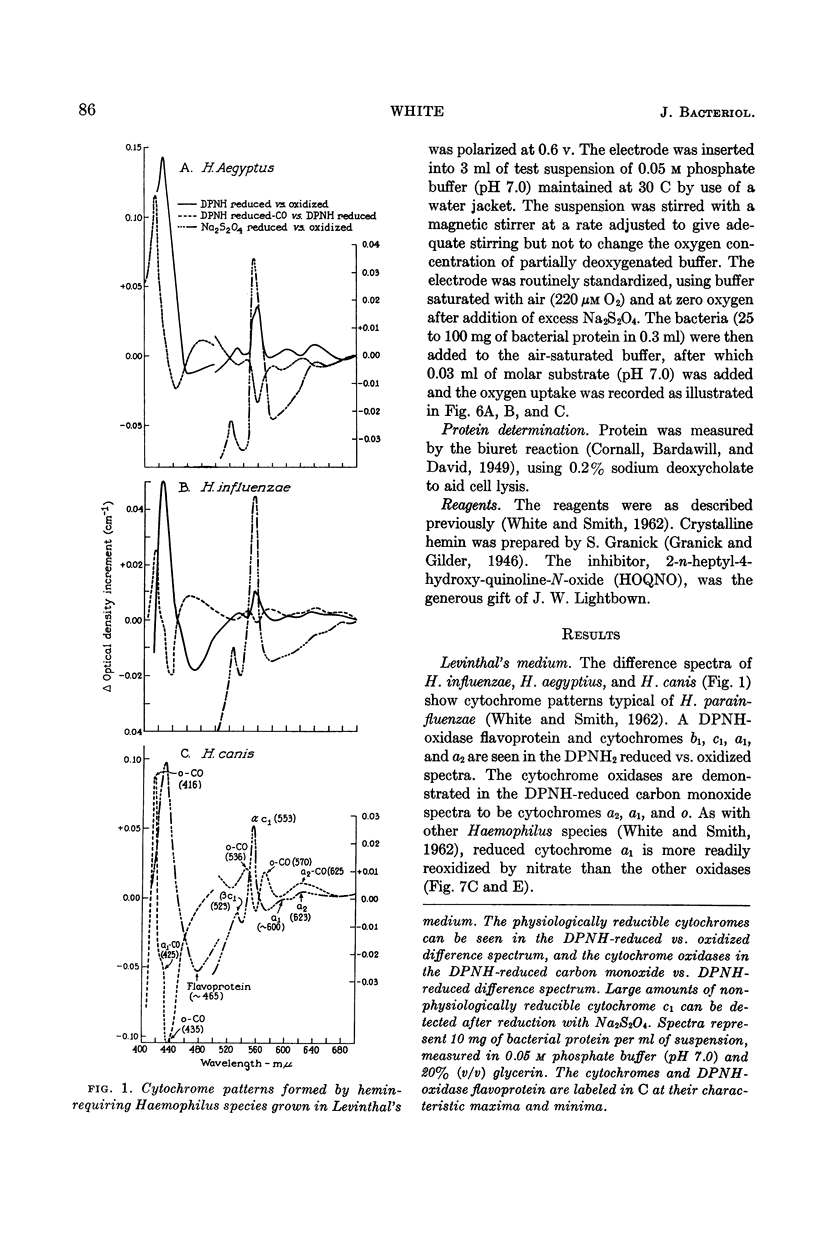
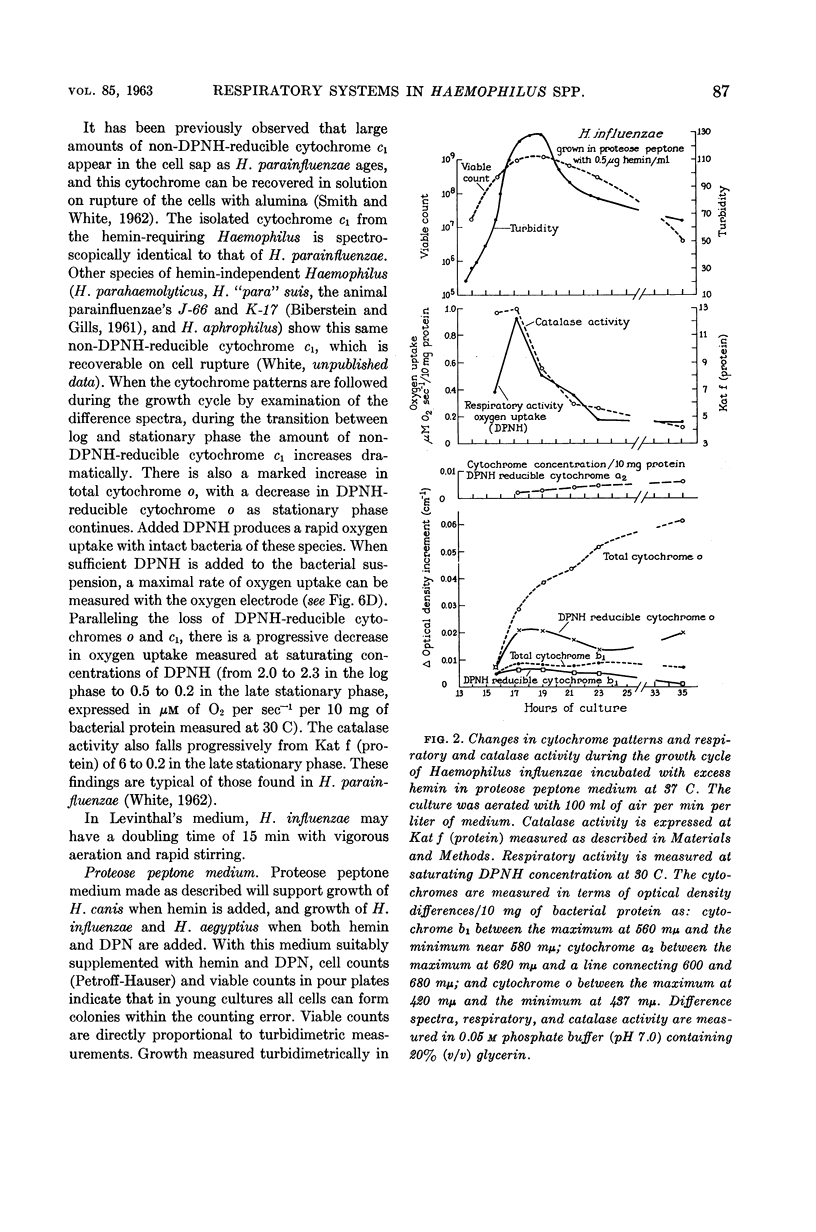
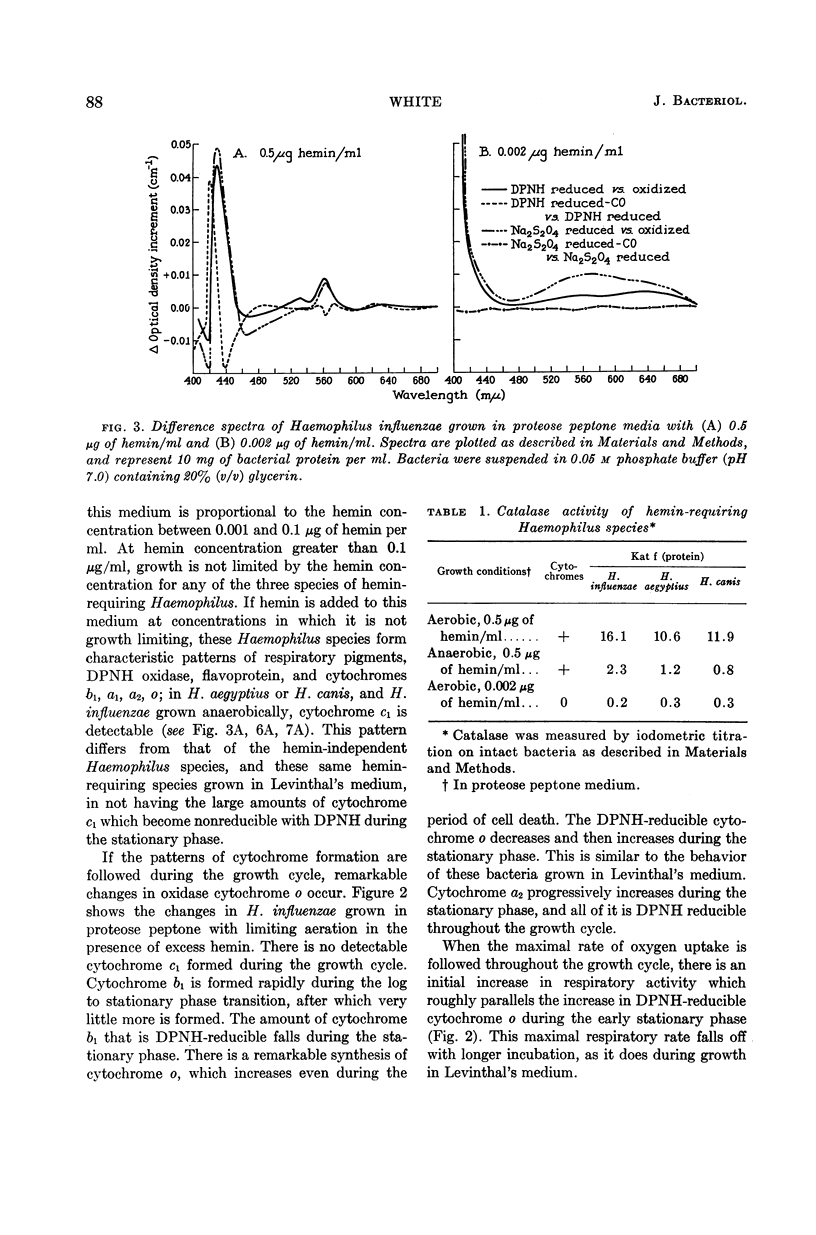
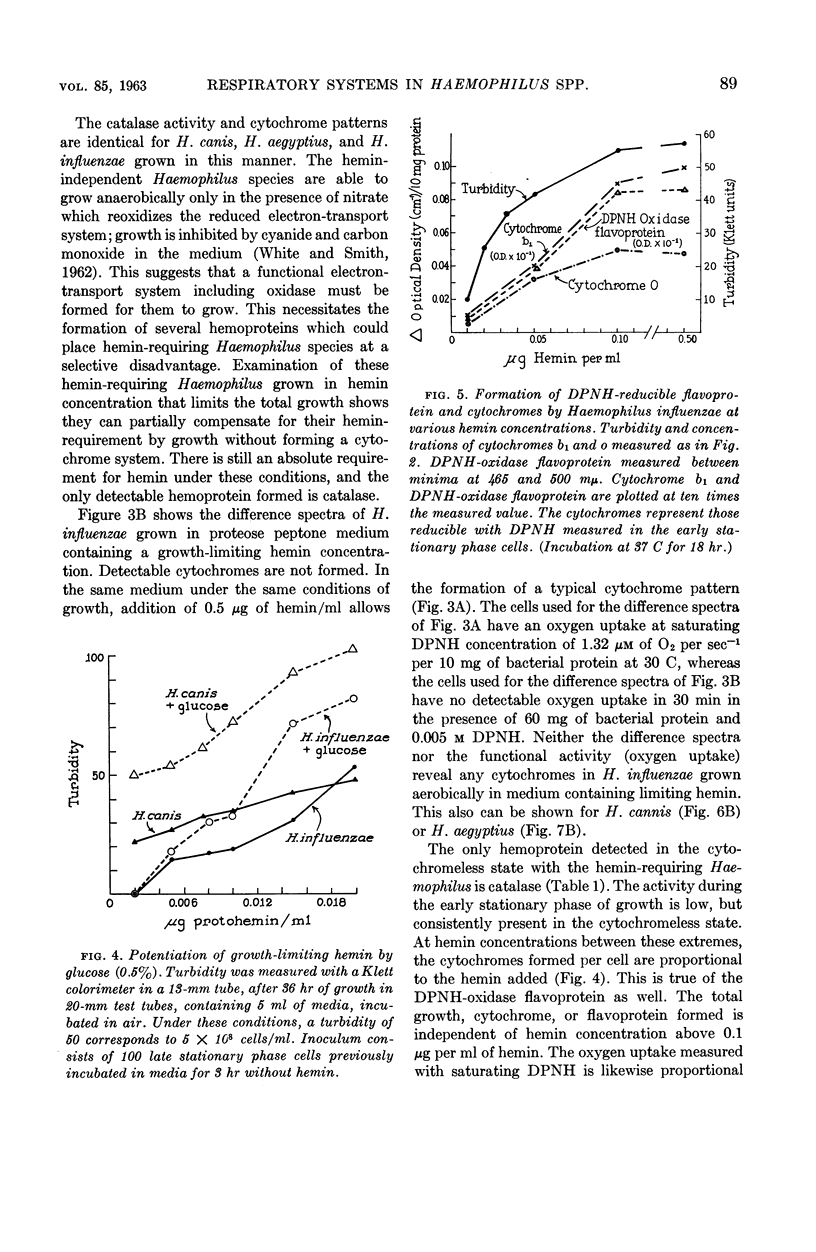
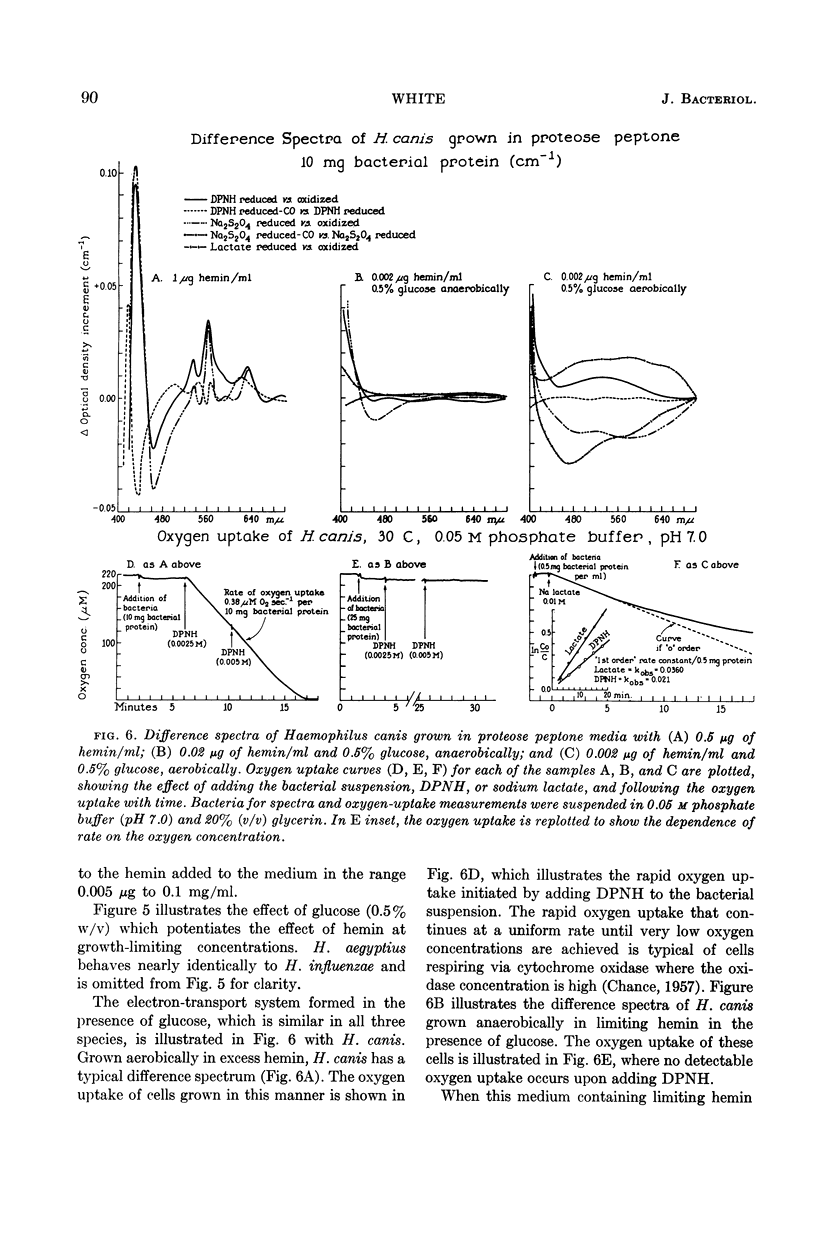
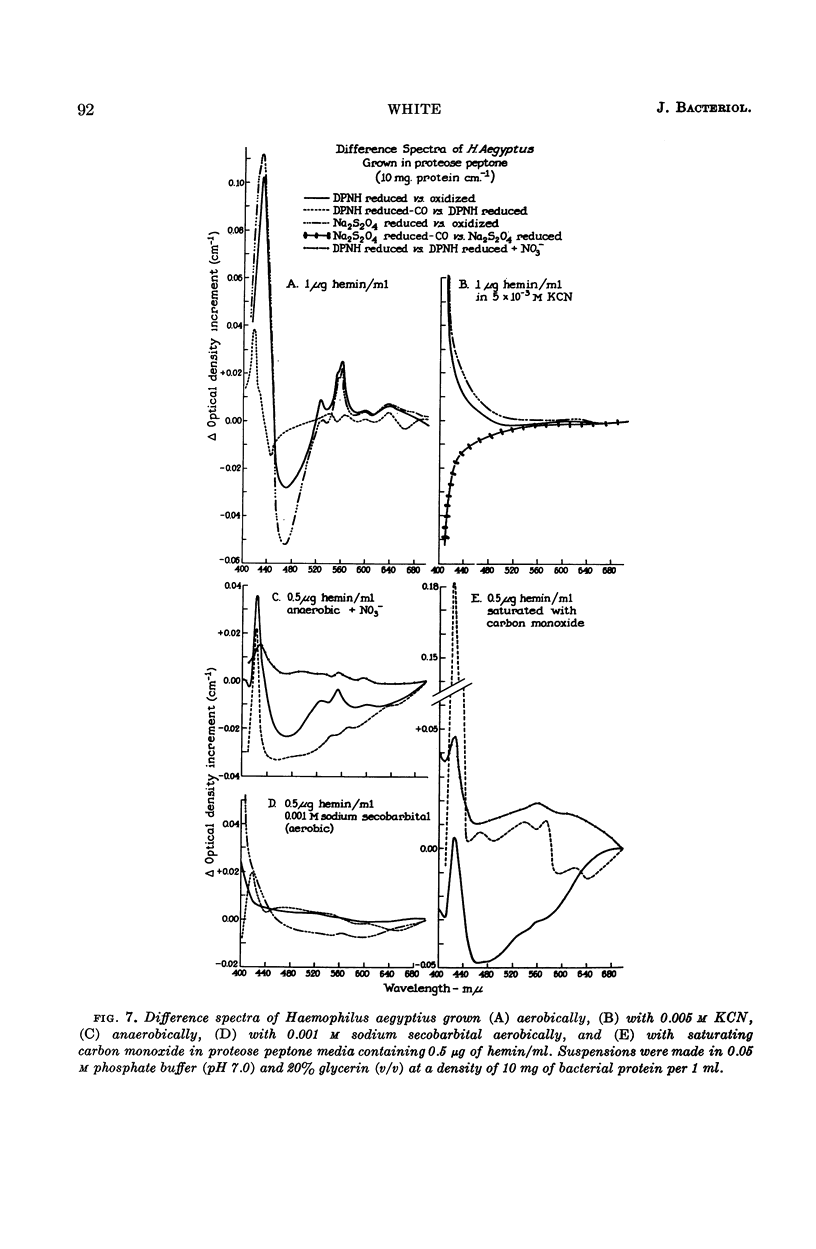
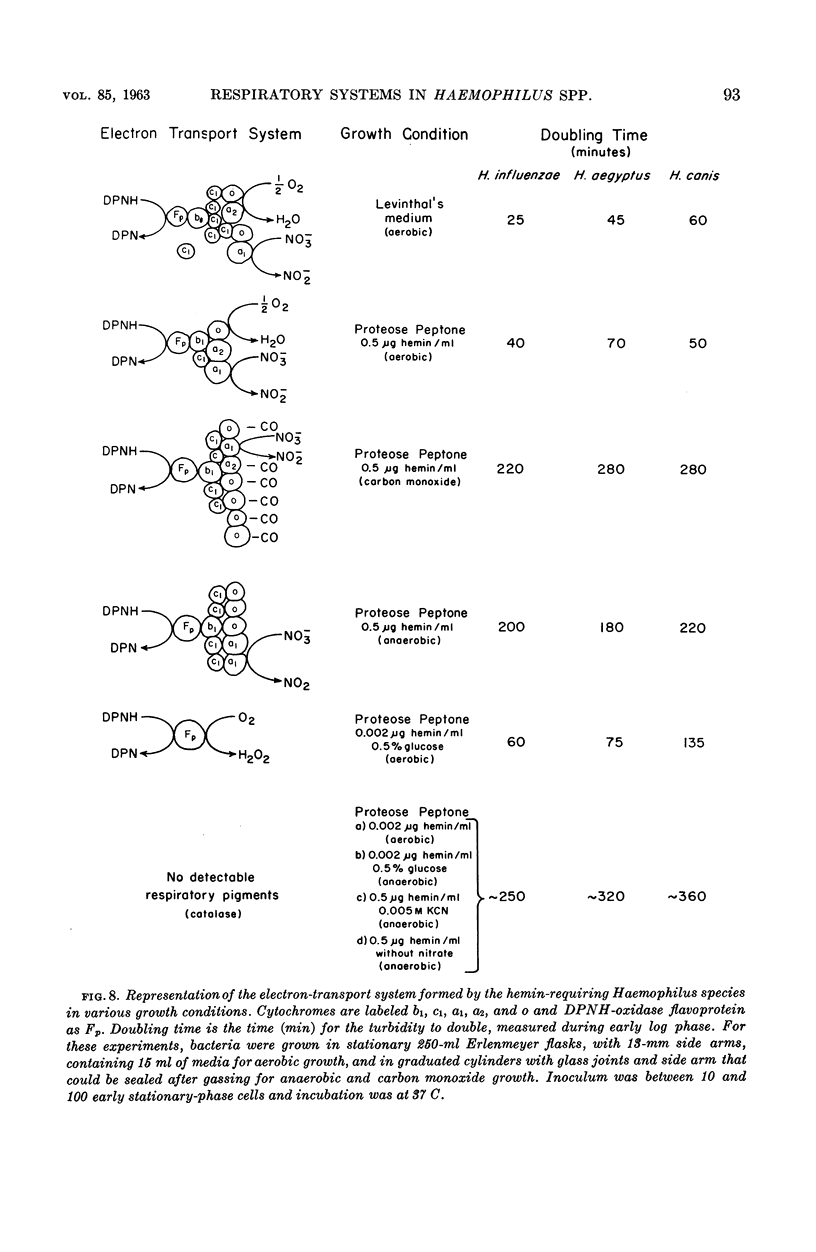
White, D. C. (Rockefeller Institute, New York, N.Y.). Respiratory systems in hemin-requiring Haemophilus species. J. Bacteriol. 85:84–96. 1963.—If grown in Levinthal's medium or in proteose peptone medium with excess hemin, Haemophilus influenzae, H. aegyptius, and H. canis (H. haemoglobinophilus) form an electron-transport system consisting of six cytochromes and two respiratory flavoproteins. In proteose peptone, these species can greatly modify the composition of their electron-transport complex. With anaerobic incubation in the presence of nitrate, they produce increased amounts of cytochrome c1 and the cytochrome oxidases a1 and o. This anaerobic pattern is greatly exaggerated by growth under carbon monoxide, in which case large concentrations of cytochrome oxidase are produced. In the presence of the inhibitor secobarbital or of growth-limiting amounts of hemin, intermediate amounts of cytochromes and respiratory flavoproteins are formed. When only small amounts of hemin are present, these species grow but form no detectable cytochrome system. Catalase is the only hemoprotein found. Under these conditions, the addition of glucose induces the formation of a lactate oxidase flavoprotein if the system is incubated aerobically. This cytochromeless state also occurs when these species are grown in KCN or anaerobically without nitrate and with excess hemin. The ability of these species to modify the composition of the electron-transport system strongly suggests that this function unit is formed from individual components. Hemin-requiring Haemophilus species have a hemin-sparing compensatory mechanism that allows growth under conditions under which hemin-independent Haemophilus species will not grow.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALEXANDER H. E., HAHN E., LEIDY G. On the specificity of the desoxyribonucleic acid which induces streptomycin resistance in Hemophilus. J Exp Med. 1956 Sep 1;104(3):305–320. doi: 10.1084/jem.104.3.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biberstein E. L., Gills M. CATALASE ACTIVITY OF HAEMOPHILUS SPECIES GROWN WITH GRADED AMOUNTS OF HEMIN. J Bacteriol. 1961 Mar;81(3):380–384. doi: 10.1128/jb.81.3.380-384.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANCE B. Cellular oxygen requirements. Fed Proc. 1957 Sep;16(3):671–680. [PubMed] [Google Scholar]
- ERNSTER L., JALLING O., LOW H., LINDBERG O. Alternative pathways of mitochondrial DPNH oxidation, studied with amytal. Exp Cell Res. 1955;(Suppl 3):124–132. [PubMed] [Google Scholar]
- GAUSE G. F., KOCHETKOVA G. V., VLADIMIROVA G. B. Biochemical changes accompanying impaired respiration in staphylococci. Nature. 1961 Jun 10;190:978–980. doi: 10.1038/190978a0. [DOI] [PubMed] [Google Scholar]
- JACKSON F. L., LIGHTBOWN J. W. Inhibition of dihydrocozymase-oxidase activity of heart-muscle preparations and of certain cell-free bacterial preparations by 2-heptyl-4-hydroxyquinoline N-oxide. Biochem J. 1958 May;69(1):63–67. doi: 10.1042/bj0690063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LASCELLES J. An assay of iron protoporphyrin based on the reduction of nitrate by a variant strain of Staphylococcus aureus; synthesis of iron protoporphyrin by suspensions of Rhodopseudomonas spheroides. J Gen Microbiol. 1956 Oct;15(2):404–416. doi: 10.1099/00221287-15-2-404. [DOI] [PubMed] [Google Scholar]
- LASCELLES J. Synthesis of tetrapyrroles by microorganisms. Physiol Rev. 1961 Apr;41:417–441. doi: 10.1152/physrev.1961.41.2.417. [DOI] [PubMed] [Google Scholar]
- LASER H. The effect of low oxygen tension on the activity of aerobic dehydrogenases. Proc R Soc Lond B Biol Sci. 1952 Oct 16;140(899):230–243. doi: 10.1098/rspb.1952.0060. [DOI] [PubMed] [Google Scholar]
- LIGHTBOWN J. W., JACKSON F. L. Inhibition of cytochrome systems of heart muscle and certain bacteria by the antagonists of dihydrostreptomycin: 2-alkyl-4-hydroxyquinoline N-oxides. Biochem J. 1956 May;63(1):130–137. doi: 10.1042/bj0630130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCARTHY B. J., HINSHELWOOD C. Variations in catalase activity during a bacterial growth cycle. Proc R Soc Lond B Biol Sci. 1959 Jan 27;150(938):13–23. doi: 10.1098/rspb.1959.0004. [DOI] [PubMed] [Google Scholar]
- RICHMOND M. H., MALLOE O. The rate of growth of Salmonella typhimurium with individual carbon sources related to glucose metabolism or to the Krebs cycle. J Gen Microbiol. 1962 Feb;27:285–297. doi: 10.1099/00221287-27-2-285. [DOI] [PubMed] [Google Scholar]
- SMITH L., WHITE D. C. Structure of the respiratory chain system as indicated by studies with Hemophilus parainfluenzae. J Biol Chem. 1962 Apr;237:1337–1341. [PubMed] [Google Scholar]
- WHITE D. C. Cytochrome and catalase patterns during growth of Haemophilus parainfluenzae. J Bacteriol. 1962 Apr;83:851–859. doi: 10.1128/jb.83.4.851-859.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE D. C., SMITH L. Hematin enzymes of Hemophilus parainfluenzae. J Biol Chem. 1962 Apr;237:1332–1336. [PubMed] [Google Scholar]



