Abstract
Climate change in the coming centuries will be characterized by interannual, decadal, and multidecadal fluctuations superimposed on anthropogenic trends. Predicting ecological and biogeographic responses to these changes constitutes an immense challenge for ecologists. Perspectives from climatic and ecological history indicate that responses will be laden with contingencies, resulting from episodic climatic events interacting with demographic and colonization events. This effect is compounded by the dependency of environmental sensitivity upon life-stage for many species. Climate variables often used in empirical niche models may become decoupled from the proximal variables that directly influence individuals and populations. Greater predictive capacity, and more-fundamental ecological and biogeographic understanding, will come from integration of correlational niche modeling with mechanistic niche modeling, dynamic ecological modeling, targeted experiments, and systematic observations of past and present patterns and dynamics.
Keywords: biogeography, climate change, paleoecology, regeneration niche
Ecology is fundamentally concerned with understanding the abundance and distribution of organisms, which bears on virtually every application of ecological knowledge from conservation and management of species populations to restoration of ecosystem functions and services. The ecological niche concept lies at the heart of these pursuits. The niche has a rich and complex history, with usage falling into 2 clusters. The Eltonian niche emphasizes resource-consumption relationships among species, whereas the Grinnellian niche focuses on environmental factors, biotic and abiotic, that influence survival, growth, and reproduction of individuals (1–3).
The Grinnellian niche underpins efforts to predict ecological and biogeographic responses to global environmental change. Correlative approaches have proliferated in the past decade (2), and are being widely applied to gauge implications of climate change. In these applications, observed patterns of species distribution and abundance are modeled empirically in multidimensional environmental space (typically comprising climatic and other physical variables), and the models are overlain onto simulated future environments (e.g., from general circulation model output) to predict future patterns (4–8). Healthy debate is underway concerning the assumptions underlying these applications (9–13).
Paleoecology, which exploits the great store of environmental and ecological history preserved in natural archives (14), has also benefited from the Grinnellian niche concept in explaining past ecological and biogeographic patterns (15–18) and inferring past climates from fossil data (19). By extending ecological observations across a broad range of earth-system states, paleoecological records can reveal fundamental phenomena and processes that would likely go unrecognized in the observational and instrumental record [e.g., novel and disappearing climates (20)], and reveal hidden or overlooked assumptions in global-change applications of niche models and other tools.
We discuss perspectives from recent paleoecological studies on the issue of forecasting ecological responses to climate change. First, we review recent findings concerning the multiscale nature of climate, emphasizing modes of variability for which detailed paleoclimatic evidence is available and which are likely to mediate ecological responses to global change in the coming centuries. We then explore implications of multiscale climate variability in the context of organismal, population, and community processes, using niche theory as a framework. Our examples emphasize invasion and population expansion of terrestrial plants, but our findings also apply to decline and extirpation, and to other organisms and ecosystems.
Nested, Multiscale Climate Variability and Change
Climate change and variability occurs at all ecologically relevant time scales, with ecologists focused on interannual to decadal time scales and paleoecologists on millennial to multimillennial scales. This has led to contrasting views of ecological dynamics. Ecologists often treat environmental variation as random fluctuations about a constant or gradually changing mean state—a convenient assumption for ecological modeling (21, 22). Paleoecologists acknowledge rapid responses to abrupt climate shifts (e.g., the late-glacial Younger Dryas Event) (23, 24), but have focused on gradual, biogeographic adjustments to orbitally driven climate changes (15, 25–28).
A new picture is emerging from high-resolution paleoclimatic and paleoecological studies that link the real-time dynamics observed in ecological studies with the long-term changes observed in the fossil record. Recent paleoclimate studies indicate that low-frequency climate trends have been punctuated by significant fluctuations (29, 30) and rapid transitions between climate states (31–33). For example, toward the end of the orbitally controlled early-Holocene warm period, between ca. 5500 and 4000 years BP, a series of abrupt climate changes spanning decades to centuries occurred throughout much of the world. These included abrupt cooling and pluvial events in Europe (34, 35), monsoon shifts in the Indian Ocean (36), and widespread century-scale droughts in North America and elsewhere (29, 33).
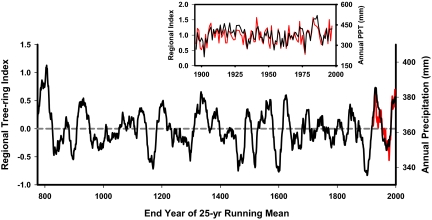
High-resolution studies of the past millennium indicate decadal and centennial climate patterns that appear nonstationary (means and moments of the distribution vary over time) from the perspective of the last century. Tree-ring studies from across the globe show drift in the magnitude and duration of wet/dry and cool/warm events. Predominantly wet or dry regimes can persist for 10 to 25 years or more, and average precipitation can vary by 10 to 30% from one decade to the next. For example, integrated moisture records from the Upper Colorado River basin (Fig. 1) reveal periods of dampened decadal variability, some with low means (900–1100 CE and 1700s CE) and others with high means (1300s CE), periods of strong multidecadal variability (800–950, 1100s, 1400–1650, 1800–2000 CE), and century-scale trends (ramp-down to the late 1200's megadrought) (37). Tree-ring records also indicate that extreme, decadal-scale wet and dry events are characteristic of the entire western United States (38), and these events are likewise embedded in lower-frequency climate variability across wide regions. Moreover, extreme climate anomalies are not randomly distributed in time. Instead, unusually dry years cluster to form long-term droughts, with wet years aggregating to form pluvial periods (39).
Fig. 1.
Reconstruction of Upper Colorado River Basin hydroclimate variability since 775 CE based on tree-ring records. Tree growth is represented as a regional index (i.e., z scores) based on ring-widths from 11 of the oldest chronologies in the basin (42). The composite tree-ring record is strongly correlated (r = 0.75) with annual precipitation in the region (see Inset). A 25-year running mean is plotted for both the tree-ring index (black) and observed annual precipitation (red). Note decadal to centennial shifts in the mean and variability, and extended wet/dry regimes; no two centuries show similar patterns.
The climate in any region integrates ocean-atmosphere interactions across the globe operating across a range of time scales. For example, the subdecadal El Niño-Southern Oscillation (ENSO) in the tropical Pacific interacts with the Pacific Decadal Oscillation poleward of 20° and the Atlantic Multidecadal Oscillation (AMO) to influence continental climate over much of North America (39, 40). Climate impacts of interannual ENSO variability may be contingent on the state of the decadal-to-multidecal AMO and PDO variability. For reasons not well understood, different combinations of ENSO, PDO, and AMO states are associated with a variety of spatial and temporal expressions of moisture and temperature across much of North America.
Paleoclimate records and modeling studies indicate that these modes of ocean variability have changed over centennial to multimillennial time scales. ENSO variability was weakened under early Holocene orbital forcing, with high variability emerging only in the last few thousand years (41). Future climate change will be the product of natural variability acting over multiple spatial and temporal scales superimposed on anthropogenic trends. The transition from climate observed in the historic record to the climate of the future will inevitably include shifts between dominant climatic modes, speeding up or slowing down anthropogenic changes and advancing or delaying the crossing of ecological thresholds (42).
Niche Dimensions and Proximal Controls: What Matters To Organisms?
Hutchinson (43, 44) envisioned the Grinnellian niche as an envelope or response surface in an n-dimensional environmental space. The value of n may be very large. Temperature, for example, comprises a large suite of variables. Its simplest expression, as an annual mean, explains many biotic patterns at regional to global scales (45, 46). Seasonal and monthly mean temperatures provide additional explanatory power in ecology and biogeography. These and other integrated measures (e.g., growing-degree days) are widely used in correlative models of species distribution (4–8), and perform well in predicting modern distribution and abundance patterns.
Proximal influence of temperature on survival and reproduction of plants and animals involves a much broader suite of variables. Most organisms have maximum and minimum temperature thresholds for survival and reproduction. Different plant species, for example, have different low-temperature thresholds, and sensitivity varies among individuals and populations depending on cold-hardening and acclimation (47). For some species, these are absolute thresholds, whereas for others, individuals can resist low temperatures for a few hours or days before they succumb. Thresholds may also change for individuals according to season and life-history stage. Thus, a proximal temperature predictor of a species range limit might consist of the probability that temperature falls below a threshold within x days after bud break, or probability of winter temperatures falling below a freezing threshold for y hours, or frequency of years (relative to mean generation time) in which summer temperatures persist below a threshold for z days. Some may be more subtle: Seedling survival of subalpine conifers may be limited by the frequency of growing-season days in which early-morning temperatures are below a low-temperature photoinhibition threshold (48).
The number of proximal variables (and hence niche dimensions) that influence species distribution and abundance may exceed practical capabilities for empirical modeling or parameterization. High dimensionality is generally dealt with in the environmental and social sciences by assuming strong covariance structure. The success of species-distribution modeling derives from strong covariance between the numerous “hidden” proximal variables and a smaller suite of easily measured or modeled variables − such as seasonal mean temperatures. Virtually all proximal temperature-related variables are strongly correlated with seasonal means and other integrated measures, because all covary along latitudinal, longitudinal, and elevational gradients at regional to global scales. This spatial covariance is conserved through time. For example, in the Northern Hemisphere, probability of subfreezing temperature after the spring equinox and the frequency of years in which winter minimum temperature drops below −10 °C will increase with latitude and elevation, whereas mean January temperature will decrease. These spatial relationships have obtained throughout the Phanerozoic, and will persist into the remote future.
Although the strength and direction (positive or negative) of spatial covariance among temperature-related variables is strongly conserved, the covariance structure (slope, intercept, dispersion) may change as atmospheric circulation patterns respond to various forcings and feedbacks (17, 18). This may reduce the predictive power and accuracy of empirical niche models when applied to past and future climate states.
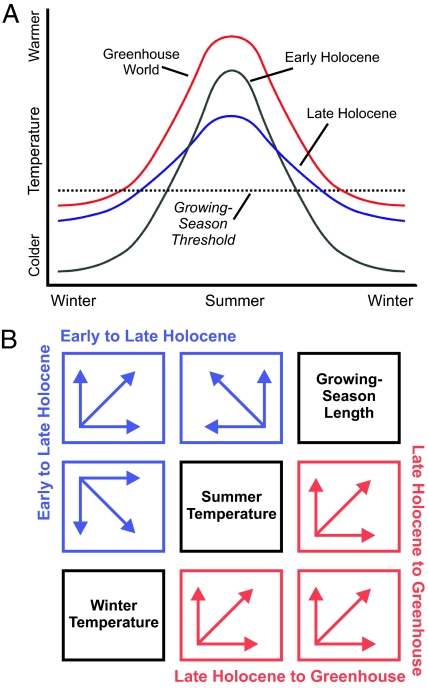
As a simple example, Milankovitch forcing in the early Holocene sharpened the seasonal temperature contrast over much of the Northern Hemisphere relative to the late Holocene (Fig. 2). Mid to late Holocene reduction of the seasonal insolation contrast led to decreased mean summer and increased mean winter temperatures. However, in many regions this transition was probably accompanied by an increase in length of the growing season (Fig. 2), fostering northward expansion of phenologically sensitive species during a period of cooling summers (49). Growing-degree days or other heat sums might also have decreased, depending on the extent of midsummer daytime temperature decrease.
Fig. 2.
Conceptual diagrams contrasting presumed midlatitude Northern Hemisphere seasonal temperatures at different times. (A) Orbital (Milankovitch) forcing in the Early Holocene resulted in warmer summers and cooler winters than today (represented by the Late Holocene). Summer temperatures decreased, but winter temperatures increased, from the Early to Late Holocene, owing to orbital forcing. As a consequence, growing-season length may have increased whereas summer temperatures decreased. Thus, although summer temperature and growing-season length are positively correlated in space, they may be negatively correlated in time. The Greenhouse World curve shows changes anticipated under greenhouse warming scenarios, in which summer and winter temperatures increase, and growing-season length is also increased. (B) Multiway plot of presumed vectors of change for summer temperature, winter temperature, and growing-season length between the early Holocene and late Holocene (blue), and between the late Holocene and the projected Greenhouse World of 2100 CE (red).
For a species that requires some minimum of consecutive frost-free days to complete its growth and reproductive cycles, mean July temperature would serve as a strong predictor of distribution and abundance today. However, mean July temperature might serve as a poor predictor of range or population shifts even in the recent past, owing to the temporal decoupling of growing-season length and mean July temperature (Fig. 2B). Looking forward into the next century, all 3 variables are predicted to change in the same direction (Fig. 2) (8), but current slopes and dispersion patterns may not be conserved, because the magnitude of change will differ among these variables, and will also vary spatially.
Moisture, another important variable, is often expressed as mean annual or seasonal precipitation or as actual or potential deficit. Like temperature, moisture comprises numerous ecologically relevant proximal variables, with covariance conserved in space but potentially evolving in time. Temperature and moisture interact, both in their spatial and temporal patterns and in their biological effects. Their biological influence is also contingent on other variables (e.g., photosynthetically active radiation, daytime and nighttime cloud cover, ambient CO2 concentrations) and vice versa. The temporal evolution of the realized environment—the shape and position of the n-dimensional cloud—yields new combinations of environmental variables and altered environmental complex-gradients (in the sense of ref. 50) (16–18).
Although the covariance structure of the multidimensional environmental space can be characterized at any given time, our capacity to use some dimensions as predictors of other dimensions may be limited under environmental change. The potential magnitude of this problem remains largely unexplored. Needed is a more comprehensive compilation of proximal climatic factors known to influence natural populations and distributions, and sensitivity analysis of their covariance under different climate regimes.
Climate Variability, the Regeneration Niche, and Range Dynamics
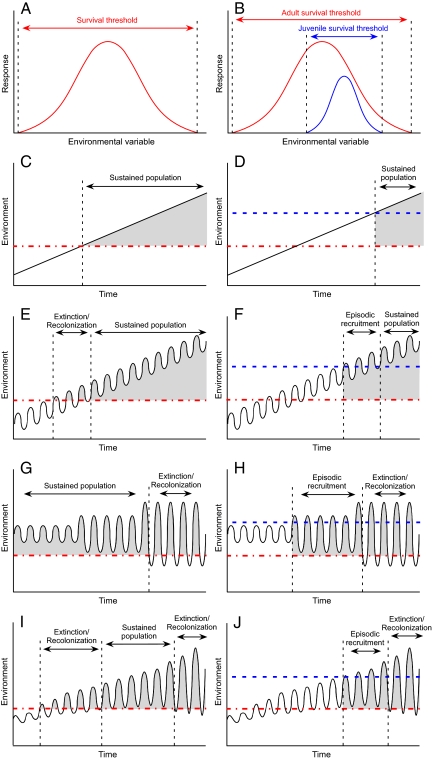
The fundamental niche for a species along one dimension is often conceptualized as a single response curve, with an optimum and thresholds beyond which individuals cannot survive, regardless of age or life-history stage (Fig. 3A). However, ecologists have long recognized that environmental responses may differ among life-history stages (51, 52), with juveniles often having narrower niches than adults (Fig. 3B). Seedlings with limited root systems, low carbon reserves, and reduced photosynthetic capacity will undergo mortality under conditions that present no difficulty for conspecific adults. Woody plants provide numerous examples in which seedlings are far less tolerant than adults of growing-season drought, flooding, and temperature extremes (53–55). Similarly, many species can reproduce sexually within a narrower range of conditions than those in which they can grow (55–57). The regeneration niche (52) describes the fundamental niche for successful reproduction, and hence is often more relevant to assessment of range limits than the adult-growth niche. The adult niche may itself undergo different phases of sensitivity. For example, young trees may be disproportionately affected by warming (58), whereas older trees may be more susceptible to severe droughts (59).
Fig. 3.
Consequences of alternative niche models for species colonization under different climate-change scenarios. (A) Conventional unitary model of ecological response (fitness, growth rate, abundance) to a continuous environmental variable (e.g., temperature, moisture). (B) Alternative model wherein adult and juvenile individuals have differing responses to the same variable, with the juvenile niche embedded within the adult niche. (C–J) plot environmental change at a site through time (black line) and its consequences under the alternative niche models. The red dash-dot line represents the survival threshold for all individuals of the species. The blue dashed/dotted line represents the survival threshold for juveniles of the species. Shaded zones represent periods when the species is capable of colonizing the site. (C and D) Expectations under monotonic increase in the environmental variable. (E and F) The environmental variable increases steadily with constant variance. (G and H) The mean remains constant, but the variance increases (shown in 3 stages). (I and J) An increasing trend in the mean along with increasing variance.
The unitary and regeneration niche models have different consequences for geographic and population responses to climate change. Under a scenario of gradual, monotonic change, the unitary niche model predicts potential colonization once the environment passes the universal survival threshold (Fig. 3C), with population increase if the environment continues to change in a favorable direction. Under the regeneration-niche model, colonization is delayed until the regeneration threshold is attained (Fig. 3D). Adding high-frequency environmental variability to a low-frequency trend under the traditional model introduces an intermediate period in which populations can colonize during favorable periods but are extirpated during unfavorable periods (Fig. 3E). Assuming source populations within effective dispersal distance, an extinction/recolonization dynamic (60) will occur until the environmental trend carries the variability beyond the survival threshold, at which point the population becomes sustainable (Fig. 3E). Under the regeneration model, colonization is delayed until fluctuations carry the environment beyond the regeneration threshold, and the population experiences a period of episodic or event-limited recruitment and expansion (Fig. 3F). If fluctuations carry the environment below the survival threshold, or if frequency of recruitment events is less than the mean generation time, extinction/recolonization dynamics will ensue until the environmental trend carries the site safely above the survival or recruitment threshold.
Even in the absence of a trend, a change in magnitude of environmental variability can influence geographic range and population size. An increase in amplitude of environmental fluctuation under the unitary model can increase the magnitude of fluctuations in population size. Sufficiently wide amplitude of environmental fluctuation can lead to periodic extirpation and recolonization (Fig. 3G), as can less dramatic fluctuations around a mean closer to the survival threshold (see Fig. 3E). Under a regeneration-niche scenario, an increase in amplitude can lead to a series of recruitment pulses which, if at higher frequency than mean generation time, can sustain and grow a population (Fig. 3H). As in the case of the unitary model, fluctuations of sufficient amplitude can lead to an extinction/recolonization phase (Fig. 3H).
The final scenario combines a monotonic trend with increasing variance, in accord with predictions of increasing climate variability in a warming world (61). Under a unitary niche model, a population can first experience extinction/recolonization dynamics, followed by sustained growth, and followed again by extinction/recolonization (Fig. 3I). The regeneration niche model can lead first to episodic recruitment and then to extinction/recolonization (Fig. 3J), similar to the pattern for increasing variance.
The precise outcome of all of these scenarios depends on the steepness of the trend, the amplitude and frequency of the variability, and the position of the respective survival thresholds. Other sequences are conceivable, but all outcomes fall into 4 general categories: no population, sustained population, episodic recruitment, and extinction/recolonization. These are potential outcomes, contingent on availability of propagule sources for initial colonization and recolonization.
Historical Contingencies and Ecological Ratchets
Historical contingencies, whereby particular events leave persistent imprints, are widely acknowledged as critical factors in evolutionary biology (62–64). A parallel concept in ecology, ecological legacy, refers to ecosystem properties that are attributable to past events (e.g., disturbances) or past system states (14, 65). Both concepts are relevant to global change ecology and biogeography. Historical contingencies in the dynamics of species distributions are inevitable given multiscale and multidimensional environmental variation, episodic climatic change, and stage-specific Grinnellian niches. Conversely, ecological and biogeographic realizations may represent legacies of previous events and realizations.
Historical contingencies can arise simply from the episodic nature of recruitment when environmental variability hovers around a juvenile survival threshold (Fig. 3 F, H, and J). Each favorable period provides opportunity for colonization of unoccupied sites, expansion of populations in occupied sites, and (ultimately) increase in propagule flux density at occupied sites for additional colonization and expansion. Environmental variability underlies a ratchet mechanism for invasion of species with persistent, long-lived individuals (e.g., woody plants). Forward motion (colonization and population expansion) proceeds during favorable periods, and persistence ensures that reverse motion (decline and extirpation) is minimized during unfavorable periods. In such systems, the number of occupied sites and local population density at any given time will be a function not of the contemporary environment, but of the cumulative number or duration of recruitment episodes elapsed since initial colonization.
Historical contingencies deriving from climate variability can be amplified by other factors. Multiscale climate variability ensures that sites will experience different magnitudes, durations, and frequencies of favorable and unfavorable periods, each potentially imparting a distinct historical signature on ecosystems. Allee effects (66) can interact with climate variability to maintain populations or accelerate invasion. Favorable recruitment events of sufficient magnitude, duration, and/or frequency can build a colonizing population to levels where density-dependent factors (outcrossing, mate encounter, microenvironment, propagule flux density) no longer limit population expansion and dispersal to new sites. In patchy landscapes, favorable climate episodes can increase size and density of colonization targets, allowing establishment of new populations (and propagule sources) via long-distance dispersal (67). Habitats or microsites suitable for species populations during adverse periods can prevent extirpation and maintain sources for subsequent colonization and expansion. Finally, climate events synchronize widespread ecological disturbances, including wildfires, windstorms, pest outbreaks, and drought dieback (59, 68–72), providing opportunities for rapid colonization by species with propagule sources in the vicinity. All of these factors can interact with climate variability to maintain net forward motion of the invasion ratchet.
These processes are well-documented in ecological and dendroecological studies of modern natural populations. In the American Southwest, decadal to multidecadal climate variation sets the tempo for episodic disturbance and recruitment in forests and woodlands, governing fuel and moisture conditions for wildfires, drought and temperature conditions for insect-induced mortality, and moisture and fire conditions for seedling recruitment (68–70, 73). Across a broader portion of the western United States, decadal to multidecadal variations have produced legacies in the form of synchronous fire disturbances (74–76) and pest outbreaks (77). In humid eastern North America, historical droughts have resulted in widespread tree mortality (78–81), and seedling recruitment of many trees is mediated by summer moisture (81–86). Ample potential exists for ecological legacies imposed by past climate events in most of the world.
Paleoecological studies document historical contingencies in Holocene range expansions. Piñon pine (Pinus edulis) colonized a mesic site in northeastern Utah by long-distance dispersal in the mid-13th Century CE, but did not expand further until a large recruitment pulse in the mid- and late 14th Century (42). These events were paced by climate variability: Initial establishment coincided with a brief wet period embedded within a series of multidecadal droughts. The last of these droughts, from ca. 1260 to 1290 CE, was followed by an extended wet period, facilitating rapid expansion (42). Thus, the modern population is contingent on the rapid 14th Century population expansion, which was itself contingent on 13th Century establishment and survival. Had the pioneer individuals not colonized, or had they succumbed during the late 13th century megadrought, the modern northern limit of the species might be much further south. Had colonization occurred centuries earlier, the species might occupy suitable habitat to the north, 50–100 km beyond its current range limit.
Holocene invasion of Utah juniper (Juniperus osteosperma) in north-central Wyoming shows similar contingencies (87). Utah juniper was established on a few sites ca. 5400 years BP, persisted without further expansion for 2600 years, and then colonized multiple sites across a broad area during a favorable period lasting 1800 years. It has persisted on those sites without further expansion for the past 1,000 years. Had the species not established a handful of isolated, persistent populations 5400 years ago, it might now occupy only a fraction of the sites where it occurs today. Conversely, had colonization not been interrupted by climate change ca. 5400 years BP, the species might occur across a much larger territory today, filling a number of gaps where suitable habitat occurs but the species is absent (87).
Colonization contingencies are also documented in humid regions. Holocene expansion of mesic trees in the western Great Lakes region was episodic, with climate-driven onset and cessation of expansion pulses (88–91). Dendroecological and pollen studies of tamarack (Larix laricina) near its northern range limits in northwest Québec show climate-driven pulses of range expansion (92).
Challenges of Prediction in an Evolving World
The earth system is destined for some degree of anthropogenic climate change in the coming decades (93). Conservation of biodiversity, maintenance of ecological services, and management of natural resources will need to adapt, requiring some level of predictive capacity. Such capacity has 2 components: ability to predict the course of climate change in coming decades, and ability to predict ecological and biogeographic responses to climate change. Land-surface/atmosphere feedbacks further require that the latter predictions inform the former. Perhaps the most confident prediction that can be made about climate change between now and 2100 CE is that it will be neither smooth nor gradual. Anthropogenically driven trends will interact with and influence natural variability, and the next century will include periods of slow change punctuated by rapid transitions. Ecological realizations of any future time—species distributions, community composition, ecosystem function—will be determined by the particular course of climate regimes, events, and transitions between now and then.
Correlational models place ecologists and biogeographers in a weak position to predict future ecological realizations (10–13). We have emphasized 4 interlinked sources of uncertainty in application of these models: multiscale climate variability, temporal decoupling of environmental covariance patterns, influence of the regeneration niche, and effects of historical contingency on ecological and biogeographic realizations. We do not question the usefulness of correlative models in gauging the potential biotic consequences of particular climate-change scenarios. However, we view their role as being primarily cautionary and illustrative, rather than predictive and prescriptive. Results of such models may be most useful as biogeographically or ecologically scaled metrics of the extent and nature of predicted climate changes. As such, they can help identify broad targets for conservation and resource planning.
In the face of changing climate and its accompanying uncertainties, ecologists, conservation biologists, and resource managers will have to identify appropriate points and scales of intervention to maintain specific ecological goods and services. Such interventions include postdisturbance manipulation, particularly in early successional stages, to ensure desirable outcomes, and transplantation of appropriate genotypes and species to foster maintenance of biodiversity and provide future goods and services. However, successful implementation of these interventions requires forecasting capacity. Interventions will be ineffective if initiated during unfavorable climate intervals, and planning will be most effective if large-scale disturbances can be anticipated in advance.
Some degree of climate forecasting months to years in advance may be within reach. Probability of future multidecadal climate-regime shifts has recently been calculated based on probability distribution functions from a 500-year AMO reconstruction (94). Decadal climate predictions may soon become routine using climate models initialized with current sea-surface temperature observations in the North Atlantic and tropical Pacific basins (95).
This climate-forecasting capacity can be invaluable in ecological management, but only if ecological forecasting ability is at a sufficiently advanced stage. Empirical modeling of the Grinnellian niche represents a valiant attempt at attaining forecasting capacity, but is by itself insufficient. Observational, experimental, and alternative modeling efforts should be accelerated and integrated (e.g., 96). Observations are critical for identifying fundamental patterns, mechanisms, and phenomena. Needed are real-time studies of the fundamental biology, natural history, and distribution of individual species and populations, dense long-term monitoring networks to identify trends and patterns in disturbance, demography, and phenology, and retrospective studies to assess the full array of ecological responses and sensitivities to past climate changes of various rates and magnitudes. Laboratory experiments, field manipulations, and unreplicated large-scale experiments provide rigorous testing of mechanisms and detailed insights into processes (97, 98). Ecological modeling, including correlative, mechanistic (12, 99–101), and dynamic approaches (102), offers opportunities for exploring consequences of scenarios and mechanisms. Implementation and integration of these diverse approaches constitutes a grand scientific challenge for ecologists.
However quickly a comprehensive research program can be implemented, ongoing and future rates of environmental change are likely to outstrip scientific capacity to predict ecological responses and societal capacity to adapt appropriately. Immediate actions to slow down the rates of global change will buy precious time to allow scientific understanding and mitigation strategies to catch up.
Acknowledgments.
We thank J.H.B., W.A. Reiners, and 2 anonymous reviewers for providing valuable comments. This work was supported by the National Science Foundation. S.T.J. thanks the organizers of the Sackler Colloquium for the opportunity to participate.
Footnotes
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “Biogeography, Changing Climates and Niche Evolution,” held December 12–13, 2008, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. The complete program and audio files of most presentations are available on the NAS web site at www.nasonline.org/Sackler_Biogeography.
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Chase JM, Leibold MA. Ecological Niches. Chicago: Univ of Chicago Press; 2003. [Google Scholar]
- 2.Soberón J. Grinnellian and Eltonian niches and geographic distributions of species. Ecol Lett. 2007;10:1115–1123. doi: 10.1111/j.1461-0248.2007.01107.x. [DOI] [PubMed] [Google Scholar]
- 3.Soberón J, Nakamura M. Niches and distributional areas: Concepts, methods, and assumptions. Proc Nat Acad Sci. 2009;106:19644–19650. doi: 10.1073/pnas.0901637106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thuiller W, Lavorel S, Araújo MB. Niche properties and geographical extent as predictors of species sensitivity to climate change. Global Ecol Biogeog. 2005;14:347–357. [Google Scholar]
- 5.Hamann A, Wang T. Potential effects of climate change on ecosystem and tree species distribution in British Columbia. Ecology. 2006;87:2773–2786. doi: 10.1890/0012-9658(2006)87[2773:peocco]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 6.Rehfeldt GE, Crookston NL, Warwell MV, Evans JS. Empirical analyses of plant-climate relationships for the western United States. Int J Plant Sci. 2006;167:123–1150. [Google Scholar]
- 7.Iverson LR, Prasad AM, Matthews SN, Peters M. Estimating potential habitat for 134 eastern US tree species under six climate scenarios. For Ecol Man. 2008;254:390–406. [Google Scholar]
- 8.Lawler JL, et al. Projected climate-induced faunal changes in the western hemisphere. Ecology. 2009;90:588–597. doi: 10.1890/08-0823.1. [DOI] [PubMed] [Google Scholar]
- 9.Graham CH, Ferrier S, Huettman F, Moritz C, Peterson AT. New developments in museum-based informatics and applications in biodiversity analysis. Trends Ecol Evol. 2004;19:497–503. doi: 10.1016/j.tree.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Dormann CF. Promising the future? Global change projections of species distributions. Basic Appl Ecol. 2007;8:387–397. [Google Scholar]
- 11.Thuiller W, et al. Predicting global change impacts on plant species' distributions: Future challenges. Persp Plant Ecol Evol Syst. 2008;9:137–152. [Google Scholar]
- 12.Morin X, Lechowicz MJ. Contemporary perspectives on the niche that can improve models of species range shifts under climate change. Biol Lett. 2008;4:573–576. doi: 10.1098/rsbl.2008.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeschke JM, Strayer DL. Usefulness of bioclimatic models for studying climate change and invasive species. Ann New York Acad Sci. 2008;1134:1–24. doi: 10.1196/annals.1439.002. [DOI] [PubMed] [Google Scholar]
- 14.National Research Council. The Geologic Record of Ecological Dynamics. Washington, DC: National Academies Press; 2005. [Google Scholar]
- 15.Webb T., III Is vegetation in equilibrium with climate? How to interpret late-Quaternary pollen data. Vegetatio. 1986;67:75–91. [Google Scholar]
- 16.Jackson ST. In: Evolution of Phanerozoic Terrestrial Ecosystems. Gastaldo RA, DiMichele WA, editors. New Haven: Paleontological Society; 2000. pp. 287–308. [Google Scholar]
- 17.Jackson ST, Overpeck JT. Responses of plant populations and communities to environmental changes of the Late Quaternary. Paleobiology. 2000;26(Suppl):194–220. [Google Scholar]
- 18.Williams JW, Jackson ST. Novel climates, no-analog communities, and ecological surprises. Frontiers Ecol Env. 2007;5:475–482. [Google Scholar]
- 19.Webb T, III, Anderson KH, Bartlein PJ, Webb RS. Late Quaternary climate change in eastern North America: A comparison of pollen-derived estimates with climate model results. Quat Sci Rev. 1998;17:587–606. [Google Scholar]
- 20.Williams JW, Jackson ST, Kutzbach JE. Projected distributions of novel and disappearing climates by 2100 AD. Proc Natl Acad Sci USA. 2007;104:5738–5742. doi: 10.1073/pnas.0606292104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chesson P, Huntly N. The roles of harsh and fluctuating conditions in the dynamics of ecological communities. Amer Nat. 1997;150:519–553. doi: 10.1086/286080. [DOI] [PubMed] [Google Scholar]
- 22.Shigesada N, Kawasaki K. Biological Invasions: Theory and Practice. Oxford: Oxford Univ Press; 1997. [Google Scholar]
- 23.Shuman B, Webb T, III, Bartlein P, Williams JW. The anatomy of a climate oscillation: Vegetation change in eastern North America during the Younger Dryas chronozone. Quat Sci Rev. 2002;21:1777–1791. [Google Scholar]
- 24.Williams JW, Post DM, Cwynar LC, Lotter AF, Levesque AJ. Rapid and widespread responses to past climate change in the North Atlantic. Geology. 2002;30:971–974. [Google Scholar]
- 25.Betancourt JL, van Devender TR, Martin PS, editors. Packrat Middens: The Last 40,000 Years of Biotic Change. Tucson, AZ: Univ of Arizona Press; 1990. [DOI] [PubMed] [Google Scholar]
- 26.Jackson ST, Overpeck JT, Webb T, III, Keattch SE, Anderson KH. Mapped plant macrofossil and pollen records of Late Quaternary vegetation change in eastern North America. Quat Sci Rev. 1997;16:1–70. [Google Scholar]
- 27.Overpeck JT, Whitlock C, Huntley B. In: Paleoclimate, Global Change and the Future. Alverson KD, Bradley RS, Pedersen TF, editors. Berlin: Springer; 2003. pp. 81–111. [Google Scholar]
- 28.Webb T, III, Shuman B, Williams JW. In: The Quaternary Period in the United States. Gillespie AR, Porter SC, Atwater BF, editors. Amsterdam: Elsevier; 2004. pp. 459–478. [Google Scholar]
- 29.Booth RK, et al. A severe centennial-scale drought in mid-continental North America 4200 years ago and apparent global linkages. Holocene. 2005;15:321–328. [Google Scholar]
- 30.Morrill C, Jacobsen RM. How widespread were climate anomalies 8200 years ago? Geophys Res Lett. 2005;32:L1970. [Google Scholar]
- 31.Mayewski PA, et al. Holocene climate variability. Quat Res. 2004;62:243–255. [Google Scholar]
- 32.Booth RK, Notaro M, Jackson ST, Kutzbach JE. Widespread drought episodes in the western Great Lakes region during the past 2000 years: Geographic extent and potential mechanisms. Earth Planet Sci Lett. 2006;242:415–427. [Google Scholar]
- 33.Shuman BN, Newby P, Donnelly JP. Abrupt climate change as a catalyst of ecological change in the Northeast U.S. throughout the past 15,000 years. Quat Sci Rev. 2009 in press. [Google Scholar]
- 34.Seppä H, Birks HJB. July mean temperature and precipitation trends during the Holocene in the Fennoscandian tree-line area: Pollen-based climate reconstructions. Holocene. 2001;11:527–539. [Google Scholar]
- 35.Magny M. Holocene climate variability as reflected by mid-European lake-level fluctuations and its probable impact on prehistoric human settlements. Quat Intl. 2004;113:65–79. [Google Scholar]
- 36.deMenocal P, Ortiz J, Guilderson T, Sarnthein M. Coherent high- and low-latitude climate variability during the Holocene warm period. Science. 2000;288:2198–2202. doi: 10.1126/science.288.5474.2198. [DOI] [PubMed] [Google Scholar]
- 37.Meko DM, et al. Medieval drought in the Upper Colorado River Basin. Geophys Res Lett. 2007;34 doi: 10.1029/2007GL029988. (10, L10705) [DOI] [Google Scholar]
- 38.Fye FK, Stahle DW, Cook ER. Paleoclimatic analogs to twentieth-century moisture regimes across the United States. Bull Amer Met Soc. 2003;84:901–909. [Google Scholar]
- 39.McCabe GJ, et al. Associations of multi-decadal sea-surface temperature variability with U.S. Drought. Quat Int. 2008 doi: 10.1016/j.quaint.2007.07.001. [DOI] [Google Scholar]
- 40.Enfield DB, Mestas-Nuñez AM, Trimble PJ. The Atlantic Multidecadal Oscillation and its relation to rainfall and river flows in the continental U.S. Geophys Res Lett. 2001;28:2077–2080. [Google Scholar]
- 41.Donders TH, Wagner-Cremer F, Visscher H. Integration of proxy data and model scenarios for the mid-Holocene onset of modern ENSO variability. Quat Sci Rev. 2008;27:571–579. [Google Scholar]
- 42.Gray ST, Betancourt JL, Jackson ST, Eddy RG. Role of multidecadal climatic variability in a range extension of pinyon pine. Ecology. 2006;87:1124–1130. doi: 10.1890/0012-9658(2006)87[1124:romcvi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 43.Hutchinson GE. Concluding remarks. Cold Spring Harbor Symp Quant Biol. 1958;22:425–427. [Google Scholar]
- 44.Hutchinson GE. An Introduction to Population Ecology. New Haven: Yale Univ Press; 1978. [Google Scholar]
- 45.Lieth H, Whittaker RH. Primary Productivity of the Biosphere. New York: Springer; 1975. [Google Scholar]
- 46.Rohde K. Latitudinal gradients in species diversity: The search for the primary cause. Oikos. 1992;65:514–527. [Google Scholar]
- 47.Larcher W. Physiological Plant Ecology. 3rd Ed. Berlin: Springer; 1995. [Google Scholar]
- 48.Germino MJ, Smith WK. Sky exposure, crown architecture, and low-temperature photoinhibition in conifer seedlings at alpine treeline. Plant Cell Env. 1999;22:407–415. [Google Scholar]
- 49.Holmgren C, Norris J, Betancourt JL. Inferences about winter temperatures and summer rains from the late Quaternary record of C4 perennial grasses and C3 desert shrubs in the northern Chihuahuan Desert. J Quat Sci. 2007;22:141–161. [Google Scholar]
- 50.Whittaker RH. Gradient analysis of vegetation. Biol Rev. 1967;49:207–264. doi: 10.1111/j.1469-185x.1967.tb01419.x. [DOI] [PubMed] [Google Scholar]
- 51.Mason HL. The principles of geographic distribution as applied to floral analysis. Madroño. 1936;3:181–190. [Google Scholar]
- 52.Grubb PJ. The maintenance of species-richness in plant communities: The importance of the regeneration niche. Biol Rev. 1977;52:107–145. [Google Scholar]
- 53.Pearson GA. Management of Ponderosa Pine in the Southwest as Developed by Research and Experimental Practice. Washington, DC: Forest Service; 1950. [Google Scholar]
- 54.Donovan LA, McLeod KW, Sherrod KC, Jr., Stumpff NJ. Response of woody swamp seedlings to flooding and increased water temperatures. (I) Growth, biomass, and survivorship. Amer J Bot. 1988;75:1181–1190. [Google Scholar]
- 55.Black RA, Bliss LC. Reproductive ecology of Picea mariana (Mill.) BSP., at tree line near Inuvik, Northwest Territories, Canada. Ecol Mon. 1980;50:331–354. [Google Scholar]
- 56.Pigott CD. In: Fruit and Seed Production. Marshall C, Grace J, editors. Cambridge: Cambridge Univ Press; 1992. pp. 203–216. [Google Scholar]
- 57.Tremblay MF, Bergeron Y, Lalonde D, Mauffette Y. The potential effects of sexual reproduction and seedling recruitment on the maintenance of red maple (Acer rubrum L.) populations at the northern limit of the species range. J Biogeog. 2002;29:365–373. [Google Scholar]
- 58.van Mantgem PJ, et al. Widespread increase of tree mortality rates in the western United States. Science. 2009;323:521–524. doi: 10.1126/science.1165000. [DOI] [PubMed] [Google Scholar]
- 59.Mueller RC, et al. Differential tree mortality in response to severe drought: Evidence for long-term vegetation shifts. J Ecol. 2005;93:1085–1093. [Google Scholar]
- 60.Holyoak M, Ray C. A roadmap for metapopulation research. Ecol Lett. 1999;2:273–275. doi: 10.1046/j.1461-0248.1999.00081.x. [DOI] [PubMed] [Google Scholar]
- 61.IPCC. Climate Change 2007: The Physical Science Basis. Cambridge: Cambridge Univ Press; 2007. [Google Scholar]
- 62.Losos JB, Jackman TR, Larson A, de Queiroz K, Rodriguez-Schettino L. Contingency and determinism in replicated adaptive radiations of island lizards. Science. 1998;279:2115–2118. doi: 10.1126/science.279.5359.2115. [DOI] [PubMed] [Google Scholar]
- 63.Taylor EB, McPhail JD. Historical contingency and ecological determinism interact to prime speciation in sticklebacks, Gasterosteus. Proc R Soc London Ser B. 2000;267:2375–2384. doi: 10.1098/rspb.2000.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vermeej GJ. Historical contingency and the purported uniqueness of evolutionary innovations. Proc Natl Acad Sci USA. 2006;103:1804–1809. doi: 10.1073/pnas.0508724103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Foster DR, Knight DH, Franklin JF. Landscape patterns and legacies resulting from large, infrequent forest disturbances. Ecosystems. 1998;1:497–510. [Google Scholar]
- 66.Taylor CM, Hastings A. Allee effects in biological invasions. Ecol Lett. 2006;8:895–900. [Google Scholar]
- 67.With KA. The landscape ecology of invasive spread. Cons Biol. 2002;16:1192–1203. [Google Scholar]
- 68.Swetnam TW, Betancourt JL. Mesoscale disturbance and ecological response to decadal climatic variability in the American Southwest. J Climate. 1998;11:3128–3147. [Google Scholar]
- 69.Swetnam TW, Allen CD, Betancourt JL. Applied historical ecology: Using the past to manage for the future. Ecol Appl. 1999;9:1189–1206. [Google Scholar]
- 70.Breshears DD, et al. Regional vegetation die-off in response to global-change type drought. Proc Natl Acad Sci USA. 2005;102:115144–115148. doi: 10.1073/pnas.0505734102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bigler C, Gavin DG, Gunning C, Veblen TT. Drought induces lagged tree mortality in a subalpine forest in the Rocky Mountains. Oikos. 2007;116:1983–1994. [Google Scholar]
- 72.Negron JF, McMillin JD, Anhold JA, Coulson D. Bark beetle-caused mortality in a drought-affected ponderosa pine landscape in Arizona, USA. For Ecol Man. 2009;257:1353–1362. [Google Scholar]
- 73.Brown PM, Wu R. Climate and disturbance forcing of episodic tree recruitment in a southwestern Ponderosa pine landscape. Ecology. 2005;86:3030–3038. [Google Scholar]
- 74.Kitzberger T, et al. Contingent Pacific-Atlantic Ocean influence on multicentury wildfire synchrony over western North America. Proc Natl Acad Sci USA. 2006;104:543–548. doi: 10.1073/pnas.0606078104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Heyerdahl EK, et al. Climate drivers of regionally synchronous fires in the inland Northwest (1651–1900) Intl J Wildl Fire. 2008;17:40–49. [Google Scholar]
- 76.Heyerdahl EK, Morgan P, Riser JP., II Multi-season climate synchronized historical fires in dry forests (1650–1900), Northern Rockies, USA. Ecology. 2008;89:705–716. doi: 10.1890/06-2047.1. [DOI] [PubMed] [Google Scholar]
- 77.Speer JH, Swetnam TW, Wickman BE, Youngblood A. Changes in pandora moth outbreak dynamics during the past 622 years. Ecology. 2001;82:679–697. [Google Scholar]
- 78.Hursh CR, Haasis FW. Effects of 1925 summer drought on southern Appalachian hardwoods. Ecology. 1931;12:380–386. [Google Scholar]
- 79.Stickel PW. Drought injury in hemlock-hardwood stands in Connecticut. J For. 1933;31:573–577. [Google Scholar]
- 80.Secrest HC, MacAloney HJ, Lorenz RC. Causes of the decadence of hemlock at the Menominee Indian Reservation, Wisconsin. J For. 1941;39:3–12. [Google Scholar]
- 81.Hough AF, Forbes RD. The ecology and silvics of forests in the high plateaus of Pennsylvania. Ecol Mon. 1943;13:299–320. [Google Scholar]
- 82.Stearns FW. The composition of the sugar maple-hemlock-yellow birch association in northern Wisconsin. Ecology. 1951;32:245–265. [Google Scholar]
- 83.Winget CH, Kozlowski TT. Yellow birch germination and seedling growth. For Sci. 1965;11:386–392. [Google Scholar]
- 84.Coffman MS. Eastern hemlock germination influenced by light, germination media, and moisture content. Michigan Bot. 1978;37:99–103. [Google Scholar]
- 85.Mladenoff DJ, Stearns F. Eastern hemlock regeneration and deer browsing in the northern Great Lakes region: A re-examination and model simulation. Cons Biol. 1993;7:889–900. [Google Scholar]
- 86.Houle G. Spatiotemporal patterns in the components of regeneration of four sympatric tree species—Acer rubrum, A. saccharum, Betula alleghaniensis and Fagus grandifolia. J Ecol. 1994;82:39–53. [Google Scholar]
- 87.Lyford ME, Jackson ST, Betancourt JL, Gray ST. Influence of landscape structure and climate variability on a late Holocene plant migration. Ecol Mon. 2003;73:567–583. [Google Scholar]
- 88.Davis MB. In: Colonization, Succession and Stability. Gray AJ, Crawley MJ, Edwards PJ, editors. Oxford: Blackwell; 1987. pp. 373–393. [Google Scholar]
- 89.Woods KD, Davis MB. Paleoecology of range limits: Beech in the Upper Peninsula of Michigan. Ecology. 1989;70:681–696. [Google Scholar]
- 90.Parshall T. Late Holocene stand-scale invasion by hemlock (Tsuga canadensis) at its western range limit. Ecology. 2002;83:1386–1398. [Google Scholar]
- 91.Booth RK, Jackson ST, Gray CED. Paleoecology and high-resolution paleohydrology of a kettle peatland in upper Michigan. Quat Res. 2004;61:1–13. [Google Scholar]
- 92.Peñalba MC, Payette S. Late-Holocene expansion of eastern larch (Larix laricina [Du Roi] K. Koch.) in northwestern Québec. Quat Res. 1997;48:114–121. [Google Scholar]
- 93.Solomon S, Plattner G-K, Knutti R, Friedlingstein P. Irreversible climate change due to carbon dioxide emissions. Proc Natl Acad Sci USA. 2009;106:1704–1709. doi: 10.1073/pnas.0812721106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Enfield DB, Cid-Cerrano L. Projecting the risk of future climate shifts. Intl J Clim. 2006;26:885–895. [Google Scholar]
- 95.Keenlyside NS, Latif M, Jungclaus J, Kornblueh L, Roeckner E. Advancing decadal-scale climate prediction in the North Atlantic sector. Nature. 2008;453:84–88. doi: 10.1038/nature06921. [DOI] [PubMed] [Google Scholar]
- 96.Strayer DL. Freshwater Mussel Ecology: A Multifactor Approach to Distribution and Abundance. Berkeley, CA: Univ of California Press; 2008. [Google Scholar]
- 97.Berkelmans R, van Oppen MJH. The role of zooxanthellae in the thermal tolerance of corals: “A nugget of hope” for coral reefs in an era of climate change. Proc Royal Soc B. 2006;273:2305–2312. doi: 10.1098/rspb.2006.3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ibáñez I, Clark JS, Dietze MC. Evaluating the source of potential migrant species: Implications under climate change. Ecol Appl. 2008;18:1664–1678. doi: 10.1890/07-1594.1. [DOI] [PubMed] [Google Scholar]
- 99.Morin X, Augspurger C, Chuine I. Process-based modeling of species' distribution: What limits temperate tree species' range boundaries? Ecology. 2007;88:2280–2291. doi: 10.1890/06-1591.1. [DOI] [PubMed] [Google Scholar]
- 100.Ibáñez I, Clark JS, LaDeau S, Hille Ris Lambers J. Exploiting temporal variability to understand tree recruitment response to climate change. Ecol Mon. 2007;77:163–177. [Google Scholar]
- 101.Morin X, Thuiller W. Comparing niche- and process-based models to reduce prediction uncertainty in species range shifts under climate change. Ecology. 2009;90:1301–1313. doi: 10.1890/08-0134.1. [DOI] [PubMed] [Google Scholar]
- 102.Prentice IC, et al. In: Terrestrial Ecosystems in a Changing World. Canadell JG, Pataki DE, Pitelka LF, editors. Heidelberg: Springer; 2007. pp. 175–192. [Google Scholar]