Abstract
Species with narrow environmental niches typically have small geographic ranges. Small range size is, in turn, often associated with low local abundance. Together, these factors should mean that ecological specialists have very small total populations, putting them at high risk of extinction. But some specialized and geographically restricted species are ancient, and some ecological communities have high proportions of rare and specialized endemics. We studied niche characteristics and patterns of distribution and abundance of terrestrial vertebrates in the rainforests of the Australian Wet Tropics (AWT) to identify mechanisms by which rare species might resist extinction. We show that species with narrow environmental niches and small geographic ranges tend to have high and uniform local abundances. The compensation of geographic rarity by local abundance is exact, such that total population size in the rainforest vertebrates of the AWT is independent of environmental specialization. This effect would tend to help equalize extinction risk for specialists and generalists. Phylogenetic analysis suggests that environmental specialists have been gradually accumulating in this fauna, indicating that small range size/environmental specialization can be a successful trait as long as it is compensated for by demographic commonness. These results provide an explanation of how range-restricted specialists can persist for long periods, so that they now form a major component of high-diversity assemblages such as the AWT.
Keywords: abundance, range size, rarity
The Australian Wet Tropics (AWT) bioregion encompasses 1.8 million ha of mixed tropical forest environments between the latitudes of 19 ° 15′ and 15 ° 30′ in northeastern Queensland, from coastal lowlands to an elevation of 1,620 m. Rainforest dominates the region, covering an area of slightly less than a million ha and distributed primarily along a series of disjunct mountain ranges with high orographic rainfall of between 1.5–9 m per year. The rainforests form an isolated and discrete ecosystem completely surrounded by dry tropical forests. Most of the rainforest in the region (≈95%) is protected in the AWT World Heritage Area declared in 1988. These rainforests are a remnant of forests that were widespread in Miocene Australia. The rainforests of the region have been subjected to a series of climate fluctuations over the Quaternary, often resulting in the rainforests being reduced to small, fragmented refugia (1–3). The various mountain ranges within the region form a number of biogeographic subregions. The biogeographic history of climatic fluctuations and its influence on the biodiversity of the region has been well studied over the last 30 years, and thus the region is well suited to macroecological research. Rainforests in the AWT bioregion form a distinct and isolated ecosystem of high diversity, especially rich in regionally endemic species. Many of these regionally endemic species have extremely small geographic ranges (4).
Species with small geographic ranges often have low population density and thereby a small total population size (number of individuals) that would presumably result in a classic “double jeopardy” high risk of extinction (5–7). These factors beg the question: How have so many highly restricted species survived in the AWT bioregion? We examined this question by using extensive data describing the range size, local abundance, and ecological specialisation of 159 species of rainforest vertebrates (≈80% of the entire rainforest species present in the region (see Dataset S1 for species list and data). Although over 200 species have been recorded in rainforest in the region (4), many of these are species for which rainforest is a suboptimal habitat. The species included in the analyses here represent the vast majority of the species assemblages present in rainforest throughout the region. We utilized more than 3,600 standardized surveys to calculate local abundances for these species, characterized environmental niches by producing bioclimatic distribution models for each species, and used Ecological Niche Factor Analysis (ENFA) (8) to derive measures of specialization and marginality of the climatic niche (see ref. 9 for full details of abundance estimates and sampling methodology). Specialization represents the narrowness of the climate niche relative to the climatic range across the region, and marginality measures how atypical is the climate niche compared with the average climate for the region. Also, we measured the degree of specialization to structural vegetation types for each species.
Results
There were strong correlations among all three measures of niche specialization (climatic specialization/marginality, r = 0.60; vegetation specialization/marginality, r = 0.50; climate specialization/vegetation specialization, r = 0.38; all P < 0.0001). Therefore, we combined them into a single variable, the first principal component from a factor analysis on all three variables. The first principal component, hereafter referred to as “specialization”, accounted for 66% of the variation in the factor analysis and was positively correlated with all three original variables (r = 0.81, 0.87, and 0.76 respectively, all P < 0.0001). We measured species abundances in 3,600 standardized censuses across many sites distributed over elevational, latitudinal, and climatic gradients across the bioregion. For each species, we calculated mean abundance and a measure of the evenness of abundance (the inverse of the coefficient of variation in abundance). For more detailed analysis of distribution and abundance data and sampling methodology, refer to VanDerWal (9).
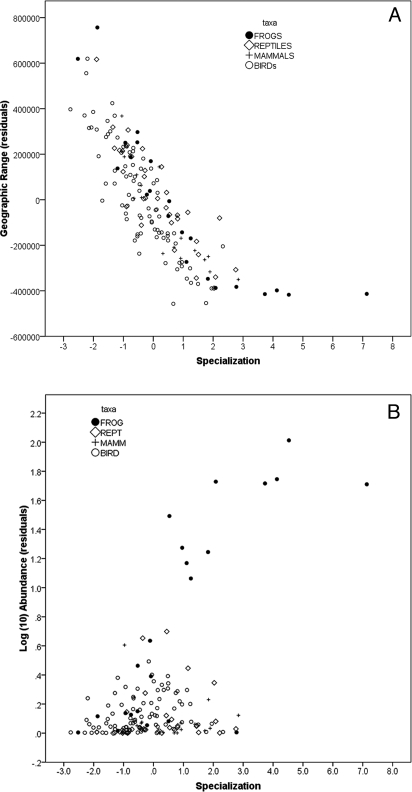
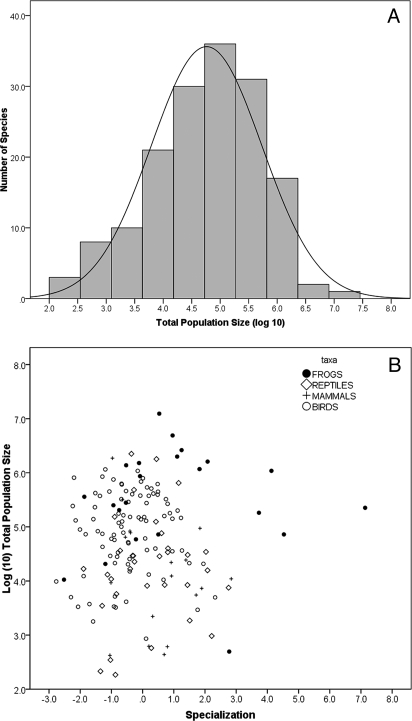
Geographic ranges of AWT vertebrates vary from 700 to 1.2 million ha with 12 species occupying less than 100,000 ha. Range size is negatively related to specialization (Fig. 1A; r = −0.82, P < 0.0001) whereas local abundance is positively correlated with specialization (Fig. 1B; r = 0.530, P < 0.0001). The latter relationship varies across taxonomic groups with frogs and birds both showing significant trends (frogs: r = 0.773, P < 0.0001; birds: r = 0.257, P = 0.017) and mammals and reptiles not being significant when examined individually (mammals: r = −0.194, P = 0.441; reptiles: r = 0.142, P = 0.430). The overall effect is to equalize total population size with respect to specialization. We estimated total population size in the AWT for each species as the product of range size and abundance; total population size is reasonably normally distributed across species and unrelated to specialization both as a whole assemblage and within separate taxonomic group (Fig. 2 A and B; all species: r = 0.02, P = 0.78). There is significant variation among major taxon groups in total population size (F3,155 = 13.41, P < 0.0001), with frogs having the largest mean populations (5.49 ± 0.18, mean and SEM of log(10) population size), followed by birds, reptiles, and then mammals (respectively, 4.91 ± 0.09; 4.23 0.15; and 4.11 ± 0.21). We found no significant relationships between specialization and total population size within any of these taxa (Fig. 2B; mammals: r = −0.199, P = 0.429; birds: r = 0.037, P = 0.734; reptiles: r = −0.106, P = 0.558; frogs r = −0.018, P = 0.936). There is a positive relationship between mean abundance and the evenness of abundance (r = 0.64, P < 0.0001), showing that species with restricted niches not only have higher local abundance, but are also more uniformly distributed within their small ranges.
Fig. 1.
Relationships of environmental specialization to geographic range size (A) and local abundance (B) among rainforest vertebrate species of the AWT. Specialization is measured as the first principal component from a factor analysis of three primary measures of niche dimensions (climate specialization, climate marginality, and vegetation specialization). Range size (ha) and abundance (/ha) are measured as residuals after controlling for effects of phylogeny (see Methods and Dataset S1).
Fig. 2.
Total population sizes of rainforest vertebrates in the AWT bioregion. (A) Distribution of total population size for 159 vertebrate species in rainforests of the AWT. The bars are empirical data, and the line shows a normal fit to these data; the empirical distribution departs only slightly from lognormal (Shapiro–Wilk W = 0.9699; P = 0.044). (B) Total population sizes of vertebrate species in relation to the degree of environmental specialization as measured by values on the first principle component derived from a factor analysis of climate specialization, climate marginality, and vegetation specialization.
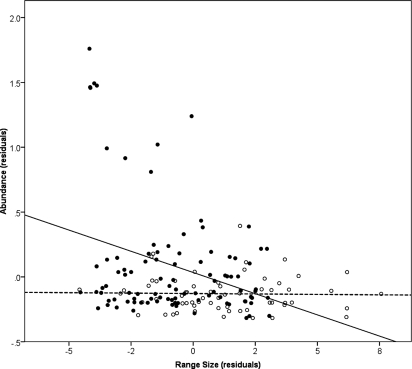
In AWT vertebrate fauna, the strong negative relationship between specialization and geographic range size, together with the positive relationship of specialization and abundance (driven by amphibians and birds), produces a weakly negative correlation between range size and abundance across all species (r = −0.32, P < 0.0001; Fig. 3). However, controlling for environmental specialization reveals a weak positive relationship between range size and abundance (r = 0.24, P < 0.01).
Fig. 3.
Relationship of abundance to range size of vertebrates in the AWT. Symbols distinguish species that are restricted to the rainforest and associated wet sclerophyll forest (filled circles) and forest generalists (open circles). The relationship is significant only in the first category of species (F1,93 = 11.57, P < 0.001). Range size (ha, × 100,000) and (log10) abundance (per ha) are measured as residuals after controlling for effects of phylogeny (see Methods).
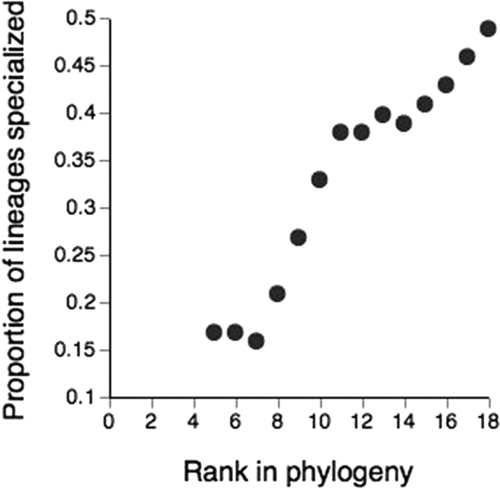
To examine whether specialized narrow-range species are more prone to extinction over time, we compiled a “best-estimate” phylogeny for the fauna and coded living species as having either high or low specialty (according to whether they were above or below the median values for specialization) and used parsimony reconstruction methods to map the distribution of these traits through the whole phylogeny. We found no evidence to suggest that narrow-range specialists are concentrated at the tips of the phylogeny (Fig. 4).
Fig. 4.
Distribution of specialization over the phylogeny of AWT vertebrates. The proportion of lineages composed of specialists is shown from the root (rank 0) to the tips of the phylogeny. Species were classified as specialists if they were above the median value of the specialization index used in Figs. 1 and 2. “Rank” refers to the number of branch points away from the root and was used as the metric for the analysis because we did not have information on branch lengths or a time scale.
Discussion
Geographic rarity should increase the probability of extinction as a result of environmental change (10, 11). Demographic rarity (low and/or patchy abundance) contributes independently to extinction risk by making species more vulnerable to stochastic population fluctuations. Species that are both geographically and demographically rare therefore face a double jeopardy of extinction and should be at especially high risk (6, 12). Extant species of vertebrates in the AWT seem to have escaped this double jeopardy by compensating for geographic rarity with higher local abundance, often combined with a more uniform distribution of abundance within their range, supporting predictions by Rabinowitz (7). The latter should enhance the resilience of the species by maintaining high demographic connectivity throughout the range.
We suggest two possible causes of the positive relationship of abundance to specialization in the assemblage as a whole and amphibians and birds in particular. The first is extinction filtering over the biogeographic history of these species; the importance of extinction filtering has been demonstrated by a variety of studies and techniques, including assemblage composition and nestedness (13–15) and phylogeographic studies by using a variety of molecular analyses [as reviewed in Moritz (16)]. Past extinctions would tend to remove specialists if they also had low and patchy abundance but small-niched species might survive if the extinction jeopardy due to environmental specialization and small range was counteracted by high and uniform abundance. This mechanism may have been powerful in the AWT because the rainforests of the region underwent repeated contractions to small refugia through Quaternary glacial cycles (1–3). Episodes of localized extinctions as rainforests contracted were followed by reformation of rainforest assemblages across the region, through a combination of expansions of species that had survived in small rainforest refuges and invasions of reconstituted rainforest environments by forest generalists that had persisted in other forest types (2, 3, 17, 18). A high proportion of the species in our study (95 of 159) are primarily restricted to rainforest (4) and must have been selected for their ability to persist for thousands of years in small and isolated refugia. The ability to persist in a refuge would be raised by specialization on the conditions of that place, combined with the demographic resilience conferred by high and uniform local abundance and/or a high dispersal ability within and across habitats. This interpretation is supported by the observation that the compensation of range and abundance, which equalizes total population size over the whole assemblage, is mainly due to the 95 species that are restricted to the rainforest and fringing wet sclerophyll forests (most of these species are regional endemics); forest generalists have larger geographic ranges within the rainforests of the AWT with moderate and less variable abundances (Fig. 3). Of course, the hypothesis assumes that the ecological patterns we have documented have been similar through evolutionary history. Unfortunately, although the fossil record documents a long period of faunal stability spanning several glacial cycles, data on relative abundances are not yet available to allow the reconstruction of temporal patterns of relative abundance for individual taxa (19).
A second mechanism that might contribute to the high and uniform abundance of small-niched species could be that, because of their restricted ranges, they have accumulated local adaptations that allow them to be more abundant, whereas in more widespread species gene flow across suboptimal environmental gradients and over long distance may restrict local adaptation (20) and thereby limit local abundance. This mechanism could operate in the absence of periodic contractions to refugia and both mechanisms might have worked together to produce the patterns we describe.
Many studies have examined relationships between geographic range and local abundance in large assemblages of species, typically finding positive correlations (21–25). The relationships between range size, abundance, and specialization shown here are significant to a long-running debate on the cause of positive range–abundance relationships (22). A widespread view is that the relationship is due to a common effect of niche breadth on both range and abundance, but the fact that the range–abundance relationship in this assemblage is weakly positive only when effects of niche dimensions are removed points to other mechanisms, such as metapopulation processes, as the cause (23, 24, 26). Komonen (27) recently demonstrated that positive abundance–distribution relationship can result if geographically restricted species are missed in the analysis. We spent a lot of time on field surveys ensuring that we included as many rare species as possible, and our results support those of Komonen (27). Our observation that specialists have high local abundance differs from many previous analyses of range–abundance relationships but may be a common pattern in assemblages, such as the AWT, that have many ancient small-ranged endemic species. Although this pattern was observed in all taxonomic groups, the compensation between small-range-size/environmental specialization and abundance was most pronounced in the frogs (Fig. 1B). Species of frogs within the Microhylidae family are among the most geographically restricted species in the region with a number of species only occurring on a single mountaintop. A previous study identified another compensatory mechanism in microhylid frogs where narrow-range specialists had a less specialized diet (28).
The hypothesis that narrow-range specialists in AWT vertebrate fauna that have high, and often uniform, local abundance are resistant to extinction is supported by several lines of evidence. Previous studies have shown that most narrow-range specialists in the region are old, predating the climatic fluctuations of the Quaternary (16). Rainforest was periodically reduced to small, fragmented refugia during these climatic fluctuations, and it therefore follows that any extant species survived these range and population contractions. The ability to maintain viable populations would be enhanced by high and uniform local abundance, especially for those species with poorer dispersal ability. Further, the phylogenetic analysis presented here, albeit crude, also supports the hypothesis that species that can compensate for small range size with high abundances have been selected for, as narrow-range specialists have been gradually accumulating in the assemblage. If specialization leads quickly to extinction, specialized lineages would be concentrated at the tips of the phylogeny. Instead, the proportion of all lineages that are specialized increases steadily from the root to the tips of the phylogeny (Fig. 4). The increase in the lineage density of specialists was due to the fact that most inferred transitions between states were from low to high specialization, rather than the reverse (25 versus 15 transitions). This reversal could only have produced the observed increase in the prevalence of specialization if the rate at which specialists lineages went extinct was less than the rate at which they arose. We recognize that a limitation of our parsimony approach is an inability to properly address uncertainty of character states at nodes. However, our analysis represents the best possible approach given the available data. Specifically, a lack of data on branch lengths prevented us from pursuing a more sophisticated supertree approach. A further limitation is the lack of fossil data preventing us from excluding the alternative possibility that specialized lineages may be newly derived from now-extinct nonspecialized ancestors.
Extant species of vertebrates in the AWT, although many species are geographically rare now and in the past were often even more restricted, seem to have persisted by compensating for geographic rarity with high local abundance and/or a more uniform distribution of abundance within their range. We suggest that a selection gradient where environmental specialization is compensated for by this combination of traits is consistent with an assemblage that has been filtered by repeated habitat contractions. Species either must survive in situ in small refugia or be sufficiently generalist to utilize a broad habitat mosaic containing a matrix of suboptimal habitat types.
Methods
Data on occurrence of species across the region was collected on intensive field surveys and collated from the literature, institutional databases, and many field biologists working in the region to produce a distribution database with over 100,000 accurate location records for vertebrate species (4). Environmental niches were modeled from presence data by using the Maxent algorithm (29), chosen because it outperforms other common ecological-niche modeling approaches (30, 31) and produces results that are relatively insensitive to sample size (31, 32). Niche models were constructed by using both climate and vegetation data. Climate variables used were annual mean temperature, temperature seasonality, maximum temperature of the warmest week, minimum temperature of the coldest week, annual precipitation, precipitation seasonality, precipitation of the driest quarter, and precipitation of the wettest quarter; all variables were extracted by using the Anuclim 5.1 software (33) and an 80-m resolution DEM (resampled from GEODATA 9 Second DEM Version 2; Geoscience Australia, http://www.ga.gov.au/). Vegetation data used in the models consisted of floristically classified broad vegetation groups at a 1:2 million resolution (34). Species occupied from 1 to 16 vegetation groups. All defaults in the modeling software were used with the exception that 36,000 background points representing a 1-km grid over the study area were used, rather than 10,000 random background points, to ensure that all environments in the study regions were captured in training the models.
Geographic ranges were estimated by projecting the modeled niches onto the study region. Models were evaluated by expert opinion, comparison with previously published and used distribution maps in the region (1, 4, 35), and by using model-evaluation statistics (mean area under the receiver operator curve (AUC) = 0.904 ± 0.081; minimum AUC value of any model used was 0.633; 88% of species AUC >0.80). These potential distributions were clipped to excise areas where expert knowledge indicated true absences. The clipping of the potential range to provide our best-possible estimate of the realized range size was based on a subregional presence/absence matrix compiled by an extensive collation of distributional data, followed by intensive targeted surveys by S.E.W. and others in the Centre for Tropical Biodiversity and Climate Change to fill gaps in both geographic and environmental space over the last 10 years, building and improving on previous publications and analyses detailing species distributions in the AWT bioregion (4, 35). In other words, the potential distributions created by the models were clipped by expert opinion and known biogeographic barriers to provide a best-available estimate of the realized (actual) distributions [as per VanDerWal (9)]. ENFA measures of marginality and specialization were estimated from the occurrence and climate data used for the distribution modeling (vegetation information was excluded as categorical variables cannot be used with ENFA). Climate data were Box-Cox transformed before analysis. ENFA was run by using the adehabitat package (v1.7.1, http://www.r-project.org) and Box-Cox transformations were done by using the companion to applied regression (CAR) package (v1.2–7, http://www.r-project.org), both within the R statistical program (v2.6.2, http://www.r-project.org). Vegetation groups for each occurrence point were extracted from a spatial vegetation layer created by Stanton (36). An index of vegetation specialization was calculated for each species based on these data and applying an inverse of Simpson's diversity index (values were reversed so that high values indicate a more specialized vegetation niche).
Species abundances were estimated from taxon-appropriate standardized survey methods at geographically unique locations across the study region since 1992. Details of the survey methods, abundance estimates, and sampling effort are provided in VanDerWal (9). In summary, the data represents >1,300 bird surveys, >900 frog surveys, >1,100 reptile surveys, and >300 mammal surveys. The sampling area at each locality varied depending on the survey technique used: amphibians (stream, 0.6 ha; terrestrial, 0.1 ha), mammals (spotlight, 4 ha; trapping grid, 0.4 ha), reptiles (active search, 0.8 ha, spotlight, 4 ha), birds (dawn census, 1.5 ha). Zero counts from surveys undertaken outside a species range can unduly reduce the mean abundance estimate for a species. We therefore excluded all surveys that fell outside of a species' geographic range as estimated by using niche models (see above).
For each taxonomic group, a best-estimate phylogeny was created by first constructing an outline structure tree at the family level and then filling in at the species level from published phylogenies. In the majority of cases, phylogenies used from the literature were the most recent available and based on molecular methods. Complete details, references and phylogenetic trees are presented in a separate publication (37). Use of disparate data sources and lack of phylogenetic relationships in many endemic species meant that it was not possible to retain information on branch lengths. Instead, branching nodes were assumed to be spaced at uniform intervals through the phylogeny.
In cases where a specific endemic species was not included in a published phylogeny, they were added in at the point where other species in the genus were placed. Similarly, subspecies were added as sister species. The expertise of Prof. Craig Moritz and Dr. Conrad Hoskin was also used to assess relationships among lesser-known taxa and endemic species based on their extensive experience with phylogenetic relationships of AWT fauna. The phylogeny was complete for species used in this analysis but did not include related species occurring outside the region, meaning that branches in the phylogeny could represent either lineage divergence in situ or entry of related species that originally arose outside the region.
We used phylogenetic eigenvector regression (38) to control for effects of phylogeny in ecological analyses. We constructed a phylogenetic distance matrix for all pairs of species by using the number of nodes along the path between species in the pair as our measure of phylogenetic distance (39). The phylogenetic distance matrix was double-centered, and a principle coordinates analysis was carried out (as per ref. 39) in the R statistical program (v2.6.2, http://www.r-project.org).
Because our phylogeny was relatively large, we conducted the analysis at the family level (n = 54 families) to control the number of significant eigenvectors. The first three eigenvectors (explaining 88.9% of the variation in pairwise distances among species) were used in further analysis. These eigenvectors were entered in statistical models to control for effects of phylogenetic location. We mapped the evolutionary history of specialization over the phylogeny by using the parsimony method in Mesquite (40). We used binary states because we were interested in determining change in representation of specialized species from the root to the tips of the phylogeny, independent of differences in the number of lineages.
Acknowledgments.
We would like to thanks many field assistants who helped collect this data over the years. Funding over the years has been kindly provided by the James Cook University Research Advancement Program, the Australian Research Council, the Queensland Government Innovation Funds, the Rainforest Cooperative Research Centre, the Marine and Tropical Science Research Facility, the Wet Tropics Management Authority, the U.S. National Science Foundation, Earthwatch Institute, National Geographic, and the Skyrail Foundation.
Footnotes
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “Biogeography, Changing Climates and Niche Evolution,” held December 12–13, 2008, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. The complete program and audio files of most presentations are available on the NAS web site at www.nasonline.org/Sackler_Biogeography.
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. E.A.H. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0901640106/DCSupplemental.
References
- 1.Nix H. Biogeography: Pattern and process. In: Nix HA, Switzer MA, editors. Rainforest Animals: Atlas of Vertebrates Endemic to Australia's Wet Tropics. 1st Ed. Vol 1. Canberra: Australian National Parks and Wildlife; 1991. pp. 11–40. [Google Scholar]
- 2.Graham CH, Moritz C, Williams SE. Habitat history improves prediction of biodiversity in rainforest fauna. Proc Natl Acad Sci USA. 2006;103:632–636. doi: 10.1073/pnas.0505754103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.VanDerWal J, Shoo LP, Williams SE. New approaches to understanding late Quaternary climate fluctuations and refugial dynamics in Australian wet tropical rain forests. J Biogeogr. 2009;36:291–301. [Google Scholar]
- 4.Williams SE. Vertebrates of the Wet Tropics Rainforests of Australia: Species distributions and biodiversity. Cairns, Australia: Rainforest-CRC; 2006. [Google Scholar]
- 5.Hubbell SP. The Unified Neutral Theory of Biodiversity and Biogeography. Princeton: Princeton Univ Press; 2001. [DOI] [PubMed] [Google Scholar]
- 6.Lawton JH, editor. Population Dynamic Principles. New York: Oxford Univ Press; 1995. [Google Scholar]
- 7.Rabinowitz D. Seven forms of rarity. In: Synge H, editor. The Biological Aspects of Rare Plant Conservation. New York: John Wiley; 1981. pp. 205–217. [Google Scholar]
- 8.Hirzel AH, Hausser J, Chessel D, Perrin N. Ecological-niche factor analysis: How to compute habitat-suitability maps without absence data? Ecology. 2002;83:2027–2036. [Google Scholar]
- 9.VanDerWal J, Shoo LP, Johnson CN, Williams SE. Abundance and the environmental niche: Environmental suitability estimated from niche models predicts the upper limit of local abundance. Am Nat. 2009;174:282–291. doi: 10.1086/600087. [DOI] [PubMed] [Google Scholar]
- 10.Futuyma D, Moreno G. The evolution of ecological specialization. Annu Rev Ecol Syst. 1988;19:201–233. [Google Scholar]
- 11.McKinney ML. Extinction vulnerability and selectivity: Combining ecological and paleontological views. Annu Rev Ecol Syst. 1997;28:495–516. [Google Scholar]
- 12.Johnson CN. Species extinction and the relationship between distribution and abundance. Nature. 1998;394:272–274. [Google Scholar]
- 13.Williams SE. Patterns of mammalian species richness in the Australian tropical rainforests: Are extinctions during historical contractions of the rainforest the primary determinants of current regional patterns in biodiversity? Wildl Res. 1997;24:513–530. [Google Scholar]
- 14.Schneider CJ, Williams SE. Effects of Quaternary climate change on rainforest diversity: Insights from spatial analyses of species and genes in Australia's Wet Tropics. In: Bermingham E, Dick CW, Moritz C, editors. Tropical Rainforests: Past, Present and Future. Chicago: Chicago Univ Press; 2005. p. 745. [Google Scholar]
- 15.Williams SE, Pearson RG. Historical rainforest contractions, localized extinctions and patterns of vertebrate endemism in the rainforests of Australia's wet tropics. Proc R Soc London Ser B. 1997;264:709–716. doi: 10.1098/rspb.1997.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moritz C, Patton JL, Schneider CJ, Smith TB. Diversification of rainforest faunas: An integrated molecular approach. Annu Rev Ecol Syst. 2000;31:533–563. [Google Scholar]
- 17.Hilbert DW, Graham A, Hopkins MS. Glacial and interglacial refugia within a long-term rainforest refugium: The wet tropics bioregion of northeast Queensland. Palaeogeogr Palaeoclimatol Palaeoecol. 2007;251:104–118. [Google Scholar]
- 18.Hopkins MS, Head J, Ash JE, Hewitt RK, Graham AW. Evidence of a Holocene and continuing recent expansion of lowland rainforest in humid, tropical north Queensland. J Biogeogr. 1996;23:737–745. [Google Scholar]
- 19.Hocknull SA, Zhao JX, Feng YX, Webb GE. Responses of Quaternary rainforest vertebrates to climate change in Australia. Earth Planet Sci Lett. 2007;264:317–331. [Google Scholar]
- 20.Kirkpatrick M, Barton NH. Evolution of a species' range. Am Nat. 1997;150:1–23. doi: 10.1086/286054. [DOI] [PubMed] [Google Scholar]
- 21.Gaston KJ, et al. Abundance-occupancy relationships. J Appl Ecol. 2000;37:39–59. [Google Scholar]
- 22.Gaston KJ, Blackburn TM, Lawton JH. Interspecific abundance-range size relationships: An appraisal of mechanisms. J Anim Ecol. 1997;66:579–601. [Google Scholar]
- 23.Brown JH. On the relationship between abundance and distribution of species. Am Nat. 1984;124:255–279. [Google Scholar]
- 24.Hanski I, Kouki J, Halkaa A. Three explanations for the positive relationship between distribution and abundance of species. In: Ricklefs R, Schluter D, editors. Historical and Geographical Determinants of Community Diversity. Chicago: Univ Chicago Press; 1993. pp. 108–116. [Google Scholar]
- 25.Blackburn TM, Cassey P, Gaston KG. Variations on a theme: Sources of heterogeneity in the form of the interspecific relationship between abundance and distribution. J Anim Ecol. 2006;75:1426–1439. doi: 10.1111/j.1365-2656.2006.01167.x. [DOI] [PubMed] [Google Scholar]
- 26.Lawton JH, Nee S, Letcher AJ, Harvey PH. Animal distributions: Patterns and processes. In: Edwards PJ, May RM, Webb NR, editors. Large-Scale Ecology and Conservation Biology. London: Blackwell Scientific; 1994. pp. 41–58. [Google Scholar]
- 27.Komonen A, Paivinen J, Kotiaho JS. Missing the rarest: Is the positive interspecific abundance-distribution relationship a truly general macroecological pattern? Biology Letters. 2009;5:492–494. doi: 10.1098/rsbl.2009.0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams Y, Williams S, Alford R, Waycott M, Johnson C. Niche breadth and geographical range: Ecological compensation for geographical rarity in rainforest frogs. Biol Lett. 2006;2:532–535. doi: 10.1098/rsbl.2006.0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phillips SJ, Anderson RP, Schapire RE. Maximum entropy modeling of species geographic distributions. Ecol Modell. 2006;190:231–259. [Google Scholar]
- 30.Elith J, et al. Novel methods improve prediction of species' distributions from occurrence data. Ecography. 2006;29:129–151. [Google Scholar]
- 31.Hernandez PA, Graham CH, Master LL, Albert DL. The effect of sample size and pecies characteristics on performance of different species distribution modeling methods. Ecography. 2006;29:773–785. [Google Scholar]
- 32.Wisz MS, et al. Effects of sample size on the performance of species distribution models. Diversity and Distributions. 2008;14:763–773. [Google Scholar]
- 33.McMahon JP, Hutchinson MF, Nix HA, Ord KD. ANUCLIM User's Guide, Version 1. Canberra, Australia: Centre for Resource and Environmental Studies, Australian National University; 1995. p. 90. [Google Scholar]
- 34.Accad A, Neldner VJ, Wilson BA, Niehus RE. Remnant vegetation in Queensland: Analysis of remnant vegetation 1997–1999-2000–2001-2003, including regional ecosystem information. Brisbane, Australia: Queensland Herbarium, Queensland Environmental Protection Agency; 2006. [Google Scholar]
- 35.Williams SE, Pearson RG, Walsh PJ. Distributions and biodiversity of the terrestrial vertebrates of Australia's Wet Tropics: A review of current knowledge. Pacific Conservation Biol. 1996;2:327–362. [Google Scholar]
- 36.Stanton JP, Stanton D. Vegetation of the Wet Tropics bioregion of Queensland. Cairns, Australia: Wet Tropics Management Authority; 2005. [Google Scholar]
- 37.Williams SE, et al. Distributions, life history characteristics, ecological specialisation and phylogeny of the rainforest vertebrates in the AWT bioregion. Ecology. 2009 in press. [Google Scholar]
- 38.Diniz JAF, De Sant'ana CER, Bini LM. An eigenvector method for estimating phylogenetic inertia. Evolution. 1998;52:1247–1262. doi: 10.1111/j.1558-5646.1998.tb02006.x. [DOI] [PubMed] [Google Scholar]
- 39.Miles DB, Dunham AE. Comparative analyses of phylogenetic effects in the life history patterns of iguanid reptiles. Am Nat. 1992;139:848–869. [Google Scholar]
- 40.Maddison WP, Maddison DR. Mesquite: A modular system for evolutionary analysis. Version 2.01. 2009. www.mesquiteproject.org.