Abstract
G. Evelyn Hutchinson more than a half century ago proposed that one could characterize the ecological niche of a species as an abstract mapping of population dynamics onto an environmental space, the axes of which are abiotic and biotic factors that influence birth and death rates. If a habitat has conditions within a species' niche, a population should persist without immigration from external sources, whereas if conditions are outside the niche, it faces extinction. Analyses of species' niches are essential to understanding controls on species' geographical range limits and how these limits might shift in our rapidly changing world. Recent developments in ecology and evolutionary biology suggest it is time to revisit and refine Hutchinson's niche concept. After reviewing techniques for quantifying niches, I examine subtleties that arise because of impacts species have on their own environments, the density-dependent modulation of how individuals experience environments, and the interplay of dispersal and temporal heterogeneity in determining population persistence. Moreover, the evolutionary record over all time scales reveals a spectrum of rates of change in species' niches, from rapid niche evolution to profound niche conservatism. Substantial challenges revolving around the evolutionary dimension of the Hutchinsonian niche include quantifying the magnitude of evolved intraspecific and clade-level variation in niches and understanding the factors that govern where along the spectrum of potential evolutionary rates any given lineage lies. A growing body of theory provides elements of a conceptual framework for understanding niche conservatism and evolution, paving the way for an evolutionary theory of the niche.
Keywords: Allee effects, ecological niche, landscape texture, niche conservatism, niche evolution
The niche concept is central to ecology (1, 2). G. Evelyn Hutchinson in a seminal essay >50 years ago (1) proposed a formalization of the niche that still resonates in the minds of ecologists. After years of quiescence, there has been a recent upsurge of interest in species' niches (2, 3), driven in part by the urgency of predicting ecological responses to rapid environmental change. In this article, after summarizing Hutchinson's niche concept and sketching empirical approaches to quantifying niches, I will point out limitations in standard articulations of the Hutchinsonian niche. Some arise from its focus on a species solely when it is rare, which can belie the impact of positive density dependence and feedback processes on population persistence; others arise from its relative neglect of temporal variation and spatial dynamics; and yet others arise from genetic variation and evolution in the niche itself. These reflections will lead to alternative and complementary niche definitions, enriching and extending Hutchinson's concept.
The Hutchinsonian Niche
Hutchinson's principal writings on the niche (1, 4) mingle consideration of the basic niche concept with issues of species coexistence. But the niche is a useful autecological construct, independent of its implications for interspecific interactions and community organization (3, 5), for instance, by providing an essential conceptual tool for understanding range limits (3). The idea, simply stated, is that one can represent the potential environments an organism faces as an abstract space, with axes corresponding to environmental factors that affect organismal performance; the niche is a mapping of population dynamics onto this space. Hutchinson's exact words (ref. 1, p. 416) were that the fundamental niche is a volume in which “every point … corresponds to a state of the environment that would permit the species … to exist indefinitely” (presumably without immigration). As we shall shortly see, there is ambiguity in the phrase “exist indefinitely.” The 1957 article concluded a Cold Harbor symposium titled “Population Studies: Animal Ecology and Demography.” The demographic perspective on the niche was later formalized (4, 6) in terms of a logistic growth parameter, the intrinsic growth rate (in continuous time) r0(x) (= b − d, per-capita birth rate minus death rate, assessed at low density), expressed as a function of a vector of environmental states x. One may compactly define the Hutchinsonian niche of a population of a given species as that niche volume wherein its r0(x) ≥ 0. Regions of niche space with r0(x) < 0 are outside the niche. (It is conventional to refer to the abstract environmental space in which population dynamics is gauged as “niche space,” and I here follow this convention.)
When analyzing species coexistence, community ecologists recognize that one must consider not just invasion conditions (expressed as zero net-growth isoclines, where r0 = 0, defining niche boundaries in spaces with axes of abundances of resources, predators, etc.), but also the impact of species on their environments (7, 8). This enriched niche concept within community ecology, embracing impacts and requirements, has yet to influence the niche models currently being developed in distributional ecology. There has also been little attention in classical niche theory to how a species' density can directly modify the demographic consequences of the environment.
Quantifying the Niche
All niche quantifications require specification of what counts as the “environment,” which may be complex and require very specific natural history information. There are three complementary approaches to niche quantification, each with strengths and weaknesses. I do not intend to comprehensively review these approaches, but rather sketch them so as to provide an empirical context for some conceptual points below.
Experimental Niche Models.
One can construct a niche model by placing a species into a range of environments and monitoring its success. An early population study by Birch (9) exemplifies this direct approach. He estimated mortality and fecundity schedules to calculate r0 for two beetle species introduced into microcosms at different food moistures and temperatures, leading to the graphical model of Fig. 1A. This niche model qualitatively explains range limits in Australia (9); along a transect into the arid interior (from X to Z in Fig. 1A), this species drops out. A recent lab study of Daphnia magna similarly characterized r0 as a function of pH and Ca2+ concentration, generating a statistical response model that helped explain distributional limits (10). In the field, one can carry out transplant experiments across range edges and assess demographic performance. When carefully conducted, such experiments are potentially a powerful tool for making inferences about niches (e.g., refs. 11–13), yet such transplant studies are scarce (14). Although surely hundreds (if not thousands) of empirical studies provide insights into species' niches, few quantify r0 across wide swaths of environmental space (as in refs. 9 and 10), particularly at biogeographic (rather than local habitat) scales where multiple niche axes are likely at play. It is clear why experimental assessments of the niche are relatively rare, because they are laborious, focus on small and manageable organisms, require a good a priori sense of relevant environmental variables, and may be difficult to run long enough to capture important, but rare, events.
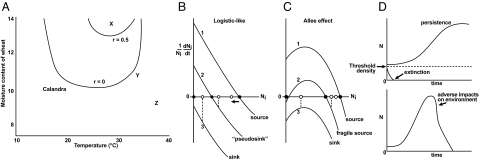
Fig. 1.
The niche and population dynamics. (A) Experimental niche model for a beetle, Calandra oryzae (L): intrinsic growth rate at different temperature and moisture values (9). The isopleth r = 0 defines the niche boundary. Site X is in the niche; sites Y and Z are not. (B) Per-capita growth rate at three locations along a gradient, expressed as functions of local density. Growth is logistic; without movement, maximal growth rate is at n = 0, and equilibrium is at carrying capacity K (black dots). Sites 1 and 2 are within the range; site 3 is outside of the range. Adding constant dispersal shifts local abundances toward the white dots; high-K sites are lowered (site 1); low-K sites (e.g., site 2) are raised above K (growth rate is negative; such sites are called “pseudosinks”); and sites (e.g., site 3) outside the niche sustain sink populations. (C) Allee effects along gradient. At low N, per-capita growth rate increases with N (this could be direct density dependence or indirect density dependence with short time lags). Site 2 has a positive, locally stable equilibrium; a population can persist even if it cannot increase when rare. Site 2 is within the population persistence niche, but outside of the establishment niche. (D) (Upper) Alternative local dynamics with Allee effects. (Lower) Local dynamics given niche destruction.
Mechanistic Niche Models.
“Bottom-up” niche models synthesize knowledge of individual performance as a function of environmental conditions into a demographic model in which parameters are functions of position in niche space (e.g., refs. 15–18). Such models are essential bridges between lower-level organismal traits and population-level predictions about species' distributions and responses to environmental change. Again, as with the “brute force” approach of conducting experiments to characterize the niche, developing these models is challenging and even the best to date have limitations, requiring field experiments or observations to estimate key parameters.
Statistical Niche Models (SNMs).
There has been dramatic growth in the development of what are often simply called “ecological niche models” (19). These take spatially explicit knowledge of species geographical distributions and suites of environmental variables and relate the two datasets by using various protocols (20, 21) to generate a species distributional or predictive habitat model portraying a species' realized niche, as expressed in its habitat breadth or geographical range (19, 22, 23). I suggest that the term ecological niche model be used broadly to encompass experimental and mechanistic process-based models of the niche as well as such statistical distributional models. Although there are limitations in this “top-down” approach (e.g., it requires a species' distribution to be well-known and near equilibrium), and many methodological issues need improvement (24–27), it has considerable value. For instance, for rare or poorly understood organisms, SNMs provide a starting point for projecting impacts of environmental change (e.g., ref. 28) and crafting efficient survey designs (25, 28). One welcome recent trend is that multiple approaches are being applied simultaneously (29, 30). SNMs, for instance, can guide site selection for experimental analyses of range limits (e.g., ref. 31), field experiments can boost confidence in SNMs and mechanistic models (32), and mechanistic models in turn can help winnow the field of potentially important variables in SNMs or experimental studies (29).
The Establishment Niche Versus the Population Persistence Niche
These empirical approaches to niche quantification all aim at defining conditions for a species to increase when rare, i.e., r0 > 0 (3), as a function of a set of environmental variables. Along a gradient across a niche boundary, as in Fig. 1A, a logistic-like model for spatial variation in local population dynamics might be depicted as in Fig. 1B. With low dispersal, a range limit along the gradient should be anchored wherever population growth shifts from positive to negative values (i.e., between sites 2 and 3). The match between Hutchinsonian niches and species' distributions will often be imperfect and for many reasons (29, 33). Hutchinson himself (1, 4) noted that species' distributions could be constrained by competition, so realized niches could be less than fundamental niches. Dispersal can create source-sink dynamics in which immigration maintains species in habitats outside of their niches (33). Conversely, dispersal limitation may prevent species from occupying perfectly suitable but isolated habitats.
But it is less widely recognized in the literature of distributional ecology that positive and negative feedbacks, including impact components of the niche (7, 8), can have large effects on distributions. Because of feedbacks, the domain of niche space where a species can establish when rare (i.e., have a positive r0) may differ, sometimes greatly, from where it can persist, if at low densities there is sufficiently strong positive density dependence, so that growth rates increase with population size. Courchamp et al. (34) argue that such Allee effects, the various reasons population growth rate can increase with increasing density, are ubiquitous. For instance, in sexual species, at low densities mating pairs may not easily form. Interspecific interactions can imply Allee effects [e.g., prey can enjoy reduced mortality because increasing numbers satiate predators; predators can glean cues from each other to find prey (35)]. A species' density thus can directly modulate how it experiences its environment. More broadly, feedback effects in complex webs of interacting species, where interspecific interactions can at times be stronger than intraspecific interactions, can lead to alternative states, and many taxa modify abiotic environments in ways that enhance their own recruitment (36), leading to indirect Allee effects. When the full niche is projected onto a subset of niche axes, such positive feedbacks can inflate the range of conditions tolerated by a species along those axes, so that populations persist in sites where the species could not invade. Fig. 1C shows a schematic model for a population with Allee effects along a gradient. Site 2 is outside of the Hutchinsonian fundamental niche; a small colonizing propagule faces extinction. But if the species surmounts a threshold density, it persists. Species can thus be found at sites with conditions outside of the Hutchinsonian niche, as assessed by r0.
To deal with this ambiguity in the original concept of the fundamental niche I suggest that one should distinguish: the establishment niche, that range of niche space in which a population has a positive intrinsic growth rate when very rare, i.e., r0 > 0, from the population persistence niche, that range of niche space in which populations above some threshold density N > 0 can persist.
Given the vicissitudes of demographic stochasticity, extinction during colonization can occur even in favorable environments within the establishment niche (37, 38). However, given enough time, with recurrent episodes of colonization attempts all but absolute dispersal barriers should be breached. By contrast, for a species to occur at locations within its population persistence niche but outside of its establishment niche, it must be present in sufficient numbers and for long enough for positive feedbacks to act and boost growth above zero. There are several ways this can happen: (i) Large dispersal pulses can push a local population above its Allee threshold, for instance because of a transient surge of productivity in a source. (ii) Dispersers from multiple sources may pool; the likelihood of this occurring depends on the spatial configuration of occupied and unoccupied sites and the severity of dispersal barriers (39). (iii) The environment might change; if the environment initially permits positive growth at low densities (as in in Fig. 1C, site 1) a trickle of immigrants should suffice for establishment. Later deterioration can create a “fragile source,” which persists although it could not establish. Positive feedbacks in the niche magnify the impact of history and the contingencies of landscape geometry in generating species distributional ranges.
Niche Destruction
So in theory, the population persistence niche of a species can include a wider range of environments, at least along some axes, than found in its establishment niche (Fig. 2A). Conversely, the establishment niche can at times exceed the persistence niche. Organisms both construct (40) and destroy their own niches. Organisms by consuming resources degrade their environments, and given time lags in resource renewal, can exceed carrying capacity and crash. A classic example is the introduction of reindeer onto St. Matthew Island, where freed of predation and with abundant food the population surged, depleted their food, and then plummeted to extinction (41). Pathogens can cause host extinctions (42), particularly where the host is abundant (43), precisely where the disease should increase most rapidly if initially rare. Such populations are initially within the establishment niche, so that populations initially increase. But then severe time-lagged negative feedbacks emerge that doom the population; these locations lie outside of the population persistence niche. In Fig. 2B, the hatched region denotes slices of niche space where a species increases when rare but then faces extinction because of its impacts (Fig. 1D).
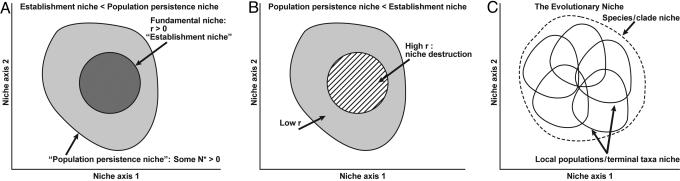
Fig. 2.
Alternative niche concepts. (A) With Allee effects, the population persistence niche > the establishment niche. (B) With niche destruction, sites with a high initial growth rate are vulnerable to extinction, so lie outside of the population persistence niche, along these axes. In plant ecology, the term persistence niche is sometimes used to denote the role of individual adult survival through disturbances in permitting population persistence, for populations that have already established (90). I am using the term population persistence niche more broadly, to emphasize distinct aspects of the niche at the population rather than individual level. (C) The evolutionary niche. This figure (adapted from ref. 64) can describe many scenarios: (i) a species with geographic variation among niches of local populations; (ii) frames of a species over time, with niches wandering over its history within broad limits (bounded niche evolution); (iii) aggregate niche of a clade, with specialists descended from an ancestral generalist; and (iv) an adaptive radiation, where from an ancestral specialist (not shown) a clade collectively wanders through much more niche space than represented in any species.
It is not clear how important these theoretical possibilities are in practice. But if a species is in approximate demographic equilibrium across its range, its realized abundances will reflect the entire suite of positive and negative feedbacks over short to long time scales that regulate its numbers. Feedback effects may in some cases be quantitatively large in governing species' distributions, but such effects have been largely neglected in all three of the approaches to quantifying niches sketched above.
Stitching the Niche
“Organisms juxtapose bits and pieces of the world and so determine what is relevant” (ref. 44, p. 33).
Species threatened by local extinction may nonetheless persist in variable environments because of mechanisms permitting survival in situ (e.g., seed banks for early successional species), or colonization permits establishment at empty sites, or dispersal in other ways moderates the impact of local harsh conditions. Classical views of the niche (4, 8) pay little attention to the interplay of temporal variation and dispersal that many species require, yet dispersal and the spatial pattern of habitat availability can vitally determine persistence (45) and thus the niche. Consider a spatially closed population with discrete generations and no density dependence in a time-varying environment, so N(t +1) = R(t)N(t) or X(t +1) = X(t) + r(t); here N(t) and R(t) are population size and recruitment per individual in generation t, and X(t) = ln(N(t)), r(t) = ln(R(t)). If environmental variation is stationary, r(t) has a constant mean r̄ and variance. When r̄ < 0, all population trajectories decline toward extinction, independent of variance and autocorrelation in r̄ (38). A natural generalization of the Hutchinsonian fundamental niche to temporally varying environments is that a habitat has conditions within the species niche if r̄ > 0, and outside it if r̄ < 0.
But characterizing the niche by r̄ alone is inadequate in spatially open populations (45). Temporal variance and autocorrelation in r, and the spatial patterning of this variation, all influence persistence. This is obvious for weedy species requiring colonization into empty sites after disturbance or pathogens that must infect fresh hosts to replace infected hosts they kill, but the point pertains to any species in spatially open and temporally varying environments. Theoretical models (46) and experiments (47) show that when local populations are linked by dispersal, a positive autocorrelation through time in local growth rates that is to a degree spatially uncorrelated can permit persistence, even if the average growth rate in each patch predicts extinction [and adaptive habitat selection further enhances persistence (48)].
By moving among favorable locations in a spatiotemporally varying environmental palette domain, organisms can stitch together viable niches. In classic metapopulations local populations go extinct, yet the entire ensemble persists via colonization into empty patches (49, 50). Such species' niches must be characterized at larger spatial scales than any single population, and landscape properties such as matrix permeability define range limits (51–53). For many species, dispersal and the spatial structure of the environment (e.g., connectedness) are integral to characterizing the niche. The literature on ecological niche models outlined above has yet to adequately address the challenges of scale, spatial and temporal heterogeneity, and dispersal-mediated processes in metapopulations and metacommunities in quantifying the niche.
The Evolutionary Niche
The ecological niche to a first approximation defines the environmental context within which adaptive evolution occurs (54) and niches themselves can evolve. I have examined Hutchinson's niche concept making the tacit assumption that a species has a niche. If this species is a single genetic clone, this assumption is reasonable, and it sometimes also holds for genetically variable species across time and space and for phylogenetic clades. The term “niche conservatism” is used when (relative to an appropriate yardstick, and to a reasonable approximation) a species' niche is constant across its range, or its evolutionary history, or when related species have not diverged from an ancestral niche (54, 55). Many examples do suggest niche conservatism (e.g., at range limits; refs. 56 and 57), often over long time scales. Yet there can be intraspecific genetic variation in the niche, fueling rapid niche evolution (58) both within species and across clades. What is needed is a conceptual framework to help understand why any given species or clade lies where it does on the spectrum from rigid stasis to fluid niche plasticity, building on the rich classical evolutionary literature on the evolution of specialization and generalization (59).
It is useful to distinguish several empirical questions one can pose about niche conservatism and evolution. Is evolution more likely to be rapid along some axes in niche space than along others? For instance, some niche variables may vary largely at biogeographical scales, whereas others such as soil pH may vary at fine scales, and niche evolution may differ as a function of the spatial scale of environmental variation. Is niche evolution “bounded” (Fig. 2C), and if so, what are the relative contributions of external forces (e.g., interspecific interactions) and internal variables (e.g., genetic architecture, life histories) to these bounds? Niche evolution may be quite different if niche axes themselves are dynamical variables responsive over short time scales to impacts of the species itself (e.g., resources that can be consumed, natural enemies that can coevolve) versus relatively static and coarse-scaled.
All of these questions can be addressed at different organizational levels.
Species-Level Questions.
Within a population, is there segregating genetic variation permitting niche evolution in response to a change in conditions? Across a species' range, do local populations have different niches? If so, is there variation along some niche dimensions, with little along others? What underlying factors (e.g., genetic, organismal, historical, community) explain interspecific differences in intraspecific niche variation? Does niche evolution often contribute to a species' ability to persist in changing environments, does the rate of niche evolution change over time, and how is such evolution influenced by plasticity?
Clade-Level Questions.
Within a given clade above the species level, do niches evolve among species, and if so, how fast and for what reasons? Niches will likely evolve as diversity unfolds, but do they wander only within a bounded domain of niche space (Fig. 2C) or instead shift in unconstrained Brownian motion (60)? There are significant methodological issues only beginning to be addressed in the study of phylogenetic niche conservatism; for instance, what is the appropriate null or neutral model for niche evolution (61)? Are there predictable patterns of niche evolution during evolutionary diversification of a clade?
Community-Level Questions.
For a given environmental change, some species or clades in a local community exposed to that change may show niche evolution, whereas others display niche conservatism. What accounts for such interspecific and interclade differences? How does community structure itself influence the scope for niche evolution?
Losos (60) has argued that many ecological traits are labile (and this is surely true), but does note that “no study to date has conducted a formal, comparative study of the niche of a clade.” Species can differ greatly in their propensities for niche evolution along any given niche axis. As an example of differences in rates of niche evolution within a plant community, along an axis of toxic heavy metal concentration in the soil on mine tailings a few centuries old in England, classic studies by A. D. Bradshaw and his students showed some species exhibited substantial niche expansion via the evolution of tolerance to the toxic soil, but many others in the same communities did not (62). Bradshaw suggested the latter simply had no genetic variation for adaptation along this particular niche axis, whereas species that expanded their niche to include the toxic soils had preexisting genetic variation for tolerance. This may answer the initial question as to why some species in the community rapidly evolved tolerance, whereas others did not, but raises a deeper problem of understanding why some species harbor genetic variation permitting evolution along some niche axes, whereas other cohabiting species do not. Evolutionary biologists often assume that characters always have genetic variation upon which natural selection can act, but there is growing evidence that in some species, genetic variation may be absent for key traits determining niches and range limits, particularly in sparse populations prone to bottlenecks, or genetic interactions among traits that may constrain responses to selection (63, 64).
Some studies strongly suggest marked intraspecific geographical niche variation. The most direct evidence comes from transplant experiments across a range of environments. Reciprocal transplants of lodgepole pine, Pinus contorta, across its thermal range in western North America have been used to construct performance curves (seedling survival and growth) as functions of the difference in temperature between sites of origin and transplantation (65). Propagules from southern populations failed to establish and/or grow in northern climes, and vice versa. The results provide a prima facie case for intraspecific geographic variation in climatic dimensions of the niche. Were a continent-wide catastrophe to wipe out all but one population, which then provided seedlings for range restoration, the geographical range initially resulting from the surviving population would be a fraction of the original range. A reviewer has suggested that the literature of applied biology may be implicitly replete with such examples of intraspecific geographical variation in niches. At a more local scale, there are some excellent examples of genotypes with sharply different niche requirements. For instance, along a thermal gradient along a channel emanating from a hot spring, different strains of the cyanobacterium Synechoccus lividus sort out in accord with different maximal thermal tolerance (66, 67). Such case studies demonstrate that a species' total ecological niche can encompass more specialized niches among local populations (Fig. 2C), and that even at a local scale there at times can be substantial genetic variation in the niche.
Explicit experimental assessments of niche variation such as these (65, 66) are still scarce, although many classic examples of rapid evolution (e.g., evolution of pesticide and antibiotic resistance) surely involve niche evolution and would repay examination through the lenses of niche models. An alternative approach is to use correlational niche models to make inferences about intraspecific niche variation. One such model for several Mexican birds found plausible instances of large intraspecific differences in the niche (68). Although nonevolutionary explanations cannot be ruled out (68, 69), this result is consistent with evolved intraspecific niche variation across species' ranges. SNMs based on the entire geographic ranges of species might thus often overestimate the niche that pertains to local populations, leading to inaccurate predictions about responses to global change. An increasing number of case studies using SNMs do suggest intraspecific niche evolution (e.g., refs. 70 and 71). Coupled with appropriate null models (60, 61), and (ideally) experimental approaches and mechanistic models, SNMs potentially provide useful tools for identifying examples of niche conservatism and quantifying niche evolution at the species and higher levels.
Mechanistic niche models can be turned into evolutionary models by assuming that model parameters are influenced by genetic variation, and that expressions for per-capita growth rates describe fitness. For this protocol to address niche evolution, one must attend to many scales of biological organization and the environmental and landscape context of evolution, from the details of genetic variation and architecture influencing each model parameter (and covariance among parameters), the “texture” of the environment in space and time, whole-organism responses (in particular demographic responses) to this texture, and community interactions and ecosystem feedbacks. Theoretical studies have made initial steps in this direction and have begun to clarify genetic and environmental settings promoting niche conservatism versus evolution (72, 73), providing provisional answers to some of the questions posed above.
Consider a species in a landscape with two kinds of habitat patches. It is well-adapted to one habitat type but disperses occasionally into the other, outside of its niche. Will the species show niche conservatism or instead niche evolution? Without genetic variation permitting adaptation to the other habitats, one, of course, expects niche conservatism. Theoretical studies suggest that even given genetic variation, however, demographic constraints may greatly slow niche evolution. Assume dispersal is pulsed and sporadic. When a colonizing propagule lands in a patch with conditions outside of the niche, its numbers will decline, but selection will often increase mean fitness; for persistence, adaptation must occur fast enough for numbers to rebound before extinction. A broad range of models lead to similar conclusions (72–75): adaptive colonization outside of the niche is unlikely into harsh sinks and is unlikely with only a few colonizing individuals. If initial fitness in the sink is low, thousands of colonizing “trials” may occur before adaptive colonization and hence niche evolution occurs, potentially implying millenial-scale conservatism (72). Thus, niche conservatism is likely if colonizing propagules are small in number and infrequent, and there are large differences in the fitness optima of different habitats, leading to very negative growth rates for colonizing propagules. Increasing “propagule pressure” conversely facilitates niche evolution, particularly if initial growth rates are only moderately low.
Different niche dimensions can interact to influence niche conservatism and evolution (54). Imagine the second habitat is outside of the niche because of predation and unfavorable abiotic conditions. If the predator is absent, these patches might now be within the niche, even though abiotic conditions are suboptimal. After establishment, the species can adapt, which may in turn allow it to reproduce sufficiently to withstand the predator, when it finally reappears. Shifts in community composition are likely strong drivers of niche evolution. Whether or not niche evolution or conservatism is observed depends both on factors intrinsic to a species (e.g., genetic variation, and how organismal function integrates different niche axes into demographic responses), and extrinsic factors (the spatiotemporal structure of the environment, including other species).
With recurrent dispersal, sink populations may be sustained outside of the niche (33, 75). Theoretical studies have clarified the manifold effects of movement on the likelihood of niche evolution (73, 76, 77). The rate of immigration influences population size in the sink; depending on the nature of density dependence, immigration can hamper adaptation (78) or facilitate it (Allee effects; ref. 79). Recurrent immigration also provides a conduit for genetic variation from the source, at times fostering niche evolution (73, 79). Experimental study of the evolution of resistance to antibiotics in Pseudomonas aeruginosa showed that for this asexual species increasing immigration facilitates adaptation to sinks; moreover, as predicted by theory, the harsher the sink, the slower is adaptation (80). However, recurrent immigration can also hamper niche evolution, if reproduction between maladapted immigrants and better-adapted residents lowers the latter's fitness (the classic scenario of gene flow vs. selection; ref. 81). The interplay of gene flow and selection can generate alternative evolutionary states (82), and emergent niche properties of a species can strongly depend on initial conditions. Details of genetic architecture matter. For instance, for alleles of small effect, increasing bidirectional movement usually facilitates adaptation to a sink (83), but for alleles of large effect, intermediate rates of movement are least favorable for their retention (84, 85).
Theoretical studies have barely scratched the surface of the universe of possible genetic architectures, landscape geometries, and demographic scenarios at play in niche evolution. In a sense, three spaces or “landscapes” must be considered simultaneously to understand niche evolution. (i) The niche itself is a kind of landscape: for a given genotype or phenotype, it is a mapping of performance (e.g., establishment, or population persistence) onto an abstract environmental space. (ii) For a given set of environmental conditions (broadly defined to include population size and composition), there may be a fitness or adaptive landscape describing fitnesses of alternative phenotypes in that environment, which determines the direction of selection. (iii) And then, there is the real landscape, the mapping of environmental states across space (e.g., as gradients, or discrete habitat patches, or fractal laces) that defines the spatial context of both population persistence and evolutionary transformation. A species' niche may readily evolve along gentle spatial gradients in the environment, yet show tight conservation in a different landscape geometry, such as abrupt transitions between the two ends of that same gradient.
Fig. 2C could also represent the summed ecological niche of a phylogenetic clade that has unfolded over time. For instance, an ancestor with a generalist niche may have spawned specialized descendents, without overall expansion of the clade's aggregate niche. Or a clade may be expanding across niche space, but within broadly defined bounds. A recent study (86) suggests that patterns of morphological evolution in the fossil record are best explained by a model of evolution with adaptive optima moving between fixed limits. Niches over evolutionary time scales may also shift, but within limits that in some clades are quite constrained, and in others are loose and almost nonexistent. One complication is that there is a strong scale dependence in assessments of niche conservatism and evolution. Anoline lizards in the West Indies show examples of both conservatism and substantial lability in the niche, assessed with SNMs developed for terrestrial environments (e.g., climate, topography; ref. 87). Yet no anoles have colonized marine environments, so along a niche axis spanning marine and terrestrial environments, the clade has been completely conservative. This may not be surprising, but if there is a marine iguana in the Galapagos, why not a marine anole in the West Indies?
Losos (60) suggests phylogenetic niche conservatism requires that species have more similar niches than expected, given a Brownian model of evolution. This is an interesting suggestion, but it has two potential weaknesses. First, two clades in a given environment might both show Brownian motion in their niches, but with different rate constants. It seems reasonable that the one evolving more slowly against the environmental template be deemed to have greater niche conservatism (88). Second, assessments of niche conservatism can be scale-dependent (89) and may be sensitive to how data are transformed for statistical purposes. Consider a linear transect across the bounded niche evolution shown in Fig. 2C. Let x be position along this axis, with edges by convention at 0 and 1, within which a species' niche wanders over evolutionary time. This bounded domain can be transformed to an infinite domain by letting y = log[x/(1 − x)]. Now assume that evolution within the clade along this new transformed axis is described by a Brownian process. If we use y as our metric, using a Brownian motion criterion, there is no niche conservatism; using x, by contrast, there is. Despite these caveats, Losos' suggestion (ref. 60 and see also refs. 61 and 88) cogently highlights the need for appropriate yardsticks for assessing the degree of conservatism in species' niches. I suggest that one cannot rigorously assess niche conservatism or evolution solely by comparing species in a clade to one another, but must somehow relate the clade as a whole to a wider domain of possibilities, defined by outgroups, or even better by the template of environmental possibilities within which evolutionary dynamics plays out. It is true that niches cannot be defined independent of organisms (44); it is also true that it makes no sense to talk about niches without reference to external environments.
A cross-cutting conclusion from the discussion of ecological subtleties in the niche concept reflected on above is that patterns of dispersal and population connectivity are not merely perturbations of species' distributions from the inherent mapping of niches onto distributions, but are subtly woven into the very fabric of the niche itself. In like manner, patterns of dispersal playing out against a shifting mosaic of spatiotemporally shifting selective pressure and demographic asymmetries surely loom large in determining whether species' niches are highly labile or instead tightly conserved over evolutionary time. Hutchinson's niche concept, 50-plus years after its formalization, when enriched with an appreciation of space, feedbacks, density-dependent impacts on persistence, and evolution, is as lively and important now, as ever.
Acknowledgments.
I thank Mike Barfield and David Hall for assistance; two reviewers, Jon Chase, Jonathan Losos, and David Ackerly for thoughtful comments; and grant NSF EF-0525751 and the University of Florida Foundation for support. This is a contribution of the joint NCEAS-NESCent working group “Mechanistic Distribution Models: Energetics, Fitness, and Population Dynamics”.
Footnotes
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “Biogeography, Changing Climates and Niche Evolution,” held December 12–13, 2008, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. The complete program and audio files of most presentations are available on the NAS web site at www.nasonline.org/Sackler_Biogeography.
The author declares no conflict of interest.
This article is a PNAS Direct Submission. D.D.A. is a guest editor invited by the Editorial Board.
References
- 1.Hutchinson GE. Concluding remarks. Cold Spring Harbor Symp. 1957;22:415–427. [Google Scholar]
- 2.Schoener TW. In: The Encyclopedia of Ecology. Levin SA, editor. Princeton: Princeton Univ Press; 2009. pp. 3–13. [Google Scholar]
- 3.Soberon J. Grinnellian and Eltonian niches and geographic distributions of species. Ecol Lett. 2007;10:1115–1123. doi: 10.1111/j.1461-0248.2007.01107.x. [DOI] [PubMed] [Google Scholar]
- 4.Hutchinson GE. An Introduction to Population Ecology. New Haven, CT: Yale Univ Press; 1978. [Google Scholar]
- 5.James FC, Johnston RF, Warner NO, Niemi G, Boecklen W. The Grinnellian niche of the Wood Thrush. Am Nat. 1984;124:17–47. [Google Scholar]
- 6.Maguire B., Jr Niche response structure and the analytical potentials of its relationship to the habitat. Am Nat. 1973;10:213–246. [Google Scholar]
- 7.Leibold MA. The niche concept revisited: Mechanistic models and community context. Ecology. 1985;76:1371–1382. [Google Scholar]
- 8.Chase JM, Leibold MA. Ecological Niches: Linking Classical and Contemporary Approaches. Chicago: Univ Chicago Press; 2003. [Google Scholar]
- 9.Birch LC. Experimental background to the study of the distribution and abundance of insects. I. The influence of temperature, moisture, and food on the innate capacity for increase of three grain beetles. Ecology. 1953;34:698–711. [Google Scholar]
- 10.Hooper HL, et al. The ecological niche of Daphnia magna characterized using population growth rate. Ecology. 2008;89:1015–1022. doi: 10.1890/07-0559.1. [DOI] [PubMed] [Google Scholar]
- 11.Moore KA. Fluctuating patch boundaries in a native annual forb: The roles of niche and dispersal limitation. Ecology. 2009;90:378–387. doi: 10.1890/07-1753.1. [DOI] [PubMed] [Google Scholar]
- 12.Angert AL, Schemske DW. The evolution of species' distributions: Reciprocal transplants across the elevation ranges of Mimulus cardinalis and. M lewisii. Evolution (Lawrence, Kans.) 2005;59:1671–1684. [PubMed] [Google Scholar]
- 13.Angert AL. Growth and leaf physiology of monkeyflowers with different altitude ranges. Oecologia. 2006;148:183–194. doi: 10.1007/s00442-006-0361-z. [DOI] [PubMed] [Google Scholar]
- 14.Geber M. To the edge: Studies of species' range limits. New Phytol. 2008;178:225–227. doi: 10.1111/j.1469-8137.2008.02414.x. [DOI] [PubMed] [Google Scholar]
- 15.Kearney M, et al. Modeling species distributions without using species distributions: The cane toad in Australia under current and future climates. Ecography. 2008;31:423–434. [Google Scholar]
- 16.Buckley LB, Roughgarden J. Effect of species interactions on landscape abundance patterns. J Anim Ecol. 2005;74:1182–1194. [Google Scholar]
- 17.Crozier L, Dwyer G. Combining population-dynamic and ecophysiological models to predict climate-induced insect range shifts. Am Nat. 2006;167:853–866. doi: 10.1086/504848. [DOI] [PubMed] [Google Scholar]
- 18.Hoogenboom MO, Connolly SR. Defining fundamental niche dimensions of corals: Synergistic effects of colony size, light, and flow. Ecology. 2008;90:767–780. doi: 10.1890/07-2010.1. [DOI] [PubMed] [Google Scholar]
- 19.Peterson AT. Predicting species' geographic distributions based on ecological niche modeling. Condor. 2001;103:599–605. [Google Scholar]
- 20.Austin M. Species distribution models and ecological theory: A critical assessment and some possible new approaches. Ecol Model. 2006;200:1–19. [Google Scholar]
- 21.Phillips SJ, Anderson RP, Schapire RE. Maximum entropy modeling of species geographic distributions. Ecol Model. 2006;190:231–259. [Google Scholar]
- 22.Guisan A, Zimmermann NE. Predictive habitat distribution models in ecology. Ecol Model. 2000;135:147–186. [Google Scholar]
- 23.Hirzel A, Le Lay G. Habitat suitability modeling and niche theory. J Appl Ecol. 2008;45:1372–1381. [Google Scholar]
- 24.Jeschke JM, Strayer DL. Usefulness of bioclimatic models for studying climate change and invasive species. Ann NY Acad Sci. 2008;1134:1–24. doi: 10.1196/annals.1439.002. [DOI] [PubMed] [Google Scholar]
- 25.Guisan A, et al. Using niche-based models to improve the sampling of rare species. Conserv Biol. 2006;20:501–511. doi: 10.1111/j.1523-1739.2006.00354.x. [DOI] [PubMed] [Google Scholar]
- 26.Seguardo P, Araujo MB, Kunin WE. Consequences of spatial autocorrelation for niche-based models. J Appl Ecol. 2006;43:433–444. [Google Scholar]
- 27.Parolo G, Rossi G, Ferrarini A. Toward improved species niche modeling: Arnica montana in the Alps as a case study. J Appl Ecol. 2008;45:1410–1418. [Google Scholar]
- 28.Thorn JS, Nijman V, Smith D, Nekaris KAI. Ecological niche modeling as a technique for assessing threats and setting conservation priorities for Asian slow lorises (Primates: Nycticebus) Divers Distrib. 2009;15:289–298. [Google Scholar]
- 29.Guisan A, et al. Making better biogeographical predictions of species' distributions. J Appl Ecol. 2006;43:386–392. [Google Scholar]
- 30.Brown KA, Spector S, Wu W. Multiscale analysis of species introductions: Combining landscape and demographic models to improve management decisions about non-native species. J Appl Ecol. 2008;45:1639–1648. [Google Scholar]
- 31.Cunningham HR, Rissler LJ, Apodaca JJ. Competition at the range boundary in the slimy salamander: Using reciprocal transplants for studies on the role of biotic interactions in spatial distributions. J Anim Ecol. 2009;78:52–62. doi: 10.1111/j.1365-2656.2008.01468.x. [DOI] [PubMed] [Google Scholar]
- 32.Ebeling SK, Welk E, Auge H, Bruelheide H. Predicting the spread of an invasive plant: Combining experiments and ecological niche models. Ecography. 2008;31:709–710. [Google Scholar]
- 33.Pulliam HR. On the relationship between niche and distribution. Ecol Lett. 2000;3:349–361. [Google Scholar]
- 34.Courchamp F, Berec L, Gascoigne J. Allee Effects in Ecology and Conservation. New York: Oxford Univ Press; 2008. [Google Scholar]
- 35.Fletcher RJ. Emergent properties of conspecific attraction in fragmented landscapes. Am Nat. 2006;168:207–219. doi: 10.1086/505764. [DOI] [PubMed] [Google Scholar]
- 36.Wilson JB, Agnew AD. Positive-feedback switches in plant communities. Adv Ecol Res. 1992;23:263–336. [Google Scholar]
- 37.MacArthur RH, Wilson EO. The Theory of Island Biogeography. Princeton: Princeton Univ Press; 1968. [Google Scholar]
- 38.Lande R, Engen S, Saether BE. Stochastic Population Dynamics in Ecology and Conservation. New York: Oxford Univ Press; 2003. [Google Scholar]
- 39.Keitt TH, Lewis MA, Holt RD. Allee dynamics, critical phenomena, and species borders. Am Nat. 2001;157:203–216. doi: 10.1086/318633. [DOI] [PubMed] [Google Scholar]
- 40.Odling-Smee FJ, Laland KN, Feldman MW. Niche Construction: The Neglected Process in Evolution. Princeton: Princeton Univ Press; 2003. [Google Scholar]
- 41.Klein DR. The introduction, increase, and crash of reindeer on St. Matthew Island. J Wildlife Manage. 1968;32:350–367. [Google Scholar]
- 42.de Castro F, Bolker B. Mechanisms of disease-induced extinction. Ecol Lett. 2005;8:117–126. [Google Scholar]
- 43.Antonovics J. The effect of sterilizing diseases on host abundance and distribution along environmental gradients. Proc R Soc London Ser B. 2009;276:1443–1448. doi: 10.1098/rspb.2008.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lewontin R, Levins R. Biology Under the Influence: Dialectical Essays on Ecology, Agriculture, and Health. New York: Monthly Review Press; 2007. [Google Scholar]
- 45.Chesson P. General theory of competitive coexistence in spatially varying environments. Theor Popul Biol. 2000;58:211–237. doi: 10.1006/tpbi.2000.1486. [DOI] [PubMed] [Google Scholar]
- 46.Roy M, Holt RD, Barfield M. Temporal autocorrelation can enhance the persistence and abundance of metapopulations comprised of coupled sinks. Am Nat. 2005;166:246–261. doi: 10.1086/431286. [DOI] [PubMed] [Google Scholar]
- 47.Matthews DP, Gonzalez A. The inflationary effects of environmental fluctuations ensure the persistence of sink metapopulations. Ecology. 2007;88:2848–2856. doi: 10.1890/06-1107.1. [DOI] [PubMed] [Google Scholar]
- 48.Schmidt KA. Site fidelity in temporally correlated environments enhances population persistence. Ecol Lett. 2004;7:176–184. [Google Scholar]
- 49.Levins R. Some demographic and genetic consequences of environmental heterogeneity for biological control. Bull Entomol Soc Am. 1969;15:237–240. [Google Scholar]
- 50.Hanski I. Metapopulation Ecology. Oxford: Oxford Univ Press; 1999. [Google Scholar]
- 51.Carter RN, Prince SD. Epidemic models used to explain biogeographical distribution limits. Nature. 1981;293:644–645. [Google Scholar]
- 52.Holt RD, et al. Theoretical models of species' borders: Single-species approaches. Oikos. 2005;108:28–46. [Google Scholar]
- 53.Harding KC, McNamara JM. A unifying framework for metapopulation dynamics. Am Nat. 2002;160:173–185. doi: 10.1086/341014. [DOI] [PubMed] [Google Scholar]
- 54.Holt RD, Gaines MS. Analysis of adaptation in heterogeneous landscapes: Implications for the evolution of fundamental niches. Evol Ecol. 1992;6:433–447. [Google Scholar]
- 55.Wiens JJ, Graham CH. Niche conservatism: Integrating evolution, ecology, and conservation biology. Annu Rev Ecol Evol. 2005;36:519–539. [Google Scholar]
- 56.Gaston KJ. The Structure and Dynamics of Geographic Ranges. Oxford: Oxford Univ Press; 2003. [Google Scholar]
- 57.Bridle JR, Vines TH. Limits to evolution at range margins: When and why does adaptation fail? Trends Ecol Evol. 2006;22:140–147. doi: 10.1016/j.tree.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 58.Pearman PB, Guisan A, Broennimann O, Randin CF. Niche dynamics in space and time. Trends Ecol Evol. 2007;23:149–158. doi: 10.1016/j.tree.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 59.Futuyma DJ, Moreno G. The evolution of ecological specialization. Annu Rev Ecol Syst. 1988;19:207–233. [Google Scholar]
- 60.Losos JB. Phylogenetic niche conservatism, phylogenetic signal and the relationship between phylogenetic relatedness and ecological similarity among species. Ecol Lett. 2008;11:995–1003. doi: 10.1111/j.1461-0248.2008.01229.x. [DOI] [PubMed] [Google Scholar]
- 61.Warren DL, Glor RE, Turelli M. Environmental niche equivalency versus conservatism: Quantitative approaches to niche evolution. Evolution (Lawrence, Kans.) 2008;62:2868–2883. doi: 10.1111/j.1558-5646.2008.00482.x. [DOI] [PubMed] [Google Scholar]
- 62.Bradshaw AD. Genostasis and the limits to evolution. Philos Trans R Soc London Ser B. 1991;333:289–305. doi: 10.1098/rstb.1991.0079. [DOI] [PubMed] [Google Scholar]
- 63.Blows MW, Hofmann AA. A reassessment of genetic limits to evolutionary change. Ecology. 2005;86:1371–1384. [Google Scholar]
- 64.Willi Y, Van Buskirk J, Hoffmann AA. Limits to the adaptive potential of small populations. Annu Rev Ecol Evol. 2006;37:433–458. [Google Scholar]
- 65.Rehfedlt GE, Ying CC, Spittlehouse DL, Hamilton DA., Jr Genetic responses to climate in Pinus contorta: Niche breadth, climate change, and reforestation. Ecol Monogr. 1999;69:375–407. [Google Scholar]
- 66.Castenholz RW. In: The Biology of Blue-Green Algae. Carr NG, Whitten BH, editors. London: Blackwell; 1973. pp. 379–414. [Google Scholar]
- 67.Colwell RK, Fuentes ER. Experimental studies of the niche. Annu Rev Ecol Syst. 1975;6:281–310. [Google Scholar]
- 68.Peterson AT, Holt RD. Niche differentiation in Mexican birds: Using point occurrence data to detect ecological innovation. Ecol Lett. 2003;6:774–782. [Google Scholar]
- 69.Murphy HT, Lovett-Doust J. Accounting for regional niche variation in habitat suitability models. Oikos. 2007;116:99–110. [Google Scholar]
- 70.Pfenninger M, Nowak C, Magnin F. Intraspecific range dynamics and niche evolution in Candidula land snail species. Biol J Linn Soc. 2007;90:303–317. [Google Scholar]
- 71.Broennimann O, et al. Evidence of climatic niche shift during biological invasion. Ecol Lett. 2007;10:701–709. doi: 10.1111/j.1461-0248.2007.01060.x. [DOI] [PubMed] [Google Scholar]
- 72.Holt RD, Barfield M, Gomulkiewicz R. In: Species Invasions: Insights into Ecology, Evolution, and Biogeography. Sax D, Stachowicz J, Gaines SD, editors. Sunderland, MA: Sinauer; 2005. pp. 259–290. [Google Scholar]
- 73.Kawecki TJ. Adaptation to marginal habitats. Annu Rev Ecol Evol. 2008;39:321–342. [Google Scholar]
- 74.Gomulkiewicz R, Holt RD. When does evolution by natural selection prevent extinction? Evolution (Lawrence, Kans) 1995;49:201–207. doi: 10.1111/j.1558-5646.1995.tb05971.x. [DOI] [PubMed] [Google Scholar]
- 75.Orr HA, Unckless RL. Population extinction and the genetics of adaptation. Am Nat. 2008;172:160–169. doi: 10.1086/589460. [DOI] [PubMed] [Google Scholar]
- 76.Holt RD, Gomulkiewicz R. How does immigration influence local adaptation? A reexamination of a familiar paradigm. Am Nat. 1997;149:563–572. [Google Scholar]
- 77.Garant D, Forde SE, Hendry AP. The multifarious effects of dispersal and gene flow on contemporary adaptation. Funct Ecol. 2007;21:434–443. [Google Scholar]
- 78.Gomulkiewicz R, Holt RD, Barfield M. The effects of density dependence and immigration on local adaptation and niche evolution in a black-hole sink environment. Theor Popul Biol. 1999;55:283–296. doi: 10.1006/tpbi.1998.1405. [DOI] [PubMed] [Google Scholar]
- 79.Holt RD, Knight TM, Barfield M. Allee effects, immigration, and the evolution of species' niches. Am Nat. 2004;163:253–262. doi: 10.1086/381408. [DOI] [PubMed] [Google Scholar]
- 80.Perron GG, Gonzalez A, Buckling A. Source-sink dynamics shape the evolution of antibiotic resistance and its pleiotropic fitness cost. Proc R Soc London Ser B. 2007;274:2351–2356. doi: 10.1098/rspb.2007.0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kirkpatrick M, Barton NH. Evolution of a species' range. Am Nat. 1997;150:1–23. doi: 10.1086/286054. [DOI] [PubMed] [Google Scholar]
- 82.Ronce O, Kirkpatrick M. When sources become sinks: Migrational meltdown in heterogeneous habitats. Evolution (Lawrence, Kans.) 2001;55:1520–1531. doi: 10.1111/j.0014-3820.2001.tb00672.x. [DOI] [PubMed] [Google Scholar]
- 83.Holt RD. Demographic constraints in evolution: Toward unifying the evolutionary theories of senescence and niche conservatism. Evol Ecol. 1996;10:1–11. [Google Scholar]
- 84.Kawecki TJ. Adaptation to marginal habitats: Contrasting influence of dispersal on the fate of rare alleles with small and large effects. Proc R Soc London Ser B. 2000;267:1315–1320. doi: 10.1098/rspb.2000.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kawecki TJ, Holt RD. Evolutionary consequences of asymmetric dispersal rates. Am Nat. 2002;160:333–347. doi: 10.1086/341519. [DOI] [PubMed] [Google Scholar]
- 86.Estes S, Arnold SJ. Resolving the paradox of stasis: Models with stabilizing selection explain evolutionary divergence on all time scales. Am Nat. 2007;169:227–244. doi: 10.1086/510633. [DOI] [PubMed] [Google Scholar]
- 87.Knouft HJ, Losos JB, Glor RE, Kolbe JJ. Phylogenetic analysis of the evolution of the niche in lizards of the Anolis sagrei group. Ecology. 2006;87:S29–S38. doi: 10.1890/0012-9658(2006)87[29:paoteo]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 88.Ackerly D. Conservatism and diversification of plant functional traits: Evolutionary rates versus phylogenetic signal. Proc Natl Acad Sci USA. 2009;106:19699–19706. doi: 10.1073/pnas.0901635106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wiens JJ. Commentary on Losos: Niche conservatism déjà vu. Ecol Lett. 2008;11:1004–1005. doi: 10.1111/j.1461-0248.2008.01238.x. [DOI] [PubMed] [Google Scholar]
- 90.Bond WJ, Midgley JJ. Ecology of sprouting in woody plants: The persistence niche. Trends Ecol Evol. 2001;16:45–51. doi: 10.1016/s0169-5347(00)02033-4. [DOI] [PubMed] [Google Scholar]