Abstract
Estimating actual and potential areas of distribution of species via ecological niche modeling has become a very active field of research, yet important conceptual issues in this field remain confused. We argue that conceptual clarity is enhanced by adopting restricted definitions of “niche” that enable operational definitions of basic concepts like fundamental, potential, and realized niches and potential and actual distributional areas. We apply these definitions to the question of niche conservatism, addressing what it is that is conserved and showing with a quantitative example how niche change can be measured. In this example, we display the extremely irregular structure of niche space, arguing that it is an important factor in understanding niche evolution. Many cases of apparently successful models of distributions ignore biotic factors: we suggest explanations to account for this paradox. Finally, relating the probability of observing a species to ecological factors, we address the issue of what objects are actually calculated by different niche modeling algorithms and stress the fact that methods that use only presence data calculate very different quantities than methods that use absence data. We conclude that the results of niche modeling exercises can be interpreted much better if the ecological and mathematical assumptions of the modeling process are made explicit.
Keywords: distributions modeling, Grinnellian niches, niche modeling, scenopoetic variables
Some of the most fundamental ideas about distributional areas of species were presented by Joseph Grinnell >90 years ago (1–3), in a series of papers describing the contrasting roles of different types of environmental factors acting at different scales. More recently, George Evelyn Hutchinson developed seminal ideas about ecological niches and their relation with areas of distribution (4, 5). However, it took much more time to develop the data and analytical techniques required to put these ideas into widespread practice. The suite of methods variously called species distribution modeling, habitat modeling, or ecological niche modeling (ENM) (6–9) all have a similar purpose: to identify places suitable for the survival of populations of a species via identification of their environmental requirements. Although, as we will discuss, strictly speaking, modeling a habitat or a distribution is not synonymous with modeling a niche, for the sake of brevity we will refer to all of these methods as ENM and will make the appropriate distinctions when necessary.
ENM has received greatly increased attention in the last 10–15 years. Essentially, it is a technique used to estimate actual or potential areas of distribution, or sets of favorable habitats for a given species, on the basis of its observed presences and (sometimes) absences. These methods relate “niches” to “areas of distribution.” The quotes are used to indicate that rigorous definitions of those concepts have not as yet been presented.
Although in the past few years the field has matured considerably, several conceptual problems still remain. It is not an exaggeration to say that no consensus exists about what it is that the different methods model (10, 11). Even without widespread agreement about terminology and concepts, researchers in the field of modeling niches, habitats, and distributions have managed to develop and apply sophisticated software to widely available databases. The resulting explosion of work focused on applying these methods to a large number of species and problems and addressing important, but mostly methodological, issues, like sensitivity to number of occurrence records (12), ratio of presences to absences (13), grain of the environmental layers (14), different types of absences (15), and other technical points (16). A smaller number of papers have focused on the fundamental ecological and mathematical issues underlying the working of ENM in any of its forms (8, 10, 11, 17–20).
In this article we focus explicitly on concepts. First, we argue in favor of restricted definitions of niche, inspired by Grinnell's early use of the term, because, at the cost of losing some generality, this step leads to operational and straightforward concepts for niches and corresponding distributional areas. Second, we use these operational niche concepts to clarify the term “niche conservatism” and propose ways of measuring it. Third, we discuss the role of biotic interactions as factors that need to be understood thoroughly for a correct appreciation of the potential and limitations of ENM in estimating distributional areas. Finally, using a probabilistic approach, we discuss major differences between several main types of ENM algorithms.
A Tale of Two Niches
The first step is to state explicitly what is meant by the word niche. Niche concepts are numerous (21). To offer two examples from recent literature, one view (21, 22) sees niche as the joint specification of requirements of resources that permit positive growth rate of a population, together with its impacts. Chase and Leibold (21) also included effects of predators, parasites, and noninteractive stressors in their definition. This niche is defined by sets of zero-growth isoclines in resource space, together with impact vectors and resource supply points. The other view (23) is niche as a subset of environmental conditions under which populations of a species have positive growth rates. These environmental dimensions mostly characterize climatic or other physical factors.
Many other meanings have been applied to the term (21). In their broadest sense, most definitions of niche intend to specify the environments that allow a population to survive, but they differ in the emphasis placed on key points. For example, the two niche concepts cited above differ in types of variables used [resources or other dynamically linked requirements, vs. relatively static conditions, which are the bionomic and scenopoetic variables of Hutchinson (5), respectively]; the abstract objects constituting niches (sets of vectors vs. regions in phase-spaces); and the spatial and temporal scales at which the definitions are meaningful (local, and commensurate with the activities of individuals vs. biogeographic, and commensurate with species' distributions). Using a single term to denote such disparate meanings considerably hinders understanding.
In this article we distinguish between Eltonian niches (24), which are spatially fine-grained and based on variables related to ecological interactions and resource consumption, and Jackson and Overpeck's meaning (23), the Grinnellian niches (24, 25). Using only noninteractive, nonconsumable scenopoetic variables as axes in the multidimensional niche space is what characterizes Grinnellian niches. Not mixing scenopoetic and bionomic axes is a simplification that departs from tradition (4, 26) and requires qualifications and caveats. We refer the reader to published literature about these details (19, 23, 24), but we stress that restricting niche definitions by type of variable leads to comparative simplicity of definitions and operations and permits the use of terabytes of freely accessible datasets characterizing scenopoetic variables.
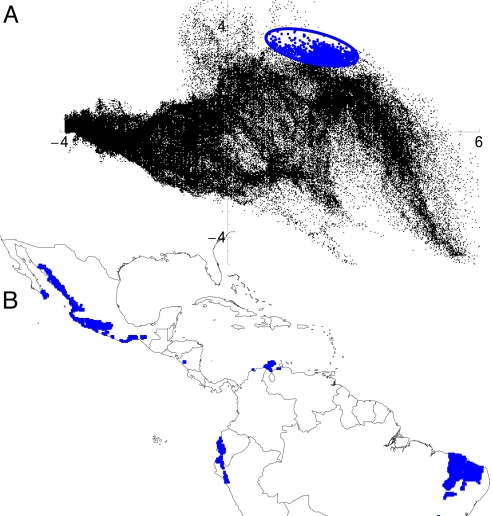
In Fig. 1, we illustrate some important concepts of Grinnellian niche theory. The cloud of points represents combinations of the two first principal components extracted from the 19 bioclimatic variables of the project WorldClim (27) across North and South America, estimated at a resolution of 10 min of arc. This set of points is a 2D view of the environmental space of Jackson and Overpeck (23), denoted by E. It is important to notice how irregular it is in both shape and internal structure. The blue ellipse represents a hypothetical fundamental niche (FN), NF, namely the set of combinations of those two variables for which the intrinsic population growth rate (the growth rate at low densities of itself and of negative interactors) of a species would be positive (24). This meaning is identical to Hutchinson's FN, if restricted to scenopoetic variables. FNs can best be calculated experimentally, or on the basis of biophysical first principles (28). The subset E ∩ NF was called by Jackson and Overpeck (23) the potential niche (PN). It is simply the part of the FN that actually exists in a given region and time, represented in Fig. 1A by the points within the ellipse. It is clear that a PN may often be substantially smaller than its corresponding FN. Finally, the realized niche (RN) is the part of the PN that the species would actually use, after the effects of competitors and predators are taken into account: it is the subset of E in which the species would have source populations even in the presence of competitors and other negative interactors. In other words, the RN corresponds to areas of existing source populations (24, 29). Besides interactions, dispersal disequilibrium can also prevent a species from fully occupying its PN (23).
Fig. 1.
The duality of environmental and geographical spaces. (A) An example of an E-space in two dimensions (the two first principal components of 19 bioclimatic variables across the Americas) at a resolution of 10 min of arc. Each of 156,932 black dots represents an existing combination of principal components. Notice the irregular shape and structure of the E-space. The blue ellipse represents a hypothetical FN. The set of blue dots inside the ellipse is the PN, which in this instance contains 2,232 elements. (B) The projection of the PN in A to geographical space. The environmental combinations contained in the PN project to four disjoint geographic areas, Mexico, Brazil, Ecuador–Peru, and Colombia. A species with FN as depicted in A would have potentially favorable conditions in every blue region in the map in B.
A duality (see ref. 77) of environmental and geographic spaces exists that was first formally expressed by Hutchinson (4). By restricting discussion to Grinnellian niches, the duality immediately becomes operational, because scenopoetic variables are easily made to correspond to cells in geographic grids. Hence, in Fig. 1 each point in the graph corresponds to a single cell in a grid (denoted by G) of 10 min of arc resolution covering the entirety of the Americas. In general, every cell in geographic space can be characterized uniquely by using enough environmental variables, so it is possible to establish one-to-one relationships between G and E. However, geographic projections of the environmental subsets can have complicated structures (8, 24). This structure is illustrated in Fig. 1, where the blue regions in the map correspond to the climates enclosed in the blue ellipse, which by definition constitute the PN. Note that regular shapes in E may correspond to rather irregular and fragmented shapes in geographical space.
A brief digression is needed to highlight the point that scaling presents some thorny issues. Ideally, grid resolution should be established by biological considerations of the size, mobility, and ecology of the species. However, considerations of data availability often become dominant (30). Also, changing the resolution of the geographic grid creates an instance of the modifiable areal unit problem (31, 32), a difficult conceptual problem in geography that in our context means that varying resolution may lead to different estimates of niches. Given this general problem, niche modelers should always report the specifics of the grids they use and the precision at which variables are measured.
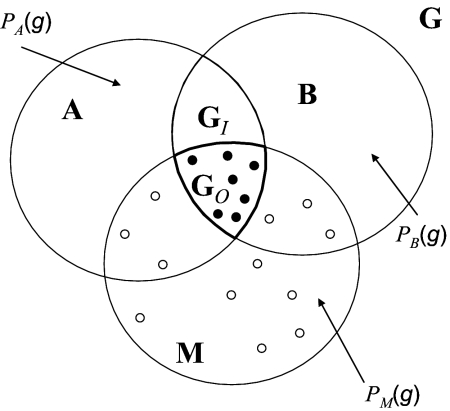
A heuristic scheme useful for analyzing the interplay between movements, abiotic, and scenopoetic environments is the BAM (biotic, abiotic, and movements) diagram (19), shown in Fig. 2. We denote by A the region in the geographic space where the PN scenopoetic conditions occur. B is the region where biotic conditions would allow existence of viable populations, determined mainly by Eltonian factors. Finally, M is the region that has been accessible to dispersal or colonization by the species over some relevant time interval. The intersection G0 = A ∩ B ∩ M represents the area actually occupied by the species, and G1 = A ∩ B ∩ Mc represents a potentially invasible area (it has the correct abiotic and biotic conditions but remains outside reach). The regions in the BAM diagram represent a static view of a complicated, spatially explicit, mutispecies model (24), but their simplicity is helpful in discussing several conceptual problems. The different subfields of niche or distributions modeling represent different approaches to estimating the regions in the BAM diagram and/or their corresponding environmental features.
Fig. 2.
A BAM diagram (24), which is an abstract representation of geographic space. Set A represents regions in space where the FN (or PN) occurs. The probability PA(g) is high for cells belonging to A. Region B represents regions where the biological conditions (competitors, predators, diseases) is favorable, and the value of PB(g) would be high for cells within B. The M region represents regions to which the species has access because of its movement and colonizing capacities and the structure of barriers and distances, within a specified period, with corresponding high values of PM(g). GO represents the actual area of distribution of the species, where abiotic and biotic conditions are favorable and within reach to dispersing individuals. GI is a potential area of distribution, invasible if the structure of M changes. ●, observations of presence; ○, observations of true absences of the species.
Evolving the Niche, or Not
Evolutionary factors are not included in the previous framework. Still, a fundamental assumption underlying many applications of ENM, and specifically in transferring niche predictions across space or in time, is that niches are “conserved” (33). Niche conservatism refers to the empirical evidence (34) and theoretical arguments (35, 36) showing that, to some extent, niches appear to evolve relatively slowly within lineages. Evidence includes phylogenetic inertia in ecological characters and the capacity to predict the geographic potential of invasions of species using data on the environments used by ancestral populations (37–42). The term “niche conservatism” is, unfortunately, too vague: what features of niche are being conserved, and what is the meaning of conservatism? One way of making the term specific and quantifiable is by measuring features of specific types of niches and studying their rates of change through time. Focus on Grinnellian niches permits these steps. Both RNs and FNs can change, but because the causes of change in RN may be ecological (i.e., release from competitors) rather than evolutionary (43) conservatism in a strict sense should refer to the FN. Because Grinnellian niches are subsets of a multidimensional space, several things can change independently in them, for example, position, size, and shape (23, 40, 42). Niche conservatism means “slow” temporal changes in the position, size, or shape of the FN. We do not know the shapes of FNs, but it may be reasonable to hypothesize that they are convex in the existing multidimensional space; hence, as a preliminary hypothesis, we visualize a FN as a multidimensional ellipsoid of the form NF(t) = [x − μ(t)]M−1(t)[x − μ(t)]T − 1, where the x is a vector of values of the scenopoetic variables being used, the vector μ(t) represents the centroid (the position) of NF(t), the matrix M(t) is the variance-covariance matrix of NF(t), and T indicates matrix transposition. The determinant of M−1(t) would be a measure of niche size, because it is proportional to the volume of the ellipsoid. With these conventions, change in size of the FN in a time interval Δt is proportional to a number (|M−1(t)| − |M−1(t + Δt)|)/Δt. The change in position per unit time is a vector: [μ(t) − μ(t + Δt)]/Δt, displaying the magnitude of change along each dimension.
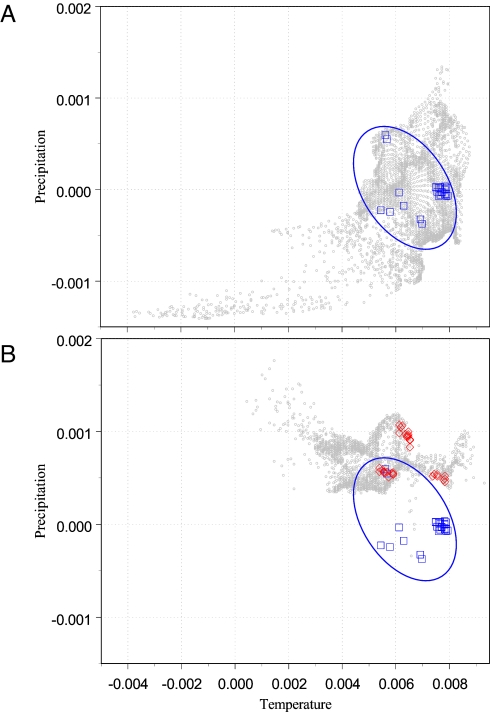
2It would desirable to define units by which to measure niche evolution (see ref. 78). Following Haldane (44), in principle, it is feasible to measure the rate of change of both size and position of the FN in terms of a proportional change per year. We illustrate this idea by using data of the prickly pear moth, Cactoblastis cactorum, a native of southern South America, which has now established populations in northern Florida (45). We use standardized mean annual temperature and annual precipitation at 10 min of arc of spatial resolution (27) to represent the niches of C. cactorum. Merely for the purpose of illustration, a simple enclosing of the observed data are used to estimate the position of C. cactorum's niche in the E-space in Argentina, ca. 1920. The same ellipsoid is then plotted in the E-space in Florida, with data of C. cactorum presences taken there in 2000. The position of the niche is seen to have changed with the invasion to the new distributional area (see Fig. 3). Notice that, for the purpose of illustration, we are using a very simple approximation to the RN of C. cactorum, rather than an unknown FN.
Fig. 3.
The same niche in two regions of E-space. (A) A subset of E-space is shown in light gray. The two dimensions are standardized mean annual temperature and annual precipitation, centered on the Argentine region where the moth C. cactorum occurs. The blue squares are reported occurrences of C. cactorum. The blue ellipse is a hypothetical representation of the FN of C. cactorum. (B) Another subset of the same E-space, now centered on the region of northern Florida that C. cactorum has invaded in the last 10 years. The units and the scale of A and B are the same. The hypothetical FN is placed exactly in its original Argentine position. The red diamonds are reported occurrences of C. cactorum in Florida. It is apparent that the structure of the E-space in the two regions is very different. C. cactorum in Florida occupies regions of similar temperature but higher precipitation than in Argentine. Whether this difference reflects different availability of climates or a true evolution in the FN of the moth cannot be determined with the available data.
The proportional rate of change in these two scenopoetic variables (standardized mean temperature and yearly precipitation) is {|Temp2000 − Temp1920|/(80 years × Temp1920)} = 1.4 × 10−3/year, and the change in the mean precipitation is {|Precip2000 − Precip1920|/(80 years × Precip1920)} = 1.29 × 10−2/year. Change has thus been 10 times faster in one of the dimensions of the niche (precipitation) than in temperature. These rates were estimated for the RN by using the centroid in E of observations in the Argentine range in 1920 versus the centroid of observations in the Florida area of expansion in the decade of 2000s. Is this shift an example of niche evolution (33, 46)? In this example, only the RN was estimated, and it may be difficult to disentangle ecological and evolutionary factors affecting changes in it (33, 40, 46). Moreover, the same FN may yield quite different PNs in different regions of the planet, even in the absence of competitive or predator release. In the case in C. cactorum actual distributions of values of precipitation and temperature in northern Florida and northern Argentina are radically different, as displayed in Fig. 3. Therefore, the same FN (i.e., same position and size), hypothetically illustrated by the blue ellipses in Fig. 3, is expressed as quite different PNs in Argentina (Fig. 3A) versus Florida (Fig. 3B). The position of the estimated niche has indeed shifted over the span of time and space (blue diamonds, Fig. 3A versus red squares, Fig. 3B), but this change may result simply from the fact that available E-space in Florida has a structure very different from that of the original in Argentina. This effect should be borne in mind when interpreting niche shifts based on correlative ENMs, because, as we will see, they estimate niches that only in very specific cases can be unequivocally identified as PN or RN.
The evolutionary meaning of changes in position versus changes in size of fundamental Grinnellian niches is different. If the centroid of the FN changes very slowly, predictions of geographic potential of invasion of new zones become more feasible. If it is just the size of the FN that changes slowly, however, and the centroid is mobile, then prediction of the potential geography of invasions becomes problematic. Stating the problem of measuring niche evolution in Grinnellian terms clarifies concepts significantly. Unfortunately, studying relative rates of change in niche size versus niche position of FNs is fraught with difficulties because FNs can only be estimated by experimental methods (28).
The Eltonian Noise Hypothesis
For many decades after Hutchinson (4) first proposed the distinction between the FNs and the RNs, it was almost axiomatic in ecological theory to assume that competitive interactions (more generally, negative interactions) would reduce FN to the RN. Is this assumption valid for Grinnellian niches (17)? There is a complicating factor. The milieu (47) of Eltonian factors represented by B is in practice very difficult to represent as static values assigned to a grid, the way that scenopoetic variables are used to construct the space E. For most species, their biological milieu is simply too fine-grained and dynamic in time and space (48) as to permit mapping it at high sampling density over an entire geographic distribution.
The immediate question, then, is how feasible is predicting distributional areas without resorting to data pertinent to B? Lack of documentation of the role of biotic interactions in ENM has been often mentioned as an important limitation to reliable predictions of species' distributions (49) and examples exist in which their inclusion improves predictions of distributions (50). Still, ENMs based entirely on scenopoetic variables have demonstrated considerable predictive value in a variety of cases (37, 51–53). To explain this apparent paradox, two extreme explanations come to mind. First, perhaps Eltonian factors correlate closely with scenopoetic variables, which thus capture an important part of the biotic signature (54). Alternatively, in some cases, Eltonian factors like competition may not affect distributions at the large extents and low resolutions characteristic of geographic distribution maps (55). We call this the Eltonian Noise Hypothesis. For example, for some species, interactions may be extremely important determinants of abundance at spatial resolutions much smaller than the coarse-grained scales typically used in ENM, but at these coarse resolutions the effect may be averaged-out, leading to simple “presence” of the species in the much larger cells of a distribution map (24, 56). In cases in which local interactions do have impacts on distributions at geographic extents (57, 58), the Eltonian Noise Hypothesis is falsified.
It is perfectly feasible to map parts of A based on species' distributional characteristics in relation to the values of coarse-grained scenopoetic variables. Such maps mostly show smoothly changing patterns, with obvious effects of elevation, slope orientation, climate patterns, etc. How would a map of B look? In one of the scarce works reporting on the spatial structure of mortality causes over the entire distribution of a species, Brewer and Gaston (54) showed that biotic mortality factors affecting populations of a leaf-miner varied significantly across localities. Thompson (48) reviewed other examples, confirming the possibility that the details and effects of bionomic interactions, such as the presence and impact of mutualists, competitors, and predators can change dramatically across the geographic distribution of a species. These findings suggest that the BAM diagram's abstract representation of B as a compact circle may be misleading. The sets A and B probably have rather contrasting spatial structures, A with long-ranged autocorrelations, and B requiring fine-grained spatial characterization. Without a much larger empirical database about such factors, the relative roles of Grinnellian and Eltonian factors in determining distributions would be difficult to assess.
No Silver Bullets
A number of recent papers have set out to compare the performance of different algorithms (among the dozens available) for estimating ecological niches (15, 59, 60). Such analysis generally resort to a few measures of performance, like the receiver operating characteristic (61), which are applied indiscriminately to diverse niche modeling methods. However, different methods calculate different objects, have different assumptions, and may use different kinds of data. The depth of these differences is perhaps not widely appreciated. In correlative approaches to modeling distributions, or their corresponding niches, the input data consist of observations of presences of the species and sometimes its absence. The lack of absence data is often regarded as simply a case of low-quality data (15); however, as we will see, the lack of absence data fundamentally alters the nature of the problem. What can and cannot be estimated is different when absence data are lacking. Moreover, there are different types of absences that should not be treated symmetrically. An absence from an inaccessible area with suitable environment is not the same as one from the reciprocal situation. If one is modeling an area of distribution (GO in Fig. 2), all absences are informative. If the objective is to model niches, absences from the region GI constitute incomplete data. Therefore, presence-only and presence–absence problems are rather different, and the algorithms (7) applied to them should be conceptually appropriate to their particularities (15).
To clarify this we resort to a probabilistic interpretation of the BAM diagram. Denote by P(Y = 1| g) the probability of presence, interpreted as presence of source populations in a randomly chosen cell g. We model presence of sources (that is, Y = 1) by using three binary random variables called I, J, K that represent random access of a site by the species, abiotic suitability, and biotic suitability, respectively. The event {Y = 1} is equivalent to {I = 1} ∩ {J = 1} ∩ {K = 1}. We use the following succinct notation for conditional probabilities that label specific segments in the BAM diagram:
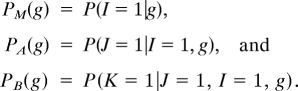 |
Thus, PM(g) is the probability that cell g belongs to the area that, in an appropriate time interval, has been accessible for the species. It can in principle be estimated by classifying cells in terms of ecological barriers, remoteness, and capacities for dispersal of a species over a given time period. For highly dispersive species in small regions, it may be the case that PM(g) = 1 for every cell g. PA(g) depends on the scenopoetic variables characterizing g, and can, in principle, be estimated experimentally (28). The values of PA(g) will be high for cells with environments within the PN. Finally, PB(g) depends on the Eltonian factors of g. This probability would be very difficult to estimate for large numbers of cells (17), but as we saw, what evidence exists indicates that it may vary dramatically over space (48, 54, 62). The Eltonian Noise Hypothesis means that PA(g) is uncorrelated with PB(g).
By a multiplication rule for conditional probabilities, we propose the following equation:
Eq. 1 relates what may be called a statistical representation of probability of presence (the left side) to a more ecological representation that is based on causal factors (the right side). Godsoe (personal communication) has arrived to a similar equation using a different reasoning.
Now, it is well known that the probability P(Y = 1|g) can be estimated directly if true absences are available (6, 63). In this case any of many multivariate regression methods (generalized linear models, generalized additive models, regression trees, logistic regression, etc.) will estimate that probability as a function of the environment in g. From P(Y = 1|g) the area of distribution (region GO in Fig. 2) is immediately available. By Eq. 1, therefore, incorporation of true-absence data allows estimation of the combined effects of scenopoetic and Eltonian variables, and dispersal, namely PB(g)PA(g)PM(g).
However, if absence information is missing, then P(Y = 1|g) cannot be estimated reliably. Application of Bayes' rule to P(Y = 1|g) allows obtaining a second equation:
P(g|Y = 1) is the probability of the observer being at g given that the species is present (63, 64), which essentially provides information on how to classify sites by their similarity to those already known as containing the species. Some methods can estimate P(g|Y = 1), but the relationship between P(g|Y = 1) and the actual probability of presence is obscured by the term P(Y = 1)/Pv(g). The so-called prevalence, P(Y = 1), cannot be estimated without absence data (61, 65), and the term Pv(g) (the probability of an observer randomly visiting cell g), is not only is seldom known, but in general should have strong spatial biases, because most biological exploration is concentrated along roads, rivers, around biological stations, etc (66, 67). Biases in visitation and detection probabilities can alter interpretation of modeling results significantly (63, 64, 68), but reasons of space prevent further discussion of this problem here.
To estimate P(g|Y = 1) Maxent and other methods resort to so-called background absences (65, 69), which are randomly sampled pseudoabsences taken from the region G. However, the existence of the term P(Y = 1)/Pv(g) prevents us from simplistically assuming that P(g|Y = 1) estimates the probability of presence. Only by assuming that Pv(g) is unbiased is it possible to suppose that Maxent estimates a quantity proportional to the probability of presence P(Y = 1|g) (64).
Another method that resorts to use background absences is genetic algorithm for rule set production (GARP) (70), a machine learning method that does not estimates probabilities. When multiple GARP models are generated and combined via consensus approaches (71) an estimate of the concordance between different stochastic solutions to an optimization problem is obtained. This number sometimes but not always correlates well with the Maxent-estimated probability P(g|Y = 1) (72). Ideally, one would expect the outputs of both Maxent and GARP to be high when the environments in a cell are similar to those in presence-observed cells, which by hypothesis should also have large values of PA(g). How well presence-only methods approximate PA(g), however, is determined by the unknown form of the sampling bias term Pv(g). Perhaps these algorithms characterize “lower bounds” to the PN, so the areas of distribution modeled by them are intermediate to GO and A (11). Unfortunately, without further information, presence-only algorithms alone do not specify exactly what area was estimated.
Finally, presence-only data (without background absences) can be used by envelope techniques like BIOCLIM (73), support vector machines (74), or similarity methods like Mahalanobis distance classification (75), which simply surround presence points in environmental space with different geometrical shapes and assume implicitly that points within the shape are also favorable to the species. Although capable of producing indices of similarity to observed environments, these methods most often just identify a subset of E that is regarded as a niche. Which niche? Probably, again, something in between the RN and the PN, which is related to the probability PA(g). In other words, presence-only envelope methods classify cells in ways that probably would have a large intersection with a classification based on PA(g). Similarly to presence-background methods then, they predict areas likely to be bounded by GO and A.
We see that different classes of methods estimate different terms of Eq. 2. It is unadvisable therefore to treat them as conceptual equivalents, to be tested only in terms of their capacity to predict independent datasets. Different methods are differently suited to different biological problems, an idea that can be stated explicitly by using Eq. 2 and a BAM diagram.
Conclusions
Grinnell was among the first to speak of niches as related to areas of distribution of species (2). He also was among the first to discuss factors affecting the shape of distributions of species (1). His analysis provides many of the elements we have discussed here, including a hierarchical view of processes, the importance of climatic variables in defining coarse-grained features of distributions, and finer-grained habitat structure and biotic interactions determining the details of the whereabouts of organisms. As we have seen, by defining Grinnellian niches according to this general philosophy, it is possible to make many concepts operational and visualize with great agility the niche-distribution duality anticipated by Hutchinson (4).
We extract several lessons from the above discussion. First, the niche-distributional area duality (77) is composed of several related, but quite distinct, objects. The FN, PN, and RN are different entities, and they correspond in explicit but complicated ways to different actual and potential distributional areas (A, GO, GI). Being specific about what niches and what areas are being studied and modeled is not pedantic nit-picking, but a simple consequence of the complexity of the subject. This lesson carries over to discussions about niche conservatism, as we saw that the term may refer to very different features of the FN, with different ecological and evolutionary properties.
The second lesson derives from the fact that different modeling algorithms estimate different parts of Eq. 2 and thus different sectors of the BAM diagram. Methods that estimate GO (these are species distribution models, in the strict sense), also permit estimating the RN and therefore may be inappropriate for study of issues of FN evolution, unless it is proven that for that particular case the potential and actual areas coincide (19), making FN and RN similar. Other methods, or different configurations of factors represented in the BAM diagram, may estimate environmental subsets closer to the FN. Therefore, specification of the ecological assumptions of the problem and selection of the modeling method should go hand by hand, as Austin (76), has suggested in a slightly different context. Ideally, independent estimations of A (mechanistically) and M (from considerations about history and/or movement patterns of the species) in tandem with ENM can lead to more rigorous estimation of the different actual and potential areas and environments in Hutchinson's duality.
Finally, the actual, physical structure of both the environmental and geographic spaces, in the present and the past, should be taken into account when interpreting the results of niche modeling. The structure of environmental space is hugely irregular, in both its boundaries and the density of points inside, and changes in time. Grinnellian niches, being subsets of these spaces as defined by the activities and physiology of species, inherit these irregularities, and interpretation of ENM exercises ignores them at its peril. Similarly, expressing the M and B regions of Fig. 2 realistically is seldom done, hindering interpretation of the results of species distributions modeling. In particular, documenting the structure of B empirically remains a serious methodological challenge.
Acknowledgments.
We thank Alma Solis for providing data on the Smithsonian Institution Argentine specimens of C. cactorum; John Madsden and Clifton Abbot for data on the observations of C. cactorum in Florida; David Wake, Elizabeth Hadly, and David Ackerly for inviting J.S. to speak at the Sackler Colloquium, providing the opportunity to express the ideas in this article to an expert audience; A. Townsend Peterson for reading the manuscript and making many thoughtful comments; and two anonymous referees for positive criticism that allowed us to improve the article. J.S. was partially supported by grants from the National Science Foundation–Experimental Program to Stimulate Competitive Research and Microsoft Research.
Footnotes
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “Biogeography, Changing Climates and Niche Evolution,” held December 12–13, 2008, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. The complete program and audio files of most presentations are available on the NAS web site at www.nasonline.org/Sackler_Biogeography.
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. E.A.H. is a guest editor invited by the Editorial Board.
References
- 1.Grinnell J. Barriers to distribution as regards birds and mammals. Am Nat. 1914;48:248–254. [Google Scholar]
- 2.Grinnell J. The niche-relationships of the California Thrasher. Auk. 1917;34:427–433. [Google Scholar]
- 3.Grinnell J. Field tests of theories concerning distributional control. Am Nat. 1917;51:115–128. [Google Scholar]
- 4.Hutchinson GE. Concluding remarks. Cold Spring Harbor Symp Quant Biol. 1957;22:415–427. [Google Scholar]
- 5.Hutchinson GE. An Introduction to Population Ecology. New Haven, CT: Yale Univ Press; 1978. [Google Scholar]
- 6.Pearce J, Boyce MS. Modeling distribution and abundance with presence-only data. J Appl Ecol. 2006;43:405–412. [Google Scholar]
- 7.Guisan A, Zimmermann N. Predictive habitat distribution models in ecology. Ecol Model. 2000;135:147–186. [Google Scholar]
- 8.Hirzel AH, Le Lay G. Habitat suitability modeling and niche theory. J Appl Ecol. 2008;45:1372–1381. [Google Scholar]
- 9.Peterson AT. Predicting species' geographic distributions based on ecological niche modeling. Condor. 2001;103:599–605. [Google Scholar]
- 10.Kearney M. Habitat, environment, and niche: What are we modeling? Oikos. 2006;115:186–191. [Google Scholar]
- 11.Jiménez-Valverde A, Lobo JM, Hortal J. Not as good as they seem: The importance of concept in species distribution modeling. Diversity Distributions. 2008;14:885–890. [Google Scholar]
- 12.Wisz MS, et al. Effects of sample size on the performance of species distribution models. Diversity Distributions. 2008;14:763–773. [Google Scholar]
- 13.Jiménez-Valverde A, Lobo JM. The ghost of unbalanced species distribution data in geographical model predictions. Diversity Distributions. 2006;12:521–524. [Google Scholar]
- 14.Araújo MB, Thuiller W, Williams PH, Reginster I. Downscaling European species atlas distributions to a finer resolution: Implications for conservation planning. Global Ecol Biogeogr. 2005;14:17–30. [Google Scholar]
- 15.Brotons L, Thuiller W, Araújo M, Hirzel A. Presence-absence versus presence-only modeling methods for predicting bird habitat suitability. Ecography. 2004;27:437–448. [Google Scholar]
- 16.Peterson AT, Papes M, Soberón J. Rethinking receiver operating characteristic analysis applications in ecological niche modeling. Ecol Model. 2008;213:63–72. [Google Scholar]
- 17.Araújo MB, Guisan A. Five (or so) challenges for species distribution modelling. Global Ecol Biogeogr. 2006;33:1677–1688. [Google Scholar]
- 18.Austin M. An ecological perspective on biodiversity investigations: Examples from Australian eucalypt forests. Ann Mo Bot Gard. 1996;85:2–17. [Google Scholar]
- 19.Soberón J, Peterson AT. Interpretation of models of fundamental ecological niches and species' distributional areas. Biodiversity Informatics. 2005;2:1–10. [Google Scholar]
- 20.Pulliam HR. On the relationship between niche and distribution. Ecol Lett. 2000;3:349–361. [Google Scholar]
- 21.Chase JM, Leibold M. Ecological Niches: Linking Classical and Contemporary Approaches. Chicago: Univ Chicago Press; 2003. [Google Scholar]
- 22.Leibold M. The niche concept revisited: Mechanistic models and community context. Ecology. 1996;76:1371–1382. [Google Scholar]
- 23.Jackson ST, Overpeck JT. Responses of plant populations and communities to environmental changes of the late Quaternary. Paleobiology. 2000;26(Suppl):194–220. [Google Scholar]
- 24.Soberón J. Grinnellian and Eltonian niches and geographic distributions of species. Ecol Lett. 2007;10:1115–1123. doi: 10.1111/j.1461-0248.2007.01107.x. [DOI] [PubMed] [Google Scholar]
- 25.James FC, Johnston RF, Warner NO, Niemi G, Boecklen W. The Grinnellian niche of the Wood Thrush. Am Nat. 1984;124:17–47. [Google Scholar]
- 26.Colwell RK, Fuentes E. Experimental studies of the niche. Annu Rev Ecol Syst. 1975;6:281–310. [Google Scholar]
- 27.Hijmans RJ, Cameron S, Parra J, Jones PG, Jarvis A. Very high-resolution interpolated climate surfaces for global land areas. Int J Climatol. 2005;25:1965–1978. [Google Scholar]
- 28.Kearney M, Porter WP. Mechanistic niche modeling: Combining physiological and spatial data to predict species' ranges. Ecol Lett. 2009;12:334–350. doi: 10.1111/j.1461-0248.2008.01277.x. [DOI] [PubMed] [Google Scholar]
- 29.Pulliam R. On the relationship between niche and distribution. Ecol Lett. 2000;3:349–361. [Google Scholar]
- 30.Guisan A, Thuiller W. Predicting species distribution: Offering more than simple habitat models. Ecol Lett. 2005;8:993–1009. doi: 10.1111/j.1461-0248.2005.00792.x. [DOI] [PubMed] [Google Scholar]
- 31.Openshaw S, Taylor PJ. The modifiable areal unit problem. In: Wrigley N, Bennet RJ, editors. Quantitative Geography: A British View. London: Routledge; 1981. [Google Scholar]
- 32.Fotheringham SA, Brundson C, Charlton M. Quantitative Geography. London: SAGE; 2000. [Google Scholar]
- 33.Broennimann O, et al. Evidence of climatic niche shift during biological invasion. Ecol Lett. 2007;10:701–709. doi: 10.1111/j.1461-0248.2007.01060.x. [DOI] [PubMed] [Google Scholar]
- 34.Huntley B, Bartlein PJ, Prentice IC. Climatic control of the distribution and abundance of beech (Fagus L. ) in Europe and North America. J Biogeogr. 1989;16:551–560. [Google Scholar]
- 35.Holt RD, Gaines MS. Analysis of adaptation in heterogeneous landscapes: Implications for the evolution of fundamental niches. Evol Ecol. 1992;6:433–447. [Google Scholar]
- 36.Holt RD, Gomulkiewicz R. In: Case Studies in Mathematical Modeling: Ecology, Physiology, and Cell Biology. Othmer HG, Adler F, Lewis M, Dillon JM, editors. Englewood Cliffs, NJ: Prentice–Hall; 1997. pp. 25–50. [Google Scholar]
- 37.Peterson AT. Predicting the geography of species' invasions via ecological niche modeling. Q Rev Biol. 2003;78:419–433. doi: 10.1086/378926. [DOI] [PubMed] [Google Scholar]
- 38.Peterson AT, Soberón J, Sánchez-Cordero V. Conservatism of ecological niches in evolutionary time. Science. 1999;285:1265–1267. doi: 10.1126/science.285.5431.1265. [DOI] [PubMed] [Google Scholar]
- 39.Losos JB. Phylogenetic niche conservatism, phylogenetic signal, and the relationship between phylogenetic relatedness and ecological similarity among species. Ecol Lett. 2008;11:995–1007. doi: 10.1111/j.1461-0248.2008.01229.x. [DOI] [PubMed] [Google Scholar]
- 40.Pearman PB, Guisan A, Broennimann O, Randin C. Niche dynamics in space and time. Trends Ecol Evol. 2007;23:149–158. doi: 10.1016/j.tree.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 41.Wiens J, Graham C. Niche conservatism: Integrating evolution, ecology, and conservation biology. Annu Rev Ecol Syst. 2005;36:519–539. [Google Scholar]
- 42.Ackerly DD. Community assembly, niche conservatism, and adaptive evolution in changing environments. Int J Plant Sci. 2003;164:S165–S184. [Google Scholar]
- 43.Colwell RK, Futuyma D. On the measurement of niche breadth and overlap. Ecology. 1971;52:567–576. doi: 10.2307/1934144. [DOI] [PubMed] [Google Scholar]
- 44.Haldane JBS. Suggestions as to a quantitaive measurment of rates of evolution. Evolution (Lawrence, Kans) 1949;3:51–56. doi: 10.1111/j.1558-5646.1949.tb00004.x. [DOI] [PubMed] [Google Scholar]
- 45.Hight SD, et al. Expanding geographical range of Cactoblastis cactorum (Lepidoptera: Pyralidae) in North America. Florida Entomol. 2002;85:527–529. [Google Scholar]
- 46.Randin CF, et al. Are niche-based models transferable in space? J Biogeogr. 2006;33:1689–1703. [Google Scholar]
- 47.McGill BJ, Enquist BJ, Weiher E, Westoby M. Rebuilding community ecology from functional traits. Trends Ecol Evol. 2006;21:179–185. doi: 10.1016/j.tree.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 48.Thompson JN. The Geographic Mosaic of Coevolution. Chicago: Univ Chicago Press; 2005. [Google Scholar]
- 49.Davis AJ, Jenkinson LS, Lawton JH, Shorrocks B, Wood S. Making mistakes when predicting shifts in species range in response to global warming. Nature. 1998;391:783–786. doi: 10.1038/35842. [DOI] [PubMed] [Google Scholar]
- 50.Heikkinen RK, Luoto M, Virkkala R, Pearson RG, Korber J-H. Biotic interactions improve prediction of boreal bird distributions at macro scales. Global Ecol Biogeogr. 2007;16:754–763. [Google Scholar]
- 51.Feria P, Peterson AT. Prediction of bird community composition based on point-occurrence data and inferential algorithms: A valuable tool in biodiversity assessments. Diversity Distributions. 2002;8:49–56. [Google Scholar]
- 52.Raxworthy CJ, et al. Predicting distributions of known and unknown reptile species in Madagascar. Nature. 2003;426:837–841. doi: 10.1038/nature02205. [DOI] [PubMed] [Google Scholar]
- 53.Sanchez-Cordero V, Martínez-Meyer E. Museum specimen data predict crop damage by tropical rodents. Proc Natl Acad Sci USA. 2000;97:7074–7077. doi: 10.1073/pnas.110489897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brewer A, Gaston KJ. The geographical range structure of the holly leaf-miner. II. Demographic rates. J Anim Ecol. 2003;72:82–93. [Google Scholar]
- 55.Prinzing A, Durka W, Klotz S, Brandl R. Geographic variability of ecological niches of plant species: Are competition and stress relevant? Ecography. 2002;25:721–729. [Google Scholar]
- 56.Pearson RG, Dawson TP. Predicting the impacts of climate change on the distribution of species: Are bioclimatic envelopes useful? Global Ecol Biogeogr. 2003;12:361–371. [Google Scholar]
- 57.Leathwick JR, Austin M. Competitive interactions between tree species in New Zealand's old-growth indigenous forest. Ecology. 2001;82:2560–2573. [Google Scholar]
- 58.Bullock JM, Edwards RJ, Carey PD, Rose RJ. Geographical separation of two Ulex species at three spatial scales: Does competition limit species' ranges? Ecography. 2000;23:257–271. [Google Scholar]
- 59.Lawler JJ, White D, Neilson RP, Blaustein AR. Predicting climate-induced range shifts: Model differences and model reliability. Global Change Biol. 2006;12:1568–1584. [Google Scholar]
- 60.Elith J, Burgman M. In: Predicting Species Occurrences: Issues of Scale and Accuracy. Scott JM, Heglund PJ, Morrison ML, editors. Washington, DC: Island; 2002. pp. 303–313. [Google Scholar]
- 61.Elith J, et al. Novel methods improve prediction of species' distributions from occurrence data. Ecography. 2006;29:129–151. [Google Scholar]
- 62.Sagarin R, Gaines S, Gaylord B. Moving beyond assumptions to understand abundance distributions across ranges of species. Trends Ecol Evol. 2006;21:524–530. doi: 10.1016/j.tree.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 63.Phillips S, et al. Sample selection bias and presence-only distribution models: Implications for background and pseudo-absence data. Ecol Appl. 2009;19:181–197. doi: 10.1890/07-2153.1. [DOI] [PubMed] [Google Scholar]
- 64.Phillips S, Dudík M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography. 2008;31:161–175. [Google Scholar]
- 65.Ward G, Hastie T, Barry S, Elith J, Leathwick JR. Presence-only data and the EM algorithm. Biometrics. 2009;65:554–563. doi: 10.1111/j.1541-0420.2008.01116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Graham C, Ferrier S, Huettman F, Moritz C, Peterson AT. New developments in museum-based informatics and applications in biodiversity analysis. Trends Ecol Evol. 2004;19:497–503. doi: 10.1016/j.tree.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 67.Bojórquez-Tapia LA, Balvanera P, Cuaron AD. Biological inventories and computer databases: Their role in environmental assessments. Environ Manage. 1994;18:775–785. [Google Scholar]
- 68.Argaez J, Christen A, Nakamura M, Soberón J. Prediction of potential areas of species distributions based on presence-only data. Environ Ecol Stat. 2005;12:27–44. [Google Scholar]
- 69.Phillips SJ, Anderson RP, Schapire RE. Maximum entropy modeling of species geographic distributions. Ecol Model. 2006;190:231–259. [Google Scholar]
- 70.Stockwell DRB, Peters DP. The GARP modeling system: Problems and solutions to automated spatial prediction. Int J Geogr Information Syst. 1999;13:143–158. [Google Scholar]
- 71.Anderson RP, Lew D, Peterson AT. Evaluating predictive models of species' distributions: Criteria for selecting optimal models. Ecol Model. 2003;162:211–232. [Google Scholar]
- 72.Papes M, Gaubert P. Modelling ecological niches from low numbers of occurrences: Assessment of the conservation status of poorly known viverrids. Diversity Distributions. 2007;13:890–902. [Google Scholar]
- 73.Busby JR, Margules CR, Austin MP. In: Nature Conservation: Cost Effective Biological Surveys and Data Analysis. Margules CR, Austin MP, editors. Melbourne, Australia: CSIRO; 1991. p. 64. [Google Scholar]
- 74.Guo Q, Kelly M, Graham CH. Support vector machines for predicting distribution of Sudden Oak Death in California. Ecol Model. 2005;182:75–90. [Google Scholar]
- 75.Farber O, Kadmon R. Assessment of alternative approaches for bioclimatic modeling with special emphasis on the Mahalanobis distance. Ecol Model. 2003;160:115–130. [Google Scholar]
- 76.Austin MP. Spatial prediction of species distribution: An interface between ecological theory and statistical modeling. Ecol Model. 2002;157:101–118. [Google Scholar]
- 77.Colwell R, Rangel T. Hutchinson's duality: The once and future niche. Proc Natl Acad Sci USA. 2009;106:19651–19658. doi: 10.1073/pnas.0901650106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ackerley D. Conservatism and diversification of plant functional traits: Evolutionary rates versus phylogenetic signal. Proc Natl Acad Sci USA. 2009;106:19699–19706. doi: 10.1073/pnas.0901635106. [DOI] [PMC free article] [PubMed] [Google Scholar]