Abstract
How biotic interactions, current and historical environment, and biogeographic barriers determine community structure is a fundamental question in ecology and evolution, especially in diverse tropical regions. To evaluate patterns of local and regional diversity, we quantified the phylogenetic composition of 189 hummingbird communities in Ecuador. We assessed how species and phylogenetic composition changed along environmental gradients and across biogeographic barriers. We show that humid, low-elevation communities are phylogenetically overdispersed (coexistence of distant relatives), a pattern that is consistent with the idea that competition influences the local composition of hummingbirds. At higher elevations communities are phylogenetically clustered (coexistence of close relatives), consistent with the expectation of environmental filtering, which may result from the challenge of sustaining an expensive means of locomotion at high elevations. We found that communities in the lowlands on opposite sides of the Andes tend to be phylogenetically similar despite their large differences in species composition, a pattern implicating the Andes as an important dispersal barrier. In contrast, along the steep environmental gradient between the lowlands and the Andes we found evidence that species turnover is comprised of relatively distantly related species. The integration of local and regional patterns of diversity across environmental gradients and biogeographic barriers provides insight into the potential underlying mechanisms that have shaped community composition and phylogenetic diversity in one of the most species-rich, complex regions of the world.
Keywords: Andes Mountains, biogeography, environmental gradients, phylogenetic β diversity
A central problem for evolutionary ecologists is to determine how contemporary and historical factors interact to influence species composition within and among communities. Recent approaches to this problem have focused on how biotic interactions and the physiological and ecological characteristics of a species (i.e., environmental filtering) have influenced local phylogenetic community structure (1, 2). However, because two communities with similar phylogenetic community structure may differ in their species composition, analyses of phylogenetic community structure alone cannot fully address how biogeographic barriers, biotic interactions, physiological constraints, and evolutionary processes, such as diversification of clades within novel environments, combine to influence the composition of local communities. Quantifying changes in the species and phylogenetic composition [i.e., the compositional β diversity (CBD) and phylogenetic β diversity (PBD)] across biogeographic barriers or along environmental gradients provides important additional information relevant to this issue (3–6). PBD measures the amount of shared phylogenetic history between two communities in the same way that CBD measures the similarity in species composition across sites. The integration of these measures can provide different insights into the ecological and evolutionary mechanisms that structure communities (5).
Here, we explore the role of biogeographic barriers and environmental filtering in structuring local community composition of hummingbirds by analyzing phylogenetic community structure, PBD and CBD using 189 hummingbird communities distributed across Ecuador. Ecuador provides an ideal setting for studying changes in community structure over space because it contains marked environmental gradients (Fig. 1). Elevation ranges from sea level to >6,000 m, providing strong temperature and precipitation gradients. A precipitation gradient also exists west of the Andes extending from the wet Chocó region in the north to the dry and seasonal Tumbesian region in the south. Finally, the topographic complexity within the Andes provides additional opportunities for isolation and diversification (7–12).
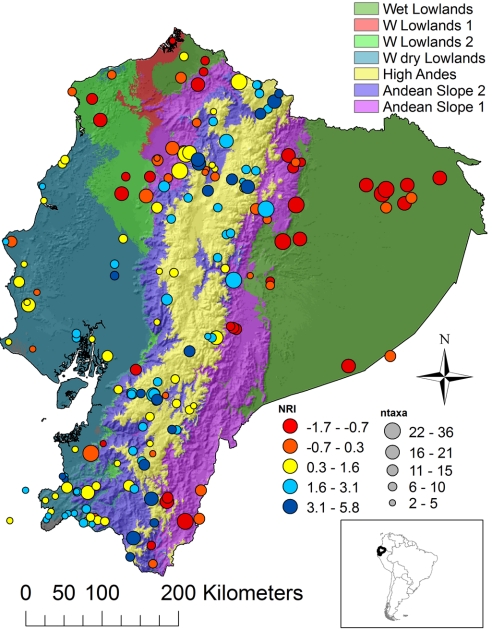
Fig. 1.
Map of Ecuador with communities and corresponding NRI values. Size of the circles is proportional to the number of species in each community (ntaxa). Background colors represent the result of an environmental classification (see Materials and Methods).
Hummingbirds are an ideal system for studying patterns of phylogenetic structure and community composition. Traditionally, higher taxonomy in hummingbirds includes nine major clades: topazes, hermits, mangoes, coquettes, brilliants, patagona, mountain gems, emeralds, and bees (13). More than 25 species can be found commonly in sympatry (e.g., refs. 14 and 15), and their flight skills and dietary specialization make them highly susceptible to environmental gradients. The hovering flight of hummingbirds represents a major metabolic and aerodynamic challenge, especially at high elevations (16, 17). Species with larger wings relative to body mass (i.e., lower wing loadings) and increased stroke amplitude often predominate at high elevations (16, 18, 19). Although coquettes and brilliants tend to have these characteristics, with individual species going as high as 5,000 m (20), species from other clades are also represented in the Andes and many exist at relatively high elevations (i.e., between 1,000 and 3,000 m; refs. 7, 21, and 22). Hummingbirds are the most specialized nectar-feeding birds in the New World and often compete aggressively for nectar resources (23–26). Bill morphology varies substantially in curvature and length and often influences what nectar resources are used (27). Wing shape and bill morphology influence flight ability and foraging efficiency and have been proposed to determine community organization (23, 24, 28, 29). Finally, traits involved in competition (i.e., bill morphology) and flight ability at high elevations (i.e., wing loading) have been used to identify the different clades within the hummingbirds (30), suggesting some level of conservatism in these traits. For instance, hermits have curved bills and relatively broad wings, brilliants have long straight bills and narrow wings, and coquettes have short straight bills and narrow wings (30).
Given the potential influence of environmental filtering at high elevations and competition on community structure, and assuming that traits involved in these processes exhibit low evolutionary lability, we can derive predictions about phylogenetic community structure along environmental gradients and biogeographic barriers in Ecuador. In communities where competition is prevalent, we expect coexisting species to be distant relatives (phylogenetic overdispersion). Alternatively, at high elevations, where filtering becomes relatively more important than competition, we expect coexisting species to be close relatives (phylogenetic clustering). Other processes might lead to similar patterns, but given our current knowledge of hummingbird ecology, these are the most likely. Mechanisms influencing diversity patterns can be further elucidated by comparing PBD and CBD (5). PBD can be predicted from CBD by using a null model where species turnover is independent of phylogeny (4). The observed PBD can be greater than that expected from the null model when the shared history between two communities is small relative to average shared history among random communities; in essence when species in the two communities are distantly related. This may occur along a strong environmental gradient if traits that influence existence along it are conserved. Alternatively, if PBD is smaller than that expected based on CBD then communities compared are composed mostly of closely related species. This should occur along environmental gradients or biogeographic barriers that promoted speciation events for a subset of the regional phylogeny.
Results and Discussion
Phylogenetic Community Structure.
Of the 189 hummingbird communities evaluated, 134 (71%) had a positive net relatedness index (NRI), which indicates that these communities were phylogenetically clustered (31). Of these, 37 (28%) were significantly different at the 0.05 level from a null expectation based on randomization of species across all communities (61 communities, 45%, significant at 0.1). Fewer communities (55 communities or 29% of total) had a negative NRI, indicating phylogenetic overdispersion or evenness. Of these, one (2%) and seven (13%) were significant at the 0.05 and 0.1 levels, respectively.
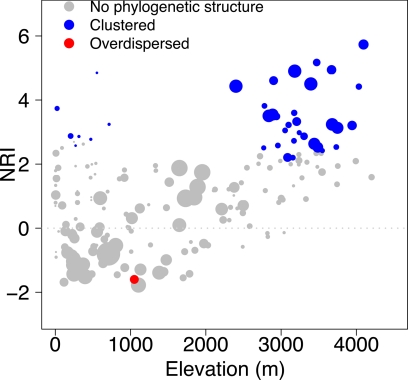
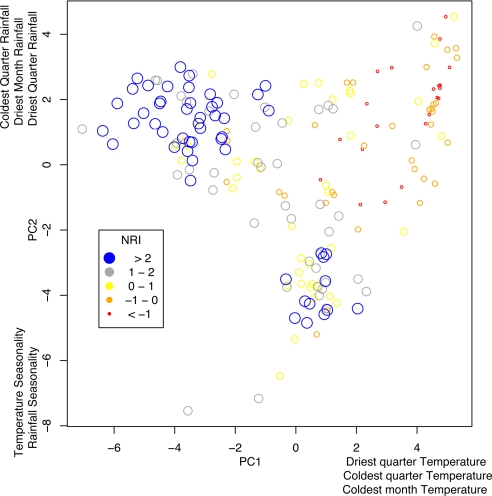
Phylogenetically clustered communities occurred either in cool, moist high-elevation Andean environments or in seasonally dry, warm environments on the western side of the Andes. Phylogenetically overdispersed communities tended to be in the warm, wet lowlands on either side of the Andes (Figs. 1 and 2). Principal components analysis (PCA) of environmental conditions across communities confirmed this result. The x axis of the PCA, which explains 41% of the variation, depicts a temperature (highly correlated with elevation) gradient; cooler sites load negatively and warmer sites load positively. The y axis, which explains 28% of the variation, represents precipitation in the dry season and annual variation in precipitation. Wetter environments load positively and drier, more seasonal environments negatively. Communities with high NRI, in relatively cool, wet high elevations along the Andes, are represented in the top left of the ordination, whereas those with high NRI from the relatively dry, warm lowlands are at the bottom of the ordination (Fig. 3). The best general additive model (GAM) explained 57% of the deviance in NRI and included a negative relationship between NRI and annual temperature, annual precipitation, and remotely sensed vegetation structure and a positive relationship with precipitation of the warmest quarter (4 months; Fig. S1 and Table S1). These results indicate that environmental variables related to temperature, precipitation, and vegetation structure are important correlates of hummingbird community structure. More broadly, the pattern of phylogenetic overdispersion in the lowlands is consistent with interspecific competition in hummingbirds in the absence of other ecologically stressful factors. Likewise, the pattern of phlyogenetic clustering at high elevations is consistent with the prediction that environmental filtering influences community structure, potentially because of metabolic and aerodynamic challenges faced by hummingbirds at high elevations. In addition, we found a similar pattern in the arid seasonal lowlands, independent of the constraints imposed by environmental conditions at high elevations.
Fig. 2.
Plot of NRI versus elevation. The size of the circle is proportional to the number of species in the community.
Fig. 3.
PCA based on climatic attributes of communities.
A shift from phylogenetic overdispersion to clustering along a stressful habitat gradient has not been commonly reported in other organisms. For instance, in Floridian oaks phylogenetic overdispersion persists along a gradient of water availability (1). Alpine plant communities show the opposite pattern to that reported here; communities along an elevational gradient became phylogenetically overdispersed at high elevations potentially because of facilitation (4). Andean hummingbirds provide an interesting comparison with existing studies of community phylogenetic structure along gradients because hummingbird flight requirements suggest there should be a strong gradient between elevation and performance. Further, our results emphasize the need to evaluate a broader range of taxonomic groups to draw generalizations about variation in community structure along gradients (32).
Competition in hummingbirds is intense and interspecific interactions are often considered important in determining the configuration of hummingbird communities (23, 26, 29). For instance, as a result of competition, communities often contain a series of species that take on different roles, such as territorials or trapliners (individuals move between a set of widely scattered flowers), where each role has a characteristic morphology (33). Likewise, different clades of hummingbirds have characteristic morphologies that influence resource use and flight ability (30). In theory, trait conservatism and competition could lead to phylogenetic overdispersion, a pattern observed here, although only in very wet lowland communities (Figs. 1 and 2). These overdispersed lowland communities had representation of eight and six of the nine hummingbird clades, in the eastern and western lowlands, respectively. Well-represented clades in these communities included those common at high elevations such as the brilliants, the lowland hermits that are confined to wet environments, and emeralds and mangos that exist in a variety of habitats (Fig. 4). At mid elevations communities have a mixture of close and distant relatives, resulting in NRI values that are close to the null expectation. However, given the empirical support for competition among hummingbirds, along with the observation that species from most major hummingbird clades have substantial representation in the Andes Mountains and lowland species often reach 2,000 m (7, 21, 22), we might have expected greater levels of phylogenetic overdispersion in communities at midelevations than observed.
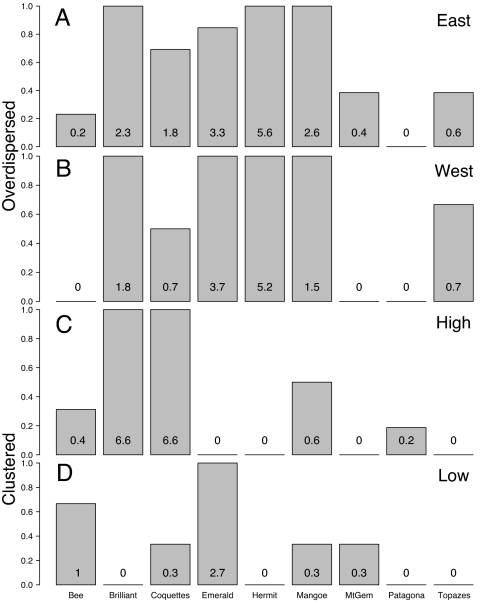
Fig. 4.
Representation of the clades of hummingbirds based on the 10th percentile of overdispersed communities that exist in the eastern (A) and western (B) moist lowlands and the 10th percentile of the clustered communities that exist in the high Andes (C) and low dry regions west of the Andes (D). The y axis represents the proportion of communities where a given clade is represented and the numbers in the bars are the mean number of species per community.
Our results show that communities in the most physiologically challenging environments in our study region, the highest elevations in the Andes and the dry isolated environments of the Tumbesian region, are phylogenetically clustered. Although species from several different clades exist at high elevations, the pattern of significant clustering >3,000 m is likely influenced by the prevalence of coquettes and brilliants (Fig. 4). Both the brilliants and coquettes have low wing loadings and increased wing-beat stroke amplitudes, which are adaptations for high-elevation flight (16, 17, 19). The coquettes also have relatively long tarsi that enable them to perch while feeding, therefore limiting expensive flight and exploiting the dominant type of inflorescences at high elevations (19, 34). In addition to clustering in high-elevation communities, a set of communities in the dry seasonal western lowlands exhibited this pattern, suggesting that environmental constraints may influence species in these communities as well. In this case, phylogenetic clustering is the result of the prevalence of emeralds in these habitats (Fig. 4). Emeralds are considered habitat generalists, and several species exist in drier habitats (7). As a result emeralds might have colonized these environments first, giving them an advantage over species from other clades. Alternatively, emeralds might have physiological adaptations, such as improved maintenance of water balance (35), that might give them an advantage over other clades in these environments. Other taxa, particularly hermits, which are common in wetter parts of the western lowlands, were not recorded in these communities, potentially because nectar resources were limited. Hermits feed extensively on Heliconia flowers (family Heliconiaceae), which are common in moist forest habitats (36, 37). Finally, although our assessment of community structure implicates environmental filtering as an important structuring mechanism, it does not mean that competition among hummingbirds is unimportant in structuring these communities, only that at the scale of this analysis, the role of filtering might be prevalent above the effect of competition (32, 38).
More broadly, analyses of community phylogenetic structure only quantify patterns, and we have interpreted these patterns by using what is known about hummingbird ecology. Nonetheless, we emphasize that other ecological, evolutionary, or stochastic factors might be important in structuring hummingbird communities. For example, other types of biotic interactions such as facilitation have been show to influence community organization in butterflies (39) and plants (40). Currently, one of the major challenges of phylogenetic structure analysis is to differentiate among alternative mechanisms that give rise to the same patterns. Further, we have focused on a regional scale of analyses in an attempt to quantify the biogeographic structure of hummingbird communities; however, more detailed analyses of clades, patterns of species co-occurrence, or specific regions could yield different insights into what factors might structure communities. Likewise, future research should evaluate how abiotic and biotic factors shape hummingbird communities by explicitly incorporating traits associated with food exploitation, competition, and flight performance into analyses of community structure.
CBD and PBD.
Phylogenetic distances among communities could be largely explained under a null model based on compositional similarity although there were a large number of comparisons that exhibited higher PBD than expected from CBD. When the identity of species in pairs of communities was randomized, most variation in PBD could be explained by CBD (R2 = 78%; Fig. S2). However, the observed values of PBD were on average greater than those predicted from this relationship (Fig. S3). To elucidate the environmental and geographic conditions that cause PBD to differ from the prediction based on CBD, we conducted partial mantel tests. CBD was the strongest predictor of PBD (r = 0.82). However, when CBD was accounted for in the model difference in altitude among sites was the next strongest predictor (r = 0.53), followed by geographic distance (r = −0.07), slope (r = −0.04), and change in habitat type (r = 0.03). All variables were significant predictors. The positive correlations of PBD to difference in elevation and habitat indicate that strong environmental gradients produce larger than expected PBD. However, greater geographic distance and occurrence on opposite sides of a biogeographic barrier (slope) resulted in lower PBD than expected.
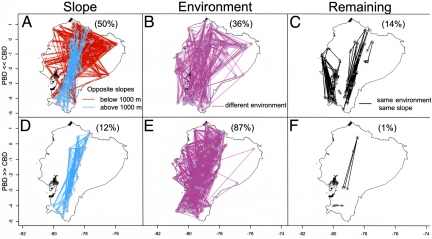
To visualize the environmental and geographic conditions that cause PBD to differ from the prediction based on CBD, we mapped community comparisons for which the discrepancy between observed and expected PBD fell into either the fifth percentile (lower than expected PBD) or the 95th percentile (higher than expected PBD). We find that a large part of comparisons (50%, n = 889) among communities where PBD is lower than expected (fifth percentile) are from either the lowlands (<1,000 m; 37% of comparisons) or highlands (>1,000 m; 13%) on opposite sides of the Andes (Fig. 5A). In contrast, there were no instances of lowland communities on the opposite sides of the Andes with a PBD greater than expected (95th percentile; Fig. 5D). This result supports the long-standing idea that the uplift of the Andes provided a significant biogeographic barrier for lowland communities, by restricting movement of lowland species to relatively episodic events (11, 41). Indeed, there are only a few species that inhabit the lowlands on both sides of the Andes and these tend to be wide-ranging species with large elevational ranges such as Florisuga mellivora, Eutoxeres aquila, and Anthracothorax nigricollis (42). Most discrepancies (87%) where PBD is greater than expected exist along the Andean slopes, suggesting that members from distinct clades exist at different points along this steep climatic and elevational gradient (Fig. 5E). Strikingly, these are mostly confined to western slopes, a pattern that would not be evident unless PBD was mapped (Fig. 5 B and E). The difference between PBD on the two slopes is likely a result of the recolonization of the humid lowlands (especially the eastern lowlands) by some lineages (i.e., Heliodoxa, Discosura, Lophornis) within the two high elevation clades, the coquettes and brilliants (7, 22), but not the dry seasonal lowlands, only present on the western side. As a result, relatively more lineages are represented at different elevations along the gradient in the eastern than the western slope of the Andes, resulting in lower PBD in the east. Finally, there are relatively few comparisons that involve communities in the same environment on the same slope (Fig. 5 C and F), indicating that within a region PBD can be predicted solely based on CBD.
Fig. 5.
Maps of community comparisons for which the discrepancy between observed and expected PBD fell into either the fifth percentile (PBD ≪ CBD) or the 95th percentile (PBD ≫ CBD). Lines are colored according to each of the following criteria: communities on opposite slopes (A and D) below (red lines) or above 1,000 m (truquoise lines), communities in different environments (B and E), and communities on the same slope and in the same environment (C and F).
By comparing PBD to CBD we were able to explore what processes might drive spatial variation in phylogenetic structure in hummingbird communities in Ecuador that were not apparent in more typical analyses of phylogenetic community structure. The uplift of the Andes produced a gradient of environments from warm lowlands to cold high elevations posing physiological challenges to hummingbirds. Altitudinal species turnover in the Andes have been documented in several species (43, 44). However, by examining multiple species in a phylogenetic framework our results move beyond analyses of pairs of species to show that entire clades replace themselves along the gradient (45). Further, by identifying which pairs of communities exhibit large discrepancies between PBD and CBD, we can begin to understand what processes determine the phylogenetic structure of hummingbird communities in Ecuador. These patterns raise interesting questions for future study as well. For example, it is not clear why the high-elevation brilliants and coquettes are not present in the dry seasonal communities of the western lowlands or why the emeralds are so prevalent in these communities.
Through the analysis of spatial variation in the structure of local communities within a regional framework we can start to evaluate the interaction between patterns caused by large-scale processes associated with biogeographic barriers and local processes such as competition and environmental filtering. Further, by quantifying phylogenetic community structure and β diversity in an explicit spatial and environmental framework we can identify environmental and geographic correlates of these patterns. More broadly, our approach should allow us to start to address long-standing questions about the mechanisms generating diversity across environmental gradients.
Materials and Methods
Community Composition.
We compiled a database of species composition of local hummingbird communities using lists from published references in peer-reviewed journals, gray literature, and nonpublished reports to environmental organizations including Bird Life International and Aves and Conservación (Dataset S1). All community locations were checked for their georeference and elevation. The taxonomy was updated to reflect the current version of the South American Classification Committee (46). The average area covered by a community was 4.2 km2 (range: 0.07 to 25), and the average elevational range covered was 299 m (range: 0 to 1,400). Finally, we only used communities that had more than one species and only included species for which there were available phylogenetic data. In total, we evaluated 189 communities including 126 species, of which 113 had available phylogenetic information. Ecuador has 132 hummingbird species.
Phylogeny.
We used a densely sampled phylogenetic estimate (166 of 324 species) for hummingbirds as the historical framework for comparative analyses. Phylogenetic relationships were inferred by using DNA sequences representing three nuclear genes [adenylate kinase intron 5 (AK1), β fibrinogen intron 7 (Bfib), and ornythin decarboxylase intron 6 (ODC)] and two mitochondrial genes [NADH dehydrogenase subunit 2 and 4 (ND2, ND4)], comprising 4,906 aligned base pairs. The phylogenetic estimate was based on partitioned Bayesian analysis (MrBayes 3.1; ref. 47) with separate partitions applied to each nuclear gene and to each codon position within the mitochondrial genes and their flanking tRNAs (for a total of 12 partitions). Appropriate substitution models for each partition were determined by using ModelTest 3.06 (48). The resulting tree was well-resolved and supported with 141 of 164 nodes receiving posterior probabilities of 95% or more. In terms of taxonomic coverage, taxa missing from our phylogeny are primarily from outside of the region of interest for this study (Ecuador). Our tree included 113 of the 126 species for which we have community occurrence data. Our data matrix included complete data for each species except Phaethornis superciliosus (missing AK1), Chalcostigma stanleyi (missing AK1), Urosticte ruficrissa (missing ODC), Thalurania fannyi (missing AK1 and ODC), and Hylocharis grayi (missing AK1, Bfib, and ODC). To produce the ultrametric tree needed for community structure analyses, we transformed the Bayesian consensus topology by using nonparametric rate smoothing (49) implemented in the program TreeEdit version 1.0a10.
Environmental Variables.
We used the following variables to describe the environment in Ecuador: annual mean temperature, mean diurnal temperature range, temperature seasonality, temperature annual range, annual precipitation, precipitation seasonality, precipitation of the warmest, and the coldest quarter from the Worldclim database (50). Remote-sensing variables relating to elevation and forest structure included: annual horizontal mean and standard deviation of Quick scatterometer (QSCAT), data that are known to be sensitive to surface roughness; annual maximum and mean leaf area index, derived from MODIS (Moderate Resolution Imaging Spectroradiometer); and MODIS tree percentage cover (51). These variables were chosen because they are relatively noncorrelated, should affect hummingbird flight and ecology, and have been informative in several recent studies on Andean birds (51). To define broad habitat types for some analyses we performed a classification based on all environmental variables by using an ISOCLUSTER analysis in ArcInfo (ArcInfo Workstation Version 8.1) and a priori specifying 20 groups.
Community Phylogenetic Structure and Environmental Correlates.
We calculated the NRI for all hummingbird communities and assessed the significance of each in relation to the distribution of 999 NRIs calculated under the null model. We used the independent swap method to generate null expectations (31, 52) and considered all species represented in the communities as the potential source pool. Positive values of these indices indicate phylogenetic clustering, and negative values indicate phylogenetic overdispersion. All analyses were done by using Phylocom version 4.01.b (53).
We conducted a PCA on a community by environmental variables matrix to explore whether communities with different NRI values inhabited different regions of multivariate environmental space. We then used the GAM procedure to evaluate the relationship between a subset of noncorrelated, informative environmental variables and NRI by using the mgcv package version 1.5 in R (54). To reduce environmental variables for input into the GAM we visually explored the variables that were correlated and conducted hierarchical partitioning to identify a set of informative variables (55).
CBD and PBD and Environmental Correlates.
We calculated CBD among communities by using the 1-Sorenson index. This index was modified to calculate PBD among communities as the proportion of branch length shared between two communities divided by the average of the sum of branch lengths of each community following ref. 4 and using PhyloSor version 0.6 (R) (also see ref. 56). We calculated expected PBD under a null model, where the species richness and turnover among communities was fixed and only the identity of the species was randomized [10 iterations, “richness model” (52)]. Next, the relationship between expected PBD and observed CBD was estimated by using ordinary least-squares regression (Fig. S3). The residuals from the observed PBD and that expected under the regression model were used to identify those comparisons where PBD was much lower (fifth percentile) or higher (95th percentile) than expected under the null model. To visualize the potential environmental and geographic causes of these discrepancies, we mapped these comparisons by drawing a line for each pair of communities being contrasted that was colored according to each of four hypotheses. The factors we expected to cause high discrepancies between expected and observed PBD were biogeographic separation (same or different side of the Andes), change in elevation, change in habitats, and geographic distance.
To further explore which factors influenced PBD we used a partial mantel test following ref. 57. In these tests, PBD was the dependent variable and the independent variables were CBD and four environmental and geographic predictors. These four predictor variables were environmental dissimilarity, scored as within the same (score of 0) or in a different habitat (score of 1; habitats followed the environmental classification above); change in elevation calculated as the absolute difference in elevation between two communities; change in slope, scored as on the same (score of 0) or on a different side (score of 1) of the Andes; and Euclidean geographic distance. The significance of each of the four predictors was calculated given all of the other predictors by using 4,000 permutations. Although mantel tests have been criticized for having low power (58), they provide useful insight in our study.
Acknowledgments.
We thank S. Baines, N. Silva, B. Tinoco, S. Gray, P. Bourdeau, D. Strubbe, and D. Padilla for stimulating discussion and comments; J. Freile, J. Hardesty, and B. Tinoco for providing community composition data for Ecuador; D. Wake, E. Hadly, and D. Ackerly for organizing the colloquium; and the following institutions for making specimens available for this study: Louisiana State University Museum of Natural Science, Academy of Natural Sciences, American Museum of Natural History, Burke Museum of Natural History, Field Museum of Natural History, National Museum of Natural History, University of Kansas Museum of Natural History, University of Michigan Museum of Zoology, Smithsonian Tropical Research Institute, and the Zoological Museum Copenhagen. This work was supported by U.S. National Science Foundation Grants DEB-0820490 (to C.H.G. and J.L.P.) and DEB-0640859 (to J.L.P.), and Danish National Science Foundation Grant J.21-03-0221 (to C.R.).
Footnotes
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “Biogeography, Changing Climates and Niche Evolution,” held December 12–13, 2008, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. The complete program and audio files of most presentations are available on the NAS web site at www.nasonline.org/Sackler_Biogeography.
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0901649106/DCSupplemental.
References
- 1.Cavender-Bares J, Ackerly DD, Baum DA, Bazzaz FA. Phylogenetic overdispersion in Floridian oak communities. Am Nat. 2004;163:823–843. doi: 10.1086/386375. [DOI] [PubMed] [Google Scholar]
- 2.Webb CO, Ackerly DD, McPeek MA, Donoghue MJ. Phylogenies and community ecology. Annu Rev Ecol Syst. 2002;33:475–505. [Google Scholar]
- 3.Tuomisto H, et al. Dissecting Amazonian biodiversity. Science. 1995;269:63–66. doi: 10.1126/science.269.5220.63. [DOI] [PubMed] [Google Scholar]
- 4.Bryant JA, et al. Microbes on mountainsides: Contrasting elevational patterns of bacterial and plant diversity. Proc Natl Acad Sci USA. 2008;105:11505–11511. doi: 10.1073/pnas.0801920105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graham CH, Fine PVA. Phylogenetic β diversity: Linking ecological and evolutionary processes across space in time. Ecol Lett. 2008;11:1265–1277. doi: 10.1111/j.1461-0248.2008.01256.x. [DOI] [PubMed] [Google Scholar]
- 6.Hardy OJ, Senterre B. Characterizing the phylogenetic structure of communities by an additive partitioning of phylogenetic diversity. J Ecol. 2007;95:493–506. [Google Scholar]
- 7.Bleiweiss R. Origin of hummingbird faunas. Biol J Linn Soc. 1998;65:77–97. [Google Scholar]
- 8.Brumfield RT, Edwards SV. Evolution into and out of the Andes: A Bayesian analysis of historical diversification in Thamnophilus antshrikes. Evolution (Lawrence, Kans) 2007;61:346–367. doi: 10.1111/j.1558-5646.2007.00039.x. [DOI] [PubMed] [Google Scholar]
- 9.Graves GR. Elevational correlates of speciation and intraspecific geographic variation in plumage in Andean forest birds. Auk. 1985;102:556–579. [Google Scholar]
- 10.Rahbek C, Graves GR. Detection of macro-ecological patterns in South American hummingbirds is affected by spatial scale. Proc R Soc London Ser B. 2000;267:2259–2265. doi: 10.1098/rspb.2000.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brumfield RT, Capparella AP. Historical diversification of birds in northwestern South America: A molecular perspective on the role of vicariant events. Evolution (Lawrence, Kans) 1996;50:1607–1624. doi: 10.1111/j.1558-5646.1996.tb03933.x. [DOI] [PubMed] [Google Scholar]
- 12.Chaves JA, Pollinger JP, Smith TB, LeBuhn G. The role of geography and ecology in shaping the phylogeography of the speckled hummingbird (Adelomyia melanogenys) in Ecuador. Mol Phylogenet Evol. 2007;43:795–807. doi: 10.1016/j.ympev.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Bleiweiss R, Kirsch JAW, Matheus JC. DNA hybridization evidence for the principal lineages of hummingbirds (Aves: Trochilidae) Mol Biol Evol. 1997;14:325–343. doi: 10.1093/oxfordjournals.molbev.a025767. [DOI] [PubMed] [Google Scholar]
- 14.Dziedzioch C, Stevens AD, Gottsberger G. The hummingbird plant community of a tropical montane rain forest in southern Ecuador. Plant Biol. 2003;5:331–337. [Google Scholar]
- 15.Robbins MB, Ridgely RS, Schulenberg TS, Gill FB. The avifauna of the Cordillera-De-Cutucu, Ecuador, with comparisons to other Andean localities. Proc Acad Natur Sci Phil. 1987;139:243–259. [Google Scholar]
- 16.Altshuler DL, Dudley R, McGuire JA. Resolution of a paradox: Hummingbird flight at high elevation does not come without a cost. Proc Natl Acad Sci USA. 2004;101:17731–17736. doi: 10.1073/pnas.0405260101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Altshuler DL, Stiles FG, Dudley R. Of hummingbirds and helicopters: Hovering costs, competitive ability, and foraging strategies. Am Nat. 2004;163:16–25. doi: 10.1086/380511. [DOI] [PubMed] [Google Scholar]
- 18.Stiles FG. Phylogenetic constraints upon morphological and ecological adaptation in hummingbirds (Trochilidae): Why are there no hermits in the paramo? Ornitol Neotrop. 2004;15(Suppl):191–198. [Google Scholar]
- 19.Stiles FG. Ecomorphology and phylogeny of hummingbirds: Divergence and convergence in adaptations to high elevations. Ornitol Neotrop. 2008;19:511–519. [Google Scholar]
- 20.Carpenter FL. Ecology and Evolution of an Andean Hummingbird (Oreotrochilus estella) Berkeley: Univ California Press; 1976. [Google Scholar]
- 21.Schuchmann KL. Family Trochilidae (Hummingbirds) Barcelona: Lunx Edicions; 1999. [Google Scholar]
- 22.McGuire JA, Witt CC, Altshuler DL, Remsen JV. Phylogenetic systematics and biogeography of hummingbirds: Bayesian and maximum-likelihood analyses of partitioned data and selection of an appropriate partitioning strategy. Syst Biol. 2007;56:837–856. doi: 10.1080/10635150701656360. [DOI] [PubMed] [Google Scholar]
- 23.Feinsinger P. Organization of a tropical guild of nectarivorous birds. Ecol Monogr. 1976;46:257–291. [Google Scholar]
- 24.Gutierrez-Zamora A. Ecological interactions and structure of a high Andean community of hummingbirds and flowers in the Eastern Andes of Colombia. Ornitol Colombiana. 2008;7:17–42. [Google Scholar]
- 25.Stiles FG. Behavioral, ecological, and morphological correlates of foraging for arthropods by the hummingbirds of a tropical wet. Condor. 1995;97:853–878. [Google Scholar]
- 26.Wolf LL, Stiles FG, Hainsworth FR. Ecological organization of a tropical, highland hummingbird community. J Anim Ecol. 1976;45:349–379. [Google Scholar]
- 27.Temeles EJ, Koulouris CR, Sander SE, Kress WJ. Effect of flower shape and size on foraging performance and tradeoffs in a tropical hummingbird. Ecology. 2009;90:1147–1161. doi: 10.1890/08-0695.1. [DOI] [PubMed] [Google Scholar]
- 28.Altshuler DL, Dudley R. The ecological and evolutionary interface of hummingbird flight physiology. J Exp Biol. 2002;205:2325–2336. doi: 10.1242/jeb.205.16.2325. [DOI] [PubMed] [Google Scholar]
- 29.Brown JH, Bowers MA. Community organization in hummingbirds: Relationships between morphology and ecology. Auk. 1985;102:251–269. [Google Scholar]
- 30.Stiles FG. Ecomorphology and phylogeny of hummingbirds: Divergence and convergence in adaptations to high elevations. Ornitol Neotrop. 2008;19:511–519. [Google Scholar]
- 31.Webb CO. Exploring the phylogenetic structure of ecological communities: An example for rain forest trees. Am Nat. 2000;156:145–155. doi: 10.1086/303378. [DOI] [PubMed] [Google Scholar]
- 32.Vamosi SM, Heard SB, Vamosi JC, Webb CO. Emerging patterns in the comparative analysis of phylogenetic community structure. Mol Ecol. 2009;18:572–592. doi: 10.1111/j.1365-294X.2008.04001.x. [DOI] [PubMed] [Google Scholar]
- 33.Feinsinger P, Colwell RK. Community organization among neotropical nectar-feeding birds. Am Zool. 1978;18:779–795. [Google Scholar]
- 34.Stiles FG. Phylogenetic constraints upon morphological and ecological adaptation in hummingbirds (Trochilidae): Why are there no hermits in the paramo? Ornitol Neotrop. 2004;15:191–198. [Google Scholar]
- 35.Bakken BH, McWhorter TJ, Tsahar E, del Rio CM. Hummingbirds arrest their kidneys at night: Diel variation in glomerular filtration rate in Selasphorus platycercus. J Exp Biol. 2004;207:4383–4391. doi: 10.1242/jeb.01238. [DOI] [PubMed] [Google Scholar]
- 36.Stiles FG. Geographical aspects of bird–flower coevolution, with particular reference to Central America. Ann Mo Bot Gard. 1981;68:323–351. [Google Scholar]
- 37.Stiles FG. Temporal organization of flowering among hummingbird foodplants of a tropical wet forest. Biotropica. 1978;10:194–210. [Google Scholar]
- 38.Helmus MR, Savage K, Diebel MW, Maxted JT, Ives AR. Separating the determinants of phylogenetic community structure. Ecol Lett. 2007;10:917–925. doi: 10.1111/j.1461-0248.2007.01083.x. [DOI] [PubMed] [Google Scholar]
- 39.Elias M, Gompert Z, Jiggins C, Willmott K. Mutualistic interactions drive ecological niche convergence in a diverse butterfly community. PLOS Biol. 2008;6:2642–2649. doi: 10.1371/journal.pbio.0060300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Valiente-Banuet A, Verdu M. Temporal shifts from facilitation to competition occur between closely related taxa. J Ecol. 2008;96:489–494. [Google Scholar]
- 41.Miller MJ, et al. Out of Amazonia again and again: Episodic crossing of the Andes promotes diversification in a lowland forest flycatcher. Proc R Soc London Ser B. 2008;275:1133–1142. doi: 10.1098/rspb.2008.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ridgely RS, Greenfield JP. The Birds of Ecuador. Ithaca, NY: Cornell Univ Press; 2001. [Google Scholar]
- 43.Dingle C, Lovette IJ, Canaday C, Smith TB. Elevational zonation and the phylogenetic relationships of the Henicorhina wood wrens. Auk. 2006;123:119–134. [Google Scholar]
- 44.Terborgh J. Bird species diversity on an Andean elevational gradient. Ecology. 1977;58:1007–1019. [Google Scholar]
- 45.Romdal TS, Rahbek C. Elevational zonation of afrotropical forest bird communities along a homogeneous forest gradient. J Biogeogr. 2009;36:327–336. [Google Scholar]
- 46.Remsen JV, Jr, et al. A Classification of the Bird Species of South America. 2008. [Accessed December 2008]. Available at www.museum.lsu.edu/∼Remsen/SACCBaseline.html.
- 47.Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- 48.Posada D, Crandall KA. MODELTEST: Testing the model of DNA substitution. Bioinformatics. 1998;14:817–818. doi: 10.1093/bioinformatics/14.9.817. [DOI] [PubMed] [Google Scholar]
- 49.Sanderson MJ. A nonparametric approach to estimating divergence times in the absence of rate constancy. Mol Biol Evol. 1997;14:1218–1231. [Google Scholar]
- 50.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Int J Climatol. 2005;25:1965–1978. [Google Scholar]
- 51.Buermann W, et al. Predicting species distributions across the Amazonian and Andean regions using remote sensing data. J Biogeogr. 2008;35:1160–1176. [Google Scholar]
- 52.Gotelli NJ, Entsminger GL. Swap algorithms in null model analysis. Ecology. 2003;84:532–535. doi: 10.1007/s004420100717. [DOI] [PubMed] [Google Scholar]
- 53.Webb CO, Ackerly DD, Kembel SW. Phylocom: Software for the analysis of phylogenetic community structure and trait evolution. Bioinformatics. 2008;24:2098–2100. doi: 10.1093/bioinformatics/btn358. [DOI] [PubMed] [Google Scholar]
- 54.Hastie T, Tibshirani R. Generalized Additive Models. London: Chapman & Hall; 1991. [DOI] [PubMed] [Google Scholar]
- 55.Walsh C, MacNally R. The hier.part: Hierarchical Partitioning. 2003 (R Project for Statistical Computing), R package version 1.0.3. [Google Scholar]
- 56.Lozupone C, Knight R. UniFrac: A new phylogenetic method for comparing microbial communities. Appl Environ Microbiol. 2005;71:8228–8235. doi: 10.1128/AEM.71.12.8228-8235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Legendre P, Borcard D, Peres-Neto PR. Analyzing β diversity: Partitioning the spatial variation of community composition data. Ecol Monogr. 2005;75:435–450. [Google Scholar]
- 58.Legendre P, Borcard D, Peres-Neto PR. Analyzing or explaining β diversity? Comment. Ecology. 2008;89:3238–3244. doi: 10.1890/07-0272.1. [DOI] [PubMed] [Google Scholar]