Abstract
Understanding the processes that create and maintain species' geographic range limits has implications for many questions in ecology, evolution, and conservation biology. Many expectations for the ecological and evolutionary dynamics of populations at the range margin rest on the concordance of geographic limits and the limits of a species' ecological niche. If range limits are coincident with niche limits, then marginal populations should have lower and/or more variable vital rates and population growth rates than central populations. Using data from 8 annual censuses of marked individuals, I investigated the demography of Mimulus cardinalis and Mimulus lewisii across the species' elevation ranges. Central and marginal populations exhibited striking demographic differences, but only for one species were differences in expected directions. Marginal populations from the M. lewisii lower elevation range limit had lower and more variable survival than central populations and appeared to be demographic sinks. In contrast, marginal populations from the M. cardinalis upper elevation limit had higher fecundity and higher population growth rates than central populations. Although the species differed with respect to central-marginal patterns, they were concordant with respect to elevation; that is, both species had higher fitness in higher reaches of their examined ranges. Potential explanations for these patterns include source-sink dynamics, with asymmetrical gene flow mediated by river currents, and climate change, with recent warming shifting the species' climatic envelopes to higher elevations. Hence, assessment of spatiotemporal variation in both demography and dispersal is necessary to fully understand the relationship between the niche and species' distributions.
Keywords: geographic range limit, lambda, population dynamics, vital rate, projection matrix
The geographic range is a fundamental unit of biogeography (1), yet our understanding of the ecological and evolutionary determinants of the placement, occupancy, extent, and limits of geographic ranges remains largely idiosyncratic and system-specific. In particular, the processes that lead to evolutionarily stable range limits remain poorly understood for most organisms. Many investigations of range limits begin by associating distribution boundaries with potentially limiting environmental variables (e.g., refs. 2 and 3). This approach has a venerable history (4–7) and was central to the early development of the niche concept (8, 9). Thus, in the broadest sense, species' distributions can be viewed as a spatial manifestation of the niche (10, 11), where the geographic range represents a mapping of fitness as a function of the abiotic and biotic environment onto a geographically varying environmental landscape and range edges arise at points along environmental continuums where births no longer exceed deaths (12, 13). Hence, quantifying variation in demographic performance across the geographic range can provide a fundamental first step toward understanding the extent to which range limits indeed represent niche limits and insight into what factors may be important in setting these limits. From an evolutionary perspective, concordance of niche limits with range boundaries implies that intraspecific niche evolution is necessary for range expansion and conversely that range limits arise because of constraints on niche evolution via natural selection (10). Thus, range edges can provide an arena for investigating adaptation, limits to adaptation, and processes that give rise to patterns of niche conservatism. Greater understanding of ecological and evolutionary dynamics at range edges is also critical for accurately predicting species' responses to a changing climate. Investigations of demographic performance across the range can provide mechanistic insight into the relationships between environment and fitness, a necessary first step for forecasting population responses to environmental changes.
Although geographic ranges would not exist without organismal movement (14), consideration of dispersal adds additional complexity to the problem of relating species' geographic distributions to the environmental landscape and the niche. Dispersal limitation may result in populations failing to occupy suitable environments (11, 15–17). For example, dispersal into formerly glaciated areas may lag behind the rate of environmental amelioration, making the present northern range limits of some temperate species transient boundaries that lie within the species' fundamental niche (15). Conversely, dispersal into unfavorable areas may cause populations to occupy environments that are outside of the species' fundamental niche, as evidenced by populations whose growth rate is below replacement level (11, 18). These sink populations may be transient or sustained by regular immigration from source populations.
Models of ecological and evolutionary dynamics at range limits highlight how complex interactions between demography and dispersal across environments may promote or hinder niche evolution at range margins (10, 12, 19–22). A net flux of migrants from the range center to the range edge may lead to gene swamping that prevents marginal populations from adapting to the local environment (19). In this scenario, dispersal from central source populations holds marginal populations in a maladapted sink state. Alternatively, dispersal may facilitate niche evolution by providing demographic rescue to maladapted marginal populations that would otherwise go extinct and by increasing genetic variation within marginal populations (12, 22, 23). In this scenario, marginal populations begin as sinks but occasionally may evolve adaptations to the sink environment with demographic and genetic input from central sources. In either case, these models yield a major inference that can be tested with empirical data, namely that range limits are stable or quasi-stable over long periods of time because evolutionary potential is limited in marginal sink populations.
Demographic observations across species' ranges are necessary to link geographic position within the range to spatiotemporal variation in fitness. Although some studies have demonstrated that certain fitness components or vital rates are lower at the range margin (e.g., refs. 24–26), many others have not found expected differences (e.g., refs. 27–29). In these latter cases, present-day range boundaries may not be at niche limits; instead, the boundaries may represent nonequilibrial edges of ranges, set by limits to dispersal over time or space. However, this interpretation is hindered because studies that do not assess lifetime fitness may fail to find differences even when they exist. Such studies may miss critical life-stage transitions that do differ between central and marginal populations. Additionally, central-marginal differences may be small at any given stage yet multiply to biologically meaningful differences at the population level. Finally, performance may be high in average years in locations where marginal populations are extant, but stochastic environmental variation may introduce extreme conditions that exceed the species' tolerance infrequently and have a disproportionate effect on long-term dynamics at the margin. Theoretical studies have illustrated that increasing variance in vital rates can yield range limits, even in the absence of consistent differences in vital rate means (12). Thus, documenting only differences in mean demographic performance may be misleading if variance is ignored. Although studies of temporal variation in abundance support the idea that population dynamics are more variable at the range margin (30–32), comparable studies of temporal variation in vital rates are limited (33) and hence we currently lack the full set of empirical evidence needed to assess demographic dynamics at geographic range limits. A deeper understanding of demographic variation across species' ranges will require studies that integrate observations across the entire life cycle and across long windows of time.
In this article, I use demographic census data collected annually from 2000 through 2007 in central and marginal populations of the native perennial herbs Mimulus cardinalis and Mimulus lewisii to examine spatiotemporal variation in demography across 2 species' ranges. This study examines the species' elevation ranges to assess differences between central and marginal populations at a tractable spatial scale. A previous investigation (34) used data from 2000 through 2003 to investigate spatial differences in population growth rates between central and marginal populations of these species. Although the riparian natural history of these species suggests that temporal environmental variation is likely to have important effects on long-term population dynamics, little temporal variation was observed in the previous short-term study (34). Here, I use a 7-year sequence to quantify temporal variation in vital rates and population growth rates and make robust inferences about mean differences between central and marginal populations. The overarching hypothesis of this study is that range limits arise at niche boundaries and are driven by changes in the means or variability of demographic fitness components. Specifically, I test the following predictions: (i) mean vital rates are lower in marginal than central populations; (ii) vital rates are more variable in marginal than central populations; (iii) population growth rates (population mean fitness) are lower and/or more variable in marginal than in central populations; and (iv) population growth rates are below replacement levels in marginal populations, indicating that marginal populations are demographic sinks.
Results
Vital Rates.
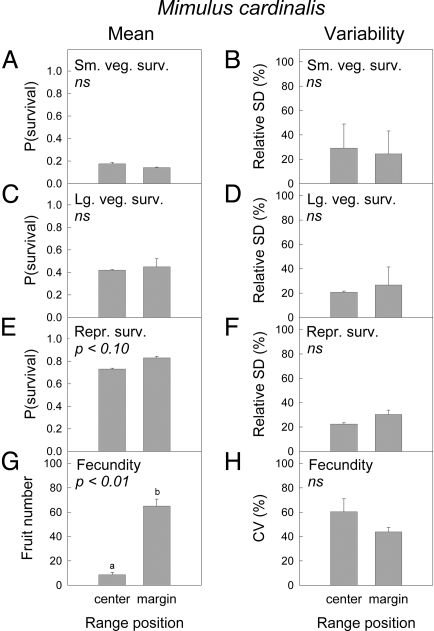
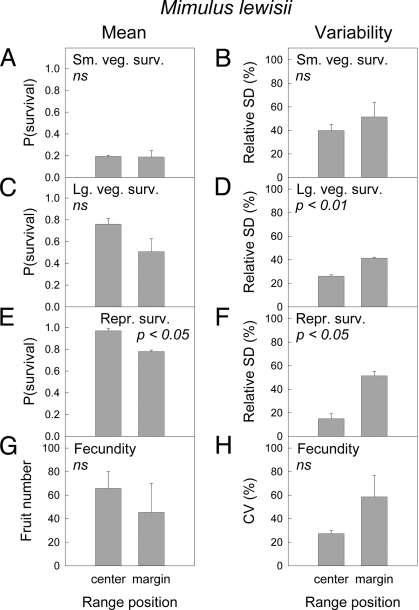
As is typical for perennial plants, the probability of annual survival increased with size and stage, from small to large vegetative plants to reproductive plants, for both M. cardinalis (Fig. 1A, C, and E) and M. lewisii (Fig. 2A, C, and E). Mean survival probability did not differ between central and marginal populations for any stage class of M. cardinalis, nor did variability of survival differ between central and marginal populations (Fig. 1 A–F). Central and marginal M. cardinalis populations did show significantly different mean fecundity (Fig. 1G). However, contrary to expectation, central populations did not have higher fecundity but instead produced ≈8-fold fewer fruits than marginal populations. Fecundity was not significantly less variable in central than in marginal populations of this species (Fig. 1H). For M. lewisii, mean survival probability of reproductive plants was significantly higher in central than in marginal populations (Fig. 2E), as predicted. Large vegetative plants showed a similar, but nonsignificant, trend in mean survival (Fig. 2C), and mean survival of small vegetative plants did not differ between central and marginal populations (Fig. 2A). For 2 of the 3 stage classes, variability in annual survival was lower in central than in marginal populations, as predicted (Fig. 2 B, D, and F). Fecundity of M. lewisii was not significantly higher (Fig. 2G) or less variable (Fig. 2H) in central than in marginal populations.
Fig. 1.
Vital rate means and relative environmental variabilities after removing sampling error (53) in central and marginal populations of M. cardinalis, based on 2000–2007 data. (A, C, E, and G) Mean values are displayed. (B, D, F, and H) Variabilities are shown. Survival values for small vegetative (A and B), large vegetative (C and D), and reproductive (E and F) plants are shown. (G and H) Fecundity values for reproductive plants are shown. Error bars show 1 SE of estimates between replicate populations at a given range position. P values are from 2-tailed t tests for central-marginal differences. ns indicates nonsignificant, P > 0.10.
Fig. 2.
Vital rate means and relative environmental variabilities after removing sampling error in central and marginal populations of M. lewisii, based on 2000–2007 data. (A, C, E, and G) Mean values are displayed. (B, D, F, and H) Variabilities are shown. Survival values for small vegetative (A and B), large vegetative (C and D), and reproductive (E and F) plants are shown. (G and H) Fecundity values for reproductive plants are shown. Error bars show 1 SE of estimates between replicate populations at a given range position. P values are from 2-tailed t tests for central-marginal differences. ns indicates nonsignificant, P > 0.10.
Population Growth Rates.
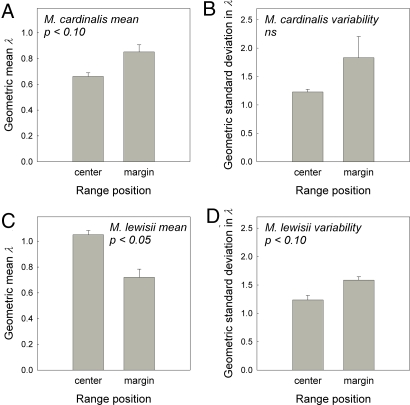
Asymptotic projections of population growth rate (λ) varied across the 7 transition intervals, from 0.47 to 0.89 for central populations and from 0.19 to 2.05 for marginal populations of M. cardinalis (Table S1). Wide confidence intervals in years with high λ estimates reflect the rare occurrence of transitions for which λ has high sensitivity, namely recruitment from seeds directly into the reproductive stage class (34). Despite some years with high λ at the margin, mean λ across all years was only marginally lower at the range center compared with the range margin for M. cardinalis (Fig. 3A). For M. lewisii, λ varied from 0.88 to 1.99 in central populations and from 0.33 to 1.31 in marginal populations across the 7 yearly transition intervals (Table S1). Mean λ of M. lewisii was significantly higher at the range center compared with the range margin (Fig. 3C). For both species, the trend for lower year-to-year variability in λ at the range center compared with the range margin was not statistically significant (Fig. 3 B and D).
Fig. 3.
Population growth rate means and temporal variabilities for central and marginal populations of M. cardinalis and M. lewisii. (A) Mean asymptotic population growth rate (λ), calculated as the geometric mean of 7 annual λ estimates, for M. cardinalis. (B) Temporal variability in λ, calculated as the geometric standard deviation in 7 annual λ estimates, for M. cardinalis. (C) Mean λ for M. lewisii. (D) Temporal variability in λ for M. lewisii. Error bars show 1 SE of estimates between replicate populations at a given range position. P values are from 2-tailed t tests for central-marginal differences. ns indicates nonsignificant, P > 0.10.
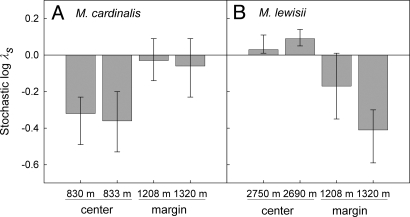
Stochastic simulations of population growth yielded results that were qualitatively similar to mean asymptotic projections. For M. cardinalis, log λs was significantly lower at the range center than at the range margin (Fig. 4A). For M. lewisii, log λs was marginally higher at the range center than at the range margin (Fig. 4B). Some populations of both species exhibited long-term growth rates that were below replacement levels (log λs < 0), consistent with the occurrence of demographic sink populations (Fig. 4). For M. cardinalis, populations whose confidence intervals around long-term stochastic growth estimates were <0 were at the range center, whereas for M. lewisii these populations were at the range margin.
Fig. 4.
Stochastic projections of population growth rate (log λs) for central and marginal populations of M. cardinalis (A) and M. lewisii (B). Error bars depict bias-corrected 95% confidence intervals, obtained from 3,000 bootstrapped datasets (57).
Discussion
Examination of detailed individual-level annual census data from natural populations has revealed marked differences in population dynamics between central and marginal areas of species' ranges. More studies have examined vital rate mean differences across the range than differences in temporal variability (33, 35, 36). Using a sequence of 7 annual transitions, this study shows that central and marginal populations differ not just in mean vital rates but in some cases also in the temporal variabilities of vital rates. These results illustrate that differences in vital rate means and variabilities accumulate into large differences in projected population growth rates, or population mean fitness, in central and marginal environments.
However, for only 1 of the 2 species examined here were the differences between central and marginal populations in the expected direction. As predicted, marginal populations of M. lewisii appear to be at niche boundaries; that is, survival probabilities were lower and more variable than in central populations; population growth rates were also significantly lower; and population growth rates at the range margin were below replacement levels, suggesting that marginal populations are demographic sinks. For M. cardinalis, however, central populations had markedly lower fecundities and lower population growth rates than did marginal populations. Paradoxically, populations at the M. cardinalis range center exhibit long-term growth rates that are below replacement levels, whereas marginal population growth rates encompass values consistent with population stability. Several other comparisons of central-marginal demographic patterns have found similarly ambiguous or unexpected results (35–39), which suggests that consideration of additional factors such as density dependence, source-sink dynamics, dispersal limitation, and disequilibrium between climate and current ranges will be necessary to fully understand the relationship between demography and distribution (39).
The difference of patterns between the 2 species is not obviously related to differences in life history or morphology; both are rhizomatous, perennial, animal-pollinated herbs that make many-seeded fruits, and phylogenetic evidence suggests that they are sister taxa (40). This finding suggests that the opposing patterns of spatial variation between central and marginal populations may not arise from intrinsic biological differences between the species, but rather may be caused by external factors. Indeed, although the species exhibit opposing patterns with respect to central-marginal differences, their patterns are the same with respect to elevation. Both species exhibit higher mean vital rates and higher population growth rates within higher elevation zones (the range center for M. lewisii, the range margin for M. cardinalis). This pattern suggests 2 possible explanations. Studies of similar riparian plant species have demonstrated that seed dispersal via downstream currents may occur over long distances and be strongly asymmetrical (41). If so, upstream populations may serve as sources for downstream populations that are chronic demographic sinks. Contrary to range limit models, where asymmetrical dispersal arises from intrinsic properties of populations (e.g., population density), this scenario posits that asymmetrical gene flow between source and sink populations arises from features of the external landscape. Asymmetrical gene flow may have similar outcomes in both cases, in that downstream populations may be ecologically rescued by upstream migrants but unable to adapt to local conditions because of cross-gradient gene flow from higher elevation environments. Genetic assignment methods offer a promising avenue for investigating this hypothesis by assessing contemporary asymmetry of gene flow (42, 43).
Recent theoretical models illustrate how dispersal barriers such as those posed by downstream currents may contribute to stable boundaries between parapatrically distributed species like M. cardinalis and M. lewisii (44). Borders may be attracted to regions where stronger competitors that would otherwise outcompete another species encounter a dispersal barrier (44). The higher population mean fitness (λ) of M. cardinalis compared with M. lewisii at middle elevation suggests the potential for competitive asymmetry between the species. However, experimental populations of M. lewisii transplanted to middle elevation in a noncompetitive environment also exhibited low mean fitness (45), suggesting that fitness of this species remains low at the range margin in the absence of competition from M. cardinalis. Experimental manipulations of density and species composition are necessary to explicitly quantify competitive interactions and their potential to influence the species' range limits.
An alternative explanation for the demographic patterns observed in this study is that present range limits, particularly of M. cardinalis, are not at equilibrium with current environments. Recent studies have documented warming trends in the study region and striking shifts of some small mammal species to higher elevations (46). The design of this study does not permit an analogous investigation of distribution shifts to higher elevations for these species. However, it is possible to consider the effect of climate trends on stationary populations at a given location. Given recent warming rates, marginal populations at middle elevation are beginning to experience temperatures that are within the range of historical temperatures at low elevation. If so, the pattern of higher population mean fitness at higher elevations is consistent with warming trends that may have shifted the species' climatic envelopes to the higher elevation zones of their current ranges. If dispersal is indeed largely downstream, then populations may be hindered in their ability to track suitable climates by dispersing to higher elevations. Temperature increases may also interact with changes in other important environmental variables. For example, changes in snowpack may have dramatic influences on all species by altering the availability of soil moisture during the growing season (47) and on riparian species in particular by also altering the magnitude of spring flooding events, which can increase both adult mortality and seedling recruitment (48). Longer time series of demographic parameters are desirable for relating variation in vital rates and population dynamics to interannual variation in climate and hydrology. Importantly, such investigations can help build a mechanistic understanding of the environmental factors that drive not only mean differences in performance but also temporal variability in performance across the range. Investigations over longer time periods will help elucidate linkages between the environmental variation, fitness, and distribution limits and ways in which changes in the environment may yield changes in distribution.
This study, like many others that use quite different approaches (e.g., refs. 16 and 49), draws on the ecological niche concept for investigating species' geographical distributions. By relating spatiotemporal variation in the environment to the fitness of individuals and the dynamics of populations, geographic distributions may be investigated as a manifestation of the niche writ large. One-to-one matching between niche and distribution limits was not observed here and should not be expected in all cases given that dispersal can introduce populations into poor quality habitats, dispersal limitation can keep populations from occupying suitable habitats, and ever-changing climates may cause distributions to be out of equilibrium with current environments. It is sobering to recognize that surveys of the same historical transects that helped to inspire Grinnell's original formulation of the niche are now revealing marked shifts in species' distributions driven by recent climate change (46). Relating the environment to fitness, abundance, and species' distributions remains a compelling ecological and evolutionary problem, with more relevance than ever given unprecedented disequilibrium between climate and species' distributions that is already underway.
Materials and Methods
Study Species.
M. cardinalis and M. lewisii (Phrymaceae) are perennial herbs that grow along seeps and stream banks in western North America. M. cardinalis occurs at low to middle elevations from southern Oregon to northern Baja California (50). M. lewisii is composed of 2 races that are separated by partial reproductive incompatibilities (40, 50, 51). Here, I study only the southern race of M. lewisii, which occurs from middle to high elevations in the Sierra Nevada Mountains of California. The range margins that are the focus of this study are the species' shared middle elevation limits between 1,200 and 1,600 m elevation in the central Sierra Nevada Mountains, where M. cardinalis reaches its upper elevation limit and M. lewisii reaches its lower elevation limit.
Study Sites.
Eight natural populations from 6 sites were monitored along an elevation transect from 830 to 2,750 m within 37.4643 and 38.0979°N latitude in Yosemite National Park, Stanislaus National Forest, and Inyo National Forest. This transect encompasses the elevation range centers and range margins for both species at a tractable spatial scale. Two sites were located at the middle-elevation range margin where populations of both species occur sympatrically (Wawona, South Fork Merced River, 37.5387°N, 119.6543°W, 1,208 m; and Carlon, South Fork Tuolumne River, 37.8152, 119.8657°W, 1,320 m). Additionally, 2 sites were at the M. cardinalis low-elevation range center (Buck Meadows, Moore Creek, 37.7770°N, 120.0635°W, 830 m; and Rainbow Pool, South Fork Tuolumne River, 37.8213°N, 120.0109°W, 833 m), and 2 sites were at the M. lewisii high-elevation range center (May Lake, Snow Creek, 37.8365°N, 119.4944°W, 2,690 m; and Warren Fork, Lee Vining River, 37.9520°N, 119.2261°W, 2,750 m).
Census Plots.
During July and August 2000, permanent census plots encompassing multiple areas suitable for all life history transitions were established at each site. Complete descriptions of plot sizes and numbers are available in ref. 34. Within each plot, every M. cardinalis and/or M. lewisii individual was uniquely identified by (x, y)-coordinates and numbered tags. Censuses were conducted each autumn from 2000 to 2007 to record annual survival, growth, reproduction, and recruitment. In total, the fates of 36,705 plants were recorded (Buck Meadows: 968; Rainbow Pool: 2,237; Wawona: 452 M. lewisii, 13,664 M. cardinalis; Carlon: 2,430 M. lewisii, 8,359 M. cardinalis; May Lake: 4,140; and Warren Fork: 4,455). For each plant, up to 20 nonflowering and 20 flowering stems were measured from the ground to the base of the last pair of expanded leaves; all remaining stems were tallied and used to estimate total stem length based on the average stem length of the 40 measured stems. Plant fecundity was estimated by multiplying the number of mature fruits on a given individual times the population mean seed number per fruit in a given year. Each fruit may contain 500–2,500 tiny seeds that cannot be counted in the field. To estimate seed number per fruit, 2 fruits were harvested each fall from each of 10 individuals growing several hundred meters downstream of the census plots. Samples of ≈200 seeds per fruit were counted under a dissecting microscope and weighed to determine the relationship between seed mass and seed number. Seed number per fruit was then estimated from total seed mass. Occasionally, seed samples could not be obtained for some population-by-year combinations, so average seed number per fruit across all other years for that population was used instead. Both species exhibit limited seed dormancy, so seed survival estimates were obtained from separate seed enclosure experiments (34).
Stage Classification.
After examining frequency distributions of stem lengths for different cohorts of plants and relationships among size, survival, and reproduction, plants were classified into the following 4 stages: seeds (both newly produced and those in the seed bank), small vegetative plants, large vegetative plants, and reproductive plants. To facilitate comparisons among sites and years, classification criteria were developed by using pooled data across all populations from 2000 to 2003 (34) and are retained here for consistency with the prior study. The boundary between small and large vegetative plants was defined as the midpoint between the median total stem length of first-year vegetative plants (i.e., seedlings) and the median total stem length of vegetative plants aged 2 and older (midpoint: M. cardinalis, 3 cm; M. lewisii, 5 cm). A seedling class based on age alone was not retained because first-year plants frequently surpassed older plants in size. The reproductive stage class was not subdivided because of differences in the size distribution of reproductive plants between sites and because survival of reproductive plants was unrelated to size.
Vital Rate Means and Variabilities.
I examined the vital rates survival and fecundity. Because of the low number of stage classes that survivors could transition among growth was not examined as a vital rate separate from survival. Annual census data were used to calculate observed vital rate means and variances. Observed variance in a vital rate includes environmentally driven (e.g., “process”) variance and sampling (e.g., “error”) variance (52, 53). Sampling variance was removed from estimates of total variance in vital rates following the methods of White (54) and Kendall (55), as given in Morris and Doak (53), for fecundity and survival, respectively. The resulting estimates of environmental variance in vital rates were then expressed on a relative basis for comparison among populations with different mean rates. Relative variability in fecundity was expressed as the coefficient of variation (corrected environmental standard deviation/mean × 100%). Because coefficient of variation is inappropriate for binomially distributed survival data, I expressed variability in survival relative to its maximum possible value (corrected environmental standard deviation/maximum standard deviation × 100%), where the maximum standard deviation is calculated as the square root of the product of the mean survival rate, p, and 1 − p (56).
Transition Matrix Models.
The projection matrix model for these analyses was a linear, time-invariant model of the form n(t + 1) = A· n(t), where n(t) is a vector of stage-classified individuals in the population at time t, n(t + 1) is the stage-classified vector of individuals at 1 time step in the future, and A is a 4 × 4 matrix of transition probabilities and stage-specific fecundities that shows how individuals in stage j at time t contribute to stage i at time t + 1. The top left-hand corner, a11, is seed dormancy, and other entries along the diagonal represent stasis in a particular vegetative class from t to t + 1. Other cells in the top row, a12–a14, are fecundities (mean number of seeds produced by a reproductive plant at time t + 1) weighted by the probability of an individual in class j at time t becoming reproductive at time t + 1. Nonreproductive stages have a nonzero contribution to the seed class if they may become reproductive within 1 annual time step. Transitions from seed to vegetative stages are represented by the first column, primarily in the second and third rows. Occasionally, spring germinants had entered the reproductive class by the time of the autumn census (nonzero value in first column, last row), in which case the top left-hand corner is a composite of seed dormancy and the seed contribution of 1-year-old reproductive plants. Transition matrices were assembled for each population and annual census interval, generating 56 matrices (4 populations × 7 years × 2 species). The dominant eigenvalue of each transition matrix is the asymptotic population growth rate, λ, a synthetic measure of population mean fitness in each environment (57). A value of λ = 1 indicates a projection of population stability, whereas a value λ <1 indicates a population that is projected to decline to extinction under the particular environmental conditions of the transition interval. Mean λ for each population was calculated as the geometric mean of the 7 annual λ estimates, and relative variability in λ was calculated as the geometric standard of the 7 annual λ estimates.
To incorporate stochastic environmental variation into long-term estimates of population growth, I used simulation models to forecast population growth across 10,000 time steps in 1,000 replicate simulations for each population. Simulations started with a population vector of 100 individuals per stage class. For each year of a given simulation run, a matrix was drawn at random from the set of 7 transition matrices and multiplied by the current population vector. Drawing matrices at random with equal probabilities preserves dependencies among matrix elements and assumes that environments are independently and identically distributed (53, 57). The stochastic log growth rate, log λs, was calculated as the arithmetic mean of log[N(t + 1)/N(t)] across all pairs of consecutive years of each simulation. Values of log λs reported here are averages from the 1,000 replicate simulations. A value of log λs = 0 indicates population stability, whereas a negative value of log λs indicates a population that is projected to decline.
Statistical Inferences.
To assess uncertainty in population projections, I used bootstrapping to calculate bias-corrected 95% confidence intervals around estimates of λ and log λs (57). Dataset bootstrapping procedures are described in detail in ref. 34. Briefly, for each population and yearly transition interval, individuals were randomly selected with replacement to generate 3,000 bootstrapped datasets of size equal to the population sample size. For each bootstrapped dataset, vegetative transition probabilities, fruit number at time t (used to estimate recruitment from the seed class), and fruit number per reproductive at time t + 1 (used to estimate contributions to the seed class) were calculated. For estimates involving seed numbers or seed dormancy, parameter estimates were drawn from cumulative probability distributions. Estimates from each bootstrap were assembled into a transition matrix for calculation of λ, and 95% confidence intervals were calculated from the distribution of 3,000 bootstrapped λ. To calculate confidence intervals around simulated log λs, simulations were repeated 3,000 times, at each time step multiplying the population vector by a matrix drawn from the bootstrapped distributions of 1 of the 7 possible transition matrices. Confidence intervals were corrected for minor differences between the observed λ or log λs and the means of the bootstrapped distributions. All matrix analyses were performed in Matlab, version 6.1 (The MathWorks). To test specific hypotheses about differences in vital rates or λs between central and marginal populations, I used 2-tailed t tests calculated in SAS, version 9.1 (SAS Institute).
Acknowledgments.
I thank D. Wake, L. Hadley, and D. Ackerly for the invitation to participate in the Sackler Colloquium on Biogeography, Climate Change, and Niche Evolution; S. Beatty, T. Croissant, and K. Hughes (Yosemite National Park), J. Haas (Stanisiaus National Forest), and L. Ford (Inyo National Forest) for guidance with permits; J. Anderson, M. Bricker, D. Grossenbacher, B. Igic, A. Smith, and A. Wilkinson for assistance with data collection; D. Schemske and C. Horvitz for helpful discussions about plant demography; and J. Paul, S. Sheth and 2 anonymous reviewers for insightful critiques of the manuscript. This work was supported by National Science Foundation Awards DEB 0206085, DEB 075660, and FIBR 0328636.
Footnotes
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “Biogeography, Changing Climates and Niche Evolution,” held December 12–13, 2008, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. The complete program and audio files of most presentations are available on the NAS web site at www.nasonline.org/Sackler_Biogeography.
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0901652106/DCSupplemental.
References
- 1.Brown JH, Stevens GC, Kaufman DM. The geographic range: Size, shape, boundaries, and internal structure. Annu Rev Ecol Syst. 1996;27:597–623. [Google Scholar]
- 2.Cumming GS. Comparing climate and vegetation as limiting factors for species ranges of African ticks. Ecology. 2002;83:255–268. [Google Scholar]
- 3.Arundel ST. Using spatial models to establish climatic limiters of plant species' distributions. Ecol Model. 2005;182:159–181. [Google Scholar]
- 4.Darwin C. On the Origin of Species by Means of Natural Selection, or, The Preservation of Favored Races in the Struggle for Life. London: J. Murray; 1859. [PMC free article] [PubMed] [Google Scholar]
- 5.Salisbury EJ. The geographical distribution of plants in relation to climatic factors. Geograph J. 1926;67:312–335. [Google Scholar]
- 6.Whittaker RH. Gradient analysis of vegetation. Biol Rev. 1967;42:207–264. doi: 10.1111/j.1469-185x.1967.tb01419.x. [DOI] [PubMed] [Google Scholar]
- 7.MacArthur RH. Geographical Ecology: Patterns in the Distribution of Species. New York: Harper and Row; 1972. [Google Scholar]
- 8.Grinnell J. Field tests of theories concerning distributional control. Am Nat. 1917;51:115–128. [Google Scholar]
- 9.Grinnell J. The niche-relationships of the California thrasher. Auk. 1917;34:427–433. [Google Scholar]
- 10.Holt RD, Gaines MS. Analysis of adaptation in heterogeneous landscapes: Implications for the evolution of fundamental niches. Evol Ecol. 1992;6:433–447. [Google Scholar]
- 11.Pulliam HR. On the relationship between niche and distribution. Ecol Lett. 2000;3:349–361. [Google Scholar]
- 12.Holt RD, Keitt TH, Lewis MA, Maurer BA, Taper ML. Theoretical models of species' borders: Single species approaches. Oikos. 2005;108:18–27. [Google Scholar]
- 13.Watkinson AR. On the abundance of plants along an environmental gradient. J Ecol. 1985;73:569–578. [Google Scholar]
- 14.Holt RD. On the evolutionary ecology of species' ranges. Evol Ecol Res. 2003;5:159–178. [Google Scholar]
- 15.Svenning JC, Normand S, Skov F. Postglacial dispersal limitation of widespread forest plant species in nemoral Europe. Ecography. 2008;31:316–326. [Google Scholar]
- 16.Laliberte E, Paquette A, Legendre P, Bouchard A. Assessing the scale-specific importance of niches and other spatial processes on beta diversity: A case study from a temperate forest. Oecologia. 2009;159:377–388. doi: 10.1007/s00442-008-1214-8. [DOI] [PubMed] [Google Scholar]
- 17.Primack RB, Miao SL. Dispersal can limit local plant distribution. Conserv Biol. 1992;6:513–519. [Google Scholar]
- 18.Revilla E, Wiegand T. Individual movement behavior, matrix heterogeneity, and the dynamics of spatially structured populations. Proc Natl Acad Sci USA. 2008;105:19120–19125. doi: 10.1073/pnas.0801725105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirkpatrick M, Barton NH. Evolution of a species range. Am Nat. 1997;150:1–23. doi: 10.1086/286054. [DOI] [PubMed] [Google Scholar]
- 20.Barton N. Adaptation at the edge of a species' range. In: Silvertown J, editor. Integrating Ecology and Evolution in a Spatial Context. London: Blackwell; 2001. pp. 365–392. [Google Scholar]
- 21.Kawecki TJ. Demography of source-sink populations and the evolution of ecological niches. Evol Ecol. 1995;9:38–44. [Google Scholar]
- 22.Holt RD, Gomulkiewicz R. How does immigration influence local adaptation? A reexamination of a familiar paradigm. Am Nat. 1997;149:563–572. [Google Scholar]
- 23.Holt RD, Barfield M, Gomulkiewicz R. Temporal variation can facilitate niche evolution in harsh sink environments. Am Nat. 2004;164:187–200. doi: 10.1086/422343. [DOI] [PubMed] [Google Scholar]
- 24.Caughley G, Grice D, Barker R, Brown B. The edge of the range. J Anim Ecol. 1988;57:771–785. [Google Scholar]
- 25.Jump AS, Woodward FI. Seed production and population density decline approaching the range-edge of Cirsium species. New Phytol. 2003;160:349–358. doi: 10.1046/j.1469-8137.2003.00873.x. [DOI] [PubMed] [Google Scholar]
- 26.Carey PD, Watkinson AR, Gerard FFO. The determinants of the distribution and abundance of the winter annual grass Vulpia ciliata ssp. ambigua. J Ecol. 1995;83:177–187. [Google Scholar]
- 27.Kellman M. Sugar maple (Acer saccharum Marsh.) establishment in boreal forest: Results of a transplantation experiment. J Biogeogr. 2004;31:1515–1522. [Google Scholar]
- 28.Yakimowski SB, Eckert CG. Threatened peripheral populations in context: Geographical variation in population frequency and size and sexual reproduction in a clonal woody shrub. Conserv Biol. 2007;21:811–822. doi: 10.1111/j.1523-1739.2007.00684.x. [DOI] [PubMed] [Google Scholar]
- 29.Alexander HM, Price S, Houser R, Finch D, Tourtellot M. Is there reduction in disease and pre-dispersal seed predation at the border of a host plant's range? Field and herbarium studies of Carex blanda. J Ecol. 2007;95:446–457. [Google Scholar]
- 30.Curnutt JL, Pimm SL, Maurer BA. Population variability of sparrows in space and time. Oikos. 1996;76:131–144. [Google Scholar]
- 31.Mehlman DW. Change in avian abundance across the geographic range in response to environmental change. Ecol Appl. 1997;7:614–624. [Google Scholar]
- 32.Williams CK, Ives AR, Applegate RD. Population dynamics across geographical ranges: Time-series analyses of three small game species. Ecology. 2003;84:2654–2667. [Google Scholar]
- 33.Nantel P, Gagnon D. Variability in the dynamics of northern peripheral versus southern populations of two clonal plant species, Helianthus divaricatus and Rhus aromatica. J Ecol. 1999;87:748–760. [Google Scholar]
- 34.Angert AL. Demography of central and marginal populations of monkeyflowers (Mimulus cardinalis and M. lewisii) Ecology. 2006;87:2014–2025. doi: 10.1890/0012-9658(2006)87[2014:docamp]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 35.Kluth C, Bruelheide H. Effects of range position, interannual variation and density on demographic transition rates of Hornungia petraea populations. Oecologia. 2005;145:382–393. doi: 10.1007/s00442-005-0141-1. [DOI] [PubMed] [Google Scholar]
- 36.Volis S, Mendlinger S, Ward D. Demography and role of the seed bank in Mediterranean and desert populations of wild barley. Basic Appl Ecol. 2004;5:53–64. [Google Scholar]
- 37.Stokes KE, Bullock JM, Watkinson AR. Population dynamics across a parapatric range boundary: Ulex gallii and Ulex minor. J Ecol. 2004;92:142–155. [Google Scholar]
- 38.Kluth C, Bruelheide H. Central and peripheral Hornungia petraea populations: Patterns and dynamics. J Ecol. 2005;93:584–595. [Google Scholar]
- 39.Purves DW. The demography of range boundaries versus range cores in eastern US tree species. Proc R Soc London Ser B. 2009;276:1477–1484. doi: 10.1098/rspb.2008.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beardsley PM, Yen A, Olmstead RG. AFLP phylogeny of Mimulus section Erythranthe and the evolution of hummingbird pollination. Evolution. 2003;57:1397–1410. doi: 10.1111/j.0014-3820.2003.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 41.Waser NM, Vickery RKJ, Price MV. Patterns of seed dispersal and population differentiation in Mimulus guttatus. Evolution. 1982;36:753–761. doi: 10.1111/j.1558-5646.1982.tb05441.x. [DOI] [PubMed] [Google Scholar]
- 42.Manel S, Schwartz MK, Luikart G, Taberlet P. Landscape genetics: Combining landscape ecology and population genetics. Trends Ecol Evol. 2003;18:189–197. [Google Scholar]
- 43.Wilson GA, Rannala B. Bayesian inference of recent migration rates using multilocus genotypes. Genetics. 2003;163:1177–1191. doi: 10.1093/genetics/163.3.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goldberg EE, Lande R. Species' borders and dispersal barriers. Am Nat. 2007;170:297–304. doi: 10.1086/518946. [DOI] [PubMed] [Google Scholar]
- 45.Angert AL, Schemske DW. The evolution of species' distributions: Reciprocal transplants across the elevation ranges of Mimulus cardinalis and M. lewisii. Evolution. 2005;59:222–235. [PubMed] [Google Scholar]
- 46.Moritz C, et al. Impact of a century of climate change on small-mammal communities in Yosemite National Park, USA. Science. 2008;322:261–264. doi: 10.1126/science.1163428. [DOI] [PubMed] [Google Scholar]
- 47.Litaor MI, Williams M, Seastedt TR. Topographic controls on snow distribution, soil moisture, and species diversity of herbaceous alpine vegetation, Niwot Ridge, Colorado. J Geophys Res Biogeosci. doi: 10.1029/2007JG000419. [DOI] [Google Scholar]
- 48.Lytle DA, Merritt DM. Hydrologic regimes and riparian forests: A structured population model for cottonwood. Ecology. 2004;85:2493–2503. [Google Scholar]
- 49.Phillips SJ, Anderson RP, Schapire RE. Maximum entropy modeling of species geographic distributions. Ecol Model. 2006;190:231–259. [Google Scholar]
- 50.Hickman JC, editor. The Jepson Manual: Higher Plants of California. Berkeley: Univ California Press; 1993. [Google Scholar]
- 51.Hiesey WM, Nobs MA, Björkman O. Experimental Studies on the Nature of Species. V. Biosystematics, Genetics, and Physiological Ecology of the Erythranthe Section of Mimulus. Washington, DC: Carnegie Institute of Washington; 1971. [Google Scholar]
- 52.Loison A, Saeligther B-E, Jerstad K, Roslashstad OW. Disentangling the sources of variation in the survival of the European dipper. J Appl Stat. 2002;29:289–304. [Google Scholar]
- 53.Morris WF, Doak DF. Quantitative Conservation Biology: Theory and Practice of Population Viability Analysis. Sunderland, MA: Sinauer; 2002. [Google Scholar]
- 54.White GC. Population viability analysis: Data requirements and essential analyses. In: Boitani L, Fuller TK, editors. Research Techniques in Animal Ecology: Controversies and Consequences. New York: Columbia Univ Press; 2000. pp. 288–231. [Google Scholar]
- 55.Kendall BE. Estimating the magnitude of environmental stochasticity in survivorship data. Ecol Appl. 1998;8:184–193. [Google Scholar]
- 56.Morris WF, Doak DF. Buffering of life histories against environmental stochasticity: Accounting for a spurious correlation between the variabilities of vital rates and their contributions to fitness. Am Nat. 2004;163:579–590. doi: 10.1086/382550. [DOI] [PubMed] [Google Scholar]
- 57.Caswell H. Matrix Population Models: Construction, Analysis, and Interpretation. 2nd Ed. Sunderland, MA: Sinauer; 2001. [Google Scholar]