Abstract
In the face of environmental change, species can evolve new physiological tolerances to cope with altered climatic conditions or move spatially to maintain existing physiological associations with particular climates that define each species' climatic niche. When environmental change occurs over short temporal and large spatial scales, vagile species are expected to move geographically by tracking their climatic niches through time. Here, we test for evidence of niche tracking in bird species of the Sierra Nevada mountains of California, focusing on 53 species resurveyed nearly a century apart at 82 sites on four elevational transects. Changes in climate and bird distributions resulted in focal species shifting their average climatological range over time. By comparing the directions of these shifts relative to the centroids of species' range-wide climatic niches, we found that 48 species (90.6%) tracked their climatic niche. Analysis of niche sensitivity on an independent set of occurrence data significantly predicted the temperature and precipitation gradients tracked by species. Furthermore, in 50 species (94.3%), site-specific occupancy models showed that the position of each site relative to the climatic niche centroid explained colonization and extinction probabilities better than a null model with constant probabilities. Combined, our results indicate that the factors limiting a bird species' range in the Sierra Nevada in the early 20th century also tended to drive changes in distribution over time, suggesting that climatic models derived from niche theory might be used successfully to forecast where and how to conserve species in the face of climate change.
Keywords: climatic niche, geographic range, elevational gradient, occupancy dynamics
Nearly a century ago, Joseph Grinnell (1) presented the concept of the ecological niche as the primary determinant of a species' range. Grinnell defined the niche as a set of environmental conditions that restricts each species, through “physiological and psychological respects,” to a geographical range where it can prosper. In particular, Grinnell (2) discussed the important role played by temperature in ultimately defining range boundaries, but noted that within the limits of physiological tolerance, numerous factors, including interspecific competition, can determine realized range boundaries. Since Grinnell, empirical explorations of species' range determinants have successfully related environmental limits to range boundaries through physiological knowledge (3). At the same time, field and laboratory experiments have demonstrated that species interactions may also limit ranges (4, 5) and climatic associations can rapidly change when species are introduced to new environments (6). Nevertheless, the concept that environmental limiting factors define the niche where a species can have a positive growth rate still remains the dominant explanation for range boundaries (7), suggesting that the spatial extent of the range for most species is approximately equal to the geographical expression of a species' niche (8).
Temporal sampling of changing environments makes it possible to measure the dynamic relationship between the environment, a species' climatic requirements, and its realized range. If ranges are shaped by physiological limitations that remain fixed over the time scale of comparison, then species ranges should also move across the landscape as averages and extremes of temperature, precipitation, and relative humidity change over short time spans (9–11). This process, by which species follow limiting environmental boundaries through geographical space to remain in a favorable climatic space, is called niche tracking (10, 12, 13). Niche tracking can occur when a local population in unfavorable climate conditions becomes extinct or when individuals colonize sites in newly favorable climates. Studies of both recent and paleontological climate change have examined niche tracking through range changes (14, 15). If species track niches limited by temperature, then they should move upward in elevation or poleward in latitude as the climate warms. A global metaanalysis of 434 species that have shifted ranges indicated that 81% of species showed this expected pattern in response to recent climate change (16). However, 19% moved in directions opposite that predicted by temperature, and many others did not change range. Studies that have explored life history factors as potential correlates of movement patterns have found no simple explanation (17), and there is little empirical evidence for why species show heterogeneous responses (15).
A more direct approach to examining the role of the niche in driving species response to climate change is necessary if these seemingly contradictory patterns are to be understood. Analyses of shifts in elevational or latitudinal range are used as proxies for shifting temperature gradients (18), yet niches can be defined by any set of abiotic factors that may or may not covary with elevation or latitude (19, 20). If the role of the niche in dynamically determining ranges is to be understood, multiple environmental facets of the niche need to be explored. Modern resurveys of areas with historical occurrence data provide unique opportunities to empirically test the role of niche tracking in driving species-specific responses to climate change (21).
Here, we use a unique dataset of changes in avian site occupancy over the past century to test the degree to which 53 bird species distributed across an elevational gradient track a two-variable environmental niche through space and time. We expect species to have responded to climate change by modifying their ranges to remain within their preexisting climatic niche. Our data come from historical (1911–1929) surveys and contemporary (2003–2008) resurveys of 82 sites along four elevational transects throughout the Sierra Nevada of California (Fig. 1). These sites have seen an average change in breeding season climate toward warmer (+0.80 ± 0.07 °C, mean ± SE) and wetter (+5.90 ± 0.57 mm) conditions, revealing an overall increase in ecosystem net primary productivity (NPP). We examined changes in climatological range and site occupancy between the two survey periods and how these changes related to the climatic niches of species. We tested whether: (i) species have, on average, tracked their climatic niche over time (i.e., climatic range centroids moving toward the niche centroid, not away from it); (ii) environmental gradients tracked by a species can be predicted a priori based on climatological factors limiting historical distributions; and (iii) colonization and extinction probabilities at sites are modeled well by a site's climatological position relative to the niche centroid.
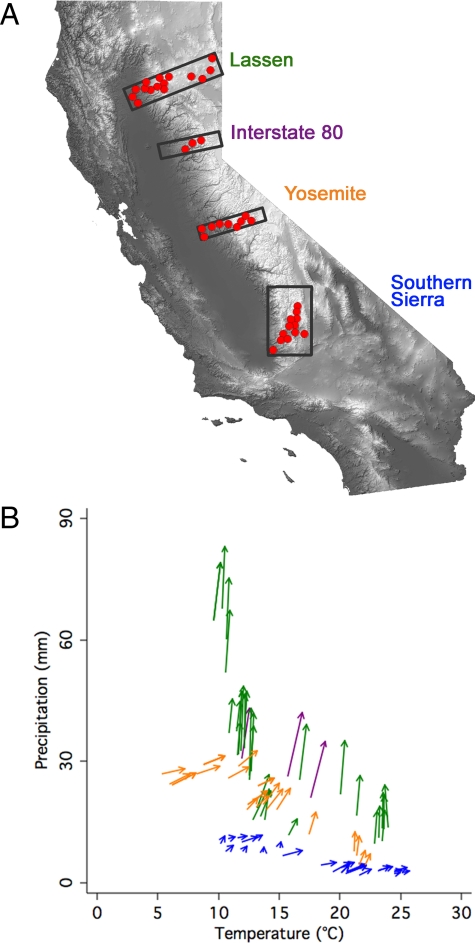
Fig. 1.
Locations of 82 bird survey sites in both geographical and climatic space. (A) Geographical locations of cross-sectional resurvey transects through the Sierra Nevada superimposed onto topography of California (higher elevations in lighter gray). Locations of neighboring survey sites (red circles) have been aggregated to provide visual clarity. The number of sites per transect from south to north are: Southern Sierra, 25; Yosemite, 24; Interstate 80, 3; and Lassen, 30. (B) Locations of resurvey sites in climate space, with arrows pointing from historical breeding season climate to modern breeding season historical climate. Color codes correspond to transect: Southern Sierra in blue, Yosemite in orange, Interstate 80 in purple, and Lassen in green.
Defining the Climatic Niche and Quantifying Niche Tracking
Hutchinson (20) formalized the idea that the niche can be partitioned into the fundamental and realized portions. However, there is disagreement on whether the fundamental niche can be inferred from species occurrences (22, 23) or can only be measured from mechanistic analyses of physiological tolerances (4, 24). Our goal here is not to define the complete n-dimensional environmental niche for each species, but to determine an approximate set of climatic conditions in which species can occur, also known as the Grinnellian niche (25). Analyses of climatic conditions throughout entire ranges of species theoretically provide suitable approximations of these conditions (26). Furthermore, niche centroids provide measures of the distributional center of favorable climatic conditions (6), avoiding the difficulties inherent in measuring and interpreting range boundaries in climate space (27, 28). For instance, both source-sink dynamics (29) and the graded response of fitness to environmental conditions (30) might blur the appearance of a hard niche boundary. Consequently, we used the average historical temperature and precipitation observed across an entire species' range, as delimited by occurrences of historical (1860–1940) museum specimens, as the centroid of its climatic niche.
Range change, and thus the ability of species to track their climatic niche, occurs at two primary spatial scales. At the scale of the site where individuals live, niche tracking during environmental change can lead to three outcomes (11) (Fig. 2A). First, the site may remain within the climatic niche of the species despite climate change, allowing individuals to continue occupying it. Second, the local environment may shift outside of the climatic niche, leading to extinction at the site through reduced survival or reproductive success or emigration. Third, the local environment may shift inside the climatic niche, allowing colonization if dispersal occurs. Depending on the time scale, the magnitude of environmental change, the size of the niche, the “hardness” of niche boundaries, and other natural history characteristics of the organism, any or all of these outcomes may be expected results of climate change at the site level. Combinations of outcomes at sites are realized geographically as contractions, expansions, or stasis at the scale of the range (11). Thus, changes in site occupancy driven by changing environmental conditions are manifested as range shifts (31).
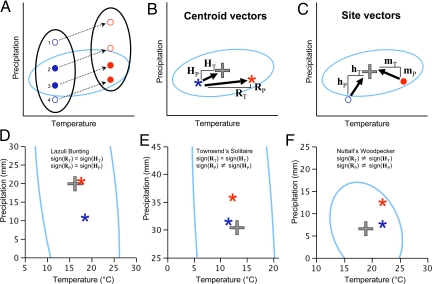
Fig. 2.
Colonization-extinction dynamics as mediated by shifting climates lead to changes in position of occupied range centroids relative to the climatic niche. (A) Four sites (circles labeled 1–4) within a geographic area (black ellipse) experience shifts in climate over time (dotted arrows), moving sites from a prior climatic position (blue circles) to a current climatic position (red circles). For a hypothetical species with a certain climatic niche defined by temperature and precipitation (light blue ellipse), a site can be unoccupied in both time periods if it remains outside the climatic niche (site 1), go extinct if the site shifts out of the climatic niche (site 2), stay occupied in both time periods if it remains inside the climatic niche (site 3), or be colonized if the site enters the climatic niche (site 4). (B) The centroids of the observed occupied ranges for a species in each time period (asterisks: blue for historic and red for current) can provide evidence of niche tracking when compared with the centroid of a species' climatic niche (gray cross). If the temperature or precipitation components of the vector from the historic range centroid to the climatic niche centroid (HT and HP, respectively) agree in sign with the corresponding climatic components of the vector from the historic range centroid to the current range centroid (RT and RP), then there is evidence for tracking for that component. (C) Individual sites can be defined by vector components describing the position of a site (e.g., site 4) either historically (hT and hP) or currently (mT and mP) relative to the climatic niche centroid. These site-specific vectors are used in combinations as covariates of colonization and extinction in occupancy models. Examples of movements of range centroids for three species show different levels of climatic niche tracking (for further details and other species, see Table S1). (D) Lazuli Bunting showed niche tracking of both temperature and precipitation, shifting to a cooler and wetter occupied range. The light blue circle is a 95% density ellipse around the full range of historic specimens that defined the climatic niche centroid. (E) Townsend's Solitaire showed niche tracking of temperature, but not precipitation. (F) Nuttall's Woodpecker tracked neither temperature nor precipitation components of the climatic niche.
At both the site and range scales, empirical data can be used to explore whether outcomes are related to climatic changes relative to the niche. Given that the climate has generally become warmer and wetter in our study region over the last century (32, 33), although with varying geographical context (Fig. 1B), a species showing geographic range stasis would exhibit movement of its climatic range toward a warmer and wetter environment. If species do track their climatic niche, then this outcome would only be expected if warmer and wetter conditions were favorable; that is, the centroid of their climatic niche is warmer and wetter than where the species occurred before environmental change. In contrast, if climatic conditions shift away from the niche centroid, vagile species might adjust their occupancy by colonizing newly favorable sites or abandoning unfavorable sites (34). This would result in an occupied range that tracks the climatic niche, despite the inertia of environmental change.
We first tested at the regional scale whether species tracked their climatic niche over time or moved independently. For each species we examined whether a temperature or precipitation shift in observed range mean (RT and RP; Fig. 2B) matched in sign with the environmental direction from the niche centroid to the observed historical range mean (HT and HP; Fig. 2B and Table S1). For example, if the climatic niche centroid is cooler and wetter (e.g., Fig. 2D) than the mean environment of the observed historical range, then an observed modern range that is also cooler and wetter than where the species was found historically would provide evidence for niche tracking on both axes.
We then examined site-specific occupancy dynamics as a predictive driver for niche tracking throughout a range. The occupancy modeling framework (35) allowed us to estimate colonization and extinction probabilities for sites, while taking into account the risk of false absences. Three models were used to test how turnover of occupancy related to the climatic position of sites relative to the climatic niche of a species (Fig. 3). In the null model (Fig. 3A), all sites have the same probability of changing occupancy status, implying that the climatic niche has no association with colonization-extinction dynamics. Alternatively, the static model assumes the magnitude of environmental shift from climate change is small relative to the distance from occupied sites to a species' climatic niche. In this case, the climatic niche does impact colonization-extinction dynamics, but without a significant change in climate, tracking of the niche across climate space is not observed. Consequently, sites in the core of the climatic niche will remain occupied, whereas peripheral sites on the margin of climatic suitability will exhibit nondirectional turnover that is typical of a range at equilibrium (7) (Fig. 3B). As the magnitude of environmental shift increases, however, the fate of sites depends on the direction of climate change and the position of that site in climate space relative to the species' climatic niche. Thus, the dynamic model assumes that sites in the core of the climatic niche mostly remain occupied, whereas peripheral site dynamics depend on whether climate is pushing the site toward or away from the core (31) (Fig. 3C). In geographic space, this directional turnover may result in a range appearing to be in nonequilibrium (7). We structured our dynamic model with five different parameterizations (see Methods), each a unique hypothesis of how colonization and extinction might be related to niche-climate dynamics.
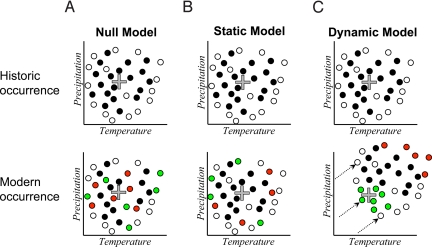
Fig. 3.
Models of site-specific change in occupancy in relation to the climatic niche. Sites are observed to be either occupied (black circles) or unoccupied (white circles), and between time periods they can either go extinct (red circles) or be colonized (green circles). (A) The null model estimates a constant probability of colonization and extinction; consequently, sites are equally likely to change occupancy status regardless of their proximity to the climatic niche centroid (gray cross). (B) The static model estimates turnover probabilities as a function of the distance from a site to the niche centroid, leading to changes in occupancy at the periphery of the climatic range. (C) The dynamic model (see Methods for six formulations) incorporates the degree to which sites have shifted in climate space over time (arrows). Thus, turnover probabilities are a function of the distance from a site to the niche centroid at present, relative to where it was in the past. This leads to a directional pattern in occupancy turnover.
Both the static model and the dynamic model represent niche tracking scenarios, but the difference between them lies in the relative role that climate change plays. If the magnitude of climatic shift is great relative to the proximity of sites to the niche centroid, we expect dynamic models to best describe turnover patterns. However, if sites have only shifted a small amount relative to their distance to the niche centroid, then a static model is likely to fit observed patterns better.
Results
Niche tracking was the overwhelming response of birds to climate change in our analysis. Of 53 focal bird species, 91% tracked either temperature or precipitation over time, and 26% of species tracked both temperature and precipitation (Table 1 and Table S1). Species tracked precipitation toward wetter conditions, but tracked temperature toward cooler or warmer conditions, depending on the species (Fig. S1 A and B).
Table 1.
Predicted climatic sensitivities for 53 bird species and the observed climate variables that species were found to track
| Predicted tracking* | Observed tracking |
|||
|---|---|---|---|---|
| Temperature | Precipitation | Both | Neither | |
| Temperature | 12 | 4 | 9 | — |
| Precipitation | 1 | 14 | 4 | 5 |
| Both | — | 3 | 1 | — |
*Predicted climatic sensitivities based on relative support of climatic variables from MaxEnt models to explain whole range distributions of species (see Methods and Table S1). Results in bold indicate comparisons where observed response matched the predicted response.
We next examined the degree to which major climatological factors limiting species' historical distributions also explained distributional changes over time. Some species showed niche sensitivity (inferred from MaxEnt models; see Methods) for both environmental parameters and others for only one. Across all species, for each environmental parameter showing niche sensitivity, we tested for agreement between predicted sensitivity and observed tracking. For these cases (n = 57), we found that 77% of a priori predictions of gradient sensitivity agreed with observed range shifts; excluding the five species that showed no niche tracking, this agreement increased to 85% (Table 1). Overall, a priori climatological sensitivities inferred from range-wide modeling were significantly associated with observed gradient tracking for both temperature (Fisher's exact test, two-tailed: P < 0.001) and precipitation (Fisher's exact test, two-tailed: P = 0.049).
Both the a priori predictions of gradient sensitivity and the observed environmental factors tracked by each species were significantly related to the average elevation occupied by a species (sensitivity: F2,50 = 75.0, P < 0.001; observations: F3,49 = 20.4, P < 0.001; data in Table 1 and Table S1). Species tracking only precipitation were centered at an average elevation of 916 m (95% C.I.: 726–1,107 m), whereas species tracking only temperature were centered at an average elevation of 1,944 m (95% C.I.: 1,701–2,186 m). Similarly, species with western United States distributions sensitive to only precipitation were centered at an average elevation of 799 m (95% C.I.: 668–932 m), compared with an average elevation of 1,904 (95% C.I.: 1,774–2,033 m) for species sensitive to only temperature. Species sensitive to or tracking both precipitation and temperature were centered at intermediate elevations (95% C.I. for sensitivity: 609–1,257 m; 95% C.I. for tracking: 1,374–1,841 m). We also observed a similar pattern showing high correlation (r = 0.923) between elevational centers of species and climatic factors limiting NPP (Table S1). Low-elevation species tended to occupy sites where NPP was limited by precipitation, high-elevation species tended to occupy sites where NPP was limited by temperature, and middle-elevation species tended to occupy sites where NPP was shaped by a combination of both climate variables. This finding suggests niche tracking may be governed by climate-induced shifts in NPP.
Site-specific models of colonization-extinction that incorporated niche components had greater Akaike's Information Criterion (AIC) weights than the null model for 50 of 53 species (Table S2). For individual species, occupancy dynamics at the site were generally best explained by static or dynamic models (e.g., Fig. S1 C–F). Averaged across all species, the null model had 0.035 model weight, compared with 0.49 for the static model, and 0.47 cumulatively for dynamic models (Table 2). Comparing the different models that incorporate the niche, a dynamic model had a greater AIC weight than the static model for 53% of species, suggesting a mixed response for dynamic versus static models. Within the class of dynamic models, we compared results from five different parameterizations (see Methods and Table 2). Of these, the directional parameterization showed generally greater support (0.18 average model weight) than any other individual dynamic parameterization. Extinction probabilities were higher (0.264 ± 0.027, mean ± SE) across all species than colonization probabilities (0.131 ± 0.017; Table S2), when estimated from null models with fixed probabilities of colonization and extinction for all sites.
Table 2.
Average strength of evidence for multiple hypotheses of site-specific niche tracking
| Model | Parameterizations of colonization (γ) and extinction (ε)* | No. of parameters† | Average AIC weight‡ |
|---|---|---|---|
| Null | logit(γ ) = β 0 logit(ε ) = β 1 | 4 | 0.035 |
| Static | logit(γ ) = β 0 + β 1 · hT + β 2 · hp logit(ε ) = β 3 + β 4 · hT + β 5 · hp | 8 | 0.492 |
| Dynamic§ | — | — | 0.473 |
| Directional¶ | logit(γ ) = β 0 + β 1 · Δ T + β 2 · Δ p logit(ε ) = β 3 + β 4 · Δ T + β 5 · Δ p | 8 | 0.177 |
| Full relative | logit(γ ) = β 0 + β 1 · δ T + β 2 · δ p logit(ε ) = β 3 + β 4 · δ T + β 5 · δ p | 8 | 0.087 |
| Precipitation only | logit(γ ) = β 0 + β 1 · δ P logit(ε ) = β 2 + β 3 · δ P | 6 | 0.062 |
| Temperature only | logit(γ ) = β 0 + β 1 · δ T logit(ε ) = β 2 + β 3 · δ T | 6 | 0.058 |
| Hybrid 1 | logit(γ ) = β 0 + β 1 · δ P logit(ε ) = β 2 + β 3 · δ T | 6 | 0.045 |
| Hybrid 2 | logit(γ ) = β 0 + β 1 · δ T logit(ε ) = β 2 + β 3 · δ P | 6 | 0.044 |
*Occupancy models also include detectability (p) and occupancy (ψ0). See Methods for details on these parameters and definitions of variables.
†Number of parameters is based on constant detectability and occupancy models.
‡Weights are averaged across results from 53 species
§AIC weight given as the sum of the weights of the following six dynamic models.
¶The binary variable Δ is given the value of 1 when the site is moving toward the niche center on an environmental axis, and 0 when the site is moving away from the niche center.
Discussion
For highly vagile species, like birds, the climatic niche can be a strong driver of responses to climate change. Our tests of niche tracking over the past 100 years showed that 48 of 53 bird species adjusted their geographic range as climate changed to move closer to their historically defined niche centroid for at least one environmental gradient (Table 1 and Table S1). Furthermore, there was strong agreement between the precipitation and temperature axes that species tracked and a priori predictions of which axes contribute most strongly to defining a species' climatic niche (Table 1 and Table S1).
Our results support the use of climatic niche modeling to predict future ranges of birds as a result of climate change (21, 28, 36). The models assume that factors limiting a species' range may also drive temporal changes to its distribution. Our results provide evidence in support of this assumption, adding to a small, but growing, body of evidence based on tests within native ranges across time (12, 13, 36, 37).
Bird species exhibited individualistic, but generally predictable, responses to temperature or precipitation shifts. This finding suggests that the highly individualistic responses of species to past and present climate change (8, 17, 38) may be explained by differing species-specific sensitivities to climatic parameters (4, 34) and the direction of climate change relative to the climatic niche. Climate change may push some sites or populations closer to the centroid of their climatic niche and other sites or populations farther away. Species might also respond more to precipitation or changes in environmental extremes than to changes in average temperature. Thus, not all species or populations should be expected to move upward in elevation or poleward in latitude (16, 39) as they respond in climate space to a shifting environment; instead, a great diversity of geographic responses should not only be predicted (40), but expected, especially in topographically complex environments (33).
All five species that did not track their climatic niche for either environmental factor (Nuttall's Woodpecker, California Thrasher, Anna's Hummingbird, Black Phoebe, and Western Scrub-Jay) inhabit low elevations and can easily exploit human-dominated areas such as urban, suburban, and agricultural ecosystems (41). In comparison, four species found in similar elevational ranges (Table S1) that avoid human-dominated areas tracked at least one environmental variable, although some species that exploit urban areas did show niche tracking (e.g., Oak Titmouse and California Towhee). The apparent association with urbanization of species that did not track their climatic niche may have implications for conservation. Species that have colonized urban, suburban, and agricultural ecosystems may be able to expand or sustain a range far from their climatic niche (either through access to key resources or use of uniquely human microclimates) and thereby escape the negative consequences of climate change.
Occupancy dynamics are the unseen mechanism behind range changes (7, 8, 31). Incorporating the climatic niche into our models of occupancy dynamics between two time periods resulted in mixed conclusions. Niche-centric models of occupancy (i.e., static and dynamic models; Fig. 3) explained transitions of site occupancy better than the null model, but evidence was equivocal as to whether climate change strongly impacted these transitions. This outcome could result from using climatic variables in defining the fundamental niche that were less important than other unexamined variables. For example, our results indicate that focal bird species may have tracked changes in temperature and precipitation through their combined influences on annual NPP, a variable implicated in a similar response to climate change in eastern North America (13). The importance of NPP could indicate that factors affecting energy availability are shaping range limits rather than, or in addition to, physiological tolerances to climatic extremes.
The role of climate change in shaping site turnover dynamics should be considered in relation to the relative magnitude of climatic shift (Fig. 2C). If climate change shifts sites in environmental space only a small amount relative to their distance from the niche centroid, then we may not expect to detect a strong signal of dynamic niche tracking. As climate change continues to shift environmental conditions of sites, more species may be likely to exhibit site turnovers leading to range change, which may be especially true, given that the magnitude of expected climate change by 2100 appears likely to exceed the observed change in the preceding century (42). Our analyses indicate that niche tracking appears widespread, albeit variable, in birds and may be the guiding principle through which we expect to see other species respond.
Methods
Observational Data and Species Ranges.
Historic and modern species observations originated from the Grinnell Resurvey Project, a large-scale multitaxa resurvey of the vertebrate fauna of the Sierra Nevada (17, 33). Historical observations were made outside of the winter (earliest was March 26 and latest was October 15, with 82% between May 1 and July 31) between 1911 and 1929 at 82 sites along four cross-sectional elevational transects (Fig. 1) as part of regular surveys or “pencil censuses” of birds by Grinnell and colleagues at the Museum of Vertebrate Zoology, University of California, Berkeley. Historical surveys were repeated at 76% of sites, with a maximum of 17 repeat surveys (median = 3). Modern observations entailed resurveys of historical sites using point counts along transects, conducted by five different observers between 2003 and 2008. Variable-distance point counts (43) lasted 7 min, and stations were placed a minimum of 250 m apart along routes that followed, as closely as possible, to historic survey paths. Sites were repeatedly surveyed a maximum of five times (median = 3) between May 4 and August 25.
Of the 240 total bird species detected during historical and modern surveys, we selected 53 focal species that matched desired criteria. First, we selected species that were restricted primarily to the western United States. Two western-restricted subspecies, formerly considered full species, “Audubon's” Yellow-Rumped Warbler (see Table S1 for taxonomy) and the “red-shafted” Northern Flicker, were also included. Second, species had to occur during both sampling eras at nine or more survey sites, with four exceptions that were added a priori for their strong association to the western United States and the Sierra Nevada: American Dipper (11 historic sites, 6 modern sites), Anna's Hummingbird (5 sites, 37 sites), California Thrasher (7 sites, 6 sites), and Pacific-Slope Flycatcher (7 sites, 10 sites). Our final group of 53 species was distributed across the elevational range (see Table S1 for average elevations of each species).
Specimen Data and the Climatic Niche.
Historical specimen data (1860–1940) used to estimate the climatic niche were assembled from museum collections accessed through ORNIS (http://olla.berkeley.edu/ornisnet). ORNIS is a data portal that facilitates easy access to >35 million unique bird records (specimen and observational) housed by 45 different providers. We downloaded all available specimen records for each species. Specimens without georeferences, or with low coordinate precision (<3 decimal places, in decimal degrees), were excluded, as were records post-1940 and specimens collected outside of the breeding season (breeding in California generally occurs between March and August for resident species and between May and July for migrants). Most specimens from most museums do not yet have estimates of georeference uncertainty (44), so uncertainty was not used as a criterion for inclusion. Obvious outliers, including vagrants or incorrect georeferences, were also excluded. With the exceptions described previously, all subspecies of each species were used, providing thorough coverage of the entire known geographic range for each species. Geographic coordinates were sorted within species, and duplicate coordinates were eliminated to reduce sampling bias. The average number of unique specimen localities for species was 148 (SD = 63). We used these historical species locations to calculate the centroid of the climatic niche for each species.
Climate Data.
Monthly mean minimum temperature, mean maximum temperature, and total precipitation were obtained from the parameter-elevation regressions on independent slopes model (PRISM). PRISM is a knowledge-based system that generates monthly by yearly climate surfaces using mathematical interpolation and expert knowledge (45, 46). PRISM data are made freely available at 2.5-arc-min spatial resolution (≈4 × 4 km), a scale reasonable for both the specimen and observational data. We used the monthly variables to compute mean estimates of temperature and precipitation during the breeding season (May through July) sampled in both eras (1910–1930 and 1986–2006). We also used estimates of historical annual mean temperature (T) and annual precipitation (P) to determine which of the two original climate variables limited NPP under the Miami model (47), where
Historical and modern climate values were extracted for locations of specimen and observational data for use in analyses. While the average breeding season precipitation in the Sierra Nevada is relatively low (maximum = 83 mm), this variable was highly correlated with average annual precipitation for our sites (r = 0.96 for historical; r = 0.94 for modern). Breeding season temperature was also highly correlated with annual mean temperature (r = 0.99 for historical; r = 0.99 for modern). Breeding season values were used for site-specific climate values, because they provided direct a priori links to changes in avian breeding season occurrence.
A Priori Hypotheses of Niche Tracking.
We used a maximum entropy technique implemented in MaxEnt (48, 49) to determine a priori whether each species' historical range-wide distribution was shaped more by temperature or precipitation. MaxEnt models were developed by using standard default settings in version 3.2.1 of the program (automatic selection of response functions; maximum number of background points, 10,000; background, conterminous United States). Climate variables were historical breeding season estimates of mean temperature and precipitation. Models were developed by using all spatially unique historical specimen localities for each species. We used the percentage contribution of each variable to the model to develop testable hypotheses of species' niche sensitivity to breeding season temperature versus precipitation. Absolute scores of contribution to variables were not directly comparable within species, so scores were standardized relative to the median contribution of each variable across all species. A species with, for example, a precipitation contribution greater than the median precipitation contribution across all species, would have precipitation selected as an a priori predictor of niche sensitivity. All species had either a temperature or a precipitation score greater than the median, and four species had both.
Occupancy Modeling.
Multiseason occupancy models (35) were built to examine site-specific occupancy dynamics as a predictive driver for niche tracking throughout a range. Multiseason occupancy models simultaneously estimate a probability of detection (p), an initial probability of occupancy (ψ0), a probability of colonization (γ), and a probability of extinction (ε) based on histories of presence and nondetection at sites over time. The strength of these models lies in being able to estimate occupancy parameters while taking into account the probability that a species was present and went undetected at each site, which is critical when dealing with historical occurrence data (17).
We fit covariates to occupancy parameters in two stages, following ref. 17. First, we ran four detectability models for each species (allowing detectability to vary by survey era or Julian day) with constant (no covariates) models for ψ0, γ, and ε. Models were compared by using AIC (50). The best detectability model (highest AIC weight, wi) for each species was used to parameterize p for all subsequent colonization and extinction models for that species.
Second, eight occupancy models were compared by using different combinations of covariates for γ and ε (35). The null model (Table 2 and Fig. 3A) had no covariates (i.e., constant probability of γ and ε). The static model (Table 2 and Fig. 3B) used the temperature and precipitation vectors hT and hP (Fig. 2C), which measure the distance from each site's historic climatic location to each species' niche centroid, as covariates for γ and ε. The dynamic models (Table 2 and Fig. 3C) were divided into two different sets based on covariates of colonization and extinction. The first set (directional dynamic) used only whether climate change pushed a site toward or away from the climatic niche centroid (represented by a binary variable, Δ). The second set (relative dynamic) used a relative distance index, δ, to examine how climate pushed a site relative to its starting and ending proximity to the climatic niche centroid. We defined:
where mT and mP are the temperature and precipitation components of the vector from the modern climate at a site to the niche centroid (Fig. 2C). This index, δ, approaches zero when a site is located very close to the climatic niche centroid and then is moved by climate change very far away. It approaches one when a site is located far away from the climatic niche and is moved by climate change to the centroid of the niche. A site that does not change distance (i.e., no climate change) from the niche mean would have a value of δ equal to 0.37 (e−1). We tested five different types of relative dynamic models (Table 2): a full model where δT and δP were covariates of both γ and ε, and the four possible combinations of either δT or δP as single covariates of γ and ε.
All eight occupancy models were compared and ranked by AIC weight, which gives an estimate of the weight of evidence from the data in support of a particular model (50). To compare directly among hypotheses, the AIC weight of each model in the model set was calculated. The cumulative weight for all dynamic models (50) was compared with the static and random models.
Acknowledgments.
We thank Andrew Rush, Allison Shultz, Teresa Feo, Pascal Title, Paul Newsam, Nadje Najar, Felix Ratcliff, Andrew Greene, Karen Rowe, and Sara Weinstein for assistance with data collection; Orien Richmond, Jen Wang, Philippe Girard, and three anonymous reviewers for helpful comments that improved earlier drafts of the article; and Michelle Koo and Philip Blumenshine for graphical help. This work, a contribution of the Grinnell Resurvey Project, was supported by National Science Foundation Grant DEB 0640859 and the Yosemite Foundation.
Footnotes
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “Biogeography, Changing Climates and Niche Evolution,” held December 12–13, 2008, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. The complete program and audio files of most presentations are available on the NAS web site at www.nasonline.org/Sackler_Biogeography.
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0901562106/DCSupplemental.
References
- 1.Grinnell J. The niche relationship of the California thrasher. Auk. 1917;34:427–433. [Google Scholar]
- 2.Grinnell J. Field tests of theories concerning distributional control. Am Nat. 1917;51:115–128. [Google Scholar]
- 3.Root TL. Energy constraints on avian distributions and abundances. Ecology. 1988;69:330–339. [Google Scholar]
- 4.Davis AJ, Lawton JH, Shorrocks B, Jenkinson LS. Individualistic species responses invalidate simple physiological models of community dynamics under global environmental change. J Anim Ecol. 1998;67:600–612. [Google Scholar]
- 5.Suttle KB, Thomsen MA, Power ME. Species interactions reverse grassland responses to changing climate. Science. 2007;315:640–642. doi: 10.1126/science.1136401. [DOI] [PubMed] [Google Scholar]
- 6.Broennimann O, et al. Evidence of climatic niche shift during biological invasion. Ecol Lett. 2007;10:701–709. doi: 10.1111/j.1461-0248.2007.01060.x. [DOI] [PubMed] [Google Scholar]
- 7.Holt RD, Keitt TH, Lewis MA, Maurer BA, Taper ML. Theoretical models of species' borders: Single-species approaches. Oikos. 2005;108:18–27. [Google Scholar]
- 8.Brown JH, Stevens GC, Kaufman DM. The geographic range: Size, shape, boundaries, and internal structure. Annu Rev Ecol Syst. 1996;27:597–623. [Google Scholar]
- 9.Peterson AT. Projected climate change effects on Rocky Mountain Great Plains birds: Generalities of biodiversity consequences. Glob Change Biol. 2003;9:647–655. [Google Scholar]
- 10.Graham RW, et al. Spatial response of mammals to late quaternary environmental fluctuations. Science. 1996;272:1601–1606. doi: 10.1126/science.272.5268.1601. [DOI] [PubMed] [Google Scholar]
- 11.Jackson ST, Overpeck JT. Responses of plant populations and communities to environmental changes of the late Quaternary. Paleobiology. 2000;26(Suppl):194–220. [Google Scholar]
- 12.Martinez-Meyer E, Peterson AT, Hargrove WW. Ecological niches as stable distributional constraints on mammal species, with implications for Pleistocene extinctions and climate change projections for biodiversity. Glob Ecol Biogeogr. 2004;13:305–314. [Google Scholar]
- 13.Monahan WB, Hijmans RJ. Ecophysiological constraints shape autumn migratory response to climate change in the North American field sparrow. Biol Lett. 2008;4:595–598. doi: 10.1098/rsbl.2008.0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Root TL, et al. Fingerprints of global warming on wild animals and plants. Nature. 2003;421:57–60. doi: 10.1038/nature01333. [DOI] [PubMed] [Google Scholar]
- 15.Parmesan C, et al. Empirical perspectives on species borders: From traditional biogeography to global change. Oikos. 2005;108:58–75. [Google Scholar]
- 16.Parmesan C, Yohe G. A globally coherent fingerprint of climate change impacts across natural systems. Nature. 2003;421:37–42. doi: 10.1038/nature01286. [DOI] [PubMed] [Google Scholar]
- 17.Moritz C, et al. Impact of a century of climate change on small-mammal communities in Yosemite National Park, USA. Science. 2008;322:261–264. doi: 10.1126/science.1163428. [DOI] [PubMed] [Google Scholar]
- 18.Parmesan C. Ecological and evolutionary responses to recent climate change. Annu Rev Ecol Evol Syst. 2006;37:637–669. [Google Scholar]
- 19.Chase JM, Leibold MA. Ecological Niches: Linking Classical and Contemporary Approaches. Chicago: Univ Chicago Press; 2003. [Google Scholar]
- 20.Hutchinson GE. Concluding remarks. Cold Spring Harbor Symp Quant Biol. 1957;22:415–427. [Google Scholar]
- 21.Wiens JJ, Graham CH. Niche conservatism: Integrating evolution, ecology, and conservation biology. Annu Rev Ecol Evol Syst. 2005;36:519–539. [Google Scholar]
- 22.Peterson AT, Soberon J, Sanchez-Cordero V. Conservatism of ecological niches in evolutionary time. Science. 1999;285:1265–1267. doi: 10.1126/science.285.5431.1265. [DOI] [PubMed] [Google Scholar]
- 23.Soberón J, Peterson AT. Interpretation of models of fundamental ecological niches and species' distributional areas. Biodiversity Informatics. 2005;2:1–10. [Google Scholar]
- 24.Kearney M, Porter WP. Mechanistic niche modeling: Combining physiological and spatial data to predict species' ranges. Ecol Lett. 2009;12:334–350. doi: 10.1111/j.1461-0248.2008.01277.x. [DOI] [PubMed] [Google Scholar]
- 25.Soberon J. Grinnellian and Eltonian niches and geographic distributions of species. Ecol Lett. 2007;10:1115–1123. doi: 10.1111/j.1461-0248.2007.01107.x. [DOI] [PubMed] [Google Scholar]
- 26.Araújo MB, Guisan A. Five (or so) challenges for species distribution modelling. J Biogeogr. 2006;33:1677–1688. [Google Scholar]
- 27.Guisan A, Thuiller W. Predicting species distribution: Offering more than simple habitat models. Ecol Lett. 2005;8:993–1009. doi: 10.1111/j.1461-0248.2005.00792.x. [DOI] [PubMed] [Google Scholar]
- 28.Kearney M. Habitat, environment, and niche: What are we modeling? Oikos. 2006;115:186–191. [Google Scholar]
- 29.Pulliam HR. On the relationship between niche and distribution. Ecol Lett. 2000;3:349–361. [Google Scholar]
- 30.Holt RD, Gaines MS. Analysis of adaptation in heterogenous landscapes: Implications for the evolution of fundamental niches. Evol Ecol. 1992;6:433–447. [Google Scholar]
- 31.Anderson BJ, et al. Dynamics of range margins for metapopulations under climate change. Proc R Soc London Ser B. 2009;276:1415–1420. doi: 10.1098/rspb.2008.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonfils C, et al. Identification of external influences on temperatures in California. Climatic Change. 2008;87:43–55. [Google Scholar]
- 33.Parra JL, Monahan WB. Variability in 20th century climate change reconstructions and its consequences for predicting geographic responses of California mammals. Glob Change Biol. 2008;14:1–17. [Google Scholar]
- 34.Araújo MB, Thuiller W, Pearson RG. Climate warming and the decline of amphibians and reptiles in Europe. J Biogeogr. 2006;33:1712–1728. [Google Scholar]
- 35.MacKenzie DI, et al. Occupancy Estimation and Modeling. Burlington, MA: Academic; 2006. [Google Scholar]
- 36.Araújo MB, Pearson RG, Thuiller W, Erhard M. Validation of species-climate impact models under climate change. Glob Change Biol. 2005;11:1504–1513. [Google Scholar]
- 37.Martinez-Meyer E, Peterson AT. Conservatism of ecological niche characteristics in North American plant species over the Pleistocene-to-recent transition. J Biogeogr. 2006;33:1779–1789. [Google Scholar]
- 38.Taper ML, Bohning-Gaese K, Brown JH. Individualistic responses of bird species to environmental change. Oecologia. 1995;101:478–486. doi: 10.1007/BF00329427. [DOI] [PubMed] [Google Scholar]
- 39.Peters RL, Darling JDS. The greenhouse effect and nature reserves. Bioscience. 1985;35:707–717. [Google Scholar]
- 40.Peterson AT, et al. Future projections for Mexican faunas under global climate change scenarios. Nature. 2002;416:626–629. doi: 10.1038/416626a. [DOI] [PubMed] [Google Scholar]
- 41.Blair RB. Land use and avian species diversity along an urban gradient. Ecol Appl. 1996;6:506–519. [Google Scholar]
- 42.Pachauri RK, Reisinger A, editors. Intergovernmental Panel on Climate Change. Climate Change 2007: Synthesis Report. Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Geneva: Intergovernmental Panel on Climate Change; 2007. p. 104. [Google Scholar]
- 43.Ralph CJ, Droege S, Sauer JR. Managing and monitoring birds using point counts: Standards and applications. In: Ralph CJ, Droege S, Sauer JR, editors. Monitoring Bird Populations by Point Counts. Washington, DC: U.S. Departure of Agriculture Forest Service; 1995. pp. 161–170. General Technical Report PSW-GTR-149. [Google Scholar]
- 44.Wieczorek J, Guo QG, Hijmans RJ. The point-radius method for georeferencing locality descriptions and calculating associated uncertainty. Int J Geogr Inf Sci. 2004;18:745–767. [Google Scholar]
- 45.Daly C, Gibson WP, Taylor GH, Johnson GL, Pasteris P. A knowledge-based approach to the statistical mapping of climate. Climate Res. 2002;22:99–113. [Google Scholar]
- 46.Daly C, Neilson RP, Phillips D. A statistical-topographic model for mapping climatological precipitation over mountainous terrain. J Appl Meteorol. 1994;33:140–158. [Google Scholar]
- 47.Leith H. In: Primary Productivity of the Biosphere. Leith H, Whittaker RH, editors. New York, NY: Springer; 1975. pp. 237–263. [Google Scholar]
- 48.Elith J, et al. Novel methods improve prediction of species' distributions from occurrence data. Ecography. 2006;29:129–151. [Google Scholar]
- 49.Phillips SJ, Anderson RP, Schapire RE. Maximum entropy modeling of species geographic distributions. Ecol Model. 2006;190:231–259. [Google Scholar]
- 50.Burnham KP, Anderson DR. Model Selection and Multimodel Inference. New York: Springer; 2002. [Google Scholar]