Abstract
The rising sea level at the end of the Pleistocene that created the islands of the Sunda Shelf in Indonesia and Malaysia provides a natural experiment in community disassembly and offers insights into the effects of body size and niches on abundance, distribution, and diversity. Since isolation, terrestrial mammal communities of these islands have been reduced by extinction, with virtually no offsetting colonization. We document three empirical patterns of disassembly, all of which are significantly different from null models of random assembly: (i) a diversity–area relationship: the number of taxa is strongly and positively correlated with island area; (ii) nested subset composition: species that occur on small islands tend to be subsets of more diverse communities inhabiting larger islands; and (iii) body size distributions: species of intermediate body sizes occur on the greatest number of islands, and smaller islands have smaller ranges of body sizes, caused by the absence of species of both very large and extremely small size. These patterns reveal the role of body size and other niche characteristics, such as habitat requirements and trophic status, in the differential susceptibility of taxa to extinction.
Keywords: allometric scaling, assembly rules, extinction, habitat fragmentation, nested subsets
Traits of organisms determine the niches of species, and niches of species shape the composition of biota. The roles of traits and associated niches in influencing the abundance, distribution, and diversity of species are reflected in community assembly rules: empirical patterns of distribution and coexistence over space and time. Since Diamond's seminal article (ref. 1 and see also refs. 2–4), especially clear examples of assembly rules have been documented for islands and insular habitats, where breakup of large contiguous environments have resulted in reduced diversity and altered taxonomic composition in the isolated fragments (e.g., refs. 5–7).
Such cases of community disassembly typically show three kinds of nonrandom patterns indicative of underlying roles of niches. First, the fragments usually exhibit strong positive correlations between the number of taxa and habitat area. Such diversity–area relationships reflect the combined effects of total population size and ecological specialization on probability of extinction. Taxa with low abundances and specialized requirements are more susceptible to extinction and survive only in the largest fragments, whereas only the few most abundant generalist taxa persist in the smallest fragments (e.g., refs. 5 and 8–10).
Second, the taxonomic composition of the fragments often shows highly nested subset structure, in which the smaller number of taxa inhabiting successively smaller fragments tends to be subsets of the richer biotas of larger fragments. This pattern implies that each taxon requires some minimal area to support sufficient populations to resist extinction, and that it can occur in all fragments of greater than threshold area (e.g., refs. 6 and 11).
Third, the habitat fragments sometimes show distinctive distributions of body sizes, with successively smaller areas having fewer taxa of extreme size. These distributions imply that body size strongly influences traits of organisms and characteristics of niches, and these in turn influence probability of extinction (12, 13). Indeed, body size is probably the single trait of paramount ecological significance, because most characteristics of organisms vary closely with size (14–17). Allometric equations or power laws of the form Y = Y0Mb describe how a trait, Y, scales with body mass, M, where Y0 is a normalization coefficient and b is the scaling exponent. In mammals, for example, (i) whole-organism metabolic rate or rate of food consumption scales as M3/4 (18, 19); (ii) rates of biomass production and maximum population growth scale as M−1/4 (14, 20, 21); (iii) lifespan, generation time, and fasting endurance time scale as M1/4 (21); and (iv) population density scales as M−3/4 (22). Thermal conductance, home range, speed of locomotion, and minimum and maximum prey size all also exhibit allometric scaling relations.
The nonvolant mammals inhabiting the islands of the continental Sunda Shelf, comprising most of the country of Indonesia and parts of Malaysia, provide an elegant “natural experiment” of the community disassembly process. Beginning at the end of the Pleistocene ≈11,000 years ago, rising sea level caused by global warming and melting of ice sheets created these islands. Before sea-level rise, each island was part of the Asian mainland and had a typical continental mammal fauna. Since being isolated, each island has experienced selective extinctions of native mammal species with little subsequent colonization, because nonvolant mammals are poor over-water dispersers (23). The contemporary taxa inhabiting each island represent an assemblage that persisted and coexisted since the late Pleistocene. Here, we focus on three kinds of empirical assembly rules: diversity–area relationships, nested subset structures, and body size distributions. These are enduring legacies of the niche relationships that have shaped the composition of these biotas since the formation of the islands.
This disassembly process offers general insights into the impact of global climate change on the geographic distributions of species and on the effects of habitat fragmentation on the extinction of species. Characterizing the empirical rules for this case of community disassembly should contribute importantly to informing conservation management and policy in a context of global, regional, and local environmental change. In particular, this case of disassembly caused by natural processes of habitat fragmentation and isolation may hold important lessons for human-caused changes, offering insights into conservation on islands and other fragmented landscapes. Similar rules of disassembly may apply even though the changes in climate and sea level that occurred at the end of the Pleistocene were much greater than have occurred so far in the present episode of global warming.
We compiled a database consisting of a list of species and their body masses for the entire terrestrial, nonvolant mammal fauna of each island of the Sunda Shelf (see Dataset 1 for data). We performed analyses at two levels of taxonomic resolution: species and genus. Paleobiologists often use the genus level of analysis, because congeneric species are often morphologically similar, are difficult to distinguish as fossils, are inferred to have similar ecological niches, and exhibit clear patterns of community composition over evolutionary time. To quantify the community disassembly process, our analyses are based on the following assumptions:
(i) A continental mammal fauna, similar to that currently inhabiting the Southeast Asian mainland, occurred on the Sunda Shelf in the Pleistocene.
(ii) The present fauna of each island was derived from a diverse ancestral species pool by extinction, after isolation of the island by rising sea levels at the end of the Pleistocene. We do not assume, however, that all of the species currently inhabiting the islands and the adjacent Southeast Asian mainland necessarily occurred on each island before sea-level rise. This would be to assume, almost certainly incorrectly, that the Pleistocene geographic ranges of all species encompassed the entire Sunda Shelf. We know, for example, that many species are currently narrowly endemic, restricted to a single island or only a portion of the largest islands, which is consistent with recent studies that suggest that climates and habitats during the Pleistocene were drier, with more savannah and less forest than at present (24, 25). Such temporal and spatial heterogeneity likely restricted the distributions of some taxa, especially forest-dwelling species at higher elevations, before isolation of islands by rising sea level.
(iii) Subsequent establishment of species caused by over-water colonization was so infrequent as to be negligible. Exceptions would be species introduced by humans during the last few centuries, and these have been identified and excluded from the analysis.
(iv) Intraspecific variation in body size among islands, although sometimes sizeable (26), is usually much less than interspecific variation and can therefore be ignored. We used a single estimate of body mass for each species.
(v) The disassembly process has undoubtedly been influenced by humans. Homo sapiens have inhabited the larger islands and probably visited the smaller ones throughout their post-Pleistocene history. Over the last 10,000 years, human impacts have increased because of the effects of population growth, agricultural development, harvesting of natural resources, and habitat modification.
(vi) The data accurately reflect the composition of the fauna of each island. Methods used in the surveys of smaller islands (mostly before 1910) did not use now-standard collection methods, pitfall traps for shrews and other methods for small arboreal species (L. Heaney, personal communication). It is possible, perhaps even likely, therefore, that some small species were not recorded on some islands where they occur. Such false absences would contribute unexplained variation and could systematically bias our estimate of the relationship between minimum body size and island area, and hence our estimate of extinction probability for the smallest species.
Results
Diversity–Area Relationship.
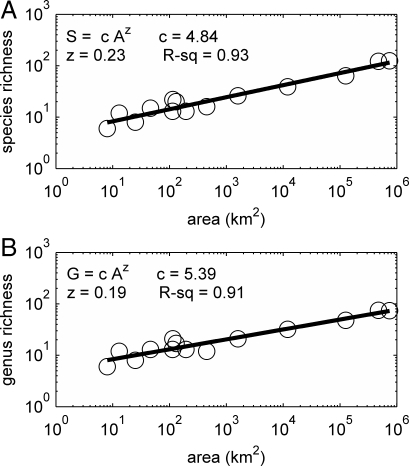
Taxonomic richness at both species and genus levels is strongly related to island area (Fig. 1). Numbers of taxa increase with island area as a power law with an exponent (z-value) of 0.23 for species richness (R2 = 0.93) and 0.19 for genus richness (R2 = 0.91). Number of species varies over an order of magnitude from <10 on the smallest islands in the database to >120 on Borneo and Sumatra. Number of genera varies ≈12-fold, from 6 to 74.
Fig. 1.
Diversity-area relationships for nonvolant, terrestrial mammals of the Sunda Shelf islands for species (A) and genera (B). Lines are ordinary least-squares regressions fitted to log-transformed data. Statistics for the resulting power laws are given.
Nested Subsets.
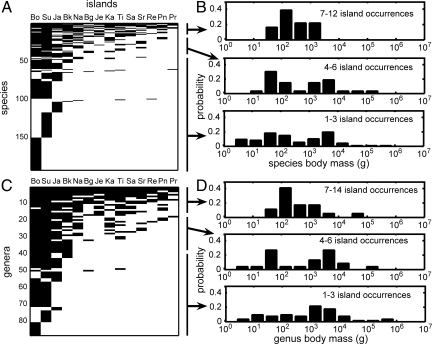
Species composition on the different islands is highly nested at both species and genus levels. The nested subset pattern is apparent when the presence–absence of each species or genus is plotted so that incidence of occurrence is ordered with respect to either species richness or island area (Fig. 2 A and C; note the relative absence of occurrences in the lower right half of the matrices). The “temperature” (T) of such matrices quantifies the departure from perfect nestedness: so that T = 0 corresponds to an “ice crystal” with perfect order, and T = 100 to “boiling water” with complete disorder. The values of T observed, T = 3.83 and 6.56 for species and genera, respectively, reflect highly nonrandom structure (deviations from three null models of random assembly are all highly significant: all P < 0.0001). Because of the strong species–area relationship, nestedness is closely associated with both species richness and island area.
Fig. 2.
The nested subset structure of the faunas of the Sunda Shelf islands as revealed by ordered presence–absence matrixes. (A and C) Presence–absence of taxa was ordered vertically by number of occurrences and horizontally by island area for species (A) and genera (C). (B and D) We plotted the associated body size distributions of species (B) and genera (D) having differing frequencies of islands occurrences. Each island and taxon is labeled by a code that references data in Dataset S1. Bo, Borneo; Su, Sumatra; Ja, Java; Bk, Bangka; Na, Natuna Besar; Bg, Banggi; Je, Jemaja; Ka, Karimata Besar; Ti, Tioman; Sa, Siantan; Sr, Sirhassan; Re, Redang; Pn, Penebangan; Pr, Perhentian Besar.
Associated with nestedness are distinctive distributions of body sizes in relation to the number of island occurrences (Fig. 2 B and D). Species and genera that occur only on a few islands have body sizes that range from very large to very small, whereas those that occur on the largest number of islands and coexist with many other species have a much narrower distribution, mostly in the range of 100 g to 1 kg.
Body Size Distributions.
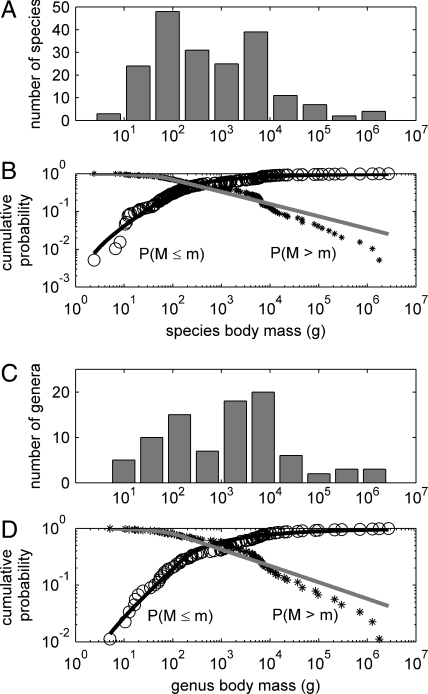
More detailed analyses of body size distributions uncover additional patterns of community assembly. Combining all species or genera for all islands reveals a wide, right-skewed frequency distribution (Fig. 3) with: (i) the smallest species being a shrew of the genus Suncus weighing 2 g and other shrews of the genus Crocidura weighing 6–11 g; (ii) species of diverse taxa clustering around a median size of 341 g; (iii) the most species-rich logarithmic size class ranging between 77 and 200 g, depending on the number or width of bins (10–15 bins); and (iv) the largest species being elephant and rhinoceros weighing >1,700 kg and occurring only on the largest islands of Borneo, Sumatra, and Java. This distribution is probably representative of the distribution of body sizes among species and genera in the source pools present on the shelf before post-Pleistocene isolation and extinction.
Fig. 3.
Frequency distributions of body sizes of species and genera of the Sunda Shelf islands. (A and C) Log-binned histograms for species and genera comprising the entire pool of species occurring on all islands. (B and D) Cumulative frequency distributions for species and genera (circles and stars) and double power law fits to these distributions using maximum-likelihood estimation (bold and gray lines). Note that the cumulative distributions plot the proportion of species larger or smaller than a given body mass, resulting in curves that cross at the median size.
Size–Incidence Relationship.
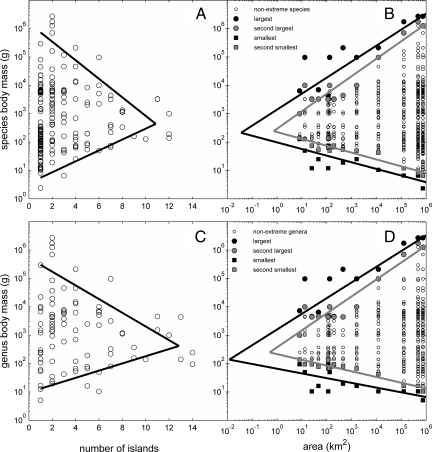
When species and genus body masses are plotted versus their respective frequencies of island occurrences, we find a triangular cloud of points converging to intermediate body sizes at high frequencies of island occurrences (Fig. 4 A and C). More quantitatively, the maximum body size, Mmax, for a given number of island occurrences, I, scales with I as Mmax ∝ e−0.75I (R2 = 0.73) at the species level and as Mmax ∝ e−0.56I (R2 = 0.61) at the genus level. The minimum body size for the species and genus level scales as Mmin ∝ e0.44I (R2 = 0.73) and Mmin ∝ e0.29I (R2 = 0.49), respectively. The scalings of extreme body sizes with the number of island occurrences intersect at intermediate body sizes of 437 and 418 g for species and genera, respectively.
Fig. 4.
Body size–incidence and body size–area relationships for mammals on the Sunda Shelf islands. (A and C) Size–incidence relationship: body mass of species and genera as functions of number of island occurrences. (B and D) Size–area relationships: body mass of species and genera as functions of island area. The bold lines are regression fits to the maximum and minimum body masses of species or genera having a given number of island occurrences (A and C) or of species or genera found on each island (B and D). The gray lines are regression fits to the second-largest and smallest body masses on each island (B and D). Note that with increasing incidence and decreasing area the body size distributions converge toward an intermediate body size in the range of 122 to 454 g.
Size–Area Relationship.
As might be expected from the above patterns, the distributions of body sizes vary systematically with island area and species richness, such that smaller islands have fewer species of extreme size (Fig. 4 B and D). The size of the largest species and second-largest species decreases with decreasing island area as a power law with exponents of 0.56 and 0.62 (R2 = 0.74 and 0.90), respectively. Likewise, the largest and second-largest genera scale with area with exponents of 0.55 and 0.60 (R2 = 0.73 and 0.90), respectively. The smallest and second-smallest body sizes decrease with island area: exponents of −0.23 and −0.24 (R2 = 0.64 and 0.88) and similarly for genera: exponents of −0.17 and −0.20 (R2 = 0.43 and 0.89), respectively. Interestingly, however, the median body masses are relatively invariant with respect to area (e.g., the exponent for species is 0.11, not significantly different from zero, P = 0.057; the exponent for genera is 0.19, P = 0.002). Consequently, extrapolating the scaling relations for these median sizes and for the smallest and largest species and genera gives lines that converge between 102 and 248 g (except for the genus maximum size–area relationship and genus median size–area relationship, which intersect at 53 g).
We evaluated the statistical significance of these scaling relationships by drawing species at random without replacement from the source pool until each island had the empirically observed number of species and repeated this exercise 10,000 times to generate expected null distributions based on random assembly (following ref. 13). These tests reject the null hypotheses for largest and second-largest species (all P ≪ 0.01 for both slopes and intercepts). In contrast, the observed slopes for the smallest and second-smallest species body size scaling relations, −0.23 and −0.24, are not significantly different from the null model based on random sampling (slopes of −0.14 and −0.16, P = 0.1089 and 0.0654 for smallest and second-smallest, respectively). The observed intercepts are, however, significantly higher than the null intercepts, 90.5 and 224.0 g, compared with the null model's 25.6 and 61.8 g (P = 0.0071 and 0.0102).
Discussion
Disassembly Rules and Niches.
The contemporary terrestrial mammal communities of the islands of the Sunda Shelf have been derived by selective extinctions of species. The roles of niches in this disassembly process are indicated by three nonrandom patterns of biodiversity.
Diversity–area relationship.
More species and genera have gone extinct on small islands than large ones, resulting in highly significant power law diversity–area relationships with z-values of 0.23 and 0.19. Using Heaney's (23) species–area relationship for nonisolated areas on the mainland of the Malay Peninsula, we estimate that the largest islands of Borneo, Sumatra, and Java retained the majority (85%, 87%, and 52%, respectively) of their Pleistocene fauna, whereas the five smallest islands have lost ≈75–88% of their species since their isolation. The areas of the islands vary by five orders of magnitude, from 8 to 743,244 km2. Such large reductions in island area can cause decreased diversity not only directly through reductions in total abundance of widespread generalist species, but also indirectly through the complete loss of certain habitat types required by specialized species. For example, the large islands have large mountains with diverse high-elevation habitats and unique resources that are completely absent on the smallest islands. So the effect of island area on diversity reflects the combined effects of abundance, specialization, and other niche attributes on extinction.
Nested subset composition.
There is a strong tendency for species and genera inhabiting smaller islands with lower diversity to be subsets of taxa occurring on larger islands with more diverse faunas. This means that each taxon has a relatively deterministic threshold area requirement. Although it is possible a posteriori to estimate a minimal island size for each species and genus from Fig. 4 B and D, we cannot say what sets these thresholds without additional detailed information on the niches of the species and the ecology of the islands. Supertramps make up one group of taxa that are conspicuous deviations from nested subset structures (1). Examples of supertramps are bird species that are excellent over-water dispersers and tend to occur only on small islands with few other species. The absence of supertramp mammals is consistent with our assumption that the mammal faunas of the Sunda Shelf islands are derived primarily by extinction with minimal over-water dispersal.
Body size distributions.
Distributions of body sizes on the islands reveal two interrelated patterns. There is a triangular body size–incidence relationship (Fig. 4 A and C). The taxa that occur on only one or a few islands include the entire range of body sizes, from shrews weighing <10 g to elephants and rhinos weighing >1,000 kg. In contrast, the taxa that occur on the largest number of islands span a much narrower range, from 100 g to 1 kg. There is a complementary triangular body size–area relationship (Fig. 4 B and D). The entire range of body sizes is represented on the largest islands, but the range becomes progressively more restricted on smaller islands. This trend is reflected in a significant decrease in the sizes of the largest and second-largest taxa and a consistent increase in the smallest and second-smallest taxa with decreasing island area. Interestingly, the median or modal size remains largely invariant with island area. The triangles in Fig. 4 depicting the body size–incidence and body size–area relations are closely coincident, converging to a similarly narrow range of 140 to 440 g for the highest incidence species and the smallest islands. We interpret these patterns to mean that a few species and genera in this special size range have generalist niches and high abundances, and these attributes have allowed the taxa to have the lowest extinction rates and hence to persist in the smallest areas and on the largest number of islands.
For the Sunda Shelf island fauna, the ecologies of the high incidence species and genera reveal the importance of niche breadth, habitat, and diet in the disassembly process. The 10 species with the highest number of island occurrences were Maxomys surifer, Callosciurus notatus, Galeopterus variegates, Macaca fascicularis, Tragulus javanicus, Rattus tiomanicus, Tupaia glis, Ratufa affinis, Petaurista petaurista, and Nycticebus coucang. Six of these are arboreal (two are gliders), two are likely semiarboreal, and only two are surface-dwelling. At least seven have omnivorous diets. It is not surprising that arboreal, omnivorous mammals have low probabilities of extinction on islands where the predominant habitat is tropical forest. It is also not surprising that dietary specialists, especially carnivores, have lower incidences reflecting higher extinction probabilities and presumably lower population densities (see ref. 27).
Allometric Relationships.
The body size–area relationship documented here for the restricted case of the Sunda Shelf islands is strikingly similar to that reported by Marquet and Taper (13) for mammals on continents, islands of the Sea of Cortéz, and American Southwest mountaintops. They observed that maximum body size decreases and minimum body size increases with decreasing landmass area, converging on a mass of 70–200 g on the smallest islands with only one terrestrial mammal species. This pattern can be readily understood from the perspective of allometry. In general, smaller mammals have lower resource requirements and thus can maintain higher population densities with lower probabilities of extinction. There is evidence, however, that the highest population densities and rates of population growth occur at intermediate body sizes of ≈100 g, rather than at the very smallest sizes (12, 28). A similar and undoubtedly related pattern is Foster's rule, the tendency of large mammals to evolve dwarf forms and small mammals to evolve giant forms when isolated on islands (29). So, there appear to be some traits unique to some intermediate-sized mammals that are reflected in their niches and expressed in high abundances, wide distributions, and evolutionary trajectories. Interestingly, the effect of body size on niche characteristics results in very different assembly rules for insular and continental mammal faunas. Local communities in nonisolated habitats on continents do not show the loss of extreme sizes and convergence toward an intermediate size as seen on islands; instead, the local communities tend to exhibit virtually the entire range of sizes found in the continent-wide pool (30, 31).
The scaling of ecologically relevant traits with body size provides a basis for exploring the disassembly rules more quantitatively and mechanistically. Here, we use the empirical ecological scaling relations reported above (see Introduction), to suggest more explicit mechanisms linking these traits, extinction probability, and body size–area relationships.
Maximum body size and extinction probability.
The data show that the maximum body mass on an island (Mmax) increases with increasing area as approximately Mmax ∝ A1/2. If we assume: (i) average species population density or number of individuals per unit area, K, scales as K ∝ M−3/4 (ref. 22; also shown for minimum viable populations of threatened and endangered species in ref. 32); (ii) island geographic ranges, G, of large species are proportional to island area, so the total island population size N ∝ GK ∝ AK ∝ AM−3/4; and (iii) for a given extinction probability, the minimum viable population size, Nmin, does not scale with mass, so Nmin ∝ M0 (as supported by a meta-analysis in ref. 33), then the minimum area required to sustain a large species, which occurs at Nmin, would scale as Amin ∝ Nmin Mmax3/4 and conversely Mmax would scale as Mmax ∝ A4/3. Note that Marquet and Taper (13) derived a similar model, using the scaling of home range area as M1, instead of population density as M−3/4, so their model predicts Mmax ∝ A1. Both predictions are significantly different from the observed A0.56. This suggests either that: (i) population densities on these islands scale not as K ∝ M−3/4, but as K ∝ MXK, where XK < −3/4; (ii) the geographic ranges of species do not include the entire areas of islands and scale sublinearly with island area, so G ∝ AXG with XG < 1; (iii) minimum viable population size increases with increasing body size, so Nmin ∝ MXN, where XN > 0; or (iv) some combination of these nonmutually exclusive possibilities, such that Mmax ∝ AXG/(XN−XK) and XG/(XN − XK) ≈ 1/2. We cannot evaluate these factors definitively, but we suspect that some combination of at least i and ii play a role, so that total population size scales less than linearly with island area and population density scales more steeply than M−3/4. These particular scaling relations could be caused in part by human impacts, including both hunting and habitat modification (e.g., forest clearing) in differentially reducing both local abundance and within-island distributions of the larger mammals.
The data show that the maximum body mass on an island (Mmax) increases with increasing area as approximately Mmax ∝ A1/2. If we assume: (i) average species population density or number of individuals per unit area, K, scales as K ∝ M−3/4 (ref. 22; also shown for minimum viable populations of threatened and endangered species in ref. 32); (ii) island geographic ranges, G, of large species are proportional to island area, so the total island population size N ∝ GK ∝ AK ∝ AM−3/4; and (iii) for a given extinction probability, the minimum viable population size, Nmin, does not scale with mass, so Nmin ∝ M0 (as supported by a meta-analysis in ref. 33), then the minimum area required to sustain a large species, which occurs at Nmin, would scale as Amin ∝ Nmin Mmax3/4 and conversely Mmax would scale as Mmax ∝ A4/3. Note that Marquet and Taper (13) derived a similar model, using the scaling of home range area as M1, instead of population density as M−3/4, so their model predicts Mmax ∝ A1. Both predictions are significantly different from the observed A0.56. This suggests either that: (i) population densities on these islands scale not as K ∝ M−3/4, but as K ∝ MXK, where XK < −3/4; (ii) the geographic ranges of species do not include the entire areas of islands and scale sublinearly with island area, so G ∝ AXG with XG < 1; (iii) minimum viable population size increases with increasing body size, so Nmin ∝ MXN, where XN > 0; or (iv) some combination of these nonmutually exclusive possibilities, such that Mmax ∝ AXG/(XN−XK) and XG/(XN − XK) ≈ 1/2. We cannot evaluate these factors definitively, but we suspect that some combination of at least i and ii play a role, so that total population size scales less than linearly with island area and population density scales more steeply than M−3/4. These particular scaling relations could be caused in part by human impacts, including both hunting and habitat modification (e.g., forest clearing) in differentially reducing both local abundance and within-island distributions of the larger mammals.
Minimum body size and extinction probability.
In small mammals weighing <100 g, Silva and Downing (28) found K ∝ M0.25. Assuming that in small mammals XG = 1 and XN = 0, then Amin should scale as Amin ∝ Mmin3/−1/4 and Mmin as Mmin ∝ A−4. The data presented above suggest that minimum body size increases with decreasing island area at a much slower rate, as approximately Mmin ∝ A−1/4, so that the minimum island area required by small species apparently increases very steeply with decreasing mass as approximately Amin ∝ Mmin3/−4. There are problems estimating this scaling relation and its statistical confidence intervals because of the relatively small number of species of extremely small size and the possibility of false absences (see above). Nevertheless, this natural experiment in community disassembly on the islands is consistent with mounting evidence that the smallest mammals are subject to powerful metabolic constraints. Their high mass-specific metabolic rates and associated requirements for food, space, and other environmental conditions place severe limitations on their life history traits, abundances, and niche breadths, which may explain their larger than expected minimum island area requirements.
The analyses and figures presented here quantify disassembly rules for mammals of the Sunda Shelf islands. Rising sea levels at the end of the Pleistocene fragmented the once continuous Southeast Asian landmass and created islands of varying size. Niche characteristics related to body size, habitat requirements, and trophic status determine minimum island areas required for persistence of species and genera. Selective extinctions of species are related to island area and body size, resulting in the distinctive body size–incidence and body size–area relationships. The patterns of disassembly on the Sunda Shelf are generally similar to those documented for other isolated landmasses. The role of niche attributes in conferring resistance to extinction and the resulting persistence of medium-sized taxa on very small islands have important implications for conservation, because habitat fragmentation is a major cause of endangerment.
Methods
We started by constructing a species list for the entire recent terrestrial, nonvolant mammal fauna of 14 well-surveyed islands. For all islands except Borneo, we used the species lists assembled by Heaney (23, 34), which we updated by incorporating more recent data on occurrences and taxonomy from Wilson and Reeder (35). For Borneo, we used Payne et al. (36) and Wilson and Reeder (35). Our list for each island includes both extant species and species that went extinct during historic times because of human impacts and excludes human-introduced species.
We constructed a database of body masses, using a single estimated value for each species. For the vast majority of species, we used the mean body mass or the average of male and female mean body masses from the “Macroecological database of mammalian body mass”, MOM Version 3.6.1 (37). For most of the species not found in MOM, we used the midpoints of the body mass range given in Walker's Mammals of the World (38). Additional data were compiled from Payne et al. (36), Alderton (39), and Wilson and Reeder (35). For the few species for which we were unable to find any published measurement of body mass, we used either: (i) the geometric mean of the body masses of extant congeneric species, because body sizes of congeners tend to be very similar (40) or (ii) when masses of congeneric species could not be found, taxon-specific allometries to estimate body mass from available body length data (following ref. 41). For genus-level analyses we used the geometric mean body masses of the congeneric species in our database.
Ideally we would have used direct data on the body mass of each population on each island, but such data are simply not available. Fortunately, the effects of insular body size evolution are small and intraspecific variation among islands is usually less than interspecific variation. L. Heaney (personal communication) suggests that intraspecific variability is at most comparable to that documented for the tri-colored squirrel (26). This idea is similar to the finding of Lomolino (29) that extant insular populations deviate from their closest relatives on continents by at most a factor of 2–3 or ± 0.50 log units. Given the 106 range in body masses across all species and the 102 to 103 range in extreme body masses, we are confident that our results are robust to the small effect of insular body size evolution.
We ordered the species and genus presence–absence matrices by area and number of island occurrences. The nested subset temperature of each matrix was calculated by using the algorithm and program of Rodriguez-Girones and Santamaria (ref. 42, but see ref. 43), an improvement over the original, seminal works of Patterson and Atmar (6, 44). We calculated P values based on three different null models, including the original null model of Atmar and Patterson (44). The calculations were based on 10,000 randomized matrices.
We used ordinary least-squares regression on log-transformed data to characterize diversity–area, size–area, and size–incidence relationships. All regression models had approximately normally distributed residuals.
We developed a null model similar to Marquet and Taper's (13) to evaluate the null hypothesis that the scaling of extreme body masses can be accounted for simply by randomly sampling from the source pool consisting of all species inhabiting Sunda Shelf islands. For each island, we drew at random and without replacement the number of species occurring on the island and recorded their associated body masses. Expected null values of scaling exponents and normalization coefficients were estimated by fitting regressions to these data. This procedure was repeated 10,000 times, producing expected distributions of parameters for the null model, and allowing one-sided tests of the null hypothesis with associated P values.
Acknowledgments.
We thank L. R. Heaney for help and advice; A. Clauset, E. B. Erhardt, M. E. Ritchie, D. Storch, W. Zuo, Program in Interdisciplinary Biological and Biomedical Sciences students, and members of J.H.B.'s and F.A. Smith lab groups at the University of New Mexico for valuable feedback; and an anonymous reviewer for helpful suggestions. J.G.O. was supported by a graduate training grant funded by the Howard Hughes Medical Institute under the Howard Hughes Medical Institute–National Institute of Biomedical Imaging Interfaces Initiative.
Footnotes
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “Biogeography, Changing Climates and Niche Evolution,” held December 12–13, 2008, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. The complete program and audio files of most presentations are available on the NAS web site at www.nasonline.org/Sackler_Biogeography.
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0901654106/DCSupplemental.
References
- 1.Diamond JM. Assembly of species communities. In: Cody ML, Diamond JM, editors. Ecology and Evolution of Communities. Cambridge, MA: Belknap; 1975. pp. 342–444. [Google Scholar]
- 2.Diamond JM. Distributional ecology of New Guinea birds: Recent ecological and biogeographical theories can be tested on the bird communities of New Guinea. Science. 1973;179:759–769. doi: 10.1126/science.179.4075.759. [DOI] [PubMed] [Google Scholar]
- 3.Grant PR. Ecological compatibility of bird species on islands. Am Nat. 1966;100:451–462. [Google Scholar]
- 4.MacArthur RH, Diamond JM, Karr JR. Density compensation in island faunas. Ecology. 1972;53:330–342. [Google Scholar]
- 5.Brown JH. Mammals on mountaintops: Nonequilibrium insular biogeography. Am Nat. 1971;105:467–478. [Google Scholar]
- 6.Patterson BD, Atmar W. Nested subsets and the structure of insular mammalian faunas and archipelagos. Biol J Linn Soc. 1986;28:65–82. [Google Scholar]
- 7.Lomolino MV, Riddle BR, Brown JH. Biogeography. Sunderland, MA: Sinauer; 2006. [Google Scholar]
- 8.Post DM, Pace ML, Hairston NG., Jr Ecosystem size determines food-chain length in lakes. Nature. 2000;405:1047–1049. doi: 10.1038/35016565. [DOI] [PubMed] [Google Scholar]
- 9.MacArthur RH, Wilson EO. An equilibrium theory of insular zoogeography. Evolution. 1963;17:373–387. [Google Scholar]
- 10.Diamond JM. Comparison of faunal equilibrium turnover rates on a tropical island and a temperate island. Proc Natl Acad Sci USA. 1971;68:2742–2745. doi: 10.1073/pnas.68.11.2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.May RM. The evolution of ecological systems. Sci Am. 1978;239:161–175. [Google Scholar]
- 12.Brown JH. Macroecology. Chicago: Univ Chicago Press; 1995. [Google Scholar]
- 13.Marquet PA, Taper ML. On size and area: Patterns of mammalian body size extremes across landmasses. Evol Ecol. 1998;12:127–139. [Google Scholar]
- 14.Peters RH. The Ecological Implications of Body Size. Cambridge, UK: Cambridge Univ Press; 1983. [Google Scholar]
- 15.Calder WA. Size, Function, and Life History. Mineola, NY: Courier Dover Publications; 1996. [Google Scholar]
- 16.McMahon TA, Bonner JT. On Size and Life. New York: Scientific American Library; 1983. [Google Scholar]
- 17.Brown JH, Gillooly JF, Allen AP, Savage VM, West GB. Toward a metabolic theory of ecology. Ecology. 2004;85:1771–1789. [Google Scholar]
- 18.Kleiber M. Body size and metabolism. Hilgardia. 1932;6:315–353. [Google Scholar]
- 19.Brody S, Proctor RC. Growth and development with special reference to domestic animals: Relation between basal metabolism and mature body weight in different species of mammals and birds. Univ Mo Agric Exp Station Res Bull. 1932;166:89–101. [Google Scholar]
- 20.Fenchel T. Intrinsic rate of natural increase: The relationship with body size. Oecologia. 1974;14:317–326. doi: 10.1007/BF00384576. [DOI] [PubMed] [Google Scholar]
- 21.Blueweiss L, et al. Relationships between body size and some life history parameters. Oecologia. 1978;37:257–272. doi: 10.1007/BF00344996. [DOI] [PubMed] [Google Scholar]
- 22.Damuth J. Population density and body size in mammals. Nature. 1981;290:699–700. [Google Scholar]
- 23.Heaney LR. Mammalian species richness on islands on the Sunda Shelf, Southeast Asia. Oecologia. 1984;61:11–17. doi: 10.1007/BF00379083. [DOI] [PubMed] [Google Scholar]
- 24.Bird MI, Taylor D, Hunt C. Palaeoenvironments of insular Southeast Asia during the Last Glacial Period: A savanna corridor in Sundaland? Q Sci Rev. 2005;24:2228–2242. [Google Scholar]
- 25.Meijaard E. Mammals of south-east Asian islands and their Late Pleistocene environments. J Biogeogr. 2003;30:1245–1257. [Google Scholar]
- 26.Heaney LR. Island area and body size of insular mammals: Evidence from the tri-colored squirrel (Callosciurus prevosti) of Southeast Asia. Evolution. 1978;32:29–44. doi: 10.1111/j.1558-5646.1978.tb01096.x. [DOI] [PubMed] [Google Scholar]
- 27.Burness GP, Diamond J, Flannery T. Dinosaurs, dragons, and dwarfs: The evolution of maximal body size. Proc Natl Acad Sci USA. 2001;98:14518–14523. doi: 10.1073/pnas.251548698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silva M, Downing JA. The allometric scaling of density and body mass: A nonlinear relationship for terrestrial mammals. Am Nat. 1995;145:704–727. [Google Scholar]
- 29.Lomolino MV. Body size evolution in insular vertebrates: Generality of the island rule. J Biogeogr. 2005;32:1683–1699. [Google Scholar]
- 30.Brown JH, Nicoletto PF. Spatial scaling of species composition: Body masses of North American land mammals. American Naturalist. 1991;138:1478–1512. [Google Scholar]
- 31.Marquet PA, Cofre H. Large temporal and spatial scales in the structure of mammalian assemblages in South America: A macroecological approach. Oikos. 1999;85:299. [Google Scholar]
- 32.Silva M, Downing JA. Allometric scaling of minimal mammal densities. Conserv Biol. 1994;8:732–743. [Google Scholar]
- 33.Traill LW, Bradshaw CJA, Brook BW. Minimum viable population size: A meta-analysis of 30 years of published estimates. Biol Conserv. 2007;139:159–166. [Google Scholar]
- 34.Heaney LR. Biogeography of mammals in SE Asia: Estimates of rates of colonization, extinction, and speciation. Biol J Linn Soc. 1986;28:127–165. [Google Scholar]
- 35.Wilson DE, Reeder DAM. Mammal Species of the World: A Taxonomic and Geographic Reference. 3rd Ed. Baltimore: Johns Hopkins Univ Press; 2005. [Google Scholar]
- 36.Payne J, Francis CM, Phillipps K. A Field Guide to the Mammals of Borneo. Kuala Lumpur, Malaysia: World Wildlife Fund; 1985. [Google Scholar]
- 37.Smith FA, et al. Body mass of late quaternary mammals. Ecology. 2003;84:3403. [Google Scholar]
- 38.Nowak RM. Walker's Mammals of the World. Baltimore: Johns Hopkins Univ Press; 1999. [Google Scholar]
- 39.Alderton D. Wild Cats of the World. United Kingdom: Blandford; 1998. [Google Scholar]
- 40.Smith FA, et al. Similarity of mammalian body size across the taxonomic hierarchy and across space and time. Am Nat. 2004;163:672–691. doi: 10.1086/382898. [DOI] [PubMed] [Google Scholar]
- 41.Silva M. Allometric scaling of body length: Elastic or geometric similarity in mammalian design. J Mammal. 1998;79:20–32. [Google Scholar]
- 42.Rodriguez-Girones MA, Santamaria L. A new algorithm to calculate the nestedness temperature of presence-absence matrices. J Biogeogr. 2006;33:924–935. [Google Scholar]
- 43.Ulrich W, Almeida-Neto M, Gotelli NJ. A consumer's guide to nestedness analysis. Oikos. 2009;118:3–17. [Google Scholar]
- 44.Atmar W, Patterson BD. The measure of order and disorder in the distribution of species in fragmented habitat. Oecologia. 1993;96:373–382. doi: 10.1007/BF00317508. [DOI] [PubMed] [Google Scholar]